Abstract
Objective
To assess the degree to which test methodology affects outcomes in clinical evaluations of walking speed.
Data Sources
Medline database and reference lists from relevant articles.
Study Selection
We conducted electronic searches by using various combinations of terms related to clinical evaluations of walking speed. Resultant abstracts were then reviewed, and the methods and results section of promising full-text articles were searched for detailed descriptions of walk-test methodologies and results. Ultimately, articles were limited to the most common participant groups, older adults (aged) and individuals with neurologic conditions (neuro). The final sample included 46 studies.
Data Extraction
Three aspects of test methodology (pace, starting protocol, distance timed) were extracted for use as independent variables. Group mean age was extracted for use as a covariate. Group mean velocity was extracted for use as the dependent variable. Data were extracted by a single investigator.
Data Synthesis
Usual and/or comfortable pace was reported nearly twice as often as fast pace in both groups. Static-start protocols were more frequently used in aged studies, whereas dynamic (ie, rolling) starts were more common in neuro studies. Distances of 6 and 10m were most common in aged and neuro studies, respectively. Multivariate analyses (analysis of covariance) showed that only pace was significantly related to the mean velocity in both groups (aged: pace, P<.01; starting protocol, P= .21; distance, P= .05; neuro: pace, P=.01; starting protocol, P=.63; distance, P=.49). However, methodology-related differences in the distribution (95% confidence intervals) of performance scores across certain clinical standards were noted within all 3 methodology variables.
Conclusions
Clinical assessments of walking velocity are not conducted uniformly. Common methodologic factors may influence the clinical interpretation of walk performances. Universal walk-test methodology is warranted to improve intergroup comparisons and the development of useful clinical criteria and consensus norms.
Keywords: Aged, Methodsm, Neurologic disorders, Rehabilitation, Review [publication type], Walking
Walking speed is a convenient measure of overall gait and functional mobility.1 Objective measures of walking speed are used as basic indicators of functional ability in clinical practice and research studies. Slow walking speed is associated with many health-related factors including physical impairments (eg, muscular strength and power), disability (eg, basic and instrumental activities of daily living), loss of independence, morbidity, and mortality.2–8
There is great variation in walk-test methodology within both clinical practice and the published literature. Although all test formats are considered valid methods for measuring the same functional attribute and have shown excellent interrater and test-retest reliabilities,9–14 there is no consensus regarding the optimal testing procedure (eg, distance, instructed pace, start protocol). This may stem from the fact that it is unknown whether these seemingly subtle differences in walk-test methodology yield clinically meaningful information.15,16 Hence, tests are likely chosen based on convenience rather than optimal efficacy. For example, if a 5-m walk is believed to be as reliable, valid, and sensitive as a 20-m test, then the 5-m protocol is more practical. It is important to note, however, that similar to other measures of functional performance, timed walk tests are subject to floor and/or ceiling effects. Ideally, test parameters should correspond to the projected range of abilities for a given study or clinical cohort.17
The purpose of this study was to assess walk-test methodology in terms of overall performance (the mean velocity) within the published literature. The implications of this study were 2-fold. First, if a relationship was found between methodology and outcomes, then that information could be used to better match test procedures with test objectives. Second, if there was no clear association between procedure and results, then it would be advantageous to adopt a common walk-test methodology to facilitate intergroup comparisons and broader standardization of norms.
METHODS
Data Sources and Selection Criteria
Data for the current study were obtained from a review of the health care literature for studies using an established distance-based measure of walking speed. Electronic searches of the MEDLINE database were conducted followed by concerted searches within the bibliographies of obtained full-text (including review) articles. Because walking speed is not one of the National Library of Medicine’s medical subjects heading terms, article abstracts and titles were searched for general descriptions of walk tests. Multiple combinations of numeric and scale descriptions of walk-test measures were used (eg, 5 m, 5 meter, 5 metre). Additional details of the literature review process were previously described.18 In all, we abstracted walk-test data from 108 full-text, peer-reviewed journal articles. Inclusion in the current analysis was limited to those studies providing necessary information for comprehensive summaries and comparisons, including patient characteristics, sample size, distance measured, instructions for pace, test protocol, and availability of walking speed mean and standard deviation (SD) values. E-mails were sent to contact authors to clarify ambiguities and/or obtain key missing methodologic information. To account for potential condition-specific differences in methodology-related performances, studies were grouped and analyzed in 2 relatively homogenous categories: older adults (aged) and people with neurologic conditions (neuro).
Data Extraction
The mean group age in years was used as a continuous variable. Instructed walking pace was coded as a dichotomous variable as follows: (1) usual and/or comfortable versus (2) fast. Testing protocol was also coded dichotomously as follows: (1) static versus (2) dynamic. Static describes the situation wherein participants stand at the starting line and timing begins as they are verbally instructed to go. Dynamic refers to protocols wherein participants begin walking before (typically 2–5m) the start line and timing begins as they cross the start line. Five studies used a turn protocol in which subjects walk a specified distance, turn around, and return to the start/end line. These studies were not included in the analysis because of the small number. The distance timed was used as a continuous variable for descriptive and correlation analyses and coded dichotomously (short [1] vs long [2]) for inclusion in the multivariate analyses. There were marked differences in the range of distances used in studies with aged and neuro participants so the following cutpoints, based on median splits, were used to distinguish short versus long criterion: aged, less than 5m versus greater than or equal to 5m, and neuro, less than 10m versus greater than or equal to 10m.
The primary outcome—walking velocity mean—for each group was recorded as a continuous variable (m/s). For intervention studies (pre- and post-test design), only baseline values were used to maintain consistency among test conditions; most studies were descriptive with only 1 (ie, baseline) test session. When participants were assigned to different groups in the same study (eg, control or intervention) and values were reported separately, we calculated a common mean and SD weighted by the sample size of each group. All data were extracted by a single investigator.
Data Synthesis
Bivariate correlations were performed to assess the underlying relationships between study parameters and outcomes. Analysis of covariance (ANCOVA) was used to evaluate differences in outcomes by test methodology after adjusting for age. The relative contribution from each study was controlled by weighting with inverse standard errors (1/SE), which gives greater weight to more precise estimates derived from larger samples.19 The main effects tested in the models were pace, protocol, and distance; age was entered as a covariate in all models. Analyses were performed separately for the 2 groups (aged, neuro) to minimize the likelihood of population-specific differences in unmeasured attributes (eg, disability level) confounding the results. SPSSa was used for all statistical analyses.
RESULTS
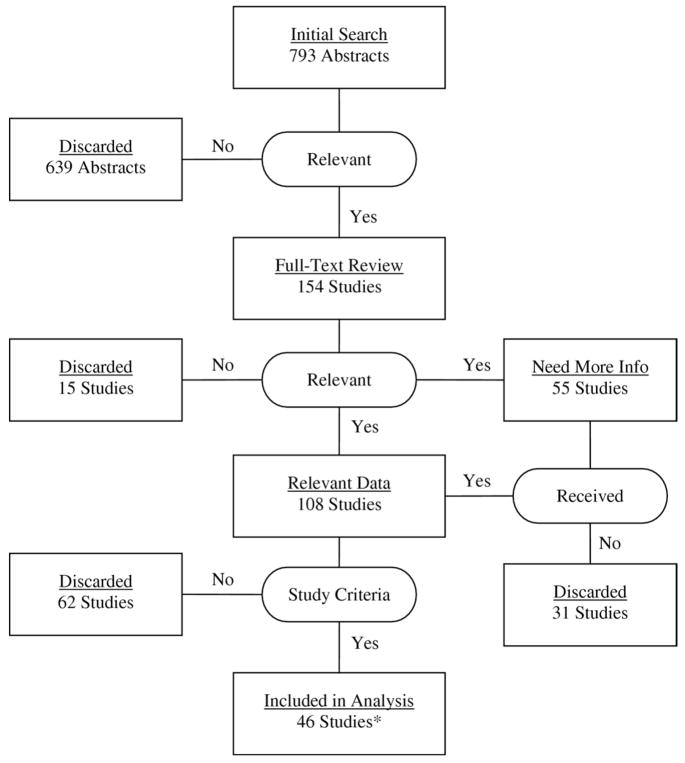
The sample included 46 studies. See figure 1 for a list of the studies included in the analyses and a detailed schematic of the study selection process.
Fig 1.
Schematic of literature search and results of study selection process. *Studies included in analysis are: Dobkin,1 Baer and Smith,2 Kuo et al,4 Ostchega et al,5 Rantanen et al,7 Rolland et al,11 van Hedel et al,13 van Loo et al,14 Salbach et al,15 Tyson and DeSouza,17 Moseley et al,20 Galvão and Taaffe,22 Henwood and Taaffe,23 Salbach et al,29 Wang et al,31 van Herk et al,32 Arnadottir and Mercer,37 Bischoff-Ferrari et al,38 Brill et al,39 Cesari et al,40 Chang et al,41 English et al,42 Gajdosik et al,43 Gold et al,44 Goldie et al,45 Herman et al,46 Kadanka et al,47 Kollen et al,48 Kressig et al,49 Meeuwsen et al,50 Miyai et al,51 Miyai et al,52 Morey and Zhu,53 Nelson et al,54 Nieuwenhuis et al,55 Pellecchia et al,56 Romberg et al,57 Taaffe et al,58 Tiedemann et al,59 Vos-Vromans et al,60 Webster et al,61 White and Petajan,62 Winchester et al,63 Witte and Carlsson,64 Wolf et al,65 and Yanagita et al.66
Some studies evaluated walking speed with more than 1 methodology (eg, both usual- and fast-pace trials) so we obtained the mean velocity data from 56 participant groups (22 aged, 34 neuro) representing a total sample size of 18,428 (16,683 aged, 1745 neuro). The numbers of groups within each methodologic variable are shown in table 1. The aged group included cohorts of older adults (age range, 54–84y) representing a broad spectrum of physical and functional abilities but without specified health conditions. The neuro group contained cohorts with the following conditions: Alzheimer’s (2 groups), developmental (1 group), multiple sclerosis (4 groups), myelopathy (1 group), Parkinson’s disease (3 groups), spinal cord injury (1 group), stroke (17 groups), stroke and/or tumor (1 group), and traumatic brain injury (TBI) (4 groups).
Table 1.
Number of Groups Within Each Methodologic Variable Included in Analyses
| Methodologic Variable | Aged | Neuro |
|---|---|---|
| Pace | ||
| Usual | 15 | 23 |
| Fast | 7 | 11 |
| Protocol | ||
| Static | 13 | 11 |
| Dynamic | 9 | 23 |
| Distance (m) | ||
| 2 | 2 | ND |
| 3 | 2 | ND |
| 4 | 4 | ND |
| 5 | ND | 6 |
| 6 | 10 | 4 |
| 8 | ND | 4 |
| 10 | 4 | 17 |
| 15 | ND | 2 |
| 30 | ND | 1 |
| Total | 22 | 34 |
Abbreviation: ND, no data.
Correlation analyses revealed that age was significantly associated with the mean velocity in both groups (aged, r=−.60; neuro, r=−.37). Pace was also significantly related to the mean velocity in both groups (aged, ρ=.49; neuro, ρ=.61). Neither protocol nor distance was significantly associated with the mean velocity.
Table 2 displays the results from the 2 ANCOVA models and observed effect sizes for all 3 independent variables. Pace (usual vs fast) was significantly related to the mean velocity in both groups, and distance (short vs long) approached statistical significance in the aged group only. Weighting by 1/SE effectively eliminated the significant bivariate association (Pearson correlation) between age and the mean velocity. In identical but not weighted models, both pace and age were significantly (P<.05) related to the mean velocity in the aged and neuro groups (data not shown).
Table 2.
Results From the ANCOVA Models.
| Source | F | df | P | Effect Size | |
|---|---|---|---|---|---|
| Aged | Corrected model | 4.012 | 4 | .018 | |
| Intercept | 7.174 | 1 | .016 | ||
| Age (y) | 0.870 | 1 | .364 | ||
| Pace | 9.424 | 1 | .007 | 1.21 | |
| Protocol | 1.676 | 1 | .213 | 0.18 | |
| Distance | 4.472 | 1 | .050 | 0.51 | |
| Error | 17 | ||||
| Total | 22 | ||||
| Neuro | Corrected model | 4.883 | 4 | .004 | |
| Intercept | 22.467 | 1 | .000 | ||
| Age (y) | 3.204 | 1 | .084 | ||
| Pace | 7.682 | 1 | .010 | 1.21 | |
| Protocol | 0.233 | 1 | .633 | 0.34 | |
| Distance | 0.494 | 1 | .488 | 0.40 | |
| Error | 29 | ||||
| Total | 34 |
NOTE. The dependent variable was the mean velocity (weighted by 1/SE from each group). Age was entered as a covariate in both models. Effect sizes were calculated for the single degree of freedom (df) comparisons (d-index) by using unadjusted values.
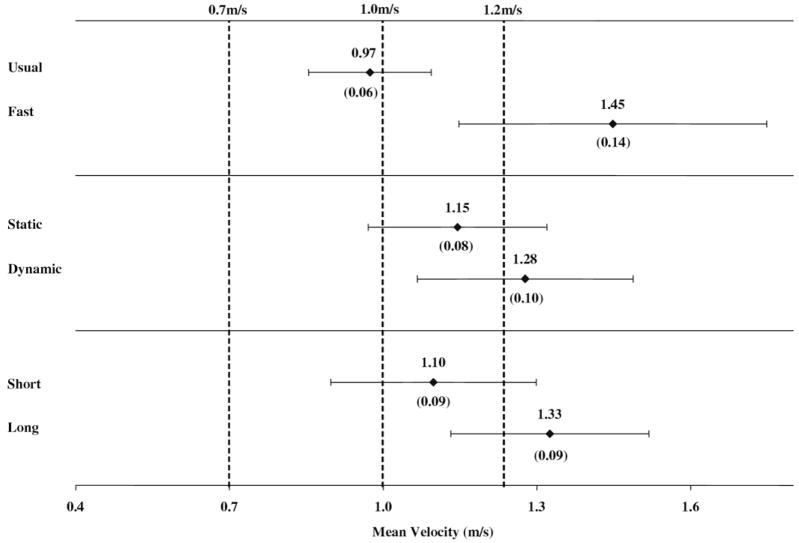
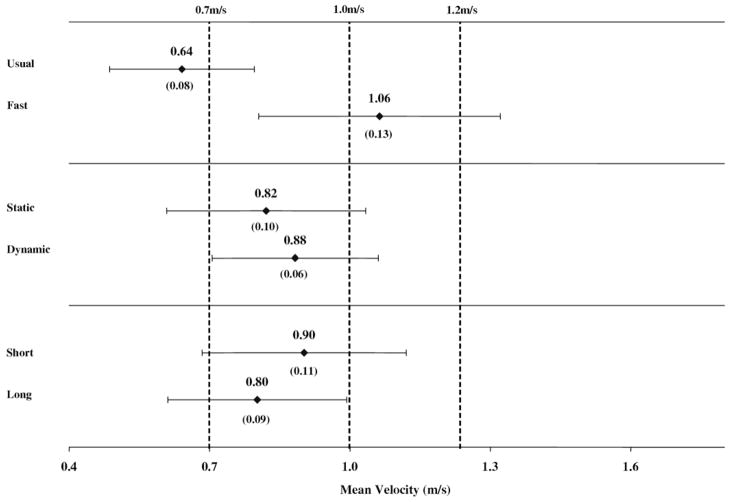
Figures 2 and 3 show the adjusted and weighted estimates (means, SEs, 95% confidence intervals) for the mean velocity within the 3 methodologic variables for the aged and neuro groups, respectively; the dashed vertical lines represent select clinical and community standards from the published literature.
Fig 2.
Age-adjusted and weighted estimates of mean velocities (SEs) and 95% confidence intervals by methodology in the aged group. The dashed vertical lines represent usual- and fast-paced standards cited in the implications section of the discussion.
Fig 3.
Age-adjusted and weighted estimates of mean velocities (SEs) and 95% confidence intervals by methodology in the neuro group. The dashed vertical lines represent usual- and fast-paced standards cited in the implications section of the discussion.
DISCUSSION
The purpose of this study was to assess the impact of walk-test methodology on walking performance within the published literature. We found that pace is the single most important methodologic factor influencing walk performance. Neither starting protocol nor distance timed showed statistically significant affects on mean walking velocities. Also, whereas age was significantly associated with the mean velocity in bivariate analysis, it did not remain significant as a covariate in the weighted ANCOVA models. This is likely a consequence of the tremendous range in sample sizes (n range, 7– 4100) in studies used in the current analysis; sample size was used as the denominator to calculate the SEs for weighting in the multivariate analysis. Additional study with more balanced (homogeneous) samples is needed to determine how age interacts with the relationship between the mean velocity and test methodology.
Pace
Differences in walking velocity between usual- and fast-paced tests within the same participant group have been well described.1,20,21 Our results substantiate that intended pace significantly impacts mean walking velocity in older adults and persons with neurologic conditions. None of the studies included in this analysis reported or discussed differences in the distribution of performance scores relative to pace. Sherrington and Lord12 did show comparable test-retest reliabilities for six-meter walk tests under comfortable and fast conditions (intraclass correlation coefficient [ICC]=.97, ICC=.94, respectively) in patients with hip fracture. Similar test-retest values were reported for TBI patients performing comfortable and fast 10-m walks (ICC=.96, ICC=.95, respectively).14 Thus, intraindividual variability appears to be unaffected by pace. Many other studies22–29 have included both pace conditions, yet differences were not compared or discussed, suggesting that many researchers include both because they are unsure which condition will show the best responsiveness. It is important to note that usual pace was used nearly twice as often as fast pace in the current analyses (see table 1) and that usual and/or comfortable pace normative values are more common in the literature than fast-pace standards.30 Dobkin1 states that both measures are important and that the difference between usual and maximum walking velocities (ie, the ability to voluntarily increase walking velocity) may be the best indicator of community-based ambulation ability.
Protocol
The dynamic start protocol effectively eliminates the acceleration phase from the timed performance.10,14,31 Removing acceleration from the timed walk protocol presumably results in faster and less variable performances. The current results could not confirm these presumptions. Overall, the dynamic protocol showed greater mean velocities compared with static-start conditions; however, these differences were not statistically significant (see figs 2, 3). In addition, starting protocol was not (significantly) correlated with the mean velocity in either group.
Static protocols were more common in the aged studies, and dynamic protocols were more common in the neuro studies (see table 1). No study was found evaluating static and dynamic starts on walking performance within the same participant group. Van Herk et al32 conducted a study comparing the time to walk 10m straight compared with 10m with a turn (2×5m) at usual pace and from a static start in patients with stroke. The mean time to complete was significantly longer under the turn protocol, and, although the difference in variability was not reported or discussed, the coefficient of variation also increased with the turn condition (.63 vs .51). The turn protocol obviously increased the degree of difficulty over the standard linear protocol, specifically the ability and time to decelerate, turn around, and accelerate again, which characterizes the expected impact that an acceleration phase (ie, static start) should have on performance relative to steady-state ambulation (ie, dynamic start).
Distance
The distance walked was marginally related to the mean velocity in the aged group, based on an α level of P less than .05, and not significantly related to the mean velocity in the neuro group (see table 2). The only study evaluating different distances within a single sample of subjects reported mixed results based on the pace of the trials. Salbach et al15 assessed 5-meter and 10-meter walk tests at both comfortable and maximum speeds in patients at 8 and 30 days poststroke. Baseline (day 8) comparisons found no differences in the mean comfortable speed between the 2 distances; however, the mean maximum speed was significantly greater over 5m compared with 10m. The 5-m walk at comfortable pace also showed better responsiveness than other pace-distance combinations. It was concluded that a 5-m walk at comfortable pace is the optimal method to assess changes in mobility in early poststroke patients because 10m may be too far for some people to maintain their speed.15 We found that 6m was the most common distance walked in studies including aged participants, and 10m was the most common distance in neuro studies (see table 1).
Implications
Walking velocity is associated with numerous indicators of physical functioning and health. Consequently, it is important to examine the results of this review in the context of clinical application. A statistically significant improvement in walking velocity, for example, may be considered meaningless to a patient and/or clinician if there is no tangible health- or function-related benefit associated with it. Conversely, nonsignificant changes may be highly valued if health-related quality of life and/or functional independence are thought to be improved. Thus, it is difficult to identify a single clinically meaningful difference (effect) that could be used as a universal standard to assess clinical improvement or, in line with the purpose of this study, to evaluate the benefit of 1 test methodology over another. Rather, as discussed in the next section and displayed in figures 2 and 3, relative effectiveness is dependent on where along the continuum of walking speed values the 2 scores lie and which criterion standard is used. Based on sample sizes in the current analysis and using a desired power level of 80% with α set at .05, it was calculated that statistical significance would be achieved at effect sizes (differences) of .87 and .69 in the aged and neuro groups, respectively. The methodology-related differences were not that large for the starting protocol and distance variables, which explain the lack of statistical significance for those variables in the ANCOVA models (see table 2).
There are a number of clinical norms (ranges) for walking velocity reported in the literature33 that are used to evaluate a person’s functional status or risk. The following are examples of usual-pace standards: 1m/s is considered “normal” for older adults without disability and velocities less than or equal to 0.7m/s are strongly associated with adverse health outcomes.34 An example of a critical value related to maximum walking performance includes the velocity necessary to cross a signaled crosswalk, which is reportedly 1.22 and 1.07m/s in the United States and United Kingdom, respectively.7,20 Thus, beyond the clinical implications and health risks associated with slow walking speed, certain velocity standards are considered essential for patients functioning outside the clinic, and these requirements vary by community and setting.35,36 Figures 2 and 3, in particular, show how test methodology need not result in statistically significant differences to potentially affect the clinical interpretation of walk performance in relation to the standards cited previously (dashed vertical lines).
Study Limitations
There are limitations in the synthesis of data from multiple studies. The most transparent shortcoming is the lack of consistent reporting of walk-test methodology in the literature. Due to time and resource restraints, only 1 author reviewed articles and extracted data for analysis. The necessity to reduce the number of variables by combining many into broad categories based on cursory similarities can conceal potentially important relationships. For example, the creation of participant groups facilitates describing and presenting the data, but it may diminish some diagnostic-specific characteristic of walking velocity. Likewise, dichotomous coding of test parameters increases the sample size of each group, improves power for statistical comparisons, and enables better interpretation of results, yet certain relationships may go unnoticed. It is unclear, for example, if subtle differences in pace instructions (eg, “fast as you can without running” vs “fast but under control” vs “fast yet safe”) result in systematic differences in a person’s performance or if the common denominator “fast” implies maximum effort, as assumed in the current study. Another limitation involves the inability to adjust and/or match for differences in impairment or disability within each test procedure category. Additionally, sex-specific walking velocities were not consistently reported in the studies reviewed, which prohibited us from accounting for this potential interaction. The objective of this study, however, was to determine if test methodology exerts a universal influence on walking performance across all study populations, not the potential effects within a particular diagnostic, sex, or age category. Based on the total sample size (N=18,428) and the variability in study types and populations included, it is reasonable to assume that people from the entire functional continuum were represented in this composite analysis.
CONCLUSIONS
Walk-test data are not measured uniformly nor is walk-test methodology reported completely. Consequently, it is difficult to compare walking performance across studies with dissimilar or unspecified test procedures.30 Although pace was the only methodologic factor that yielded consistent and statistically significant differences in walking velocity, the results suggest that all 3 methodologic factors evaluated may influence the clinical interpretation (meaningfulness) of a group’s and/or individual’s performance based on select published standards. Additional study of within-group or between-matched (eg, for impairment and/or disability) groups is necessary to verify the results of this review and analysis. In the meantime, it appears that tests at a practical distance (eg, 4 – 6m) and from a static start should be promoted with pace being determined by the underlying objective of the assessment and/or intervention. Most importantly, this study identifies the value of reporting the details of the basic methodology in the published literature, even for widely acknowledged standardized tests. Better reporting would systematically facilitate intergroup comparisons and the development of broader and more meaningful norms.
Acknowledgments
Supported by the National Institutes of Health (grant nos. K02-AG019736, T32-HD007539, K01-HD046682) and the National Institute on Disability and Rehabilitation Research (grant no. H133P040003).
Footnotes
Presented to the American Congress of Rehabilitation Medicine, October 5, 2007, Washington, DC.
Version 14; SPSS Inc, 233 S Wacker Dr, 11th Fl, Chicago, IL 60606.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
References
- 1.Dobkin BH. Short-distance walking speed and timed walking distance: redundant measures for clinical trials? Neurology. 2006;66:584–6. doi: 10.1212/01.wnl.0000198502.88147.dd. [DOI] [PubMed] [Google Scholar]
- 2.Baer G, Smith M. The recovery of walking ability and subclassification of stroke. Physiother Res Int. 2001;6:135–44. doi: 10.1002/pri.222. [DOI] [PubMed] [Google Scholar]
- 3.de Rekeneire N, Visser M, Peila R, et al. Is a fall just a fall: correlates of falling in healthy older persons. The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:841–6. doi: 10.1046/j.1365-2389.2003.51267.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuo HK, Leveille SG, Yen CJ, et al. Exploring how peak leg power and usual gait speed are linked to late-life disability: data from the National Health and Nutrition Examination Survey (NHANES), 1999–2002. Am J Phys Med Rehabil. 2006;85:650–8. doi: 10.1097/01.phm.0000228527.34158.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostchega Y, Dillon CF, Lindle R, Carroll M, Hurley BF. Isokinetic leg muscle strength in older Americans and its relationship to a standardized walk test: data from the national health and nutrition examination survey 1999–2000. J Am Geriatr Soc. 2004;52:977–82. doi: 10.1111/j.1532-5415.2004.52268.x. [DOI] [PubMed] [Google Scholar]
- 6.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–7. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 7.Rantanen T, Guralnik JM, Izmirlian G, et al. Association of muscle strength with maximum walking speed in disabled older women. Am J Phys Med Rehabil. 1998;77:299–305. doi: 10.1097/00002060-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Willen C, Stibrant SK, Ekman C, Grimby G. How is walking speed related to muscle strength? A study of healthy persons and persons with late effects of polio. Arch Phys Med Rehabil. 2004;85:1923–8. doi: 10.1016/j.apmr.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Green J, Forster A, Young J. Reliability of gait speed measured by a timed walking test in patients one year after stroke. Clin Rehabil. 2002;16:306–14. doi: 10.1191/0269215502cr495oa. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy CJ, Oldham JA. The reliability, validity and responsiveness of an aggregated locomotor function (ALF) score in patients with osteoarthritis of the knee. Rheumatology (Oxford) 2004;43:514–7. doi: 10.1093/rheumatology/keh081. [DOI] [PubMed] [Google Scholar]
- 11.Rolland YM, Cesari M, Miller ME, Penninx BW, Atkinson HH, Pahor M. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52:972–6. doi: 10.1111/j.1532-5415.2004.52267.x. [DOI] [PubMed] [Google Scholar]
- 12.Sherrington C, Lord SR. Reliability of simple portable tests of physical performance in older people after hip fracture. Clin Rehabil. 2005;19:496–504. doi: 10.1191/0269215505cr833oa. [DOI] [PubMed] [Google Scholar]
- 13.van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehabil. 2005;86:190–6. doi: 10.1016/j.apmr.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 14.van Loo MA, Moseley AM, Bosman JM, de Bie RA, Hassett L. Test-re-test reliability of walking speed, step length and step width measurement after traumatic brain injury: a pilot study. Brain Inj. 2004;18:1041–8. doi: 10.1080/02699050410001672314. [DOI] [PubMed] [Google Scholar]
- 15.Salbach NM, Mayo NE, Higgins J, Ahmed S, Finch LE, Richards CL. Responsiveness and predictability of gait speed and other disability measures in acute stroke. Arch Phys Med Rehabil. 2001;82:1204–12. doi: 10.1053/apmr.2001.24907. [DOI] [PubMed] [Google Scholar]
- 16.Ostir GV, Kuo YF, Berges IM, Markides KS, Ottenbacher KJ. Measures of lower body function and risk of mortality over 7 years of follow-up. Am J Epidemiol. 2007;166:599–605. doi: 10.1093/aje/kwm121. [DOI] [PubMed] [Google Scholar]
- 17.Tyson SF, DeSouza LH. Reliability and validity of functional balance tests post stroke. Clin Rehabil. 2004;18:916–23. doi: 10.1191/0269215504cr821oa. [DOI] [PubMed] [Google Scholar]
- 18.Graham JE, Ostir GV, Fisher SR, Ottenbacher KJ. Assessing walking speed in clinical research: a systematic review. J Eval Clin Pract. doi: 10.1111/j.1365-2753.2007.00917.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper H, Hedges LV. The handbook of research synthesis. New York: Russell Sage Foundation; 1994. [Google Scholar]
- 20.Moseley AM, Lanzarone S, Bosman JM, et al. Ecological validity of walking speed assessment after traumatic brain injury: a pilot study. J Head Trauma Rehabil. 2004;19:341–8. doi: 10.1097/00001199-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Riley PO, DellaCroce U, Kerrigan DC. Effect of age on lower extremity joint moment contributions to gait speed. Gait Posture. 2001;14:264–70. doi: 10.1016/s0966-6362(01)00133-3. [DOI] [PubMed] [Google Scholar]
- 22.Galvão DA, Taaffe DR. Resistance exercise dosage in older adults: single- versus multiset effects on physical performance and body composition. J Am Geriatr Soc. 2005;53:2090–7. doi: 10.1111/j.1532-5415.2005.00494.x. [DOI] [PubMed] [Google Scholar]
- 23.Henwood TR, Taaffe DR. Improved physical performance in older adults undertaking a short-term programme of high-velocity resistance training. Gerontology. 2005;51:108–15. doi: 10.1159/000082195. [DOI] [PubMed] [Google Scholar]
- 24.McDermott MM, Liu K, Ferrucci L, et al. Physical performance in peripheral arterial disease: a slower rate of decline in patients who walk more. Ann Intern Med. 2006;144:10–20. doi: 10.7326/0003-4819-144-1-200601030-00005. [DOI] [PubMed] [Google Scholar]
- 25.McDermott MM, Liu K, Guralnik JM, et al. Functional decline in patients with and without peripheral arterial disease: predictive value of annual changes in levels of C-reactive protein and D-dimer. J Gerontol A Biol Sci Med Sci. 2006;61:374–9. doi: 10.1093/gerona/61.4.374. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Guralnik JM, Ferrucci L, et al. Functional decline in lower-extremity peripheral arterial disease: associations with comorbidity, gender, and race. J Vasc Surg. 2005;42:1131–7. doi: 10.1016/j.jvs.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 27.McDermott MM, Liu K, Guralnik JM, Martin GJ, Criqui MH, Greenland P. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg. 1998;28:1072–81. doi: 10.1016/s0741-5214(98)70034-5. [DOI] [PubMed] [Google Scholar]
- 28.McDermott MM, Liu K, Guralnik JM, et al. The ankle brachial index independently predicts walking velocity and walking endurance in peripheral arterial disease. J Am Geriatr Soc. 1998;46:1355–62. doi: 10.1111/j.1532-5415.1998.tb06001.x. [DOI] [PubMed] [Google Scholar]
- 29.Salbach NM, Mayo NE, Wood-Dauphinee S, Hanley JA, Richards CL, Côté R. A task-orientated intervention enhances walking distance and speed in the first year post stroke: a randomized controlled trial. Clin Rehabil. 2004;18:509–19. doi: 10.1191/0269215504cr763oa. [DOI] [PubMed] [Google Scholar]
- 30.Bohannon RW, Andrews AW, Thomas MW. Walking speed: reference values and correlates for older adults. J Orthop Sports Phys Ther. 1996;24:86–90. doi: 10.2519/jospt.1996.24.2.86. [DOI] [PubMed] [Google Scholar]
- 31.Wang RY, Yen L, Lee CC, Lin PY, Wang MF, Yang YR. Effects of an ankle-foot orthosis on balance performance in patients with hemiparesis of different durations. Clin Rehabil. 2005;19:37–44. doi: 10.1191/0269215505cr797oa. [DOI] [PubMed] [Google Scholar]
- 32.van Herk I, Arendzen JH, Rispens P. Ten-metre walk, with or without a turn? Clin Rehabil. 1998;12:30–5. doi: 10.1191/026921598667081596. [DOI] [PubMed] [Google Scholar]
- 33.Oberg T, Karsznia A, Oberg K. Basic gait parameters: reference data for normal subjects, 10–79 years of age. J Rehabil Res Dev. 1993;30:210–23. [PubMed] [Google Scholar]
- 34.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–9. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 35.Ringsberg KA, Gärdsell P, Johnell O, Jónsson B, Obrant KJ, Sernbo I. Balance and gait performance in an urban and a rural population. J Am Geriatr Soc. 1998;46:65–70. doi: 10.1111/j.1532-5415.1998.tb01015.x. [DOI] [PubMed] [Google Scholar]
- 36.Robinett CS, Vondran MA. Functional ambulation velocity and distance requirements in rural and urban communities. A clinical report. Phys Ther. 1988;68:1371–3. doi: 10.1093/ptj/68.9.1371. [DOI] [PubMed] [Google Scholar]
- 37.Arnadottir SA, Mercer VS. Effects of footwear on measurements of balance and gait in women between the ages of 65 and 93 years. Phys Ther. 2000;80:17–27. [PubMed] [Google Scholar]
- 38.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–8. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 39.Brill PA, Probst JC, Greenhouse DL, Schell B, Macera CA. Clinical feasibility of a free-weight strength-training program for older adults. J Am Board Fam Pract. 1998;11:445–51. doi: 10.3122/jabfm.11.6.445. [DOI] [PubMed] [Google Scholar]
- 40.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–80. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 41.Chang M, Cohen-Mansfield J, Ferrucci L, et al. Incidence of loss of ability to walk 400 meters in a functionally limited older population. J Am Geriatr Soc. 2004;52:2094–8. doi: 10.1111/j.1532-5415.2004.52570.x. [DOI] [PubMed] [Google Scholar]
- 42.English CK, Hillier SL, Stiller K, Warden-Flood A. The sensitivity of three commonly used outcome measures to detect change amongst patients receiving inpatient rehabilitation following stroke. Clin Rehabil. 2006;20:52–5. doi: 10.1191/0269215506cr877oa. [DOI] [PubMed] [Google Scholar]
- 43.Gajdosik RL, Vander Linden DW, McNair PJ, Williams AK, Riggin TJ. Effects of an eight-week stretching program on the passive-elastic properties and function of the calf muscles of older women. Clin Biomech (Bristol, Avon) 2005;20:973–83. doi: 10.1016/j.clinbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Gold SM, Schulz H, Monch A, Schulz KH, Heesen C. Cognitive impairment in multiple sclerosis does not affect reliability and validity of self-report health measures. Mult Scler. 2003;9:404–10. doi: 10.1191/1352458503ms927oa. [DOI] [PubMed] [Google Scholar]
- 45.Goldie PA, Matyas TA, Evans OM. Deficit and change in gait velocity during rehabilitation after stroke. Arch Phys Med Rehabil. 1996;77:1074–82. doi: 10.1016/s0003-9993(96)90072-6. [DOI] [PubMed] [Google Scholar]
- 46.Herman S, Kiely DK, Leveille S, O’Neill E, Cyberey S, Bean JF. Upper and lower limb muscle power relationships in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2005;60:476–80. doi: 10.1093/gerona/60.4.476. [DOI] [PubMed] [Google Scholar]
- 47.Kadanka Z, Bednarik J, Vohanka S, et al. Conservative treatment versus surgery in spondylotic cervical myelopathy: a prospective randomised study. Eur Spine J. 2000;9:538–44. doi: 10.1007/s005860000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kollen B, Kwakkel G, Lindeman E. Hemiplegic gait after stroke: is measurement of maximum speed required? Arch Phys Med Rehabil. 2006;87:358–63. doi: 10.1016/j.apmr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Kressig RW, Wolf SL, Sattin RW, et al. Associations of demographic, functional, and behavioral characteristics with activity-related fear of falling among older adults transitioning to frailty. J Am Geriatr Soc. 2001;49:1456–62. doi: 10.1046/j.1532-5415.2001.4911237.x. [DOI] [PubMed] [Google Scholar]
- 50.Meeuwsen IB, Samson MM, Duursma SA, Verhaar HJ. Tibolone does not affect muscle power and functional ability in healthy postmenopausal women. Clin Sci (Lond) 2002;102:135–41. [PubMed] [Google Scholar]
- 51.Miyai I, Fujimoto Y, Ueda Y, et al. Treadmill training with body weight support: its effect on Parkinson’s disease. Arch Phys Med Rehabil. 2000;81:849–52. doi: 10.1053/apmr.2000.4439. [DOI] [PubMed] [Google Scholar]
- 52.Miyai I, Fujimoto Y, Yamamoto H, et al. Long-term effect of body weight-supported treadmill training in Parkinson’s disease: a randomized controlled trial. Arch Phys Med Rehabil. 2002;83:1370–3. doi: 10.1053/apmr.2002.34603. [DOI] [PubMed] [Google Scholar]
- 53.Morey MC, Zhu CW. Improved fitness narrows the symptom-reporting gap between older men and women. J Womens Health (Larchmt) 2003;12:381–90. doi: 10.1089/154099903765448899. [DOI] [PubMed] [Google Scholar]
- 54.Nelson ME, Layne JE, Bernstein MJ, et al. The effects of multidimensional home-based exercise on functional performance in elderly people. J Gerontol A Biol Sci Med Sci. 2004;59:154–60. doi: 10.1093/gerona/59.2.m154. [DOI] [PubMed] [Google Scholar]
- 55.Nieuwenhuis MM, Van TH, Sorensen PS, Ravnborg M. The six spot step test: a new measurement for walking ability in multiple sclerosis. Mult Scler. 2006;12:495–500. doi: 10.1191/1352458506ms1293oa. [DOI] [PubMed] [Google Scholar]
- 56.Pellecchia MT, Grasso A, Biancardi LG, Squillante M, Bonavita V, Barone P. Physical therapy in Parkinson’s disease: an open long-term rehabilitation trial. J Neurol. 2004;251:595–8. doi: 10.1007/s00415-004-0379-2. [DOI] [PubMed] [Google Scholar]
- 57.Romberg A, Virtanen A, Ruutiainen J, et al. Effects of a 6-month exercise program on patients with multiple sclerosis: a randomized study. Neurology. 2004;63:2034–8. doi: 10.1212/01.wnl.0000145761.38400.65. [DOI] [PubMed] [Google Scholar]
- 58.Taaffe DR, Newman AB, Haggerty CL, et al. Estrogen replacement, muscle composition, and physical function: The Health ABC Study. Med Sci Sports Exerc. 2005;37:1741–7. doi: 10.1249/01.mss.0000181678.28092.31. [DOI] [PubMed] [Google Scholar]
- 59.Tiedemann A, Sherrington C, Lord SR. Physiological and psychological predictors of walking speed in older community-dwelling people. Gerontology. 2005;51:390–5. doi: 10.1159/000088703. [DOI] [PubMed] [Google Scholar]
- 60.Vos-Vromans DC, de Bie RA, Erdmann PG, van Meeteren NL. The responsiveness of the ten-meter walking test and other measures in patients with hemiparesis in the acute phase. Physiother Theory Pract. 2005;21:173–80. doi: 10.1080/09593980500212920. [DOI] [PubMed] [Google Scholar]
- 61.Webster KE, Merory JR, Wittwer JE. Gait variability in community dwelling adults with Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:37–40. doi: 10.1097/01.wad.0000201849.75578.de. [DOI] [PubMed] [Google Scholar]
- 62.White AT, Petajan JH. Physiological measures of therapeutic response to interferon beta-1a treatment in remitting-relapsing MS. Clin Neurophysiol. 2004;115:2364–71. doi: 10.1016/j.clinph.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Winchester P, Kendall K, Peters H, Sears N, Winkley T. The effect of therapeutic horseback riding on gross motor function and gait speed in children who are developmentally delayed. Phys Occup Ther Pediatr. 2002;22:37–50. [PubMed] [Google Scholar]
- 64.Witte US, Carlsson JY. Self-selected walking speed in patients with hemiparesis after stroke. Scand J Rehabil Med. 1997;29:161–5. [PubMed] [Google Scholar]
- 65.Wolf SL, Catlin PA, Gage K, Gurucharri K, Robertson R, Stephen K. Establishing the reliability and validity of measurements of walking time using the Emory Functional Ambulation Profile. Phys Ther. 1999;79:1122–33. [PubMed] [Google Scholar]
- 66.Yanagita M, Willcox BJ, Masaki KH, et al. Disability and depression: investigating a complex relation using physical performance measures. Am J Geriatr Psychiatry. 2006;14:1060–8. doi: 10.1097/01.JGP.0000224364.70515.12. [DOI] [PubMed] [Google Scholar]