Abstract
Objective
To investigate the association between cholesterol lowering interventions and risk of death from suicide, accident, or trauma (non-illness mortality).
Design
Meta-analysis of the non-illness mortality outcomes of large, randomised clinical trials of cholesterol lowering treatments.
Studies reviewed
19 out of 21 eligible trials that had data available on non-illness mortality.
Interventions reviewed
Dietary modification, drug treatment, or partial ileal bypass surgery for 1-10 years
Main outcome measure
Deaths from suicides, accidents, and violence in treatment groups compared with control groups.
Results
Across all trials, the odds ratio of non-illness mortality in the treated groups, relative to control groups, was 1.18 (95% confidence interval 0.91 to 1.52; P=0.20). The odds ratios were 1.28 (0.94 to 1.74; P=0.12) for primary prevention trials and 1.00 (0.65 to 1.55; P=0.98) for secondary prevention trials. Randomised clinical trials using statins did not show a treatment related rise in non-illness mortality (0.84, 0.50 to 1.41; P=0.50), whereas a trend toward increased deaths from suicide and violence was observed in trials of dietary interventions and non-statin drugs (1.32, 0.98 to 1.77; P=0.06). No relation was found between the magnitude of cholesterol reduction and non-illness mortality (P=0.23).
Conclusion
Currently available evidence does not indicate that non-illness mortality is increased significantly by cholesterol lowering treatments. A modest increase may occur with dietary interventions and non-statin drugs.
Introduction
Many people are now trying to lower their serum cholesterol concentrations in order to prevent coronary heart disease. Modification of diet is the first line intervention for hypercholesterolaemia, but as it has limited efficacy,1,2 a rapidly growing number of people are taking cholesterol lowering drugs.3 As with all broadly prescribed, preventive treatments, it is important to establish that long term cholesterol reduction does not have severe adverse effects. Ten years ago, evidence supporting the efficacy of lowering cholesterol concentrations in prevention of coronary heart disease was just beginning to accumulate. At that time, data from randomised clinical trials raised concerns that reducing cholesterol concentrations might increase cancer mortality and deaths from suicides, accidents, and violence (non-illness mortality).4–6
A powerful class of cholesterol lowering drugs has been introduced in the past decade—hydroxymethylglutaryl coenzyme A reductase inhibitors, or statins. Several large clinical trials have shown that these drugs reduce major cardiovascular events by 20-30%.7 The trials also indicated that treatment with a statin for five to six years does not affect mortality from cancer. Nevertheless, the carcinogenic properties of cholesterol lowering drugs have been noted in some laboratory research,8 and the potential effect of statins on rates of cancer in humans requires further study and longer follow up.
Whether non-illness mortality increases with cholesterol reduction also remains unclear.9–11 Associations have been reported between low serum cholesterol concentrations and non-illness mortality,12 suicidal behaviour,13–15 violent crime,16 and impulsive aggression and personality disorders.17–19 However, a recent case-control study found that neither fatal nor non-fatal injuries were related to use of cholesterol lowering drugs.20 Although suicides, accidents, and trauma are a leading cause of premature death, they are relatively uncommon among participants in clinical trials. This hinders the study of treatment effects and may be due to underrepresentation of people at risk of death from these causes (by exclusion of people with a history of mood disorder, other psychiatric illness, substance misuse, or antisocial behaviour). A 1990 meta-analysis of 103 deaths due to suicide or violence among men participating in large primary prevention trials found that non-illness mortality was increased significantly by cholesterol lowering diets and drugs.4 This quantitative review re-evaluates the potential effects of cholesterol interventions on non-illness mortality, adding outcomes reported among women, data from secondary prevention trials, and the findings of recent clinical trials of statins. We included intervention specific analyses because statins and other treatments have been found to differ significantly in their effects on non-coronary heart disease mortality.21
Methods
Selection and description of studies
We included clinical trials of cholesterol lowering treatments in which participants were randomly assigned to a cholesterol lowering intervention or a control group, the mean serum cholesterol concentration in the control group remained relatively stable (⩽5% variation) during the trial, and other interventions (such as antihypertensive drugs or advice on stopping smoking or stress reduction) were not administered preferentially to participants in the treatment group. We included only trials that were designed to measure effects of treatment on clinical events and mortality. Trials intended to study treatment effects on serum lipid concentrations and tolerability and those examining preclinical outcomes (angiographic findings) typically lack procedures for following subjects who withdraw from the study and independent monitors to adjudicate cause of death.
We identified studies by using the ancestry approach (locating previous studies cited in reference lists of already identified studies and published review articles) and computer based literature searches of Medline from 1966 to March 2000. The search strategy paired the term “controlled clinical trial” with each of the following: “cholesterol,” “diet (fat restricted),” and “anticholesterolemic drugs.”
The computerised literature searches identified 275 references, but only a few were clinical trials of cholesterol reduction. Together with trials accumulated by the ancestry approach, we had a total of 64 trials for consideration. Twenty one met our inclusion criteria (see BMJ's website for list of trials and details). The most common reasons for exclusion were the use of multifactorial risk interventions and studies not designed to monitor clinical events and cause specific mortality. The Veterans Administration high density lipoprotein cholesterol trial was not included because it did not study the effects of cholesterol lowering. We extracted data from the primary publication, supplemented by subsequent reports when available.22,23
The reports of 15 of the 21 eligible trials contained data on non-illness mortality. We sought data from the authors for the other six trialsw9 w11 w13 w14 w20 w21 and for two trials for which non-illness mortality data were incomplete.w1 w3 Investigators provided previously unpublished non-illness mortality data for four trials.w9 w11 w13 w14 Therefore, we were able to include data from 19 of the 21 trials meeting the meta-analysis selection criteria. The two trials for which non-illness mortality was not available (both secondary prevention trials of clofibrate) and the two with incomplete results were comparatively small, together accounting for only 3% of the trial data in terms of patient years of observation.
Analysis of data
For each trial, we constructed 2×2 contingency tables enumerating the number of non-illness related deaths (accidents, violence, and suicide) and the number of participants not dying of non-illness causes separately for the intervention and control groups. These tables were combined according to the Mantel-Haenszel procedure,24 as modified by Yusuf and Peto25 and previously described by us.4 This analysis generates a summary odds ratio and associated 95% confidence interval for non-illness mortality in the treated participants relative to controls.
We assessed the association of non-illness mortality with the average amount of cholesterol reduction through a weighted regression model. The dependent variable in the regression analyses was the log odds ratio of non-illness mortality for the intervention group versus the control group across the individual studies. To include studies containing groups with no deaths from non-illness causes, we assigned a value of 0.5 for the number of deaths in these groups. We performed a sensitivity analysis to examine the degree to which the results changed when a value of 0.01 or 0.1 was assigned as the number of deaths for these groups. Additionally, we fitted a model excluding the five studies that contained groups with no deaths from non-illness causes. These changes did not substantially affect the results. Analysis of the residuals found no evidence of any violation of the assumptions in the regression model.
Results
The table gives the non-illness mortality for the included trials. Trials were classified as primary prevention if criteria for participants' eligibility did not include a history of coronary heart disease; all or most participants in these studies were free of heart disease at entry. Secondary prevention trials were those consisting exclusively of people with clinically evident coronary heart disease. Most participants were men aged between 40 and 70 years. The primary prevention trials had a total of 42 500 participants and secondary prevention trials enrolled 28 204 participants. All but one trial assigned an equal number of participants to the intervention and control groups. The cholesterol lowering intervention was dietary in five trials, pharmacological in 13 trials, and surgical in one trial. Five of six trials published after 1990 used a statin as the cholesterol lowering intervention. Each treatment successfully lowered the average serum cholesterol concentration in the intervention group, relative to corresponding control conditions, although the mean reduction varied appreciably across studies (3.5% to 26%). Together, these studies generated about 338 000 patient years of randomised clinical trial data.
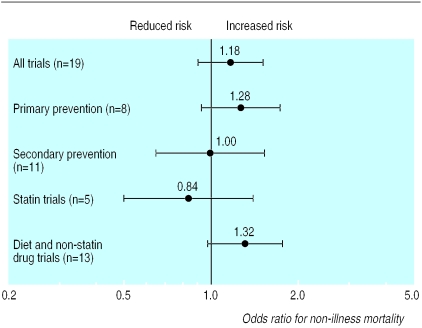
The figure shows the odds ratios for non-illness mortality across trials in the treated groups compared with control subjects. The χ2 test of heterogeneity among trial findings was not significant (P>0.05). The overall odds ratio across trials was not significantly different from 1 (1.18, 95% confidence interval 0.91 to 1.52; P=0.20). Subgroup analyses were conducted on primary and secondary prevention trials separately, and on the basis of previous meta-analyses, clinical trials using statins were separated from those using other cholesterol lowering interventions. These analyses also found no significant increases in the odds of non-illness mortality associated with cholesterol reduction. In the 13 trials of dietary therapy or non-statin drugs there was an increase in death from suicide and trauma in the treatment group compared with the control group, although this finding was not significant (odds ratio 1.32, 0.98 to 1.77; P=0.06). Finally, in a weighted regression model, the odds ratio of non-illness mortality in the treatment group versus the control group in each of the trials was not related to the mean cholesterol reduction (β= −0.04 (SE 0.03), P=0.23).
Discussion
In this updated meta-analysis we found that deaths from suicides, accident, and violence were not significantly increased among participants randomised to a cholesterol lowering intervention compared with those in the control groups. This was true for both primary and secondary prevention trials when they were analysed separately. Additionally, we found no association between the size of the magnitude of cholesterol reduction and the likelihood of death from suicide, accident, or trauma.
Although this analysis included a total of 215 people who died of non-illness causes, deaths from suicide, accidents and trauma are uncommon in randomised clinical trials of cholesterol reduction, and were less common than would be expected from population data. Among control participants in the two large American trials conducted in the 1990s,w8 w18 mortality from suicide and other injuries was 25 per 100 000 person years compared with an age and sex specific normative population rate of about 68 per 100 000.26 Therefore, we should consider whether selection of participants may have influenced the results of these studies. Clinical trials use rigorous eligibility criteria that either explicitly or indirectly exclude people with histories of mental disorder or substance misuse.w17 As a result, the trials exclude many people who may be particularly susceptible to the psychological effects of medical treatments. Whether selection bias influences reported treatment effects on non-illness mortality is difficult to determine, but the small number of deaths per trial may affect interpretation of our results. The confidence interval for the odds ratio across all 19 studies, for instance, straddles 1 and includes, at the upper boundary, a potential 50% increase in non-illness mortality.
The absence of a significant effect of treatment on non-illness mortality alone does not exclude the possibility of cholesterol reduction having any adverse effects on psychological wellbeing or quality of life. Nevertheless, randomised controlled trials suggest that statins do not increase new psychiatric diagnoses and hospital admissions or non-fatal trauma27,28 and may not adversely affect sleep29 or self reported quality of life, mood, hostility, or anger expression.30–32 These findings are clinically important because statins are now the most reliable and widely prescribed cholesterol lowering drugs. Statins have, however, been reported to reduce cognitive performance.30,33
Treatments for hypercholesterolaemia have varying effects on lipids and the rest of the body,34,35 and these treatments may therefore affect the brain or behaviour differently.36 Interestingly, risk of stroke falls during treatment in high risk patients taking statins but not in patients taking other cholesterol lowering interventions.37 Earlier meta-analyses of cholesterol lowering diets and non-statin drug treatment found treatment related increases in non-illness mortality.4,5,9 This effect is only marginally significant when recent trial data are included. Data on other behavioural outcomes during treatment with non-statin interventions are also mixed and incomplete. Studies in primates have found that consumption of low fat and low cholesterol diets potentiates aggressive behaviour and decreases social affiliation.38,39 In humans, several dietary interventions have been found to be free of adverse effects on mood but, like statins, may induce subtle cognitive decrements.40,41
What is already known on this topic
Cholesterol lowering treatments reduce cardiovascular mortality and morbidity
These treatments may affect mortality from other causes
What this study adds
Overall, cholesterol lowering treatments do not affect risk of death from suicide, accident, or trauma
Statins did not adversely affect non-illness mortality, but the effect of other treatments is less clear
Supplementary Material
Figure.
Odds ratios and 95% confidence intervals of non-illness mortality in treated groups, relative to controls, in randomised clinical trials of cholesterol reduction. Statins were used in two primary prevention and four secondary prevention trials
Table.
Mean cholesterol reduction and non-illness mortality in randomised clinical trials of cholesterol reduction
| Trial (year published) | Primary or secondary prevention | Treatment | No of subjects randomised
|
Median follow up (years) | Mean cholesterol reduction (%)* | Non-illness mortality
|
Odds ratio† (95%CI) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Treated | Control | Treated | Control | |||||||
| Los Angeles Veterans Administration study (1969)‡ | Primary | Diet | 424 | 422 | 8.0 | 12.7 | 4 | 0 | — | |
| World Health Organization cooperative trial (1978)§ | Primary | Clofibrate | 5331 | 5296 | 5.3 | 9.0 | 24 | 25 | 0.95 (0.54 to 1.67) | |
| Colestipol-Upjohn study (1978)¶ | Primary | Colestipol | 548 | 546 | 1.9 | 9.6 | 2 | 0 | — | |
| Lipid Research Clinics coronary primary prevention trial (1984) | Primary | Choletyramine | 1906 | 1900 | 7.4 | 8.5 | 11 | 4 | 2.75 (0.92 to 8.26) | |
| Helsinki heart study (1987) | Primary | Gemfibrozil | 2051 | 2030 | 5.0 | 9.6 | 10 | 4 | 2.48 (0.81 to 7.63) | |
| Minnesota coronary study (1989) | Primary | Diet | 4541 | 4516 | 1.1 | 13.6 | 33 | 28 | 1.17 (0.71 to 1.94) | |
| West of Scotland coronary prevention study (1995) | Primary | Pravastatin | 3302 | 3293 | 4.9 | 20.0 | 5 | 6 | 0.83 (0.25 to 2.72) | |
| Air Force/Texas coronary atherosclerosis prevention study (1998) | Primary | Lovastatin | 3304 | 3301 | 5.2 | 19.3 | 1 | 3 | 0.33 (0.04 to 2.87) | |
| Oslo diet-heart study (1966)** | Secondary | Diet | 206 | 206 | 5.0 | 13.9 | 0 | 0 | — | |
| MRC soybean oil trial (1968) | Secondary | Diet | 199 | 194 | 4.0 | 16 | 0 | 0 | — | |
| Scottish Society of Physicians clofibrate trial (1971)** | Secondary | Clofibrate | 350 | 367 | 1.7 | 16 | 1 | 1 | 1.05 (0.07 to 16.86) | |
| Coronary drug project (niacin, clofibrate) (1975) | Secondary | Niacin, clofibrate | 1119, 1103 | 2789 | 6.2 | 9.9, 6.5 | 8, 5 | 15 | 1.09 (0.52 to 2.29) | |
| Stockholm ischaemic heart disease study (1988)** | Secondary | Clofibrate and niacin | 279 | 276 | 5.0 | 13 | 1 | 0 | — | |
| Diet and reinfarction trial (1989)** | Secondary | Diet | 1018 | 1015 | 2.0 | 3.5 | 1 | 1 | 1.00 (0.06 to 15.97) | |
| Partial ileal bypass surgery (1990) | Secondary | Surgery | 421 | 417 | 9.7 | 23.3 | 3 | 3 | 0.99 (0.2 to 4.94) | |
| Helsinki heart study—ancillary study (1993) | Secondary | Gemfibrozil | 311 | 317 | 5.0 | 8.5 | 1 | 1 | 1.02 (0.06 to 16.41) | |
| Scandinavian simvastatin survival study (1994) | Secondary | Simvastatin | 2221 | 2223 | 5.4 | 26 | 6 | 7 | 0.86 (0.29 to 2.55) | |
| Cholesterol and recurrent events trial (1996) | Secondary | Pravastatin | 2081 | 2078 | 5.0 | 20 | 8 | 4 | 2.00 (0.62 to 6.50) | |
| Long term intervention with pravastatin in ischaemic disease (1998) | Secondary | Pravastatin | 4512 | 4502 | 6.1 | 18 | 6 | 11 | 0.54 (0.2 to 1.45) | |
Change in cholesterol concentration in intervention group during trial relative to control group.
Not reported for trials with zero deaths in either the treatment or control group.
Data on non-illness mortality available for years 6-8 only: 318 intervention and 317 control subjects during that time.
Mortality data by “intention to treat” reported in 1992.23
Only data for men available for inclusion in analyses.
Unpublished non-illness mortality data obtained from trial investigators in early 2000.
Footnotes
Funding: This work was supported in part by United States National Institutes of Health grants HL46328 and HL40962.
Competing interests: None declared.
Details of the trials included in the meta-analysis and their full references are available on the BMJ's website
References
- 1.Hunninghake DB, Stein EA, Dujovne CA, Harris WS, Feldman FB, Miller VT, et al. The efficacy of intensive dietary therapy alone or combined with lovastatin in outpatients with hypercholesterolemia. N Engl J Med. 1993;328:1213–1219. doi: 10.1056/NEJM199304293281701. [DOI] [PubMed] [Google Scholar]
- 2.Tang JL, Armitage JM, Silagy CA, Fowler GH, Neil HAW. Systematic review of dietary intervention trials to lower blood total cholesterol in free-living subjects. BMJ. 1998;316:1213–1218. doi: 10.1136/bmj.316.7139.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter C, Jones R, Corr L. Time trend analysis and variations in prescribing lipid lowering drugs in general practice. BMJ. 1998;317:1134–1135. doi: 10.1136/bmj.317.7166.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muldoon MF, Manuck SM, Matthews KM. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301:309–314. doi: 10.1136/bmj.301.6747.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davey Smith G, Pekkanen J. Should there be a moratorium on the use of cholesterol lowering drugs? BMJ. 1992;304:341–348. doi: 10.1136/bmj.304.6824.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kritchevsky SB, Kritchevsky D. Serum cholesterol an cancer risk: an epidemiologic perspective. Annu Rev Nutr. 1992;12:391–416. doi: 10.1146/annurev.nu.12.070192.002135. [DOI] [PubMed] [Google Scholar]
- 7.Ross SD, Allen IE, Connelly JE, Korenblat BM, Smith ME, Bishop D, Luo D. Clinical outcomes in statin treatment trials: a meta-analysis. Arch Int Med. 1999;159:1793–1802. doi: 10.1001/archinte.159.15.1793. [DOI] [PubMed] [Google Scholar]
- 8.Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA. 1996;275:55–60. [PubMed] [Google Scholar]
- 9.Muldoon MF, Rossouw JE, Manuck SB, Glueck CJ, Kaplan JR, Kaufmann PG. Low or lowered cholesterol and risk of death from suicide and trauma. Metabolism. 1993;42(suppl 1):45–56. doi: 10.1016/0026-0495(93)90259-q. [DOI] [PubMed] [Google Scholar]
- 10.Ryman A. Cholesterol, violent death, and mental disorder. BMJ. 1994;309:425–426. doi: 10.1136/bmj.309.6952.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golomb BA. Cholesterol and violence: is their a connection? Ann Intern Med. 1998;128:478–487. doi: 10.7326/0003-4819-128-6-199803150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs D, Blackburn H, Higgins M, Reed D, Iso H, McMillian G, et al. Report of the conference on low blood cholesterol: mortality associations. Circulation. 1992;86:1046–1060. doi: 10.1161/01.cir.86.3.1046. [DOI] [PubMed] [Google Scholar]
- 13.Zureik M, Coubon D, Ducimetiere P. Serum cholesterol concentration and death from suicide in men: Paris prospective study I. BMJ. 1996;313:649–651. doi: 10.1136/bmj.313.7058.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunugi H, Takei N, Aoki H, Nanko S. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41:196–200. doi: 10.1016/S0006-3223(95)00672-9. [DOI] [PubMed] [Google Scholar]
- 15.Garland M, Hickey D, Corvin A, Golden J, Fitzpatrick P, Cunningham S, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77–83. doi: 10.1192/bjp.177.1.77. [DOI] [PubMed] [Google Scholar]
- 16.Golomb BA, Stattin H, Mednick S. Low cholesterol and violent crime. J Psychiat Res (in press). [DOI] [PubMed]
- 17.New AS, Sevin EM, Mitropoulou V, Reynolds D, Novotny SL, Callahan A, et al. Serum cholesterol and impulsivity in personality disorder. Psychiatry Res. 1999;85:145–150. doi: 10.1016/s0165-1781(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 18.Hillbrand M, Spitz RT, Foster HG. Serum cholesterol and aggression in hospitalized male forsensic patients. Behav Med. 1995;18:33–43. doi: 10.1007/BF01857703. [DOI] [PubMed] [Google Scholar]
- 19.Freedman DS, Byers T, Barrett DH, Stroup NE, Eaker E, Monroe-Blum H. Plasma lipid levels and psychologic characteristics in men. Am J Epidemiol. 1995;141:507–517. doi: 10.1093/oxfordjournals.aje.a117465. [DOI] [PubMed] [Google Scholar]
- 20.Bovbjerg VE, Siscovick DS, Psaty BM, McCann BS, Koepsell TD, Raghunathan TE, et al. Lipid-lowering medication and risk of injury. J Clin Epidemiol. 1999;52:1197–1200. doi: 10.1016/s0895-4356(99)00125-0. [DOI] [PubMed] [Google Scholar]
- 21.Bucher HC, Griffith LE, Guyatt GH. Systematic review on the risk and benefit of different cholesterol-lowering interventions. Arterioscler Thromb Vasc Biol. 1999;19:187–195. doi: 10.1161/01.atv.19.2.187. [DOI] [PubMed] [Google Scholar]
- 22.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 23.Heady JA, Morris JN, Oliver MF. WHO clofibrate/cholesterol trial: clarifications. Lancet. 1992;340:1405–1406. doi: 10.1016/0140-6736(92)92588-7. [DOI] [PubMed] [Google Scholar]
- 24.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 25.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control. Overall injury (excluding adverse-event-related) deaths and rates per 100,000 for persons aged 55-64 in 1994-1997. www.cdc.gov/ncipc/data/us9794/oieaer.htm (accessed 4 October 1999).
- 27.West of Scotland Coronary Prevention Study Group. The effects of pravastatin on hospital admission in hypercholesterolemic middle-aged men. J Am Coll Cardiol. 1999;33:909–915. [PubMed] [Google Scholar]
- 28.Pedersen TR, Berg K, Cook TJ, Foergeman O, Haghfelt T, Kjekshus J, et al. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian simvastatin survival study. Arch Intern Med. 1996;156:2085–2092. [PubMed] [Google Scholar]
- 29.Ehrenberg BL, Lamon-Fava S, Corbett KE, McNamara JR, Dallal GE, Schaefer EJ. Comparison of the effects of pravastatin and lovastatin on sleep disturbances in hypercholesterolemic subjects. Sleep. 1999;22:117–121. doi: 10.1093/sleep/22.1.117. [DOI] [PubMed] [Google Scholar]
- 30.Wardle J, Armitage J, Collins R, Wallendszus K, Keech A, Lawson A. Randomised placebo controlled trial of effect on mood of lowering cholesterol concentration. BMJ. 1996;313:75–78. doi: 10.1136/bmj.313.7049.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, et al. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. 2000;108:538–547. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 32.Downs JR. HMG CoA reductase inhibitors and quality of life. JAMA. 1993;269:3107–3108. doi: 10.1001/jama.269.24.3107. [DOI] [PubMed] [Google Scholar]
- 33.Roth T, Richardson GR, Sullivan JP, Lee RM, Merlotti L, Roehrs T. Comparative effects of pravastatin and lovastatin on nighttime sleep and daytime performance. Clin Cardiol. 1992;15:426–432. doi: 10.1002/clc.4960150607. [DOI] [PubMed] [Google Scholar]
- 34.Polmaki A, Malminiemi K, Metsa-Ketela T. Enhanced oxidizability of ubiquinol and α-tocopherol during lovastatin treatment. FEBS Letters. 1997;410:254–283. doi: 10.1016/s0014-5793(97)00609-1. [DOI] [PubMed] [Google Scholar]
- 35.Rise P, Colombo C, Galli C. Effects of simvastatin on the metabolism of polyunsaturated fatty acids and on glycerolipid, cholesterol, and denovo lipid synthesis in THP-1 cells. J Lipid Res. 1997;38:1298–1307. [PubMed] [Google Scholar]
- 36.Walsh KM, Albassam MA, Clarke DE. Subchronic toxicity of atorvastain, a hydroxymethylglutaryl-coenzyme A reductase inhibitor, in beagle dogs. Toxicol Pathol. 1996;24:468–476. doi: 10.1177/019262339602400409. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan JR, Muldoon MF, Manuck SB, Mann JJ. Assessing the observed relationship between low cholesterol and violence-related mortality: implications for suicide risk. Ann N Y Acad Sci. 1997;836:57–80. doi: 10.1111/j.1749-6632.1997.tb52355.x. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan JR, Fontenot MB, Manuck SB, Muldoon MF. Influence of dietary lipids on agonistic and affiliative behavior in Macaca fascicularis. Am J Primatol. 1996;38:333–347. doi: 10.1002/(SICI)1098-2345(1996)38:4<333::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan JR, Manuck SB, Fontenot MB, Muldoon MF, Shively SA, Mann JJ. The cholesterol-serotonin hypothesis: interrelationships among dietary lipids, central serotonergic activity, and antagonistic behavior in monkeys. In: Hillbrand M, Spitz RT, editors. Lipids and human behavior. Washington, DC: American Psychological Association; 1997. pp. 139–166. [Google Scholar]
- 40.Wardle J, Rogers P, Judd P, Taylor M, Rapoport L, Green M, et al. Randomized trial of the effects of cholesterol-lowering dietary treatment on psychological function. Am J Med. 2000;108:547–553. doi: 10.1016/s0002-9343(00)00330-2. [DOI] [PubMed] [Google Scholar]
- 41.Kretsch MJ, Green MWK, Fong AKH, Elliman NA, Johnson HL. Cognitive effects of a long-term weight reducing diet. Int J Obesity. 1997;21:14–21. doi: 10.1038/sj.ijo.0800353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.