Abstract
The molecular mechanisms governing acquired tumor resistance during radiotherapy remain to be elucidated. In breast cancer patients, overexpression of HER2 (human epidermal growth factor receptor 2) is correlated with aggressive tumor growth and increased recurrence. In the present study, we demonstrate that HER2 expression can be induced by radiation in breast cancer cells with a low basal level of HER2. Furthermore, HER2-postive tumors occur at a much higher frequency in recurrent invasive breast cancer (59%) compared to the primary tumors (41%). Interestingly, NF-κB is required for radiation-induced HER2 transactivation. HER2 was found to be co-activated with basal and radiation-induced NF-κB activity in radioresistant but not radiosensitive breast cancer cell lines after long-term radiation exposure, indicating that NF-κB-mediated HER2 overexpression is involved in radiation-induced repopulation in heterogeneous tumors. Finally, we found that inhibition of HER2 resensitizes the resistant cell lines to radiation. Since HER2 is shown to activate NF-κB, our data suggest a loop-like HER2-NF-κB-HER2 pathway in radiation-induced adaptive resistance in breast cancer cells.
INTRODUCTION
In the clinic, radiation therapy is a powerful anti-cancer modality. Recent data suggest that radiation-induced stress response and gene expression with an adaptive resistance may severely compromise the effectiveness of radiation (1). Although radiation-induced genomic instability and by-stander effects are known to modulate cell radiosensitivity (2), specific signaling networks causing the adaptive radio-resistance in tumor cells remain to be elucidated. In mammalian cells, different protein expression patterns can be induced by ionizing radiation, suggesting that the fate of an irradiated cell may be controlled by a specific survival signaling network (3, 4). A tumor may consist of several specific cell subpopulations that may respond differently to therapeutic irradiation (5). Long-term observations of irradiated cell populations reveal a variety of cell fates (6). This wide inconsistency in radiosensitivity of a given tumor cell population suggests that heterogeneity in the signal transduction response could represent a mechanism for the development of adaptive tumor resistance.
HER2 belongs to the EGFR (epidermal growth factor receptor) family and plays an important role in cell proliferation through homodimerization or formation of heterodimers with EGFR and HER3 (7). Although HER2 overexpression, either through gene amplification or dysregulation, has been identified in many other human cancers, about 30% of human breast cancers overexpress HER2 (8). In addition to HER2-mediated cell transformation (9), the HER2-induced tumor aggressive phenotype has been linked with enhanced activity of proliferative signaling (10, 11), EGFR induction, cell cycle checkpoint dysregulation (12), and ubiquitination-mediated p53 degradation (13). HER2 is being studied extensively as a therapeutic target (14, 15). However, to further improve HER2-targeted therapy, it is essential to determine whether HER2 is inducible by anti-cancer modalities and whether the induced HER2 overexpression is responsible for acquired radioresistance. The stress-responsive transcription factor NF-κB is activated by a variety of cytotoxic conditions through phosphorylation of the inhibitor by IκB kinase (IKK) (16, 17), which is believed to be a critical factor in enhancing cell survival after irradiation (18–22). NF-κB activation through the PI3K/Akt pathway is also a major downstream event of HER2 overexpression (7,23, 24). Although NF-κB activation can decrease cellular radiosensitivity (21,25), the exact correlation between NF-κB activation and HER2 overexpression in tumor adaptive radioresistance is unclear.
We have studied breast cancer cell lines, mouse xenograft tumors, and recurrent invasive breast cancers, and we have found that radiation-induced HER2 up-regulation by NF-κB regulation is causally linked to the adaptive radioresistance. NF-κB-mediated HER2 overexpression is tightly associated with the heterogeneous pattern of radioresistance detected in the surviving cells of breast cancer cell lines treated with long-term fractionated γ radiation. NF-κB inhibitor (IMD-0354), NF-κB p65 siRNA, or HER2 siRNA inhibits HER2 overexpression and reverses the radioresistant phenotype of the radioresistant cell lines. Our results suggest a potential approach to prevent and resensitize therapy-resistant breast tumors by targeting NF-κB/HER2 pathways.
METHODS AND MATERIALS
Cell Culture
Cells of the human breast cancer cell lines MCF-7 and MDA-MB-231 were purchased from ATCC (Manassas, VA). MCF+FIR cells were obtained as described previously (21, 24). Cells of the MCF-7 cell line stably transfected with HER2 (MCF-7/HER2) were kindly provided by Dr. D. J. Slamon (University of California Los Angeles). MCF-7, MCF+FIR and MCF-7/HER2 cells were maintained in Eagle’s minimum essential medium (EMEM) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT), 5% sodium pyruvate, 5% non-essential amino acid, penicillin (100 U/ml), and streptomycin (100 µg/ml) in a humidified incubator (95% air/5% CO2) at 37°C. MDA-MB-231 cells were maintained in EMEM medium supplemented with 10% FBS, 5% nonessential amino acid, 1% penicillin and 1% streptomycin.
Derivation of the Heterogeneous MDA+FIR Cell Population
To establish a MDA-MB-231 cell population that survives a long-term therapeutic fractionated irradiation, we followed the in vitro irradiation procedure described previously (21). The radiation treatment was started with cells cultured in T-75 flasks (1 × 107 cells) with a total dose of 30 Gy γ rays (1 Gy per fraction, five times per week for 6 weeks). Parental MDA-MB-231 cells were treated with the same procedure except that they were sham-irradiated and passaged with the irradiated cells as sham-irradiated controls. Both irradiated-treated and sham-irradiated control MDA-MB-231 cells were passaged every 7 days before the 10th irradiation and were passaged every 10 days after the 10th irradiation (fewer MDA-MB-231 cells were plated to achieve a similar passage number for further experiments). Cells were fed with fresh MDA-MB-231 cell medium every 3 days. Two weeks after the final irradiation, a group of cell clones isolated from the irradiated MDA-MB-231 cell population (MDA+FIR) were cultured individually and passaged in the same medium, and all experiments were performed during 4 to 10 passages after the establishment of individual clones. All irradiations were conducted at room temperature using a GR-12 irradiator to expose cells to 60Co γ rays (dose rate, 2.3 Gy/min; U.S. Nuclear Corp., Burbank, CA).
Real-Time PCR
Total RNAs were prepared from cultured cells using Trizol Reagent (Invitrogen, Carlsbad, CA). Total RNA (2 µg) was reverse-transcribed to cDNA using an AMV reverse transcriptase kit (Promega, Madison, WI). Real-time PCR was performed using the BioRad My IQ real-time PCR system and the BioRad SYBR Green supermix following the manufacturer’s protocol (BioRad, Hercules, CA). The quantitative RT-PCR primers were as follows: HER2, 5′ GGAGAACCCCGAGTACTTGAC 3′ (sense) and 5′ GTTCTCTGCCGTAGGTGTCC 3′ (antisense); GAPDH, 5′ GGACTCATGACCACA GTCCAT 3′ (sense) and 5′ GTTCAGCTC AGGGATGACCTT 3′ (antisense). The cycle conditions for the PCR were one cycle of 3 min at 95°C, 45 cycles of 30 s at 95°C, 30 s at the 60°C, and 30 s at 72°C. Data were expressed as arbitrary units.
Western Blotting
Total cell lysates (20 µg) were separated by SDS-PAGE and blotted onto PVDF membranes. Each membrane was incubated with specific primary antibody overnight at 4°C followed by the horseradish peroxidase-conjugated secondary antibody and then visualized using the ECL Western blotting detection system (Amersham, Arlington Heights, IL). HER2 antibody (MS-730-P) was purchased from Lab Vision Corporation (Fremont, CA); p65 and β-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA) and Sigma Chemical Co. (St. Louis, MO), respectively.
Irradiation of Mouse Xenograft Tumor
A standard cell inoculation was used to generate mouse breast cancer xenograft tumors with MDA-MB-231 cells following the protocol approved by the committee for animal research at Purdue University. At the age of 7 weeks, mice were injected with 5 × 106 MDA-MB-231 cells in the axilla. Mice were euthanized before the tumor volume reached 3500 mm3. Three weeks after the last cell inoculation, the average size of the MDA-MB-231 xenograft is 2300 mm3. The mice were divided into two groups: one received sham-irradiated and the other received γ rays (2 Gy/day, dose rate: 2.3 Gy/min, for 3 times, total dose 6 Gy) from the GR-12 irradiator. The mice were anesthetized by injection of ketamine before irradiation. Twenty-four hours after the last radiation treatment, mice were killed humanely and tumor tissues were prepared for different experiments.
Immunohistochemistry
Formalin-fixed paraffin-embedded xenograft tumor sections were deparaffined in xylene twice for 5 min each, incubated in 100% ethanol for 5 min, and then hydrated by placing in 90, 80 and 70% ethanol for 5 min each. After the slides were washed with PBST (PBS with 0.1% Tween-20) for 5 min, they were incubated with permeabilization solution (0.2% Triton X-100 in PBS) for 15 min at room temperature, followed by three washes with PBST. Then the slides were blocked with 2% BSA in PBST for 1 h at room temperature followed by three washes. The slides were incubated with anti-mouse HER2 primary antibody overnight at 4°C followed by three washes and then incubation with the secondary anti-mouse antibody conjugated with Texas Red (Jackson Immuno-Research Laboratories, West Grove, PA) for 1 h at room temperature. The slides were then incubated with DAPI (300 nM in PBS) for 5 min at room temperature followed by two washes and then were sealed and analyzed with a Nikon microscope (Eclipse, E1000M).
Analysis of HER2 Expression in Recurrent Breast Cancers
The pathology database was searched for invasive breast cancer, and cases with clinical follow-up were retrieved after obtaining Institutional Review Board approval (Emory University IRB#: 901–2004). Formalin-fixed, paraffin-embedded tissue sections (5 µM) were prepared. Immunohistochemical staining was performed using the Herceptest kit (DakoCytomation) after heat-induced epitope retrieval. Moderate to strong membrane staining in more than 10% of the tumor cells was considered positive for HER2 expression. Fluorescence in situ hybridization (FISH) was performed using a PathVysion kit (Vysis, Inc.) according to the manufacturer’s instructions. A centromere 17:HER2 probe signal ratio of more than 2.2 obtained from 30 interphase nuclei was considered positive for HER2 gene amplification.
Chromatin Immunoprecipitation (ChIP) Assay
MCF-7 and MDA-MB-231 cells were cultured for 24 h before treatment with γ rays or NF-κB inhibitors (2 µM IMD-0354 for 5 h or mutant IκBα transient transfection), and soluble chromatin was crosslinked with 1% formaldehyde at 37°C for 10 min. Cell extracts were prepared and sonicated to obtain DNA fragments with sizes between 0.2 and 0.7 kb. Protein-DNA complexes were immunoprecipitated using anti-p65 (5 µg, sc-372; Santa Cruz), anti-p50 (5 µg, 06-886; Upstate), anti-p52 (5 µg, sc-848X; Santa Cruz), and anti-c-Rel (5 µg, sc-1827X; Santa Cruz) antibodies or IgG control, respectively. DNA was purified and used for PCR with primers specific for the gene promoter region encompassing the NF-κB binding site. The following primers were used: HER2 (A), 5′ GAG TGG CAGCCTAGGGAATTTACT 3′ (forward) and 5′ TATACTTCCTCAAGCAGCCCTCC 3′ (reverse); IκBα, 5′ GTAGCACCCATTAG AAACACTTC (forward) 3′ and 5′ TTCTTGTTCACTG ACTTCCCAA TA 3′ (reverse); GAPDH, 5′ GGACTCATGACCACAGTCCAT 3′ (forward) and 5′ GTTCAGCTCAGGGATGACCTT 3′ (reverse); B (the sequence around 1.4 kb upward of NF-κB binding site), 5′ AGGCCCCTGTTTCTCAACTCCCTA 3′ (forward) and 5′ GTATAGCTGCATTCTT GGCTGGGG 3′ (reverse).
Transfection and Luciferase Assay
Plasmid containing the HER2 promoter region (pGL2-basic-HER2) was a gift from Dr. Mien-Chie Hung at the University of Texas M.D. Anderson Cancer Center. The pGL2-basic-HER2-ΔNF-κB, in which the NF-κB binding site (gggacgaccc; −364 to −355) was deleted from the HER2 promoter region, was amplified by PCR with Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA) with the primer sequences: 5′ GGCGTCCCGGCGCTAGGAAGGCCTGCGCAGAGAG 3′ (sense) and 5′ CTC TCTTCGCGCAGGCCTTCCTAGCGCCGGGACGCC 3′ (anti-sense). Transfection of pGL2-basic-HER2 or pGL2-basic-HER2-ΔNF-κB luciferase reporter plasmid was described previously (26). Luciferase activity was measured with Luminometer (Promega, Madison, WI). For normalization of the reporter transfection efficiency, the total protein concentration of lysates was measured with the BCA Protein Assay kit (Pierce, Rockford, IL) with BSA as the standard.
IMD-0354 and Mutant IκB Treatment
IMD-0354 (Sigma), an efficient IKKβ inhibitor that inhibits the activation of NF-κB through IκBα phosphorylation (27), was used to block NF-κB activation (2 µM for 5 h incubation). The mutant IκBα (S32A, S36A) conjugated plasmid was used to transiently inhibit NF-κB activation by retaining NF-κB in the cytoplasm. Cells grown in complete medium with or without IMD-0354 incubation or mutant IκBα transfection were sham-irradiated or exposed to 5 Gy γ rays. NF-κB or HER2 activity and radiation sensitivity were measured by a luciferase assay and clonogenic survival assays, respectively. Western blotting was performed to measure the expression of HER2 with or without treatment.
siRNA-Mediated Target Gene Inhibition
siRNA targeting HER2 or p65 mRNAs was designed and synthesized with the Silencer siRNA Construction Kit (Ambion, Austin, TX). The primers used to synthesize the siRNAs were as follows: HER2, 5′ AACAGGTAGGTCAGTTCCAGGCCTGTCTC 3′ (sense) and 5′ AACCTGGAACTCACC TACCTGCCTGTCTC 3′ (antisense); p65, 5′ AAGGTGGGAAACTCATCATAGCCTGTCTC 3′ (sense) and 5′ AACTATGATG AGTTTCCCACCCCTGTCTC 3′ (antisense). Cells were seeded to achieve 30–50% confluence on the day of transfection. Transient transfection of siRNA was performed using Lipofectamine™ RNAiMAX reagent (Invitrogen). In brief, cells were seeded in 35-mm plates and cultured in antibiotic-free medium for 24 h and then were transfected with various concentrations of siRNAs. Scrambled RNA Duplex (Ambion, Austin, TX) served as the control. HER2 expression in transiently transfected cells was analyzed by Western blotting 60 h after transfection. All transfectants were maintained in antibiotic-free complete medium until collection for further analysis.
Clonogenic Survival Assay
Standard radiation clonogenic survival assays were performed as described previously (24) after exposure to various doses of γ rays. Different numbers of cells were seeded in 60-mm dishes after treatments with IMD-0354 (2 µM for 5 h incubation), p65 siRNA (20 nM for 60 h), or HER2 siRNA (20 nM for 60 h) with or without radiation. Each treatment was performed in three dishes, and all experiments were repeated in triplicate. The treated and control cells were cultured for 14 days, and colonies with more than 50 cells were scored and normalized to the plating efficiency of each cell line.
Statistics
The significance of differences between groups was analyzed using the two-tailed Student’s t test.
RESULTS
HER2 Expression in Irradiated Cells and in Recurrent Invasive Breast Cancers
Figure 1A–C shows that HER2 transactivation detected by mRNA levels was enhanced in breast cancer MCF-7 (Fig. 1A) and MDA-MB-231 (Fig. 1B) cells 24 h after exposure to a single dose of 5 Gy. The MCF-7/HER2 transfectants that were induced to overexpress HER2 by stable gene transfection (24) did not show a similar endogenous HER2 gene activation (Fig. 1C), indicating that breast cancer cells low in or lacking HER2 expression are sensitive to radiation-mediated HER2 gene activation. Consistent with this result, 5 Gy of γ rays significantly induced HER2 protein levels in MCF-7 and MDA-MB-231 cells, which normally express low levels of HER2, but not in the positive control MCF-7/HER2 cells exposed to radiation (Fig. 1D). In addition, compared to the wild type MCF-7 cells, the MCF+FIR cells (MCF-7 cells surviving a long-term fractionated irradiation) (21) showed increased HER2 protein levels (Fig. 1E) that was similar to that of MCF-7/HER2 cells. HER2 induction was further confirmed in in vivo irradiated mouse xenograft tumors of MDA-MB-231 cells treated with radiation (3 × 2 Gy γ rays separated by 24 h). Immunohistochemistry (Fig. 1F; Supplementary Fig. S1) and Western blotting (Fig. 1G) analysis of HER2 revealed an obvious HER2 induction in irradiated tumors compared to the sham-irradiated control tumor. We then studied HER2 expression rate in primary (102 cases) and recurrent invasive (78 cases) tumors from a group of clinically diagnosed breast cancer patients (total cases 180). As shown in Fig. 1H, HER2-postive tumors detected by immunohistochemistry were detected more frequently in the recurrent tumors (59%, 46/78) than in the primary (41%, 42/102) breast cancers. There was no difference in HER2 gene amplification in primary (33%, 48/144) and recurrent (34%, 23/67) tumors detected by the standard FISH analysis that is based on the HER2 gene copy numbers. Collectively, these results suggest that HER2 overexpression is linked with adaptive tumor resistance.
FIG. 1.
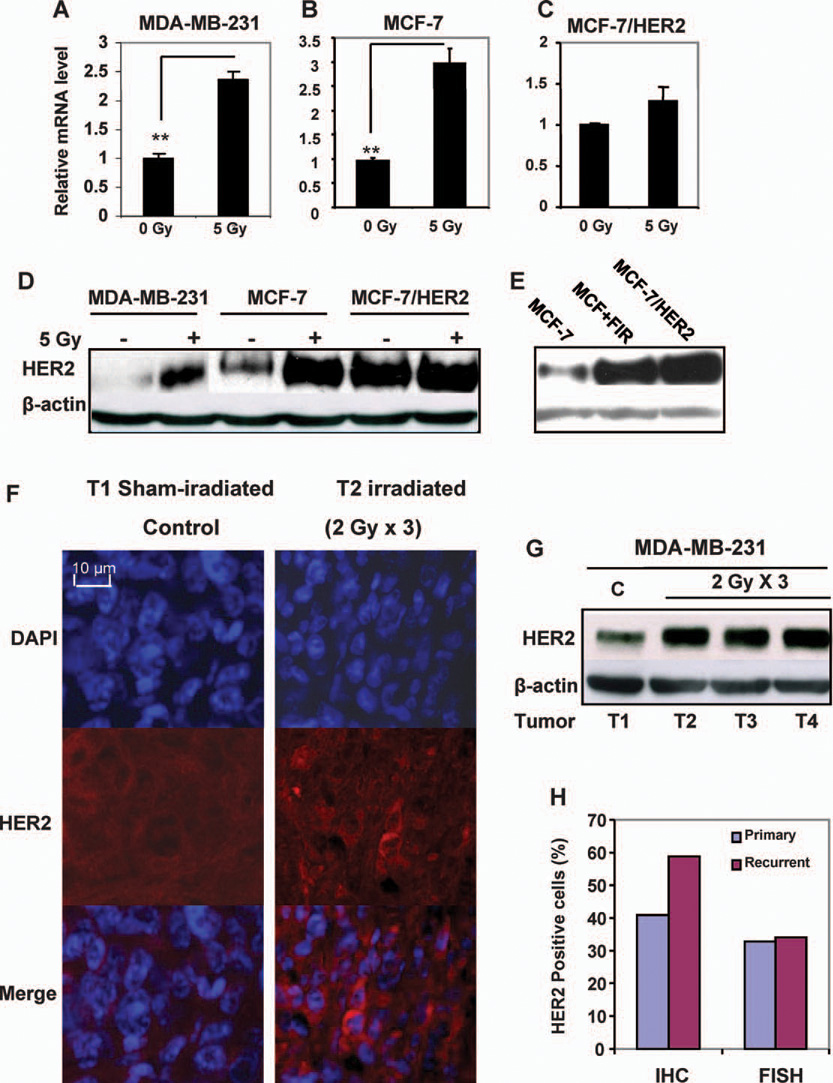
HER2 expression was induced in breast cancer cells by γ radiation. Panels A–C: HER2 mRNA levels were increased in MDA-MB-231 (panel A) and MCF-7 (panel B) breast cancer cells but not in MCF-7/HER2 cells (panel C). Total RNA purified from cells 24 h after exposure to 5 Gy of γ rays (n = 3; mean ± SE; **P < 0.01). Panels D and E: HER2 protein levels were enhanced in irradiated (5 Gy γ rays) MDA-MB-231 and MCF-7 cells (panel D) as well as in the radioresistant population that survived long-term fractionated irradiation (MCF+FIR; panel E) (21) but not in HER2-overexpressing MCF-7/HER2 cells measured by Western blot analysis. Panel F: HER2 expression was induced in irradiated MDA-MB-231 xenograft tumors (3 × 2 Gy; total tumor dose 6 Gy) detected by HER2 immunohistochemistry (red) with DAPI nuclear staining (blue) 24 h after irradiation (T1 = sham-irradiated control; additional HER2 immunohistochemistry data can be found in Supplementary Fig. S1). Panel G: Western blot of HER2 in MDA-MB-231 control (C) and irradiated xenografts 24 h after irradiation. Panel H: Increased frequency of HER2-positive breast cancer cells in human recurrent invasive breast cancers compared to primary tumors analyzed by immunohistochemistry (IHC) and FISH.
Radiation Enhanced the Recruitment of NF-κB to HER2 Promoter
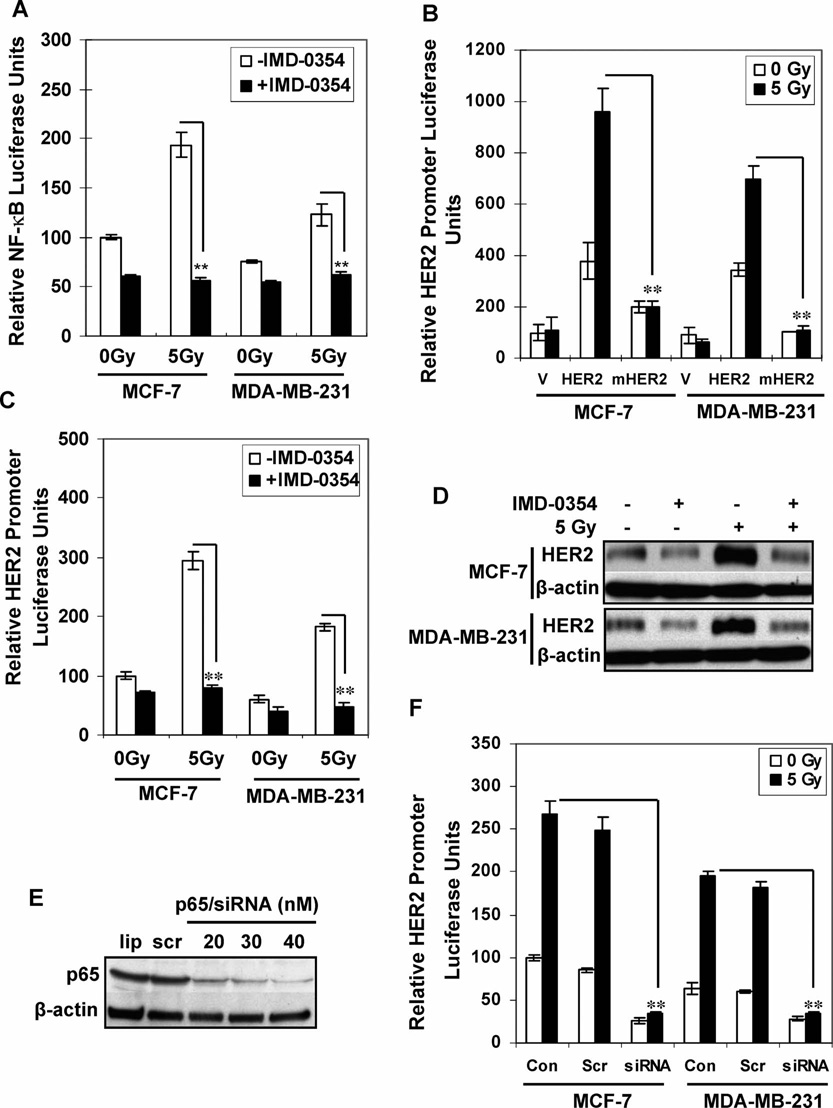
NF-κB plays a central role in radiation resistance (28,29). To determine whether radiation-induced NF-κB regulates HER2 expression, we analyzed the HER2 promoter region by searching the Transcription Element Search System (TESS) database. The NF-κB binding site (gggacgaccc; −364 to −355) was found in the human HER2 promoter region (30). We then designed two fragments of HER2 promoter for ChIP analysis (A and B in Fig. 2A). Fragment A was from −494 to −200 encompassing the NF-κB binding site (−364 to −355), and fragment B was located 1.4 kb upward of A without NF-κB binding site as a negative control. The ChIP results (Fig. 2B) revealed that p65 and p50, which form the major NF-κB complex, were detected in fragment A but not fragment B of the HER2 promoter. The other NF-κB subunits p52 and c-Rel were negligible in the remainder of the MCF-7 and MDA-MB-231 cells. The recruitment of p65 and p50 but not p52 and c-Rel to the HER2 promoter was significantly enhanced 16 h after exposure to 5 Gy. In contrast to another NF-κB-regulated promoter, radiation enhanced the recruitment of all the NF-κB subunits to IκBα promoter, a known NF-κB target gene (31). These results suggest that the p65/p50 complex of NF-κB (32) is specifically involved in radiation-induced HER2 overexpression. Recruitment of p65/p50 to the HER2 promoter was also confirmed in MDA-MB-231 xenograft tumors irradiated in vivo using the IκBα promoter as the positive control (Fig. 2C). Figure 2D shows that inhibition of NF-κB by transfection of mutant IκBα (mIκBα) or treatment with IMD-0354 (2 µM, 5 h), a selective IKKβ inhibitor (27), dramatically reduced radiation-induced recruitment of p65/p50 to the promoters of HER2 and the control IκBα. Figure 2E shows the estimated values of reduced NF-κB binding to the promoters of HER2 and IκBα. Thus the NF-κB p65/p50 complex is specifically required for radiation-induced HER2 transactivation.
FIG. 2.
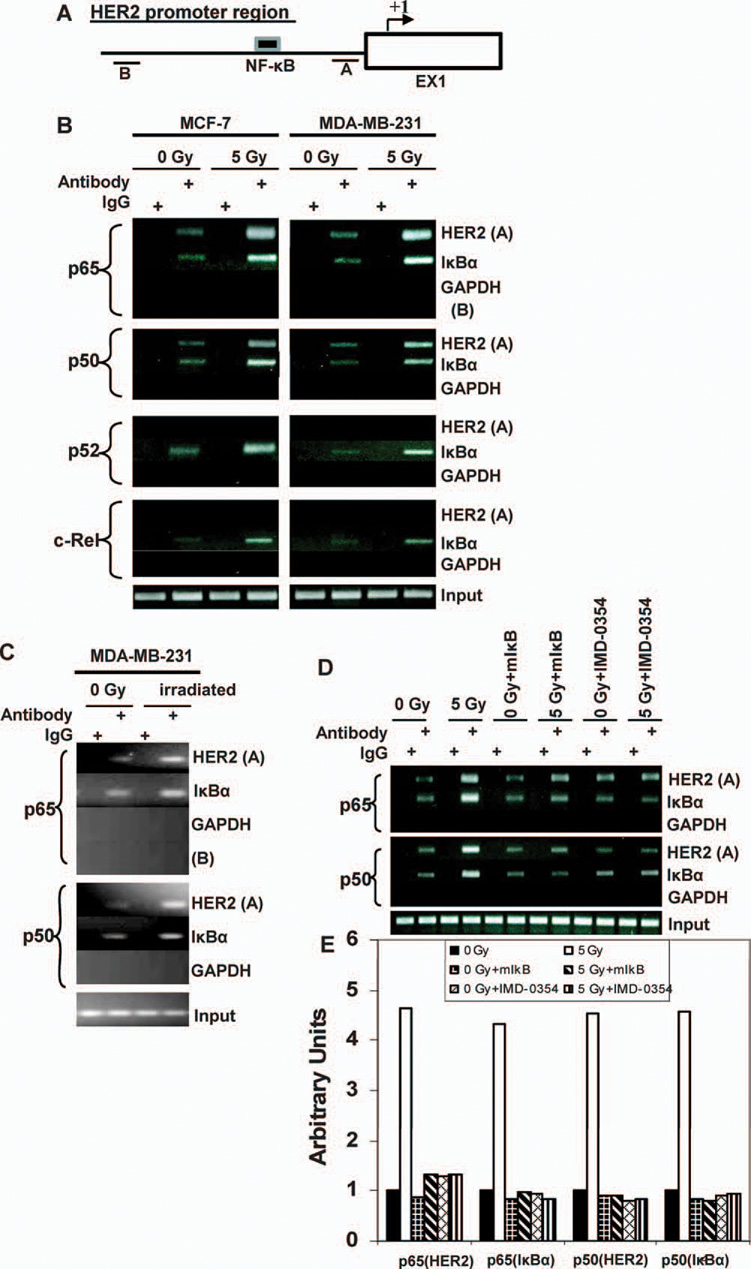
Gamma-radiation exposure enhanced the recruitment of NF-κB to HER2 promoter. Panel A: Schematic of the HER2 promoter locus of two fragments (A and B) studied by ChIP assays. Panel B: Chromatin of control and irradiated MCF-7 and MDA-MB-231 cells was immunoprecipitated with antibodies to p65, p50, p52 and c-Rel or mouse IgG. Both fragments A and B were amplified by PCR, and total chromatin (total input) was positive control for PCR. The PCR fragment of the IκBα promoter region (−1134/−902) and GAPDH were also included as positive and negative controls, respectively. Panel C: Sham-irradiated and γ-irradiated MDA-MB-231 xenografts were examined for recruitment of p65 and p50 to the HER2 promoter (fragment A) and IκBα promoter (NF-κB positive control) by ChIP assays. Fragment B l was included as the negative control. Panel D: MCF-7 cells were preincubated with 2 µM NF-κB inhibitor, IMD-0354 or DMSO (as solvent control) for 5 h or were transiently transfected with mutant IκBα before exposure to 5 Gy or sham irradiation. Recruitment of p65 and p50 to the HER2 promoter (fragment A) and IκBα promoter (NF-κB positive control) were examined by ChIP assays. Panel E: The reduction of binding of NF-κB to the HER2 and IκBα promoters by different inhibitors was estimated by densitometry normalized to the input band.
NF-κB Activation is Required for Radiation-Induced HER2 Overexpression
MCF-7 and MDA-MB-231 cells transfected with NF-κB luciferase reporters were treated with 2 µM IMD-0354 for 5 h before irradiation with 5 Gy. IMD-0354 dramatically reduced both basal and radiation-induced NF-κB activity (Fig. 3A). Compared to wild-type HER2 promoter activity, radiation-induced luciferase activity of pGL2-enhancer-HER2-ΔNF-κB (with a deleted NF-κB binding site) was absent (Fig. 3B). Figure 3C shows that IMD-0354 reduced both basal and radiation-induced HER2 promoter activity, and, consistent with the inhibited reporter activity, HER2 expression was reduced by IMD-0354 (Fig. 3D). NF-κB inhibition by p65 siRNA (20–40 nM) significantly blocked p65 expression (Fig. 3E), and in turn, p65 siRNA effectively inhibited radiation-induced HER2 promoter transactivation (Fig. 3F). Inhibition of NF-κB by mutant mIκB transfection also reduced radiation-induced HER2 protein expression (Supplementary Fig. S2).
FIG. 3.
NF-κB is required for radiation-induced HER2 expression. Panel A: MCF-7 and MDA-MB-231 cells transfected with NF-κB-driven luciferase reporters were treated with IMD-0354 (2 µM) or DMSO for 5 h before exposure to 5 Gy or sham irradiation. Luciferase activities were determined 24 h after irradiation (n = 3; mean ± SE; **P < 0.01). Results are shown as the percentage of the value for untreated MCF-7 cells. Panel B: pGL2 luciferase plasmid (V), HER2-controlled luciferase reporter (HER2), or HER2-controlled luciferase reporter with deleted NF-κB binding site (mHER2) was transfected into MCF-7 and MDA-MB-231 cells. Luciferase activity was measured 24 h after exposure to 5 Gy or sham irradiation (n = 3; mean ± SE; **P < 0.01). Panels C and D: MCF-7 and MDA-MB-231 cells transfected with HER2 luciferase reporters were treated with IMD-0354 (2 µM) or DMSO for 5 h before exposure to 5 Gy or sham irradiation. Luciferase activities were determined 24 h after irradiation (panel C; n = 3; mean ± SE; **P < 0.01). Panel D: Western blot analysis of HER2 levels 24 h after irradiation. Panels E and F: Western blot analysis of MCF-7 cells treated with scramble siRNA or p65 siRNA for 60 h (panel E; lip = transfectants reagent only; scr = 20 nM scrambled siRNA; siRNA = p65 siRNA), and luciferase activity was measured 24 h after irradiation. Results are shown as a percentage of the value for untreated MCF-7 cells (panel F; n = 3; mean ± SE; **P < 0.01).
NF-κB-Mediated HER2 Overexpression is Linked to Radioresistance
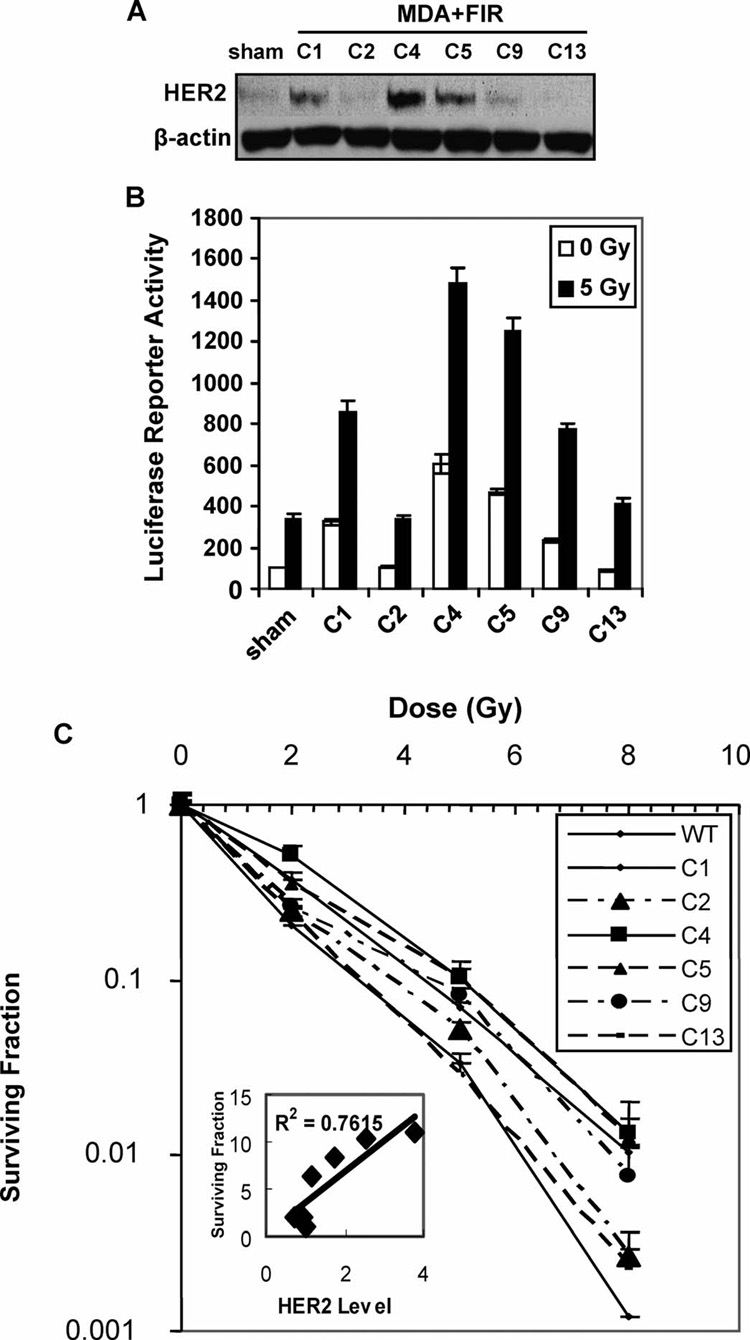
Tumor repopulation after fractionated irradiation has been linked to the failure of treatment by radiotherapy (1) due to the heterogeneous radiosensitivity among cancer cells (33). To determine whether HER2 overexpression is associated with the radioresistance in the heterogeneous cell population, we tested HER2 expression in a breast cancer cell population (MDA+FIR) derived from MDA-MB-231 cells after long-term fractionated γ-ray treatments (1 Gy/day for 30 fractions, total dose 30 Gy). A striking difference in HER2 expression levels was detected among the cloned cell lines isolated from the MDA+FIR cell population (C1, C2, C4, C5, C9 and C13) (Fig. 4A). Both the basal and radiation-induced NF-κB activities were elevated predominantly in the cell lines with higher HER2 expression (Fig. 4B). Furthermore, an enhanced clonogenic survival of the cloned cell line was tightly correlated to NF-κB activity and HER2 protein levels (Fig. 4C). The dose-modifying factor (DMF) at 10% isosurvival was 1.0 for C13, 1.1 for C2, 1.3 for C1 and C9, and 1.7 for C4 and C5, which was significantly increased with the activation of the NF-κB/HER2 pathway. Two of six MDA+FIR cell lines (C2 and C13) showed no induction of HER2 expression and demonstrated similar radiosensitivity to the sham-irradiated parental MDA-MB-231 cells as measured by clonogenic survival. Four MDA+FIR cell lines (C1, C4, C5 and C9) showed an enhanced HER2 expression with a significantly higher survival rate.
FIG. 4.
NF-κB-mediated HER2 expression is associated with the survival of different MDA-MB-231 cell populations after radiation exposure. Panel A: Western blot of HER2 expression in sham-irradiated MDA-MB-231 cells (sham) and in cells of six cloned cell lines isolated from the MDA+FIR cell population. Panel B: NF-κB transactivation of sham-irradiated MDA-MB-231 cells (sham) and in cells of six cloned MDA+FIR cell lines with or without exposure to 5 Gy γ rays. The luciferase activities are shown as a percentage of the value for sham-irradiated cells. Panel C: Clonogenic survival of sham-irradiated MDA-MB-231 cells (sham) and cells of six cloned MDA+FIR cell lines irradiated with of γ rays (inset: correlation between HER2 protein levels and clonogenicity of MDA+FIR cell lines; n = 3; mean ± SE).
Radiosensitization by NF-κB/HER2 Inhibition
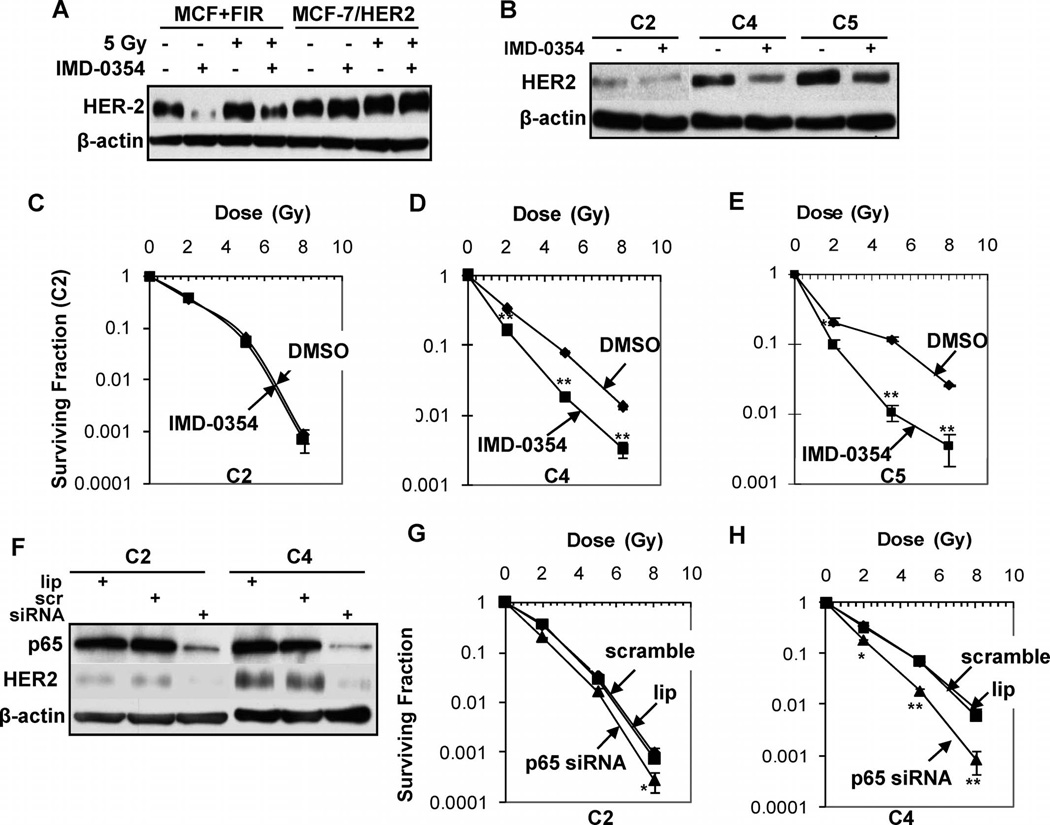
IMD-0354 inhibited the expression of HER2 in MCF+FIR cells, but relatively less inhibition was observed in MCF-7/HER2 cells (Fig. 5A), confirming that the radiation-induced endogenous HER2 expression was controlled by NF-κB. IMD-0354 efficiently reduced the overexpression of HER2 in the radioresistant cell lines C4 and C5 but not the radiosensitive C2 cells (Fig. 5B). Consistent with the HER2 reduction, IMD-0354 reduced the clonogenicity of MCF+FIR but not MCF-7/HER2 cells (Supplementary Fig. S3). In addition, pretreatment with IMD-0354 significantly inhibited the clonogenicity of radioresistant HER2-overexpressing MDA+FIR cell lines C4 and C5 but did not affect survival of the relatively radiosensitive C2 cells (Fig. 5C–E). To inhibit NF-κB directly, we designed and tested a human p65 siRNA that effectively blocked its target p65 as well as HER2 expression in the C2 and C5 MDA+FIR cells (Fig. 5F). p65 siRNA-mediated HER2 inhibition significantly radiosensitized the C4 cells with high HER2 expression (Fig. 5G) and, in contrast, did not affect the radiosensitivity of the relatively radiosensitive C2 cells with low HER2 expression (Fig. 5H). These results demonstrate that NF-κB is a potential therapeutic target for radiosensitization of HER2-overexpressing breast cancer cells.
FIG. 5.
Radiosensitization of HER2-overexpressing cells by NF-κB inhibition. Panels A and B: Inhibition of HER2 overexpression by IMD-0354 (2 µM for 5 h) in MCF+IR and radioresistant (C4, C5) cells but not in MCF-7/HER2 cells and relatively radiosensitive (C2) MDA+IR cells. Panels C–E: Clonogenic survival of cells of MDA+IR cell lines C2 (panel C), C4 (panel D), and C5 (panel E) pretreated with the NF-κB inhibitor IMD-0354 (2 µM, 5 h) or DMSO before radiation exposure (n = 3; mean ± SE; **P < 0.01 compared to DMSO-treated cells). Panel F: Inhibition of HER2 expression in cells of the radioresistant MDA+FIR cell lines C2 and C4 by NF-κB p65 siRNA. Panels G and H: Clonogenic survival of cells of the radioresistant MDA+FIR cell lines C2 (panel G) and C4 (panel H) treated with NF-κB p65 siRNA (20 nM for 60 h before irradiation; n = 3; mean ± SE; **P < 0.01).
Radiosensitization by HER2 siRNA
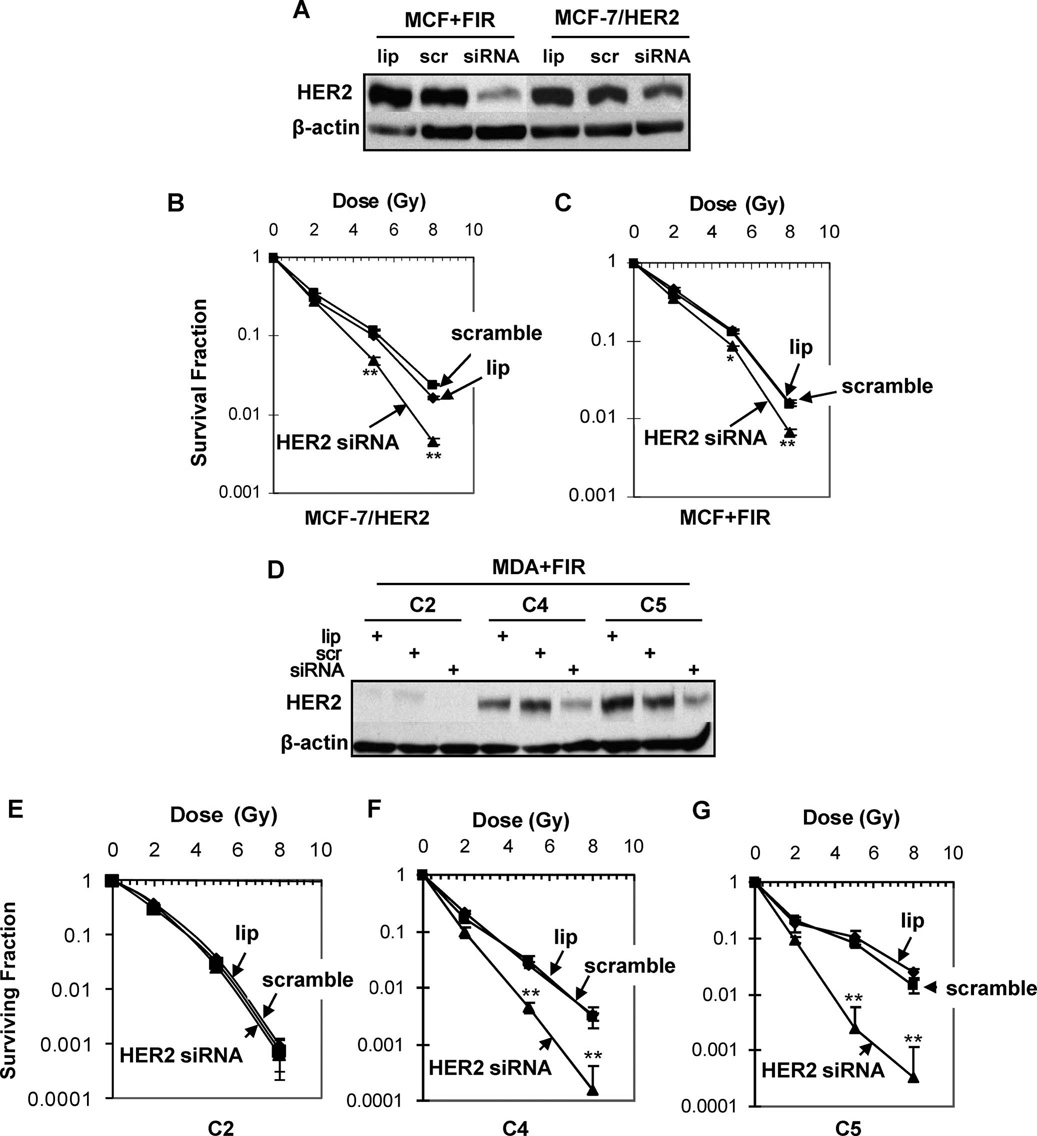
To further explore the radiosensitization induced by inhibiting the NF-κB/HER2 pathway, we determined whether direct inhibition of HER2 overexpression by siRNA can sensitize the radioresistant MDA+FIR cells. We first tested and confirmed the efficiency of a HER2 siRNA fragment in breast cancer SKBr3 cells that showed a high endogenous HER2 expression level (data not shown). A similar efficiency of HER2 inhibition was detected in MCF+FIR and MCF-7/HER2 cells treated with 20 nM of HER2 siRNA for 60 h (Fig. 6A). Radiosensitivity (as determined by clonogenic survival) was enhanced by siRNA-mediated HER2 inhibition in both MCF-7/HER2 (Fig. 6B) and MCF+FIR cells (Fig. 6C). Treatment with 20 nM of HER2 siRNA for 60 h inhibited the HER2 overexpression in the radioresistant cell lines C4 and C5 but not in the relatively radiosensitive cell line C2 (Fig. 6D). However, the radiosensitization was much greater in the radioresistant HER2-overexpressing cell lines C4 and C5 compared to the HER2-low-expressing C2 cells (which showed almost no sensitization) and the heterogeneous MCF+FIR cells (which showed very limited radiosensitization) (Fig. 6E–G). Thus targeting HER2 may be an effective mechanism to resensitize radioresistant breast cancer cells expressing high levels of HER2.
FIG. 6.
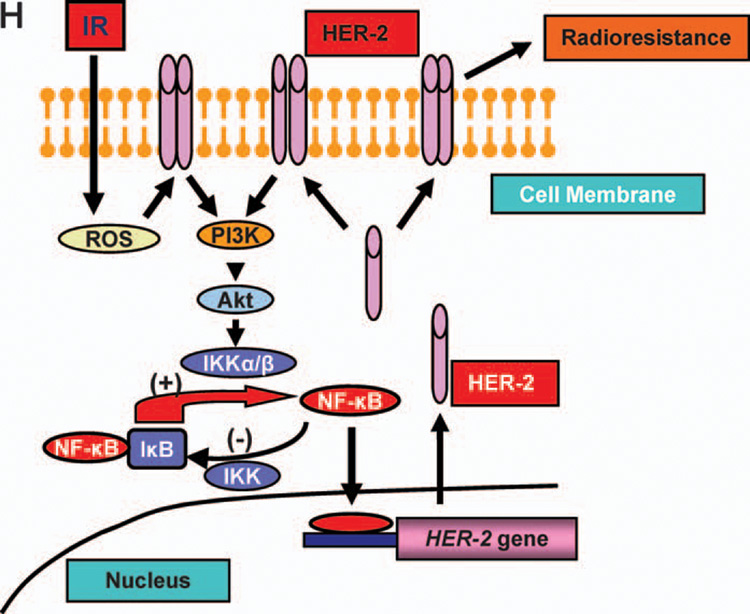
Radiosensitization by HER2 siRNA. Panel A: Inhibition of HER2 expression in MCF+IR and MCF-7/HER2 cells by 20 nM HER2 siRNA (lip =transfection reagent; scr =20 nM scrambled siRNA; siRNA = HER2 siRNA). Panels B and C: Clonogenic survival of MCF-7/HER2 (panel B) and MCF+IR (panel C) cells treated with HER2 siRNA (20 nM for 60 h) and then irradiated (points, mean; n = 3; bars, SE; **P < 0.01, *P < 0.05 compared to the scramble siRNA control). Panel D: Inhibition of HER2 expression in radioresistant (C4, C5) and radiosensitive (C2) MDA+FIR cells treated with HER2 siRNA or scrambled siRNA (20 nM for 60 h). Panels E–G: Clonogenic survival of radiosensitive C2 (panel E) and radioresistant C4 (panel F) and C5 (panel G) cells treated with HER2 siRNA (20 nM for 60 h before irradiation; n = 3; mean ± SE; **P < 0.01). Panel H: Schematic representation of radiation-induced loop-like HER2-NF-κB-HER2 pathway in radiation-induced adaptive resistance. IR, radiation.
DISCUSSION
Consistent with the data reported previously on adaptive radioresistance (33), Fig. 4 shows a heterogeneous radioresistant population in MDA-MB-231 breast cancer cells that survived fractionated doses of radiation. These results support the concept that tumor radiation response is linked with a specific subpopulation of cells, perhaps having the phenotype of cancer stem cells (44). Tumor repopulation has been linked with adaptive resistance (1), and HER2-positive breast cancer patients show a higher rate of recurrence after combined treatment with surgery and radiation (34). Our present data raise a critical concern about adaptive radioresistance in breast cancer patients who receive radiotherapy treatments to reduce the risk of local recurrence after conservative surgery (35).
Our biopsy data (Fig. 1H) suggest that HER2-positive breast cancer cells are detected more frequently in breast cancer patients with recurrent invasive tumors than in the primary tumors. Interestingly, the incidence of the HER2 gene amplification detected by FISH does not show any difference when recurrent invasive tumors are compared with primary tumors (Fig. 1H). These results suggest that HER2 gene copy number may not be a critical factor contributing to recurrence and metastases. In contrast, the enhanced HER2 protein levels detected by immunohistochemistry analysis suggest that HER2 transcriptional activation may be a key factor in the development of HER2-mediated resistance to therapy and recurrence. Thus we speculate that HER2 gene transactivation rather than HER2 gene copy number is related to tumor heterogeneity or the radioresistant phenotype of stem/progenitor cells. Breast cancer cells expressing the CSC marker CD44 (CD44+) but not CD24 (CD24−/low) are more tumorigenic (36); this is further supported by recent data indicating that CSCs are linked with treatment failure and tumor recurrence (37,38). Bao et al.reported that glioma stem cells are able to promote radioresistance by enhancing DNA damage repair (39) and Phillips et al. indicated that CD44+/CD24−/low cancer-initiating cells are more radioresistant (40). These new findings shed light on the conceptual paradigm of how cancer stem cells or cancer-initiating cells contribute to acquired radioresistance. Further clinical study is warranted to confirm the radioresistant phenotype and CSC-mediated tumor repopulation.
It is well known that HER2 overexpression is associated with aggressive tumor growth and poor prognosis of breast cancer (41). The present study on irradiated breast cancer cells and tumor xenografts as well as biopsy tissues from recurrent breast cancers reveals that NF-κB-mediated HER2 overexpression may play an essential role in the development of adaptive radioresistance and recurrence. Our results suggest that HER2 is a DNA-damage effector gene that plays a role in the pro-survival signaling network. Interestingly, NF-κB-mediated HER2 overexpression was found to be tightly correlated with the survival of heterogeneous cell lines subjected to long-term fractionated radiation treatment (Fig. 4). The heterogeneous radioresistance in the MDA+FIR cell population established in this study demonstrates new evidence of tumor repopulation during and/or after radiation treatment. Our data imply that NF-κB-mediated HER2 overexpression may increase the chance for breast cancer cells to escape the lethal effect of fractionated doses of radiation. In addition, the striking heterogeneity observed in the surviving MDA-MB-231 populations may suggest the following two possibilities: (1) Radiation induces genomic instability that specifically activates the NF-κB/HER2 pathway to enhance cell survival; (2) radiation selects the radioresistant stem/progenitor cells with a high background NF-κB/HER2 activity, causing radiation-induced tumor repopulation due to enhanced overall clonogenic survival. Either way, the NF-κB/HER2 activity-mediated advantage in cell survival should be the focus of further investigation.
A recent clinical report suggests that radiotherapy during breast maturation (such as radiotherapy for Hodgkin’s lymphoma or other pediatric solid tumors) can be a risk factor for the development of HER2-positive breast carcinomas (42). Our observations after in vivo irradiation of breast cancer cells provide the evidence that HER2 overexpression can be induced in HER2-low or -absent breast cancer cells through radiation-induced NF-κB activation. Cao et al. showed that IκBα is required for HER2 gene activation in carcinogen-induced tumor formation (43), supporting our observation that NF-κB plays a key role in HER2 overexpression. Two distinct NF-κB signaling pathways have been suggested in response to different cytotoxic stimuli (32), i.e., the classical pathway mediated mainly by the p65/p50 dimer and the alternative pathway mediated mainly by the RelB/p52 dimer (44). Using the ChIP assay, we demonstrated that NF-κB-mediated HER2 gene transactivation occurs through the classical pathway, since p65/p50 but not other NF-κB subunits are required for HER2 promoter activation (Fig. 2B). Based on these observations, it appears that the p65/p50 complex-mediated HER2 transactivation may provide potential therapeutic targets to sensitize HER2-positive breast cancer cells.
Another important implication of the current results is the loop-like activation pathway of NF-κB/HER2 signaling in breast cancer radioresistance. We have reported that NF-κB activity is increased in MCF-7 cells after long-term radiation treatment (45) and that forced overexpression of HER2 enhances the radiation-induced NF-κB activity, which in turn promotes radioresistance by activating a series of pro-survival genes (24). It is well documented that NF-κB is activated by radiation (46, 47), and NF-κB is now shown to bind directly to the HER2 promoter, resulting in HER2 overexpression and tumor adaptive radioresistance. In addition, we and others have reported that HER2 is able to activate the basal and radiation-induced NF-κB activity through the activation of PI3K/Akt (24, 48), which further induces HER2 overexpression. This feed-forward loop-like HER2-NF-κB-HER2 pathway may be specifically activated in long-term radiation-treated radioresistant breast cancer cells or breast CSCs to cause adaptive tumor resistance. A schematic presentation of the HER2-NF-κB-HER2 loop in adaptive radioresistance is proposed in Fig. 6H. We speculate that activation of this pathway results in the failure of DNA-damaging anti-cancer modalities against HER2- negative tumors.
In summary, we report here a novel finding that breast cancer cells lacking or with low HER2 expression may become resistant to therapy due to enhanced HER2 gene expression. HER2 is induced by exposure to radiation through NF-κB-mediated gene activation, and NF-κB-mediated HER2 overexpression is tightly associated with enhanced clonogenic survival and tumor repopulation. Our results suggest that breast cancer cure rates may be enhanced by targeting the NF-κB/HER2 pathway of radiation-resistant recurrent tumors.
Supplementary Material
Supplementary Figs. 1–3: http://dx.doi.org/10.1667/RR1472.1.S1.
ACKNOWLEDGMENTS
We thank M. C. Hung at University of Texas M.D. Anderson Cancer Center for providing the HER2 promoter-luciferase reporter, D. J. Slamon at University of California Los Angeles for providing the MCF-7/HER2 cells, and S. Liu at Purdue University for assistance in the animal experiments. This work was supported by the grants from the National Cancer Institute (R01 101990), Department of Energy Low Dose Radiation Research Program Biological and Environmental Research (DE-FG02-03ER63634), and Purdue Cancer Center/Indiana Elks Foundation.
REFERENCES
- 1.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat. Rev. Cancer 5. 2005;5:516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 2.Morgan WF. Genomic instability and bystander effects: a paradigm shift in radiation biology? Mil. Med. 2002;167:44–45. [PubMed] [Google Scholar]
- 3.Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, Ortiz KL, Sieck LK, Isaacs JS, Gius D. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res. 2003;63:8984–8995. [PubMed] [Google Scholar]
- 4.Amundson SA, Grace MB, McLeland CB, Epperly MW, Yeager A, Zhan Q, Greenberger JS, Fornace AJ., Jr Human in vivo radiation-induced biomarkers: gene expression changes in radiotherapy patients. Cancer Res. 2004;64:6368–6371. doi: 10.1158/0008-5472.CAN-04-1883. [DOI] [PubMed] [Google Scholar]
- 5.Dewey WC, Ling CC, Meyn RE. Radiation-induced apoptosis: relevance to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995;33:781–796. doi: 10.1016/0360-3016(95)00214-8. [DOI] [PubMed] [Google Scholar]
- 6.Forrester HB, Vidair CA, Albright N, Ling CC, Dewey WC. Using computerized video time lapse for quantifying cell death of X-irradiated rat embryo cells transfected with c-myc or c-Ha-ras. Cancer Res. 1999;59:931–939. [PubMed] [Google Scholar]
- 7.Olayioye MA. Update on HER-2 as a target for cancer therapy: intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res. 2001;3:385–389. doi: 10.1186/bcr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 9.Muthuswamy SK. ErbB2 makes beta 4 integrin an accomplice in tumorigenesis. Cell. 2006;126:443–445. doi: 10.1016/j.cell.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Pinkas-Kramarski R, Shelly M, Guarino BC, Wang LM, Lyass L, Alroy I, Alimandi M, Kuo A, Moyer JD, Yarden Y. ErbB tyrosine kinases and the two neuregulin families constitute a ligand-receptor network. Mol. Cell. Biol. 1998;18:6090–6101. doi: 10.1128/mcb.18.10.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzahar E, Moyer JD, Waterman H, Barbacci EG, Bao J, Levkowitz G, Shelly M, Strano S, Pinkas-Kramarski R, Yarden Y. Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. EMBO J. 1998;17:5948–5963. doi: 10.1093/emboj/17.20.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Coregulation of epidermal growth factor receptor/human epidermal growth factor receptor 2 (HER2) levels and locations: quantitative analysis of HER2 overexpression effects. Cancer Res. 2003;63:1130–1137. [PubMed] [Google Scholar]
- 13.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 2001;3:973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 14.Xing X, Wang SC, Xia W, Zou Y, Shao R, Kwong KY, Yu Z, Zhang S, Miller S, Hung MC. The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat. Med. 2000;6:189–195. doi: 10.1038/72294. [DOI] [PubMed] [Google Scholar]
- 15.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 16.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 18.Gius D, Botero A, Shah S, Curry HA. Intracellular oxidation/reduction status in the regulation of transcription factors NF-kappaB and AP-1. Toxicol. Lett. 1999;106:93–106. doi: 10.1016/s0378-4274(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Hu YC, Dong S, Fan M, Tamae D, Ozeki M, Gao Q, Gius D, Li JJ. Co-activation of ERK, NF-kappaB, and GADD45beta in response to ionizing radiation. J. Biol. Chem. 2005;280:12593–12601. doi: 10.1074/jbc.M410982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-kappaB is the answer—role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 21.Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol. Cell. Biol. 2003;23:2362–2378. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giedzinski E, Rola R, Fike JR, Limoli CL. Efficient production of reactive oxygen species in neural precursor cells after exposure to 250 MeV protons. Radiat. Res. 2005;164:540–544. doi: 10.1667/rr3369.1. [DOI] [PubMed] [Google Scholar]
- 23.Pianetti S, Arsura M, Romieu-Mourez R, Coffey RJ, Sonenshein GE. Her-2/neu overexpression induces NF-kappaB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IkappaB-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene. 2001;20:1287–1299. doi: 10.1038/sj.onc.1204257. [DOI] [PubMed] [Google Scholar]
- 24.Guo G, Wang T, Gao Q, Tamae D, Wong P, Chen T, Chen WC, Shively JE, Wong JY, Li JJ. Expression of ErbB2 enhances radiation-induced NF-kappaB activation. Oncogene. 2004;23:535–545. doi: 10.1038/sj.onc.1207149. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Shen B, Xia L, Khaletzkiy A, Chu D, Wong JY, Li JJ. Activation of nuclear factor kappaB in radioresistance of TP53-inactive human keratinocytes. Cancer Res. 2002;62:1213–1221. [PubMed] [Google Scholar]
- 26.Li JJ, Rhim JS, Schlegel R, Vousden KH, Colburn NH. Expression of dominant negative Jun inhibits elevated AP-1 and NF-kappaB transactivation and suppresses anchorage independent growth of HPV immortalized human keratinocytes. Oncogene. 1998;16:2711–2721. doi: 10.1038/sj.onc.1201798. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka A, Muto S, Konno M, Itai A, Matsuda H. A new IkappaB kinase beta inhibitor prevents human breast cancer progression through negative regulation of cell cycle transition. Cancer Res. 2006;66:419–426. doi: 10.1158/0008-5472.CAN-05-0741. [DOI] [PubMed] [Google Scholar]
- 28.Curry HA, Clemens RA, Shah S, Bradbury CM, Botero AG, Goswami P, Gius D. Heat shock inhibits radiation-induced activation of NF-kappaB via inhibition of I-kappaB kinase. J. Biol.Chem. 1999;274:23061–23067. doi: 10.1074/jbc.274.33.23061. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed KM, Li JJ. NF-kappa B-mediated adaptive resistance to ionizing radiation. Free Radic. Biol. Med. 2008;44:1–13. doi: 10.1016/j.freeradbiomed.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tal M, King CR, Kraus MH, Ullrich A, Schlessinger J, Givol D. Human HER2 (neu) promoter: evidence for multiple mechanisms for transcriptional initiation. Mol. Cell. Biol. 1987;7:2597–2601. doi: 10.1128/mcb.7.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 32.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed KM, Dong S, Fan M, Li JJ. Nuclear factor-kappaB p65 inhibits mitogen-activated protein kinase signaling pathway in radioresistant breast cancer cells. Mol. Cancer Res. 2006;4:945–955. doi: 10.1158/1541-7786.MCR-06-0291. [DOI] [PubMed] [Google Scholar]
- 34.Haffty BG, Brown F, Carter D, Flynn S. Evaluation of HER-2 neu oncoprotein expression as a prognostic indicator of local recurrence in conservatively treated breast cancer: a case-control study. Int. J. Radiat. Oncol. Biol. Phys. 1996;35:751–757. doi: 10.1016/0360-3016(96)00150-2. [DOI] [PubMed] [Google Scholar]
- 35.Soderlund K, Perez-Tenorio G, Stal O. Activation of the phosphatidylinositol 3-kinase/Akt pathway prevents radiation-induced apoptosis in breast cancer cells. Int. J. Oncol. 2005;26:25–32. [PubMed] [Google Scholar]
- 36.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 38.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr. Opin. Genet. Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 40.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 41.Liang K, Jin W, Knuefermann C, Schmidt M, Mills GB, Ang KK, Milas L, Fan Z. Targeting the phosphatidylinositol 3-kinase/Akt pathway for enhancing breast cancer cells to radiotherapy. Mol. Cancer Ther. 2003;2:353–360. [PubMed] [Google Scholar]
- 42.Castiglioni F, Terenziani M, Carcangiu ML, Miliano R, Aiello P, Bertola L, Triulzi T, Gasparini P, Camerini T, Tagliabue E. Radiation effects on development of HER2-positive breast carcinomas. Clin. Cancer Res. 2007;13:46–51. doi: 10.1158/1078-0432.CCR-06-1490. [DOI] [PubMed] [Google Scholar]
- 43.Cao Y, Luo JL, Karin M. IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc. Natl. Acad. Sci. USA. 2007;104:15852–15857. doi: 10.1073/pnas.0706728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Xia L, Lee ML, Khaletskiy A, Wang J, Wong JYC, Li JJ. Effector genes altered in MCF-7 human breast cancer cells after exposure to fractionated ionizing radiation. Radiat. Res. 2001;155:543–553. doi: 10.1667/0033-7587(2001)155[0543:egaimh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 47.Schieven GL, Kirihara JM, Myers DE, Ledbetter JA, Uckun FM. Reactive oxygen intermediates activate NF-kappa B in a tyrosine kinase-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn tyrosine kinases in human lymphocytes. Blood. 1993;82:1212–1220. [PubMed] [Google Scholar]
- 48.Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J. Biol. Chem. 2000;275:8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–3: http://dx.doi.org/10.1667/RR1472.1.S1.