Summary
Hormone signaling is important in a number of disease states, and hormone receptors are effective therapeutic targets. PGRMC1 (progesterone receptor membrane component 1) is a member of a multi-protein complex that binds to progesterone and other steroids, as well as pharmaceutical compounds. In spite of its name, PGRMC1 shares homology with cytochrome b5-related proteins rather than hormone receptors, and heme binding is the sole biochemical activity of PGRMC1. PGRMC1 and its homologues regulate cholesterol synthesis by activating the P450 protein Cyp51/lanosterol demethylase, and the cholesterol synthetic pathway is an important target in cardiovascular disease and in treating infections. PGRMC1 binding partners include multiple P450 proteins, PAIR-BP1, Insig, and an uncharacterized hormone/drug-binding protein. PGRMC1 is induced in a spectrum of cancers, where it promotes cell survival and damage resistance, and PGRMC1 is also expressed in the nervous system and tissues involved in drug metabolism, cholesterol synthesis and hormone synthesis and turnover. One of the appealing features of PGRMC1 and its associated protein complex is its affinity for steroids and drugs. Together with its biological role in promoting tumor survival, PGRMC1 is an attractive target for therapeutic intervention in cancer and related malignancies.
Keywords: progesterone, P450 proteins, cholesterol, carcinogenicity, chemotherapy
1.Introduction
Cell growth and proliferation are driven by multiple processes, and cells employ intricate signaling networks to link extracellular signals to changes in metabolism. In cancer and other proliferative disorders, these pathways become deregulated, and the proteins that drive these pathways are targets for therapeutic intervention. This review is about a novel family of proteins that have characteristics of hormone receptors and are co-activators of important steps in lipid metabolism. PGRMC1 may link extracellular signals to P450 activation, and the PGRMC1-associated ligand-binding function provides opportunities for therapeutic intervention.
PGRMC1 (progesterone receptor membrane component 1) is a member of a multi-protein progesterone-binding complex (Meyer et al., 1996; Peluso et al., 2008b). Thus, PGRMC1 has also been named Hpr6.6 [human membrane progesterone receptor (Gerdes et al., 1998)]. However, PGRMC1 does not bind directly to progesterone (Min et al., 2005) and has no homology with steroid receptors (Mifsud and Bateman, 2002), nuclear or membrane-associated. The only known biochemical function of PGRMC1 is binding to heme (Ghosh et al., 2005; Crudden et al., 2006), and PGRMC1 shares key structural motifs with cytochrome b5 (Mifsud and Bateman, 2002), a heme binding protein that activates cytochrome P450 proteins (Schenkman and Jansson, 2003). Indeed, PGRMC1 binds and activates P450 proteins (Min et al., 2004; Min et al., 2005; Hughes et al., 2007), which metabolize drugs, hormones and lipids. Notably, the same structural components of PGRMC1 that are required for heme binding are also required for its association with a progesterone-binding partner (Peluso et al., 2008b).
In unicellular eukaryotes, PGRMC1 homologues interact with P450 proteins to synthesize sterols and protect cells from DNA damage (Hand et al., 2003; Mallory et al., 2005a; Craven et al., 2007; Hughes et al., 2007; Thompson et al., 2007). In multicellular organisms, PGRMC1 binds to P450 proteins and has additional binding partners, patterns of transcription and pro-survival activities (Cahill, 2007; Peluso et al., 2008b). These are indicated in Figure 1. Mammals also have two additional PGRMC1 family members, called neudesin (Kimura et al., 2008) and PGRMC2/Dg6 (Gerdes et al., 1998), about which little is known. PGRMC1 is induced by a variety of non-genotoxic carcinogens, including dioxin, and its mouse and rat homologues are also called 25-Dx [25 kDa dioxin-inducible protein (Selmin et al., 1996; Krebs et al., 2000)]. Finally, PGRMC1 is expressed in the rat adrenal, and has been named IZA1 [inner zone antigen (Raza et al., 2001; Min et al., 2004)]. However, the sequence of rat IZA1 is distinct from that of 25-Dx at the carboxy-terminus, particularly with regard to the last 46 amino acids of 25-Dx (Min et al., 2004). The PGRMC1 yeast homologues are all named Dap1 [damage-associated protein (Hand et al., 2003; Hughes et al., 2007)].
Fig. 1.
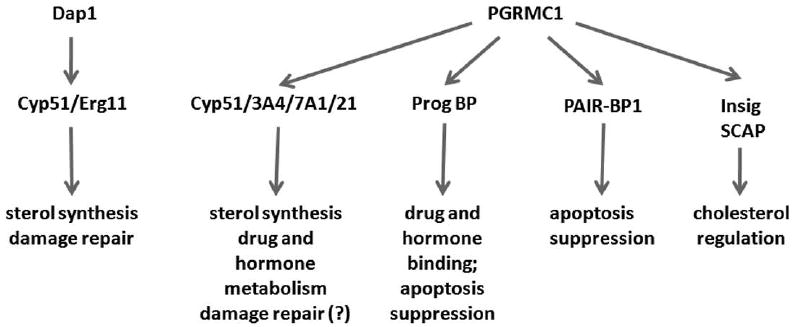
Conserved and divergent functions of PGRMC1 proteins in evolution. In unicellular organisms, Dap1 binds and activates the P450 protein Cyp51, promoting sterol synthesis and damage resistance. In mammals, PGRMC1 binds to multiple P450 proteins, suggesting additional functions in drug and hormone metabolism. PGRMC1 also binds to an unknown progesterone binding protein, to PAIR-BP1 and to Insig and SCAP.
In the past several years, a number of labs have shown that PGRMC1 is induced in tumors, including hormone-responsive tumors. Disrupting PGRMC1 in tumors inactivates pro-survival signaling and sensitizes cells to DNA damage, including damage caused by widely used chemotherapeutic drugs (Crudden et al., 2006; Peluso et al., 2008a). The putative structure for PGRMC1 contains a prominent ligand binding cleft (Figure 2), and PGRMC1 associates with a hormone/drug binding sub-unit (see below). Together, these properties suggest a structure that could be targeted, and genetic evidence suggests that PGRMC1 inhibition could improve the outcome of genotoxic chemotherapy and hormonal anti-cancer therapies. PGRMC1 targeting could also be used as an anti-infective approach in combination with P450-inhibiting anti-fungal drugs.
Fig. 2.
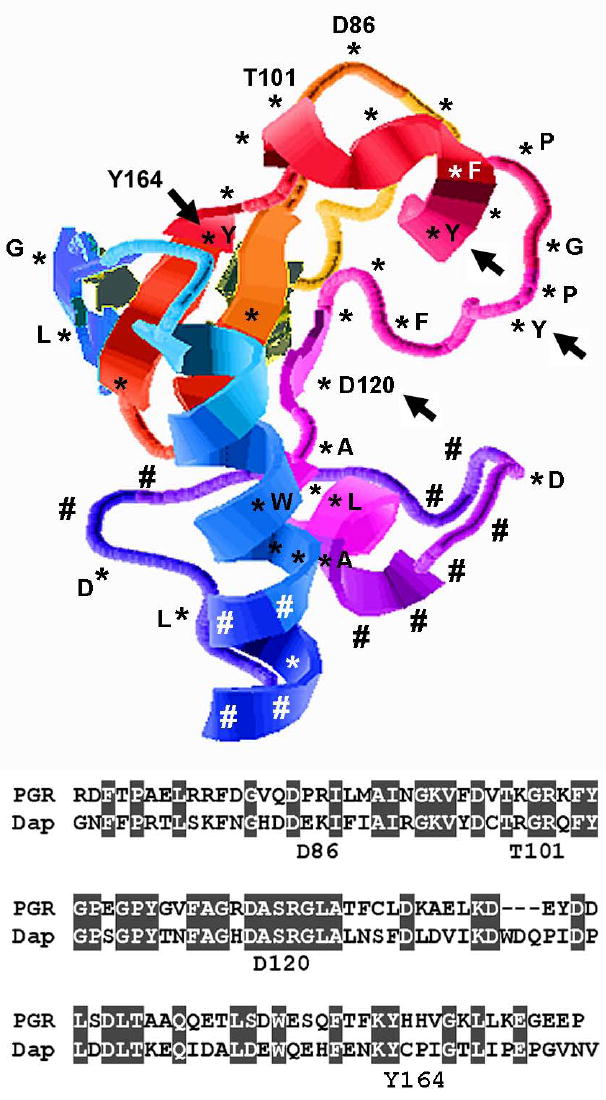
A model for the structure of residues 71-171 of PGRMC1. Asterisks indicate identical residues between PGRMC1 and its yeast homologue, Dap1, while pound signs indicate key non-conserved residues. The sequences of the two proteins are shown below with the identical sequences shaded in gray. The positions of four residues are indicated as a reference point. PGRMC1 residues Asp120, Tyr107, Tyr113 and Tyr164 are required for heme binding in PGRMC1, and the residues that are analogous to Asp120 and Tyr164 are required for heme binding in Dap1.
2.PGRMC1 and cholesterol synthesis
The cholesterol synthetic pathway is an important target for treating cardiovascular disease and inhibiting fungal infections. PGRMC1 likely regulates cholesterol synthesis in two ways. First, PGRMC1 binds and activates the P450 protein Cyp51/lanosterol demethylase (Hughes et al., 2007), which catalyzes an essential reaction in the sterol synthetic pathway (Lepesheva and Waterman, 2007). This finding was derived directly from research in the fungi Saccharomyces cerevisiae and Schizosaccharomyces pombe, where the PGRMC1 homologue, called Dap1, activates Cyp51 (Hand et al., 2003; Mallory et al., 2005a; Craven et al., 2007; Hughes et al., 2007). However, Dap1-Cyp51 binding has not been demonstrated in S. cerevisiae, and the two proteins only partially overlap in their localization (Craven et al., 2007).
Cyp51 is the target for the azole class of antifungal drugs, which are widely used to treat yeast infections, and Dap1 mediates resistance to azole drugs by activating Cyp51 (Hand et al., 2003; Mallory et al., 2005a; Hughes et al., 2007). Dap1 also regulates a second P450 protein, called Erg5, in the cholesterol synthetic pathway (Mallory et al., 2005a), suggesting a general role for Dap1 in P450 activation. As in yeast, human PGRMC1 binds to Cyp51, and human embryonic kidney cells depleted for PGRMC1 have a partial arrest in cholesterol synthesis (Hughes et al., 2007).
In addition to activating Cyp51, PGRMC1 binds to the proteins Insig (insulin-induced gene) and Scap [SREBP cleavage activating protein (Suchanek et al., 2005)], which span the endoplasmic reticulum and sense cholesterol levels. SREBP (sterol regulatory element binding protein) is a transcription factor which exists in a precursor form in the endoplasmic reticulum (Goldstein et al., 2006; Hughes et al., 2007). Insig inhibits the processing and activation of SREBP by binding to Scap (Yang et al., 2002; Goldstein et al., 2006). When Insig is itself inhibited, Scap is released to escort SREBP to the Golgi for processing (Goldstein et al., 2006). The biological role of PGRMC1 in regulating Insig and Scap is unclear, and whether Insig and Scap are part of the P450 or progesterone-binding complexes associated with PGRMC1 is not known.
3.PGRMC1 and DNA damage
3.1 Damage sensitivity phenotypes in yeast
Yeast cells lacking the PGRMC1 homologue, Dap1, are sensitive to DNA damage (Hand et al., 2003; Mallory et al., 2005a; Craven et al., 2007; Hughes et al., 2007; Thompson et al., 2007) due to a failure in a repair process. Like PGRMC1, Dap1 binds to heme (Ghosh et al., 2005; Mallory et al., 2005a; Thompson et al., 2007), and heme binding is essential for damage resistance (Mallory et al., 2005a). Furthermore, exogenous heme or over-expression of the heme synthetic pathway can overcome the requirement for Dap1 in damage resistance (Mallory et al., 2005a; Craven et al., 2007). A second study raised the possibility that heme binding is not required for Dap1-mediated damage resistance (Thompson et al., 2007), but this analysis was performed in a strain harboring a mutation in a key heme-responsive pathway (Gaisne et al., 1999), complicating the interpretation. DNA damage triggers the activation of cell cycle checkpoints, but Dap1 does not alter the cell cycle checkpoint (Hand et al., 2003).
There is a gap in our knowledge regarding the exact mechanism through which Dap1 mediates damage resistance. One important point is that the functions of Dap1 in ergosterol synthesis and damage resistance are distinct. Both the ergosterol synthetic defect and damage sensitivity in dap1Δ cells can be suppressed by hyper-expression of Cyp51 (Mallory et al., 2005a). But hyper-expression of the heme biosynthetic enzymes 5-aminolevulinate synthase and 5-aminolevulinate dehydratase completely suppress the dap1Δ damage resistance phenotype, even though they have no effect on azole drug susceptibility (Craven et al., 2007). Thus, a potential mechanism is that some chemicals may damage heme, heme precursors or P450 metabolites, and that elevated heme synthesis provides a mechanism of damage resistance. In support of this model, one study suggested that heme is a target of methylating agents (Lum et al., 2004), some of which are used in cancer chemotherapy. We conclude that, in cells treated with damaging agents, Dap1 may play a greater role in heme maintenance than sterol synthesis.
3.2 Chemotherapy sensitivity in human tumor cells
Because of the role of Dap1 in damage resistance, our lab tested whether PGRMC1 has an analogous function in cancer cells. PGRMC1 was inhibited by expression of a dominant-negative, heme binding-deficient mutant or by siRNA, and either treatment sensitized breast cancer cells to the chemotherapeutic drugs doxorubicin and camptothecin (Crudden et al., 2006). These drugs are inhibitors of topoisomerase II and topoisomerase I, respectively. Peluso, et al. reported similar results in ovarian cancer cells treated with cisplatin (Peluso et al., 2008a). PGRMC1 expression is induced by chemotherapy (Mallory et al., 2005b; Crudden et al., 2006) and in mouse cells with short telomeres (Franco et al., 2005), which suffer chromosomal damage during senescence and crisis. Together these results suggest that PGRMC1 induction is a consequence of DNA damage, and PGRMC1 plays a role in suppressing damage-induced cell death in cancer cells. In theory, PGRMC1 could be targeted by antagonists of the PGRMC1 complex in combination with chemotherapy to improve its tumoricidal activity, and targeting the larger PGRMC1 complex may have the same effect.
4.Interactions between PGRMC1 and P450 proteins
In yeast and humans, PGRMC1 binds directly to P450 proteins. Dap1 binds to Cyp51/lanosterol demethylase in yeast, and PGRMC1 binds to Cyp51, Cyp3A4, Cyp7A1/cholesterol 7α-hydroxylase and Cyp21A2/21-hydroxylase in humans (Hughes et al., 2007). This implies a role for PGRMC1 in cholesterol synthesis, drug and hormone metabolism and bile acid synthesis. Indeed, monoclonal antibodies to PGRMC1 block the 21-hydroxylation of progesterone in rat adrenal tissue (Laird et al., 1988), and PGRMC1 activates Cyp21 when the two proteins are co-expressed (Min et al., 2004; Min et al., 2005), indicating that PGRMC1 promotes progesterone turnover. As in yeast, human PGRMC1 is required for the Cyp51-catalyzed step in cholesterol synthesis (Hughes et al., 2007). PGRMC1 and its homologues have been likened to “helping hands for P450 proteins” (Debose-Boyd, 2007), and PGRMC1 is highly expressed in the rodent and human liver (Selmin et al., 1996; Gerdes et al., 1998; Krebs et al., 2000; Raza et al., 2001), suggesting that PGRMC1 may contribute to multiple P450-mediated pathways.
P450 proteins require a reductase partner, and Dap1 (the yeast PGRMC1 homologue) has reducing activity (Thompson et al., 2007), suggesting that PGRMC1 class proteins may be more than “helping hands”. PGRMC1 is also related to cytochrome b5, an important co-activator of numerous P450 reactions (Schenkman and Jansson, 2003). However, Dap1 binds to heme through a penta-coordinate mechanism (Thompson et al., 2007), which is distinct from that of cytochrome b5, and hyper-expression of cytochrome b5 could not complement loss of the DAP1 gene in yeast (Mallory et al., 2005a), suggesting that their functions do not overlap. Furthermore, Dap1 has a much higher affinity for ferric heme than ferrous heme (Thompson et al., 2007), suggesting that Dap1 would release heme during a redox cycle. Thus, the precise mechanism through which Dap1 activates P450 proteins is unclear.
Like Dap1, PGRMC1 binds to heme, although its relative affinity for ferric and ferrous heme and its reducing activity have not been determined. The putative structure of PGRMC1, from the protein database, predicts a prominent groove at the center of the protein (Figure 2). The Asp120 residue, which is required for heme binding (Crudden et al., 2006), is at the center of the groove, and a proposed phosphorylation site for the Abl tyrosine kinase (Cahill, 2007) is located near the opening of the groove (Figure 2, right, the tyrosine residue of the GPY). The Tyr164 residue, which corresponds to the yeast Tyr138 site, is required for heme binding in S. cerevisiae Dap1 (Thompson et al., 2007) and S. pombe Dap1 (Hughes et al., 2007). The Tyr107 and Tyr113 residues (indicated by arrows in the GPY site and adjacent to it) are required for heme binding in rat PGRMC1/IZA1 (Min et al., 2004), and all of these residues localize to the same central groove of Pgrmc1 as Asp120 (Figure 2). Dap1 and PGRMC1 have similar roles in cholesterol synthesis and damage resistance, and this is reflected in the conserved residues that surround the central groove (Figure 2, conserved residues are indicated by asterisks). In this depiction, residues that are different in Dap1 and PGPMC1 cluster at one end of the protein and are indicated by pound signs in Figure 2.
5. PGRMC1 and cancer
5.1 PGRMC1 expression in clinical tumor samples
PGRMC1 is over-expressed in breast tumors and in cancer cell lines from the colon, thyroid, lung, and cervix (Crudden et al., 2005). In ovarian cancer, PGRMC1 expression increased in advanced stage tumors, and PGRMC1 was homogeneously expressed within the tumors (Peluso et al., 2008a). Microarray analyses have also detected PGRMC1 expression in colon, lung, ovarian and breast tumors (Shridhar et al., 2001; Difilippantonio et al., 2003; Irby et al., 2005; Dressman et al., 2006). In breast cancer, PGRMC1 was part of a protein signature that predicts hypoxia (Dressman et al., 2006), which is reminiscent of its induction during hypoxia in S. pombe (Hughes et al., 2007). Conversely, the PGRMC1 promoter is hyper-methylated in ovarian cancer (Wei et al., 2006).
One P450-mediated pathway to which PGRMC1 could contribute is the synthesis and metabolism of estrogen and progesterone. Both hormones are synthesized from cholesterol via steps requiring multiple P450 proteins, including P450scc (Cyp11A1), Cyp17 and Cyp19 (aromatase). Estrogen (E2 and E1) is hydroxylated by Cyp1A1, Cyp1A2, Cyp3A4 and Cyp1B1 to form active metabolites 2-hydroxyestradiol (2-OH-E2) and 4-hydroxyestradiol (4-OH-E2). Both 2-OH-E2 and 4-OH-E2 are further oxidized into carcinogenic compounds estrogen-2,3-quinone and estrogen-3,4-quinone (Cavalieri et al., 2000; Bruno and Njar, 2007). While it is intriguing to speculate whether PGRMC1 could contribute to hormone synthesis, signaling and turnover, its biological role in these pathways remains to be elucidated.
5.2 A six gene carcinogenicity signature includes PGRMC1
PGRMC1 is also implicated as a biomarker in other steps in cancer progression. Nie, et al. identified a carcinogenicity signature in rats, which included PGRMC1 and five other genes, in response to 52 known carcinogenic compounds (Nie et al., 2006). Hokaiwado, et al. performed a similar analysis and identified PGRMC1, among others (Hokaiwado et al., 2004). PGRMC1 was originally cloned as 25-Dx based on its induction by the non-genotoxic carcinogen 2,3,7,8-tetrachlorodibenzo-p-dioxin in the rat liver (Selmin et al., 1996), which also induces P450 proteins such as Cyp1A1 (Nebert and Bausserman, 1970). It is unclear how P450 proteins like Cyp1A1 trigger tumor formation, although P450-mediated oxidative damage is a candidate mechanism (Albro et al., 1978). It is notable that human PGRMC1 promotes cell death in cancer cells after oxidative damage (Hand and Craven, 2003), possibly due to its activation of P450 proteins, activates the pro-survival protein kinase Akt and inactivates the cell death-associated protein IκB (Hand and Craven, 2003), suggesting multiple avenues for regulating cell survival.
Interestingly, PGRMC1/25-Dx/mPR induction by TCDD is gender-specific, so that PGRMC1 expression is induced in male rats but repressed in females (Selmin et al., 2005). TCDD induces liver tumors in females but not in males (Kociba et al., 1978), suggesting that gender-specific expression patterns may contribute to TCDD hepatocarcinogenicity. PGRMC1/25-Dx expression is repressed by progesterone and estrogen in murine neurons (Krebs et al., 2000), and it is possible that these hormones repress PGRMC1 in the liver following dioxin treatment. Thus, it is tempting to speculate that PGRMC1 could have a signaling function in male liver tissue that contributes to the early stages of cancer progression.
6.Is PGRMC1 a progesterone receptor?
There is a long-standing link between PGRMC1 and progesterone signaling. However, PGRMC1 was originally identified in liver microsomes (Meyer et al., 1996), is expressed similarly in males and females (Krebs et al., 2000), and has homologues in organisms that do not synthesize progesterone. Purified PGRMC1 does not bind to progesterone (Min et al., 2005), and the vast majority of PGRMC1 is not localized to the plasma membrane (Nolte et al., 2000; Crudden et al., 2005; Peluso et al., 2008a). Recent reviews appear to have reached a consensus that PGRMC1 is not by itself a progesterone receptor (Cahill, 2007; Losel et al., 2007). Thus, the name Hpr6.6 for human progesterone receptor is not accurate.
PGRMC1-associated progesterone binding requires an unknown protein that is associated only in partially purified PGRMC1 preparations (Peluso et al., 2008b). PGRMC1-associated progesterone binding is functionally important in cancer cells because progesterone inhibits apoptosis in granulosa cells, and this anti-apoptotic activity requires PGRMC1 (Peluso et al., 2006; Peluso et al., 2008b). However, it is unclear how PGRMC1 transduces anti-apoptotic signaling by progesterone. PAIR-BP1 (plasminogen activator inhibitor 1 RNA binding protein) binds to PGRMC1, but a PGRMC1 mutant that binds to PAIR-BP1 does not co-purify with progesterone-binding activity (Peluso et al., 2008b), suggesting that PAIR-BP1 and progesterone binding are not functionally linked.
The PGRMC1-associated progesterone-binding activity has a similar affinity for corticosterone, testosterone and cortisol (Meyer et al., 1996), suggesting limited specificity in its hormone binding. Remarkably, PGRMC1 associated progesterone binding can be competed with haloperidol, an anti-psychotic drug, with a 20 nM Ki (Meyer et al., 1998). Progesterone binding was also inhibited with fluphenazine, carbetapentane citrate and R(-)-N-(3-phenyl-1-propyl)-1-phenyl-2 aminopropane HCl (PPAP HCl) among others (Meyer et al., 1998), suggesting that the PGRMC1 complex is a mixed-function steroid/drug-binding complex (S/D-BP, Figure 3A). One intriguing model is that the PGRMC1-S/D-BP complex could bind to progesterone (Figure 3A), transmit a signal, and then switch to a complex with Cyp21, which metabolizes progesterone (Figure 3B). In theory, the same complex could bind to other steroids or xenobiotic compounds and shuttle the compounds to other P450 proteins. However, we emphasize that this model is entirely speculative. Interestingly, the heme-binding deficient mutant D120G is deficient in the formation of the PGRMC1-S/D-BP complex (Peluso et al., 2008b), suggesting a role for the heme-binding crevice in this complex.
Fig. 3.
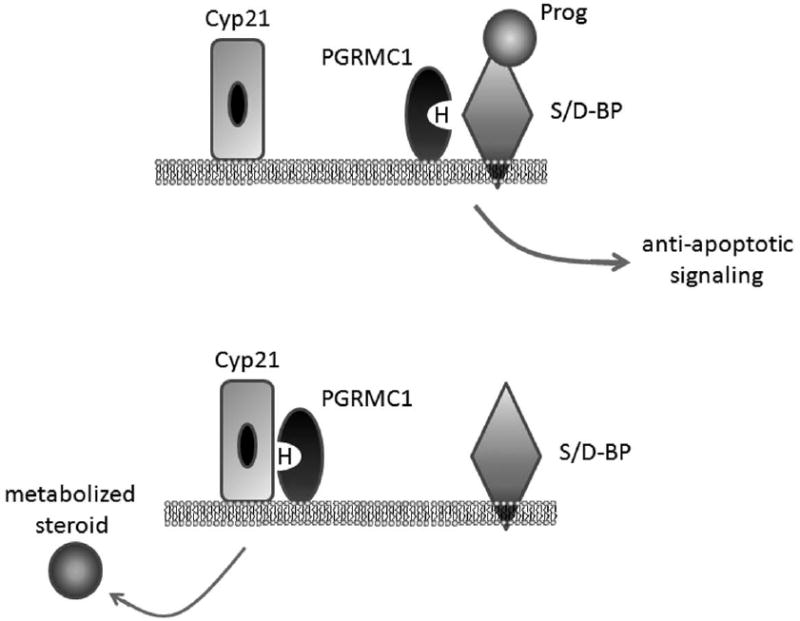
A proposed model for PGRMC1 in progesterone signaling and metabolism. PGRMC1 is part of a progesterone binding complex in which it binds to an uncharacterized steroid/drug binding protein (S/D-BP) that also has affinity for corticosterone, testosterone, cortisol and haloperidol. Progesterone binding by this complex has anti-apoptotic activity that requires PGRMC1. Heme (H) is the ligand for PGRMC1, and heme binding is required for its anti-apoptotic function. Cyp21/21-hydroxylase also binds to PGRMC1 and metabolizes progesterone to 11-deoxycorticosterone.
While the exact function of PGRMC1 in progesterone signaling is not known, progesterone clearly regulates PGRMC1 expression. In the ovary, PGRMC1 expression is induced by progesterone (Nilsson et al., 2006), and in the corpus luteum, its expression increases during pregnancy (Cai and Stocco, 2005). However, in the simian endometrium, PGRMC1 is down-regulated by progesterone (Ace and Okulicz, 2004), as it is in the mouse hypothalamus (Krebs et al., 2000). The results suggest that the hormonal regulation of PGRMC1 expression is tissue-specific.
7.PGRMC1 expression in the brain
PGRMC1/25-Dx is expressed in various areas of the brain (hypothalamic area, circumventricular organs, ependymal cells of the lateral ventricles, meninges), particularly in structures involved in CSF production and in osmoregulation (Meffre et al., 2005). PGRMC1/25-Dx is expressed at a basal level in the cerebellum when rats are born, and the mRNA and protein level of PGRMC1 are increased neonatally and decreased thereafter in both male and female rats, without significant difference between the genders (Sakamoto et al., 2004). PGRMC1 is expressed mainly at Purkinje and external granule cells in the cerebellum of neonate rats, whereas in the adult cerebellum, PGRMC1 is expressed in Purkinje cells (Sakamoto et al., 2004). Cerebellar Purkinje cells are the sites of de novo synthesis of progesterone from cholesterol, where the steroids promote dendritic growth, synaptogenesis and spinogenesis.
Progesterone is neuroprotective in a number of different model systems for brain lesions (Schumacher et al., 2007), and PGRMC1 is induced by progesterone after traumatic brain injury (Labombarda et al., 2003). In addition, pseudopregnant rats have increased expression of 25-Dx (Meffre et al., 2005), which also corresponds with neuroprotection. However, it is unclear whether PGRMC1 directly regulates neuroprotection and what its mechanism might be. One intriguing clue is the neurotrophic activity of the PGRMC1 family member neudesin, which is secreted (Kimura et al., 2008). Neudesin binds to heme and directly stimulates MAP kinase and Akt signaling in cultured neurons (Kimura et al., 2008). However, the receptor system for neudesin remains to be characterized, and it is unclear whether a portion of PGRMC1 might be secreted in a similar manner.
8.Other phenotypes associated with PGRMC1
Some phenotypes that are readily detectable in yeast have not been analyzed in detail in mammals. In S. cerevisiae, dap1Δ mutants are deficient in endocytosis (Hand et al., 2003). The phenotype was detected by measuring dye uptake, and the majority of sterol pathway mutants have increased dye uptake (Bard et al., 1978), probably due to membrane defects that allow the dye to leach into the cells. In contrast, dap1Δ mutants have decreased dye uptake, suggesting that Dap1 contributes to endocytosis. This phenotype suggests a role for Dap1 in membrane uptake and/or intracellular trafficking. Indeed, Dap1 localizes to an endosomal fraction (Craven et al., 2007) and contributes to the transport or storage of iron in yeast (Craven et al., 2007). It is unclear whether this endocytosis phenotype is conserved in humans, but the localization of PGRMC1 to the endoplasmic reticulum or to punctuate cytoplasmic sites overlaps with that of the yeast homologue (Nolte et al., 2000; Crudden et al., 2005; Peluso et al., 2008a; Peluso et al., 2008b).
In addition to regulating endocytosis, PGRMC1 has a role in regulating protein kinase-associated signaling (Hand and Craven, 2003) in which PGRMC1 increases Akt activation. Akt is phosphorylated by the PDK1 protein kinase, and there is a putative PDK1 binding region on PGRMC1 (Cahill, 2007). The exact mechanism through which PGRMC1 activates Akt is unknown, but recent work from our laboratory suggests a role for PGRMC1 in membrane-associated signaling in cancer cells (Rohe, et al., manuscript in preparation). The ability of PGRMC1 to activate signaling resembles that of yeast dap1Δ mutants, which have a defect in membrane-associated signaling from a G-protein coupled receptor cascade for the yeast α-factor mating pheromone (Hand et al., 2003). Interestingly, there is some indirect evidence that PGRMC1 binds to caveolin (Bramley et al., 2002), which controls signaling through a number of receptor complexes (Okamoto et al., 1998; Carpenter, 2000; Zajchowski and Robbins, 2002). Thus, one potential model through which PGRMC1 regulates signaling is that a PGRMC1-caveolin complex contributes to the secretion, stability or activation of signaling proteins at the cell membrane.
Another area of research that began in model organisms involves the transcriptional regulation of Dap1 and PGRMC1. In S. pombe, the DAP1 gene is under the control of the SREBP (sterol regulatory element binding protein) homologue SRE1, particularly under hypoxic growth conditions (Hughes et al., 2007), raising the possibility that PGRMC1 is similarly regulated by SREBP. Promoter mapping software predicts a sterol regulatory element in the PGRMC1 promoter, but it is unclear whether this site is active. Because PGRMC1 binds to Insig and Scap (Suchanek et al., 2005), one intriguing model is that PGRMC1 could be part of a feedback mechanism in regulating SREBP processing. However, at present, this is only speculation.
Conclusions
PGRMC1 is induced in a spectrum of cancers, where it promotes cell survival and damage resistance. In spite of its name, PGRMC1 shares homology with cytochrome b5-related proteins rather than hormone receptors, and heme binding is the sole biochemical activity of PGRMC1. The PGRMC1 binding partners that promote cell survival are not known, but may include P450 proteins, PAIR-BP1, Insig, and an uncharacterized hormone/drug-binding protein. PGRMC1 regulates cholesterol synthesis, which is important in controlling membrane-associated signaling, and the downstream targets of PGRMC1 include the serine/threonine kinase Akt. PGRMC1 is also expressed in neuronal tissue and tissues involved in cholesterol synthesis and hormone synthesis and turnover. One of the appealing features of PGRMC1 and its associated protein complex is its apparent access to steroids and drugs. Together with its biological role in promoting tumor survival, PGRMC1 appears to be an attractive target for therapeutic intervention in cancer and related malignancies.
Acknowledgments
We are grateful to Clay Adams Condley and Martin Bard for helpful discussions about PGRMC1. This work was supported in part by the American Cancer Society, grant number 85-001-19-IRG, the NIH grant COBRE P20 RR 15592 and BIRCWH (Building Interdisciplinary Research Careers in Women’s Health) grant K12DA 14040-06.
Abbreviations
- Insig
insulin-induced gene
- PAIR-BP1
plasminogen activator inhibitor mRNA binding protein
- PGRMC1
progesterone receptor membrane component 1
- Scap
SREBP cleavage activating protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ace CI, Okulicz WC. Microarray profiling of progesterone-regulated endometrial genes during the rhesus monkey secretory phase. Reprod Biol Endocrinol. 2004;2:54. doi: 10.1186/1477-7827-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro PW, Corbett JT, Harris M, Lawson LD. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on lipid profiles in tissue of the Fischer rat. Chem Biol Interact. 1978;23:315–330. doi: 10.1016/0009-2797(78)90093-5. [DOI] [PubMed] [Google Scholar]
- Bard M, Lees ND, Burrows LS, Kleinhans FW. Differences in crystal violet uptake and cation-induced death among yeast sterol mutants. J Bacteriol. 1978;135:1146–1148. doi: 10.1128/jb.135.3.1146-1148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley TA, Menzies GS, Rae MT, Scobie G. Non-genomic steroid receptors in the bovine ovary. Domest Anim Endocrinol. 2002;23:3–12. doi: 10.1016/s0739-7240(02)00140-6. [DOI] [PubMed] [Google Scholar]
- Bruno RD, Njar VC. Targeting cytochrome P450 enzymes: a new approach in anti-cancer drug development. Bioorg Med Chem. 2007;15:5047–5060. doi: 10.1016/j.bmc.2007.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill MA. Progesterone receptor membrane component 1: An integrative review. J Steroid Biochem Mol Biol. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Cai Z, Stocco C. Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology. 2005;146:5522–5532. doi: 10.1210/en.2005-0759. [DOI] [PubMed] [Google Scholar]
- Carpenter G. The EGF receptor: a nexus for trafficking and signaling. Bioessays. 2000;22:697–707. doi: 10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents--DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- Craven RJ, Mallory JC, Hand RA. Regulation of iron homeostasis mediated by the heme-binding protein Dap1 (damage resistance protein 1) via the P450 protein Erg11/Cyp51. J Biol Chem. 2007;282:36543–36551. doi: 10.1074/jbc.M706770200. [DOI] [PubMed] [Google Scholar]
- Crudden G, Chitti RE, Craven RJ. Hpr6 (heme-1 domain protein) regulates the susceptibility of cancer cells to chemotherapeutic drugs. J Pharmacol Exp Ther. 2006;316:448–455. doi: 10.1124/jpet.105.094631. [DOI] [PubMed] [Google Scholar]
- Crudden G, Loesel R, Craven RJ. Overexpression of the cytochrome p450 activator hpr6 (heme-1 domain protein/human progesterone receptor) in tumors. Tumour Biol. 2005;26:142–146. doi: 10.1159/000086485. [DOI] [PubMed] [Google Scholar]
- Debose-Boyd RA. A helping hand for cytochrome p450 enzymes. Cell Metab. 2007;5:81–83. doi: 10.1016/j.cmet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Difilippantonio S, Chen Y, Pietas A, Schluns K, Pacyna-Gengelbach M, Deutschmann N, Padilla-Nash HM, Ried T, Petersen I. Gene expression profiles in human non-small and small-cell lung cancers. Eur J Cancer. 2003;39:1936–1947. doi: 10.1016/s0959-8049(03)00419-2. [DOI] [PubMed] [Google Scholar]
- Dressman HK, Hans C, Bild A, Olson JA, Rosen E, Marcom PK, Liotcheva VB, Jones EL, Vujaskovic Z, Marks J, Dewhirst MW, West M, Nevins JR, Blackwell K. Gene expression profiles of multiple breast cancer phenotypes and response to neoadjuvant chemotherapy. Clin Cancer Res. 2006;12:819–826. doi: 10.1158/1078-0432.CCR-05-1447. [DOI] [PubMed] [Google Scholar]
- Franco S, Canela A, Klatt P, Blasco MA. Effectors of mammalian telomere dysfunction: a comparative transcriptome analysis using mouse models. Carcinogenesis. 2005;26:1613–1626. doi: 10.1093/carcin/bgi107. [DOI] [PubMed] [Google Scholar]
- Gaisne M, Becam AM, Verdiere J, Herbert CJ. A ‘natural’ mutation in Saccharomyces cerevisiae strains derived from S288c affects the complex regulatory gene HAP1 (CYP1) Curr Genet. 1999;36:195–200. doi: 10.1007/s002940050490. [DOI] [PubMed] [Google Scholar]
- Gerdes D, Wehling M, Leube B, Falkenstein E. Cloning and tissue expression of two putative steroid membrane receptors. Biol Chem. 1998;379:907–911. doi: 10.1515/bchm.1998.379.7.907. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Thompson AM, Goldbeck RA, Shi X, Whitman S, Oh E, Zhiwu Z, Vulpe C, Holman TR. Spectroscopic and biochemical characterization of heme binding to yeast Dap1p and mouse PGRMC1p. Biochemistry. 2005;44:16729–16736. doi: 10.1021/bi0511585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Hand RA, Craven RJ. Hpr6.6 protein mediates cell death from oxidative damage in MCF-7 human breast cancer cells. J Cell Biochem. 2003;90:534–547. doi: 10.1002/jcb.10648. [DOI] [PubMed] [Google Scholar]
- Hand RA, Jia N, Bard M, Craven RJ. Saccharomyces cerevisiae Dap1p, a novel DNA damage response protein related to the mammalian membrane-associated progesterone receptor. Eukaryot Cell. 2003;2:306–317. doi: 10.1128/EC.2.2.306-317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokaiwado N, Asamoto M, Tsujimura K, Hirota T, Ichihara T, Satoh T, Shirai T. Rapid analysis of gene expression changes caused by liver carcinogens and chemopreventive agents using a newly developed three-dimensional microarray system. Cancer Sci. 2004;95:123–130. doi: 10.1111/j.1349-7006.2004.tb03192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AL, Powell DW, Bard M, Eckstein J, Barbuch R, Link AJ, Espenshade PJ. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007;5:143–149. doi: 10.1016/j.cmet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Irby RB, Malek RL, Bloom G, Tsai J, Letwin N, Frank BC, Verratti K, Yeatman TJ, Lee NH. Iterative microarray and RNA interference-based interrogation of the SRC-induced invasive phenotype. Cancer Res. 2005;65:1814–1821. doi: 10.1158/0008-5472.CAN-04-3609. [DOI] [PubMed] [Google Scholar]
- Kimura I, Nakayama Y, Yamauchi H, Konishi M, Miyake A, Mori M, Ohta M, Itoh N, Fujimoto M. Neurotrophic activity of neudesin, a novel extracellular heme-binding protein, is dependent on the binding of heme to its cytochrome b5-like heme/steroid-binding domain. J Biol Chem. 2008;283:4323–4331. doi: 10.1074/jbc.M706679200. [DOI] [PubMed] [Google Scholar]
- Kociba RJ, Keyes DG, Beyer JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CN, Barnard SD, Hummel RA, Humiston CG. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol Appl Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci U S A. 2000;97:12816–12821. doi: 10.1073/pnas.97.23.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labombarda F, Gonzalez SL, Deniselle MC, Vinson GP, Schumacher M, De Nicola AF, Guennoun R. Effects of injury and progesterone treatment on progesterone receptor and progesterone binding protein 25-Dx expression in the rat spinal cord. J Neurochem. 2003;87:902–913. doi: 10.1046/j.1471-4159.2003.02055.x. [DOI] [PubMed] [Google Scholar]
- Laird SM, Vinson GP, Whitehouse BJ. Monoclonal antibodies against rat adrenocortical cell antigens. Acta Endocrinol (Copenh) 1988;119:420–426. doi: 10.1530/acta.0.1190420. [DOI] [PubMed] [Google Scholar]
- Lepesheva GI, Waterman MR. Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim Biophys Acta. 2007;1770:467–477. doi: 10.1016/j.bbagen.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losel RM, Besong D, Peluso JJ, Wehling M. Progesterone receptor membrane component 1-Many tasks for a versatile protein. Steroids. 2007 doi: 10.1016/j.steroids.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Lum PY, Armour CD, Stepaniants SB, Cavet G, Wolf MK, Butler JS, Hinshaw JC, Garnier P, Prestwich GD, Leonardson A, Garrett-Engele P, Rush CM, Bard M, Schimmack G, Phillips JW, Roberts CJ, Shoemaker DD. Discovering modes of action for therapeutic compounds using a genome-wide screen of yeast heterozygotes. Cell. 2004;116:121–137. doi: 10.1016/s0092-8674(03)01035-3. [DOI] [PubMed] [Google Scholar]
- Mallory JC, Crudden G, Johnson BL, Mo C, Pierson CA, Bard M, Craven RJ. Dap1p, a heme-binding protein that regulates the cytochrome P450 protein Erg11p/Cyp51p in Saccharomyces cerevisiae. Mol Cell Biol. 2005a;25:1669–1679. doi: 10.1128/MCB.25.5.1669-1679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory JC, Crudden G, Oliva A, Saunders C, Stromberg A, Craven RJ. A novel group of genes regulates susceptibility to antineoplastic drugs in highly tumorigenic breast cancer cells. Mol Pharmacol. 2005b;68:1747–1756. doi: 10.1124/mol.105.016519. [DOI] [PubMed] [Google Scholar]
- Meffre D, Delespierre B, Gouezou M, Leclerc P, Vinson GP, Schumacher M, Stein DG, Guennoun R. The membrane-associated progesterone-binding protein 25-Dx is expressed in brain regions involved in water homeostasis and is up-regulated after traumatic brain injury. J Neurochem. 2005;93:1314–1326. doi: 10.1111/j.1471-4159.2005.03127.x. [DOI] [PubMed] [Google Scholar]
- Meyer C, Schmid R, Scriba PC, Wehling M. Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur J Biochem. 1996;239:726–731. doi: 10.1111/j.1432-1033.1996.0726u.x. [DOI] [PubMed] [Google Scholar]
- Meyer C, Schmieding K, Falkenstein E, Wehling M. Are high-affinity progesterone binding site(s) from porcine liver microsomes members of the sigma receptor family? Eur J Pharmacol. 1998;347:293–299. doi: 10.1016/s0014-2999(98)00103-4. [DOI] [PubMed] [Google Scholar]
- Mifsud W, Bateman A. Membrane-bound progesterone receptors contain a cytochrome b5-like ligand-binding domain. Genome Biol. 2002;3:RESEARCH0068. doi: 10.1186/gb-2002-3-12-research0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min L, Strushkevich NV, Harnastai IN, Iwamoto H, Gilep AA, Takemori H, Usanov SA, Nonaka Y, Hori H, Vinson GP, Okamoto M. Molecular identification of adrenal inner zone antigen as a heme-binding protein. Febs J. 2005;272:5832–5843. doi: 10.1111/j.1742-4658.2005.04977.x. [DOI] [PubMed] [Google Scholar]
- Min L, Takemori H, Nonaka Y, Katoh Y, Doi J, Horike N, Osamu H, Raza FS, Vinson GP, Okamoto M. Characterization of the adrenal-specific antigen IZA (inner zone antigen) and its role in the steroidogenesis. Mol Cell Endocrinol. 2004;215:143–148. doi: 10.1016/j.mce.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Bausserman LL. Genetic differences in the extent of aryl hydrocarbon hydroxylase induction in mouse fetal cell cultures. J Biol Chem. 1970;245:6373–6382. [PubMed] [Google Scholar]
- Nie AY, McMillian M, Parker JB, Leone A, Bryant S, Yieh L, Bittner A, Nelson J, Carmen A, Wan J, Lord PG. Predictive toxicogenomics approaches reveal underlying molecular mechanisms of nongenotoxic carcinogenicity. Mol Carcinog. 2006;45:914–933. doi: 10.1002/mc.20205. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Stanfield J, Skinner MK. Interactions between progesterone and tumor necrosis factor-alpha in the regulation of primordial follicle assembly. Reproduction. 2006;132:877–886. doi: 10.1530/REP-06-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte I, Jeckel D, Wieland FT, Sohn K. Localization and topology of ratp28, a member of a novel family of putative steroid-binding proteins. Biochim Biophys Acta. 2000;1543:123–130. doi: 10.1016/s0167-4838(00)00188-6. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Liu X, Saunders MM, Claffey KP, Phoenix K. Regulation of ovarian cancer cell viability and sensitivity to cisplatin by progesterone receptor membrane component-1. J Clin Endocrinol Metab. 2008a;93:1592–1599. doi: 10.1210/jc.2007-2771. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone’s antiapoptotic action. Endocrinology. 2006;147:3133–3140. doi: 10.1210/en.2006-0114. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Romak J, Liu X. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone’s antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology. 2008b;149:534–543. doi: 10.1210/en.2007-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza FS, Takemori H, Tojo H, Okamoto M, Vinson GP. Identification of the rat adrenal zona fasciculata/reticularis specific protein, inner zone antigen (IZAg), as the putative membrane progesterone receptor. Eur J Biochem. 2001;268:2141–2147. doi: 10.1046/j.1432-1327.2001.02096.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Ukena K, Takemori H, Okamoto M, Kawata M, Tsutsui K. Expression and localization of 25-Dx, a membrane-associated putative progesterone-binding protein, in the developing Purkinje cell. Neuroscience. 2004;126:325–334. doi: 10.1016/j.neuroscience.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Schenkman JB, Jansson I. The many roles of cytochrome b5. Pharmacol Ther. 2003;97:139–152. doi: 10.1016/s0163-7258(02)00327-3. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Stein DG, De Nicola AF. Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol Ther. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Selmin O, Lucier GW, Clark GC, Tritscher AM, Vanden Heuvel JP, Gastel JA, Walker NJ, Sutter TR, Bell DA. Isolation and characterization of a novel gene induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Carcinogenesis. 1996;17:2609–2615. doi: 10.1093/carcin/17.12.2609. [DOI] [PubMed] [Google Scholar]
- Selmin O, Thorne PA, Blachere FM, Johnson PD, Romagnolo DF. Transcriptional activation of the membrane-bound progesterone receptor (mPR) by dioxin, in endocrine-responsive tissues. Mol Reprod Dev. 2005;70:166–174. doi: 10.1002/mrd.20090. [DOI] [PubMed] [Google Scholar]
- Shridhar V, Lee J, Pandita A, Iturria S, Avula R, Staub J, Morrissey M, Calhoun E, Sen A, Kalli K, Keeney G, Roche P, Cliby W, Lu K, Schmandt R, Mills GB, Bast RC, Jr, James CD, Couch FJ, Hartmann LC, Lillie J, Smith DI. Genetic analysis of early- versus late-stage ovarian tumors. Cancer Res. 2001;61:5895–5904. [PubMed] [Google Scholar]
- Suchanek M, Radzikowska A, Thiele C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat Methods. 2005;2:261–267. doi: 10.1038/nmeth752. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Reddi AR, Shi X, Goldbeck RA, Moenne-Loccoz P, Gibney BR, Holman TR. Measurement of the Heme Affinity for Yeast Dap1p, and Its Importance in Cellular Function. Biochemistry. 2007 doi: 10.1021/bi7013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SH, Balch C, Paik HH, Kim YS, Baldwin RL, Liyanarachchi S, Li L, Wang Z, Wan JC, Davuluri RV, Karlan BY, Gifford G, Brown R, Kim S, Huang TH, Nephew KP. Prognostic DNA methylation biomarkers in ovarian cancer. Clin Cancer Res. 2006;12:2788–2794. doi: 10.1158/1078-0432.CCR-05-1551. [DOI] [PubMed] [Google Scholar]
- Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, Brown MS. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- Zajchowski LD, Robbins SM. Lipid rafts and little caves. Compartmentalized signalling in membrane microdomains. Eur J Biochem. 2002;269:737–752. doi: 10.1046/j.0014-2956.2001.02715.x. [DOI] [PubMed] [Google Scholar]


