Abstract
Profound impairment in social interaction is a core symptom of autism, a severe neurodevelopmental disorder. Deficits can include a lack of interest in social contact and low levels of approach and proximity to other children. In this study, a three-chambered choice task was used to evaluate sociability and social novelty preference in five lines of mice with mutations in genes implicated in autism spectrum disorders. Fmr1tm1Cgr/Y (Fmr1−/y) mice represent a model for fragile X, a mental retardation syndrome that is partially co-morbid with autism. We tested Fmr1−/y mice on two genetic backgrounds, C57BL/6J and FVB/N-129/OlaHsd (FVB/129). Targeted disruption of Fmr1 resulted in low sociability on one measure, but only when the mutation was expressed on FVB/129. Autism has been associated with altered serotonin levels and polymorphisms in SLC6A4 (SERT), the serotonin-transporter gene. Male mice with targeted disruption of Slc6a4 displayed significantly less sociability than wildtype controls. Mice with conditional overexpression of Igf-1 (Insulin-like growth factor-1) offered a model for brain overgrowth associated with autism. Igf-1 transgenic mice engaged in levels of social approach similar to wildtype controls. Targeted disruption in other genes of interest, En2 (Engrailed 2) and Dhcr7, was carried on genetic backgrounds that demonstrated low levels of exploration in the choice task, precluding meaningful interpretations of social behavior scores. Overall, results show that loss of Fmr1 or Slc6a4 gene function can lead to deficits in sociability. Findings from the fragile X-model suggest that the FVB/129 background confers enhanced susceptibility to consequences of Fmr1 mutation on social approach.
Keywords: autism spectrum disorders, endophenotype, Engrailed, Fmr1, fragile X, Sert, Slc6a4, sociability
Introduction
Autism is a severe neurodevelopmental disorder characterized by abnormal social interaction and communication, restricted and unusual interests, and aberrant repetitive behavior (American Psychiatric Association 2000). Twin studies have demonstrated a strong genetic component for disease etiology (Bailey et al. 1995, Folstein & Rosen-Sheidley 2001, Steffenburg et al. 1989). However, genetic analyses to determine specific heritable factors underlying susceptibility for autism have suggested that the majority of cases involve the interaction between multiple genes, as well as possible environmental factors (Abrahams & Geschwind 2008, Freitag 2007, Polleux & Lauder 2004). One approach for the study of polygenic clinical disorders is to determine specific endophenotypes, or measurable, simplified indexes of complex disease phenotypes, that may be associated with a single gene or a limited number of genes (Braff et al. 2008, Gottesman & Gould 2003). Identification of endophenotypes may provide functional markers for disease diagnosis and classification, and for the genetic dissection of disease symptomatology. Recent investigations using endophenotyping approaches in neuropsychiatric disorders have included assessments for neuropsychological function or cognition as heritable, quantifiable markers for broader domains of impairment (Boonstra et al. 2008, da Rocha et al. 2008, Gur et al. 2007, Horan et al. 2008).
While mouse models cannot fully recapitulate diverse behavioral elements of complex neuropsychiatric disorders such as autism, engineered mutations in mouse lines provide a way to investigate the association between candidate endophenotypes and specific genes or signaling pathways implicated in the human disease (Hranilovic & Bucan 2001). The following studies utilized genetic mouse models to investigate social approach deficits as a possible endophenotype for the broad domain of abnormal social function in autism. Mutant lines were selected for alterations in genes linked to heritable, biochemical, or neuropathological aspects of autism. Our hypothesis was that one or more of these mouse lines would demonstrate deficient social approach, and thus provide a link between a single gene (Fmr1, Slc6a4, Igf-1, En2, or Dhcr7) and a quantifiable social endophenotype for more global social impairment.
In humans, fragile X syndrome is associated with mental retardation, physical abnormalities, and autistic symptoms (Hagerman et al. 1986). The disease is caused by disrupted function of the FMR1 (Fragile X Mental Retardation 1) gene, which has been modeled in the Fmr1−/y mouse (Bakker et al. 1994). Numerous studies have provided evidence for significant parallels between alterations observed in children with fragile X and abnormal behavior in Fmr1−/y mice, including deficits in attention and learning (Bakker et al. 1994, Kooy et al. 1996, Moon et al. 2006, Paradee et al. 1999), changes in reactions to sensory stimuli (Chen & Toth 2001, Frankland et al. 2004), and abnormal social responses (McNaughton et al. 2008, Mineur et al. 2006, Spencer et al. 2005). One interesting feature of this mouse model is that the phenotype of Fmr1 loss-of-function may be dependent upon the genetic background. For example, researchers have proposed that the C57BL/6J background confers resistance to effects of Fmr1 deficiency on spatial learning, while FVB/N-129/OlaHsd (FVB/129) leads to greater susceptibility (Dobkin et al. 2000, see also Paradee et al. 1999). The direction of neuroanatomical changes in Fmr1-null mice, such as labeling of mossy fiber terminals, can also be dependent on background strain (Ivanco & Greenough 2002, Mineur et al. 2002), suggesting that modifier genes play an important role in Fmr1-related phenotypes. The present report evaluates Fmr1−/y mice on both C57BL/6J and FVB/129 backgrounds for alterations in social approach.
Several lines of evidence support the involvement of dysregulated serotonergic signaling in autism, such as repeated findings of platelet hyperserotonemia in the disorder (Anderson et al. 1990, Hranilovic et al. 2007, Mulder et al. 2004, Piven et al. 1991, Whitaker-Azmitia 2005). Studies in autism populations have identified possible candidate genes in serotonergic pathways, including the serotonin transporter (SLC6A4 or SERT) (Brune et al. 2006, Devlin et al. 2005, Sutcliffe et al. 2005, Tordjman et al. 2001). One study examining gene expression profiles in monozygotic twins discordant for symptoms of autism found that expression of SLC6A4 was significantly reduced in the twin with greater symptom severity (Hu et al. 2006). Mice with disruptions of Slc6a4 function have an abnormal behavioral phenotype, including low levels of exploration and reduced social interactions (Holmes et al. 2002, 2003, Kalueff et al. 2006, 2007a,, Kalueff et al. b; see Murphy & Lesch 2008 for review), suggesting that these mice may also demonstrate deficient social approach in a choice task.
Both cross-sectional and longitudinal studies have shown that a subset of autistic children demonstrate age-dependent brain overgrowth (Courchesne et al. 2001, 2003, Hazlett et al. 2005). Brain overgrowth at an early age can be modeled by the conditional overexpression of Igf-1 (Insulin-like growth factor-1) in brain, which induces significant increases in brain volume during the embryonic and early postnatal period (Popken et al. 2004). In comparison to wildtype controls, nestin-Igf-1 transgenic mice exhibit an almost 30% greater brain size, without concomitant changes in overall body weight (Popken et al. 2004). Interestingly, Mills et al. (2007) reported both greater head circumference and higher levels of plasma IGF-1 in children with autism and autism spectrum disorder (ASD), compared to normal controls. The correlation between head size and IGF-1 levels was highly significant in the autism/ASD group, but not in the control group. The present studies investigated whether conditional overexpression of Igf-1 was associated with autism-like social behavior in mice.
Mice deficient for Engrailed-2 (En2), a gene crucial for normal development of the cerebellum, were also assessed. Several studies in human populations have reported that variants of En2 may confer risk for ASDs (Benayed et al. 2005, Brune et al. 2008, Gharani et al. 2004, Wang et al. 2008), although not all findings have been positive (Zhong et al. 2003). Researchers have observed parallels between neuroanatomical changes in brain of En2−/− mice and alterations in cerebellar structure reported in autistic children (Kuemerle et al. 2007, Murcia et al. 2005). Lastly, we examined a genetic mouse model for Smith-Lemli-Opitz syndrome (SLOS), a disease with a markedly high co-occurrence with autism (Bukelis et al. 2007, Sikora et al. 2006, Tierney et al. 2001). SLOS arises from mutations in DHCR7 (7-dehydrocholesterol reductase 7), the last enzyme in the cholesterol biosynthesis pathway, with subsequent disruption of cholesterol synthesis. During the prenatal period, Dhcr7-null mice have overt increases in serotonin immunoreactivity (Waage-Baudet et al. 2003). Unfortunately, the loss of Dhcr7 function is lethal in mice (Fitzky et al. 2001). Our study evaluated heterozygous animals (Dhcr7tm1Gst/+ or Dhcr7+/−), which have a mild reduction in the Dhcr7 enzyme.
An important issue for interpreting results from social approach tests is that low preference for the social partner may be associated with changes in activity and/or anxiety-like behavior (Kalueff et al. 2007a, Moy et al. 2007, Spencer et al. 2005). In the present studies, information from one or more control measures, including motor coordination, activity levels in an open field, and anxiety-like behavior in an elevated plus maze, was considered in the interpretation of results from the social approach assays.
Materials and Methods
Animals
Fmr1+/y and −/y mice for testing and for breeding pairs were obtained from Dr. William T. Greenough (Beckman Institute, University of Illinois, Urbana-Champaign, Il) and shipped to the University of North Carolina (UNC; Chapel Hill, North Carolina). The Fmr1-null allele was placed on two strain backgrounds: C57BL/6J (B6.129P2-Fmr1tm1Cgr (Grossman et al. 2006, McKinney et al. 2005, Miyashiro et al. 2003; originally described in Bakker et al. 1994)); and FVB.129P-Fmr1tm1Cgr (FVB/129; a sighted strain, as described in Errijgers et al. 2007, Irwin et al. 2002). The C57BL/6J mice were derived from original C57BL/6J × FVB/N × 129/OlaHsd mice, backcrossed for 6 generations to C57BL/6J mice (McKinney et al. 2005, Miyashiro et al. 2003). The FVB/129 mice were originally derived from 129/OlaHsd embryonic stem (ES) cells and backcrossed to FVB/N for multiple generations (Dobkin et al. 2000, Ivanco & Greenough 2002). Subjects for Cohort 1 of the present studies were sent from the Greenough laboratory, and started testing at 13–14 weeks of age; subjects for Cohort 2 were obtained by wildtype male × female heterozygote within-strain crosses at UNC, and started testing at 6–8 weeks of age. Only males were used in the behavioral assays. Number of litters, number of subjects (offspring of litters), and other characteristics are given in Table 1.
Table 1.
Control measures in Fmr1 mouse lines. Data shown are means ± SEM for body weight, percent time in and percent entries into the open arms of an elevated plus maze, total arm entries on the plus maze, and latency to fall from an accelerating rotarod
| % Open arm | |||||||
|---|---|---|---|---|---|---|---|
| # Litters | N | Body weight (g) | time | entries | Total entries | Latency on rotarod (s) | |
| Cohort 1a | |||||||
| C57BL/6J | 8 | ||||||
| Fmr1+/y | 10 | 27±1 | 20±5 | 23±2 | 23±1 | Not tested | |
| Fmr1−/y | 16 | 29±1 | 27±2 | 31±3 | 25±2 | Not tested | |
| FVB/129 | 8 | ||||||
| Fmr1+/y | 14 | 28±1 | 32±4 | 37±4 | 40±4 | Not tested | |
| Fmr1−/y | 13 | 32±1* | 39±6 | 39±4 | 28±3* | Not tested | |
| Cohort 2b | |||||||
| C57BL/6J | 9 | ||||||
| Fmr1+/y | 13 | 21±1c | 23±4 | 24±3 | 25±2 | 222±15 | |
| Fmr1−/y | 24 | 24±1 | 18±2 | 22±2 | 26±1 | 227±15 | |
| FVB/129 | 13 | ||||||
| Fmr1+/y | 31 | 24±1d | 26±3 | 28±2 | 28±2 | 147±14 | |
| Fmr1−/y | 26 | 26±1 | 26±4 | 25±3 | 27±2 | 139±14 | |
Source: Dr. William Greenough, University of Illinois.
Source: Bred at the University of North Carolina.
Body weight means from 10 +/y and 14 −/y mice.
Body weight means from 24 +/y and 17 −/y mice.
p<0.05, comparison to +/y group.
Slc6a4 mice (+/+, +/−, and −/−) were male and female littermate offspring bred at UNC from pairs provided by Dr. Dennis L. Murphy (Laboratory of Clinical Science, NIMH, Bethesda, MD). Mice had been backcrossed onto a C57BL/6 background for 12–15 generations (see Holmes et al. 2003) from an original mixed background (129/P1ReJ (ES cells), C57BL/6J, and CD-1; Bengel et al. 1998, Salichon et al. 2001). Subjects were taken from 11 litters, and were 6–8 weeks in age at the beginning of testing. Subject numbers for the Slc6a4 mice, as well as the three other mutant mouse lines described below, are given in Table 2.
Table 2.
Control measures in Slc6a4, Igf-1, En2, and Dhcr7 mouse lines. Data shown are means ± SEM for body weight, percent time in and percent entries into the open arms of an elevated plus maze, total arm entries on the maze, latency to fall from a rotarod, latency to find buried food and percent of group finding the food in a test for olfactory ability.
| % Open arm | Olfactory test | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Body weight (g) | time | entries | Total entries | Rotarod latency (s) | latency (s) | % group | |
| Slc6a4 | ||||||||
| Males | ||||||||
| Slc6a4+/+ | 8 | 25±1 | 8±2 | 18±4 | 26±4 | 198±31 | 115±41 | 100% |
| Slc6a4+/− | 13 | 23±1 | 6±1 | 15±2 | 26±2 | 162±22 | 272±101 | 77% |
| Slc6a4−/− | 9 | 23±2 | 6±1 | 14±2 | 21±4 | 134±23 | 100±30 | 100% |
| Females | ||||||||
| Slc6a4+/+ | 8 | 18±1 | 9±3 | 14±3 | 30±3 | 146±18 | 57±11 | 100% |
| Slc6a4+/− | 13 | 18±1 | 7±3 | 14±3 | 25±2 | 139±20 | 162±68 | 92% |
| Slc6a4−/− | 12 | 20±1 | 5±1 | 10±3 | 23±3 | 147±9 | 266±78 | 92% |
| Igf-1 | ||||||||
| Males | ||||||||
| Igf-1+/+ | 7 | 23±1 | 4±1 | 9±2 | 20±4 | 171±36 | 184±121 | 86% |
| Igf-1Tg | 6 | 24±1 | 1±1 | 4±2 | 19±2 | 145±10 | 117±85 | 100% |
| Females | ||||||||
| Igf-1+/+ | 10 | 20±1 | 3±1 | 7±2 | 26±3 | 180±33 | 199±89 | 90% |
| Igf-1Tg | 13 | 20±0.4 | 2±1 | 5±1 | 23±2 | 155±16 | 139±72 | 92% |
| En2+/+ | 11 | 21±1 | 4±1 | 6±2 | 17±2 | 123±16 | 690±106 | 36% |
| En2−/− | 8 | 20±1 | 4±3 | 6±4 | 12±1 | 129±12 | 581±137 | 50% |
| Dhcr7+/+ | 8 | 17±1 | 7±1 | 13±2 | 24±1 | 175±12 | 327±94 | 100% |
| Dhcr7+/− | 12 | 18±1 | 7±2 | 12±3 | 23±2 | 195±12 | 356±99 | 83% |
Igf-1 mice (+/+ and Tg) were male and female littermate offspring bred at UNC. The nestin/Igf-1 transgenic (Tg) mice were created on a C57BL/6 background using standard microinjection methods by the Mutant Mouse Resource Center at UNC (Popken et al. 2004). Lines were initiated by breeding the C57BL/6 transgenic heterozygous mice to C57BL/6 wildtype mice (Charles River Laboratories, Wilmington, MA). The Igf-1Tg mice used in the present study were heterozygous for the transgene (Igf-1 mice homozygous for the transgene die in utero (Popken et al. 2004)). Subjects were taken from 7 litters, and were 2–4 months in age at the beginning of testing.
En2+/+ and En2tm1Alj/tm1Alj (En2 −/−) mice were male littermate offspring bred at UNC from pairs provided by Dr. Karl Herrup (Rutgers, The State University of New Jersey, Nelson Biological Laboratories, Piscataway, NJ). En2−/− mice carried a null allele derived from D3 129/Sv ES cells (Joyner et al. 1989,1991, Millen et al. 1994), with the mutation transferred from a 129S2/SvPas background to a 129/S1 background (Gerlai et al. 1996, Kuemerle et al. 2007). Subjects were taken from 5 litters, and were 5-6 weeks in age at the beginning of testing.
Dhcr7 mice (+/+ and +/−) were male littermate offspring on a 129/SvEv background (Waage-Baudet et al. 2003), obtained from pairs bred at UNC. The mutation was produced by targeted disruption of the coding sequence of the last Dhcr7 exon, the proposed active site of the human gene (Fitzky et al. 2001). Subjects were taken from 6 litters, and were 5–7 weeks in age at the beginning of testing.
Mice from each study were separated by strain and sex, and housed in ventilated plastic tub cages, with free access to water and Purina 5058 chow. The housing room had a 12-hr light/dark cycle (lights off at 7:00 p.m.). For groups bred at UNC, genotyping was conducted from tail tissue by PCR. Testing methods were designed to minimize pain and discomfort in the mice. All procedures were conducted in strict compliance with the policies on animal welfare of the National Institutes of Health and UNC (stated in the “Guide for the Care and Use of Laboratory Animals,” Institute of Laboratory Animal Resources, National Research Council, 1996 edition). All procedures were approved by the UNC Institutional Animal Care and Use Committee.
Behavioral Testing
Order of testing for each group was: Fmr1 mice, Cohort 1: 1) elevated plus maze, 2) test for sociability. Fmr1 mice, Cohort 2: 1) elevated plus maze, 2) activity in an open field, 3) rotarod, 4) social approach test. Slc6a4, Igf-1, En2, and Dhcr7 mice: 1) neurobehavioral screen and home cage observation, 2) activity in an open field, 3) rotarod, 4) social approach test, 5) buried food test for olfactory ability, 6) elevated plus maze. Only 1 procedure was conducted per day. Detailed descriptions of these tests have been previously published (Moy et al. 2007).
Control measures
Home cage behaviors
Observations of mice in their home cages were taken at 3 different time points: 8:00 AM, 12:00 noon, and 7:00 PM. Records were taken by a human experimenter for 20 min at each time point, for a total of 60 min of home cage observation. Two hours before the noon observation, 1 white cotton nestlet square (Ancare Corp., Bellmore, NY) was added to each cage, in order to assess nest-building behavior. The evening observation was conducted 10 min before and 10 min after the lights had gone off, using red light illumination. Records were taken for nestlet shredding (amount shredded), nest building and structure (flattened nest, short walls, spherical nest), sleeping in huddles (percent of mice in huddle), activity, fighting, and any aberrant behaviors, such as tremor or seizures, or possible stereotyped responses, such as repeated “jack-hammer” jumping or cage-lid flipping. When possible, records included individual subject identification (based on a simple ear punch system) for mice that remained outside of a huddle, or that showed unusual responses. Scoring included percent of cages observed with a nest, percent of mice observed huddling, and percent of mice showing aberrant behavior.
General health and neurological reflexes
Mice were evaluated for general health, including body weight, appearance of fur and whiskers, body posture, and normality of gait. Reflexive reactions to a gentle touch from a cotton swab to the whiskers on each side of the face, the approach of the cotton swab to the eyes, and the sound from a metal clicker (Preyer reflex) were assessed. Animals were observed for the visual placing reflex (forepaw extension when lowered toward a visible surface), and for ability to grasp a metal grid with forepaws and hindpaws.
Elevated plus-maze test for anxiety-like behavior
Mice were given one 5-min trial on the plus-maze, which had 2 closed arms, with walls 40 cm in height, and 2 open arms. The maze was elevated 50 cm from the floor, and the arms were 21 cm long. Animals were placed on the center section (9.5 cm × 9.5 cm), and allowed to freely explore the maze. Arm entries were defined as all four paws entering an arm. Entries and time in each arm were recorded during the trial by a human observer via computer coding. Percent open arm time was calculated as 100 × (time spent on the open arms/(time in the open arms + time in the closed arms)). Percent open arm entries was calculated using the same formula.
Open field
Exploratory activity in a novel environment was assessed in a photocell-equipped automated open field (40 cm × 40 cm × 30 cm; Versamax system, Accuscan Instruments). Parameters included ambulation (total distance traveled), rearing movements, and time spent in the center region of the chamber. Activity chambers were contained inside sound-attenuating boxes, equipped with houselights and fans.
Rotarod performance
Mice were assessed for balance and motor coordination on an accelerating rotarod (Ugo-Basile, Stoelting Co., Wood Dale, Il). Revolutions per minute (rpm) were set at an initial value of 3, with a progressive increase to a maximum of 30 rpm across the 5-min test session. Each animal was given a test session consisting of 2 trials, with 45 seconds between each trial. Latency to fall, or to rotate off the top of the turning barrel, was measured by the rotarod timer.
Olfactory test following food deprivation
Several days before the olfactory test, an unfamiliar food (Froot Loops, Kellogg Co., Battle Creek, MI) was placed overnight in the home cages of the subject mice, in order to avoid food neophobia on the day of testing. 16–20 hours before the test, all food was removed from the home cage. On the day of the test, each mouse was placed in a large, clean tub cage (46 cm L × 23.5 cm W × 20 cm H), containing 3 cm deep paper chip bedding (Canbrands Product, Moncton NB, Canada), and allowed to explore for 5 min. The animal was removed from the cage, and 1 Froot Loop was buried in the cage bedding, approximately 1 cm below the surface of the litter. The subject mouse was then returned to the cage for a 15 min test. Measures were taken of latency to find the buried food.
Sociability and preference for social novelty
Fmr1 mice were tested in a non-automated 3-chambered box, with measures taken by a human observer blind to mouse genotype (Duncan et al. 2004, Moy et al. 2004). All other mutant lines were tested in an automated 3-chambered box (Moy et al. 2007, Nadler et al. 2004). Dividing walls had retractable doorways allowing access into each chamber. The automated box had photocells embedded in each doorway to allow quantification of entries and duration in each chamber of the social test box. The chambers of the apparatus were cleaned with water and dried with paper towels between each trial. At the end of each test day, the apparatus was sprayed with 70% ethanol and wiped clean with paper towels.
The choice test had three 10-min phases: A) Habituation. The test mouse was first placed in the middle chamber and allowed to explore, with the doorways into the 2 side chambers open. Each of the 2 sides contained an empty wire cage (11 cm H, 10.5 bottom diameter, bars spaced 1 cm apart; Galaxy Cup, Spectrum Diversified Designs, Inc., Streetsboro, Ohio). B) Sociability. After the habituation period, the test mouse was enclosed in the center compartment of the social test box, and an unfamiliar mouse (stranger 1; an adult C57BL/6J male) was enclosed in one of the wire cages and placed in a side chamber. The location for stranger 1 alternated between the left and right sides of the social test box across subjects. Following placement of stranger 1, the doors were re-opened, and the subject was allowed to explore the entire social test box. Measures were taken of the amount of time spent in each chamber and the number of entries into each chamber by the automated testing system. In addition, a human observer scored time spent sniffing each wire cage, using a computer keypad and software (Johns et al. 1998). C) Preference for social novelty. At the end of the sociability test, each mouse was further tested for preference to spend time with a new stranger. A new unfamiliar mouse was placed in the wire cage that had been empty during the previous session. The test mouse then had a choice between the first, already-investigated mouse (stranger 1) and the novel unfamiliar mouse (stranger 2). The same measures were taken as with the sociability test.
Cohort 1 of Fmr1 mice was tested for sociability, but not for social novelty preference. For this single set of mice, the test for sociability involved a choice between a side containing the unfamiliar stranger, and an empty side (without the empty wire cage present for the other groups). The measure for sniffing was not taken for the first cohort.
Statistical analysis
Data from each mutant mouse line were first analyzed using 1-way or 2-way ANOVAs (analysis of variance) or repeated measures ANOVAs, with the factor targeted mutation (genotype) and, for the Fmr1 lines, background strain (C57BL/6J or FVB/129). The repeated measures included week of testing (for body weight), time during the open field test, rotarod trial, and chamber side in the social approach test. These analyses determined main effects of genotype, background strain, and the repeated measure, and interactions between the different factors. In the Fmr1 lines, each overall ANOVA was followed by separate analyses within each background strain, in order to further examine effects of the Fmr1 genotype. The Slc6a4 and Igf-1 mouse lines included both males and females; separate analyses were conducted for each sex. Significant effects of altered genotype found in the ANOVAs were further explored using post-hoc Fisher’s PLSD (protected least-significant difference) tests to determine differences between group means. For all comparisons, significance was set at p < 0.05.
Separate analyses were used to determine levels of social preference within each experimental group. Sociability and social novelty preference were evaluated using within-genotype repeated measures ANOVAs, with the factor of chamber side (e.g., stranger 1 side or the opposite side). For all comparisons, significance was set at p < 0.05.
Results
Control measures in Fmr1 mice
Fmr1 genotype effects on body weight, elevated plus maze and rotarod performance
Overall, no differences in the control measures were found between wildtype and Fmr1−/y mice on the C57BL/6J background. Significant effects of Fmr1 genotype on body weight and one measure from the plus maze were observed in the FVB/129 lines, but only in the first cohort (Table 1). In this case, the mutant mice on the FVB/129 background weighed more than the control mice at the beginning of testing [post-hoc tests following a repeated measures ANOVA, main effect of Fmr1 genotype, F(1,49)=15.16, p=0.0003; Fmr1 genotype × strain interaction, F(1,49)=7.28, p=0.0096]. This same mutant group made significantly fewer entries than wildtype controls on the elevated plus maze [post-hoc analyses following two-way ANOVA, main effect of Fmr1 genotype, F(1,49)=10.45, p=0.0022; Fmr1 genotype × strain interaction, F(1,49)=6.16, p=0.0165]. There were no effects of Fmr1 loss on the percent time and entries on the open arms of the maze, indicating that anxiety-like behavior was similar in the mutant and control mice. Similarly, Fmr1 deficiency had no effects on latency to fall from the rotarod.
Strain effects on elevated plus maze and rotarod performance
Two-way ANOVAs indicated significant effects of background strain in the first cohort for two measures on the elevated plus maze, percent time [F(1,49)=8.71, p=0.0048] and percent entries [F(1,49)=10.19, p=0.0025], reflecting generally higher percentages in the FVB/129 groups, in comparison to the C57BL/6J groups. In the second cohort, strain had a significant main effect on rotarod performance, with the C57BL/6J groups having longer latencies [repeated measures ANOVA across trials, F(1,90)=28.14, p<0.0001].
Open field exploration
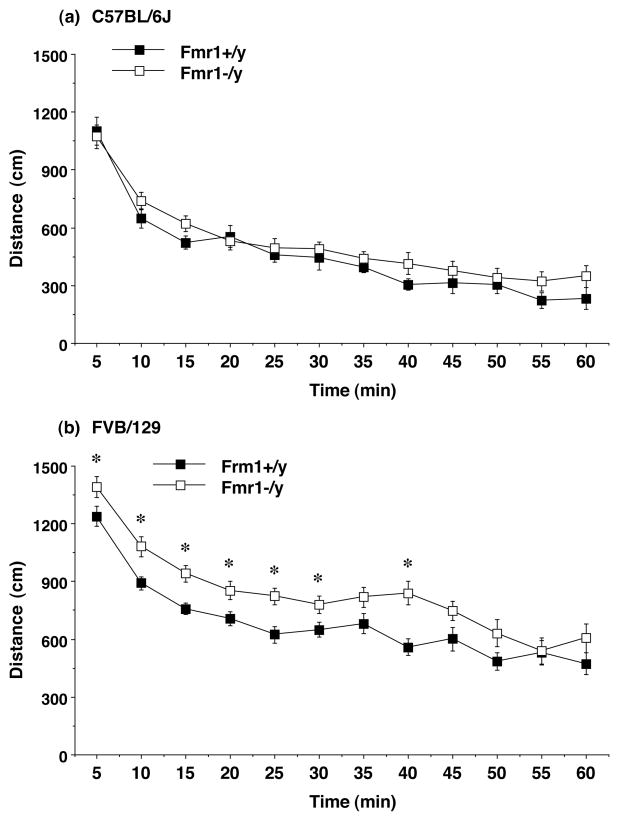
The second cohort group was further assessed for activity levels in a novel open field (Figure 1). The Fmr1−/y mice on the FVB/129 background had higher levels of distance traveled during most intervals of the 1-hour test [post-hoc tests following a repeated measures ANOVA, main effect of Fmr1 genotype, F(1,90)=7.1, p=0.0091; main effect of strain, F(1,90)=47.46, p<0.0001]. Deficiency of Fmr1 did not have significant effects on rearing movements or time spent in the center region of the open field (data not shown).
Figure 1. Open field locomotion in Fmr1 mouse lines from Cohort 2.
Significant increases in distance traveled were seen in the Fmr1-null mice on an FVB/129, but not a C57BL/6J, background. Activity was assessed by a 1-hour trial in an open field chamber. Data shown are mean ± SEM. *p<0.05.
Social approach in Fmr1 mice
Social preference in the sociability assay
Both the mice obtained from the University of Illinois and the group of mice bred at the University of North Carolina demonstrated a similar pattern in the choice task (Figure 2a,b). A significant preference for spending time in the side of the test box containing the stranger mouse, versus the opposite side, was observed in Fmr1+/y and Fmr1−/y mice on the C57BL/6J background; however, on the FVB/129 background, only the Fmr1+/y mice had significant sociability [post-hoc tests following within-group repeated measures ANOVA, main effects of side (the repeated measure) for Cohort 1, F(1,49)=86.23, p<0.0001; Cohort 2, F(1,90)=47.06, p<0.0001]. In both cohort groups, the Fmr1−/y mice on the FVB/129 background failed to demonstrate a significant preference for proximity to an unfamiliar mouse.
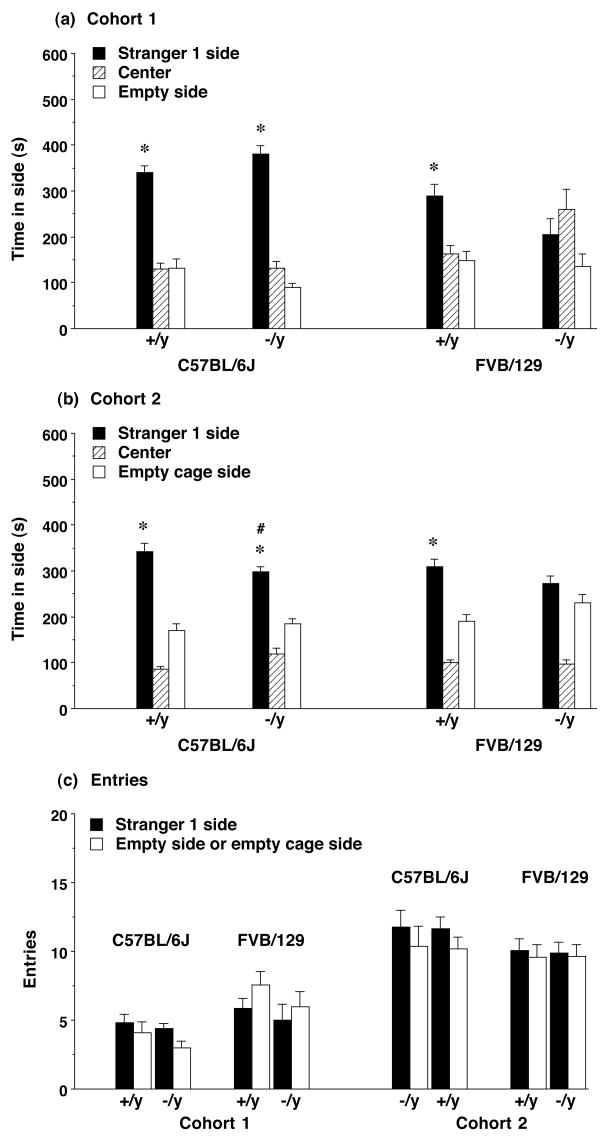
Figure 2. Time spent in each side during the test for sociability in (a) Cohort 1 and (b) Cohort 2 of the Fmr1 mouse lines, and (c) numbers of entries during the test.
Fmr1-null mice on the FVB/129 background, from both cohorts, did not have a significant preference for proximity to stranger 1. The loss of Fmr1 did not have significant effects on number of entries in either background strain. Side choice for Cohort 1 included an empty side (without any wire cage); for Cohort 2, an empty cage side. Data shown are mean + SEM. * p<0.05, within-group comparison, stranger 1 side different from empty (Cohort 1) or empty cage (Cohort 2) side. # p<0.05, comparison with same measure in +/y mice with C57BL/6J background.
Fmr1 genotype and background strain effects in the sociability assay
Repeated measures ANOVAs indicated significant group differences in amount of time spent in the two side chambers in the first cohort [main effect of strain, F(1,49)=9.34, p=0.0036, and a three-way interaction between Fmr1 genotype, strain, and side that approached significance; F(1,49)=3.85, p=0.0554], and in the second cohort [Fmr1 genotype × side interaction, F(1,90)=4.32, p=0.0406]. Further analyses indicated significant group differences for time spent in the side with the stranger mouse [Cohort 1, main effect of strain, F(1,49)=19.01, p<0.0001, and Fmr1 genotype × strain interaction, F(1,49)=5.65, p=0.0214; Cohort 2, main effect of Fmr1 genotype, F(1,90)=5.63, p=0.0198]. Post-hoc tests showed that, in Cohort 2, the Fmr1−/y mice on the C57BL/6J background spent less time in proximity to stranger 1 than the wildtype mice.
Entries in the sociability assay
There were no significant effects of Fmr1 genotype on numbers of entries during the test for sociability (Figure 2c). Therefore, the differences in social preference were not due to a lack of exploration in the Fmr1−/y mice on the FVB/129 background. In both cohort groups, there were significant effects of strain on numbers of entries [repeated measures ANOVA; Cohort 1, main effect of strain, F(1,49)=8.34, p=0.0057, and strain × side interaction, F(1,49)=9.07, p=0.0041; Cohort 2, strain × side interaction, F(1,90)=5.66, p=0.0195].
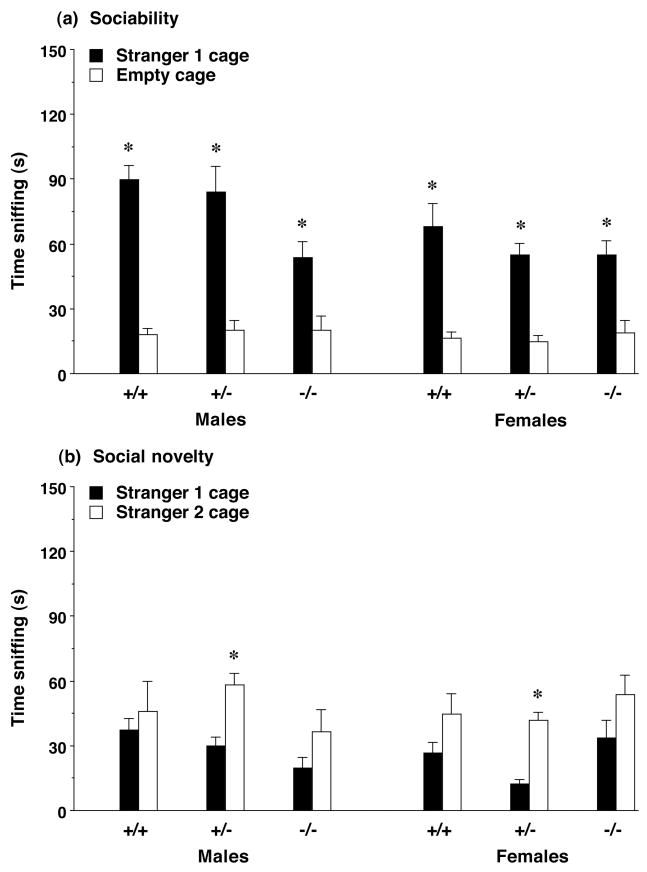
Sniffing during the sociability assay
For the second cohort of mice, measures were taken of sniffing at each cage during the test (Figure 3a). All of the experimental groups demonstrated a preference for sniffing the cage containing the unfamiliar stranger, versus sniffing the empty cage. Although an overall repeated measures ANOVA indicated a significant Fmr1 genotype × side interaction for the sniff measure [F(1,88)=6.21, p=0.0146], separate analyses for each side did not reveal any other significant effects of Fmr1 genotype.
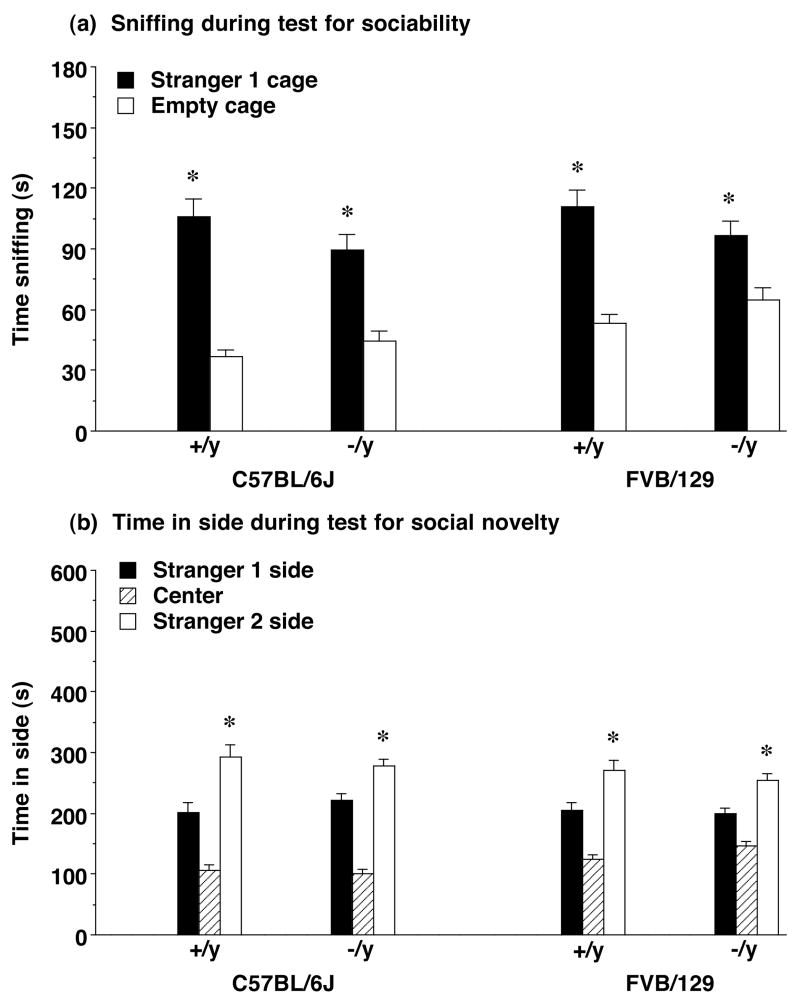
Figure 3. (a) Time spent sniffing each cage during the test for sociability and (b) time spent in each side during the test for social novelty preference in Fmr1 mouse lines from Cohort 2.
All groups had a significant preference for the wire cage containing an unfamiliar mouse, stranger 1, in comparison to an empty cage (a), and a significant preference for proximity to the more-novel stranger 2 (b). Data shown are mean + SEM. * p<0.05, within-group comparison, stranger 1 side different from opposite side.
Preference for social novelty
Cohort 2 was further tested for social approach toward a second novel stranger, in comparison to the first stranger mouse (Figure 3b). In this assay, a second unfamiliar mouse (stranger 2) was placed in the cage that had been empty during the sociability assay. No effects of Fmr1 genotype were evident for time spent with stranger 2, versus time spent with the first stranger, although there was a main effect of strain [repeated measures ANOVA; F(1,90)=13.76, p=0.0004]. Overall, the FVB/129 lines tended to spend less time than the C57BL/6J lines in the two side chambers.
Control measures in Slc6a4, Igf1, En2, and Dhcr7 mouse lines
As shown in Table 2, there were no differences between wildtype and mutant mice within each study for body weight, anxiety-like behavior on the elevated plus maze, motor coordination on an accelerating rotarod, or performance in the buried food test for olfactory ability. The home cage observations and neurobehavioral screen did not reveal any overt changes in huddling behavior, motor ability, or simple reflexive responses in any of the mouse lines (data not shown).
Social approach in Slc6a4 mice
Time spent in each side during the sociability and social novelty assays
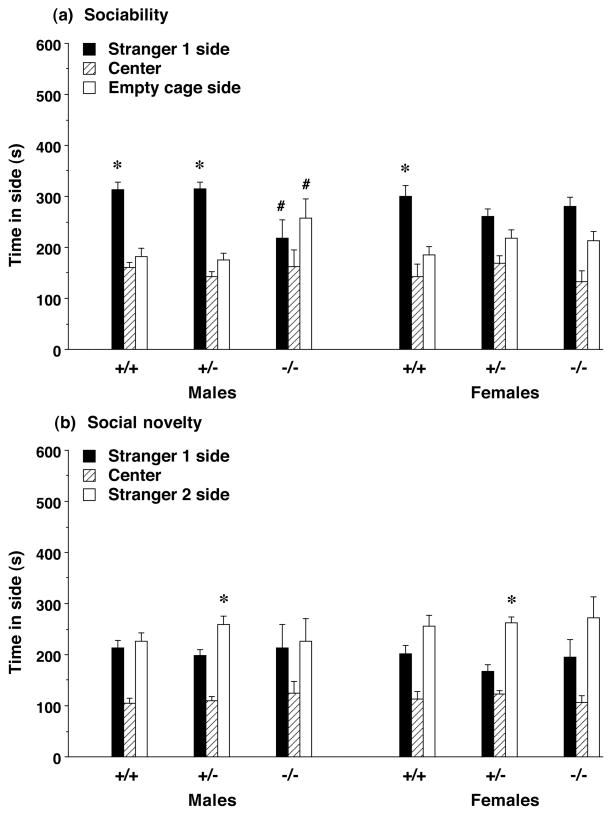
Neither the male Slc6a4−/− mice, nor the female Slc6a4+/− or −/− mice, demonstrated significant preference for spending time with the stranger mouse in the test for sociability (Figure 4a) [post-hoc tests following repeated measures ANOVAs, main effect of side for males, F(1,27)=8.95, p=0.0059; females, F(1,30)=14.98, p=0.0005]. Repeated measures ANOVAs conducted on the data from the male experimental groups indicated a significant Slc6a4 genotype × side interaction [F(2,27)=5.12, p=0.013]. Significant main effects of Slc6a4 genotype were observed for time spent in the side with the stranger [F(2,27)=5.62, p=0.0091] and time spent in the side with the empty cage [F(2,27)=3.81, p=0.0349]. Fisher’s PLSD tests revealed that the Slc6a4−/− males spent less time in the stranger side, and more time in the empty cage side, than either the Slc6a4+/+ or +/− males. There were no significant effects of Slc6a4 genotype in the female mice during the test for sociability, or in either the male or female mice during the test for social novelty preference (Figure 4b).
Figure 4. Time spent in each side by Slc6a4 mice during the tests for (a) sociability and (b) preference for social novelty.
Neither male nor female Slc6a4-null mice, or female heterozygous mice, had a significant preference for proximity to stranger 1. Data shown are mean + SEM for each group. * p<0.05, within-group comparison, stranger 1 side different from empty cage side (a) or stranger 2 side (b). # p<0.05, comparison with same measure in both +/+ and +/− male mice.
Sniffing and entries during the social approach test
All of the experimental groups demonstrated a significant preference for sniffing at the cage containing the stranger mouse, versus the empty cage (Figure 5a) [post-hoc tests following repeated measures ANOVAs, main effect of side for males, F(1,26)=72.26, p<0.0001; females, F(1,30)=98.62, p<0.0001]. In the male groups, there was a non-significant trend for reduced sniffing at the stranger mouse cage by the Slc6a4-null mice [repeated measures ANOVA, main effect of Slc6a4 genotype, F(2,26)=2.86, p=0.0751]. Overall, there were no significant effects of Slc6a4 genotype on the measure of sniffing during the sociability assay, or during the subsequent test for social novelty preference (Figure 5b), in either the male or female mice. No group differences were observed for number of entries into the side chambers during the social approach tests (see Table 3 for entries during the sociability test), suggesting that the lack of social preference in the Slc6a4−/− mice could not be attributed to general hypoactivity and a failure to explore.
Figure 5. Time spent sniffing each cage by Slc6a4 mice during the tests for (a) sociability and (b) preference for social novelty.
During the test for sociability, all groups had a significant preference for the cage containing stranger 1. Data shown are mean + SEM for each group. * p<0.05, within-group comparison, stranger 1 side different from empty cage side (a) or stranger 2 side (b).
Table 3.
Entries in the test for sociability and activity measures in a novel open field. Data shown are means ± SEM for entries into the side containing a stranger mouse or an empty cage, and for total distance traveled, rearing movements, and time spent in the center region during a 5-min activity test
| Stranger | Empty cage | Distance (cm) | Rears | Center time (s) | |
|---|---|---|---|---|---|
| Slc6a4 | |||||
| Males | |||||
| Slc6a4+/+ | 12±1 | 12±1 | 1408±134 | 50±4 | 26±3 |
| Slc6a4+/− | 11±1 | 9±1 | 1197±135 | 39±4 | 26±4 |
| Slc6a4−/− | 10±2 | 10±2 | 1376±110 | 38±4 | 29±5 |
| Females | |||||
| Slc6a4+/+ | 13±3 | 13±3 | 1433±109 | 41±4 | 24±4 |
| Slc6a4+/− | 12±1 | 12±1 | 1646±148 | 40±3 | 25±5 |
| Slc6a4−/− | 10±1 | 10±2 | 1614±103 | 38±4 | 27±4 |
| Igf-1 | |||||
| Males | |||||
| Igf-1+/+ | 11±2 | 9±2 | 1173±161 | 28±4 | 28±9 |
| Igf-1Tg | 10±1 | 9±2 | 1215±78 | 31±5 | 24±7 |
| Females | |||||
| Igf-1+/+ | 15±1 | 14±1 | 1409±165 | 30±3 | 35±5 |
| Igf-1Tg | 14±1 | 14±1 | 1505±196 | 30±3 | 36±6 |
| En2+/+ | 2±0.6 | 2±1.0 | 783±154 | 0.3±0.1 | 3±2 |
| En2−/− | 1±0.5 | 1±0.4 | 733±154 | 0.0±0.0 | 2±1 |
| Dhcr7+/+ | 4±1 | 5±1 | 510±128 | 1.9±1.0 | 4±2 |
| Dhcr7+/− | 5±1 | 5±1 | 740±87 | 2.8±1.4 | 4±2 |
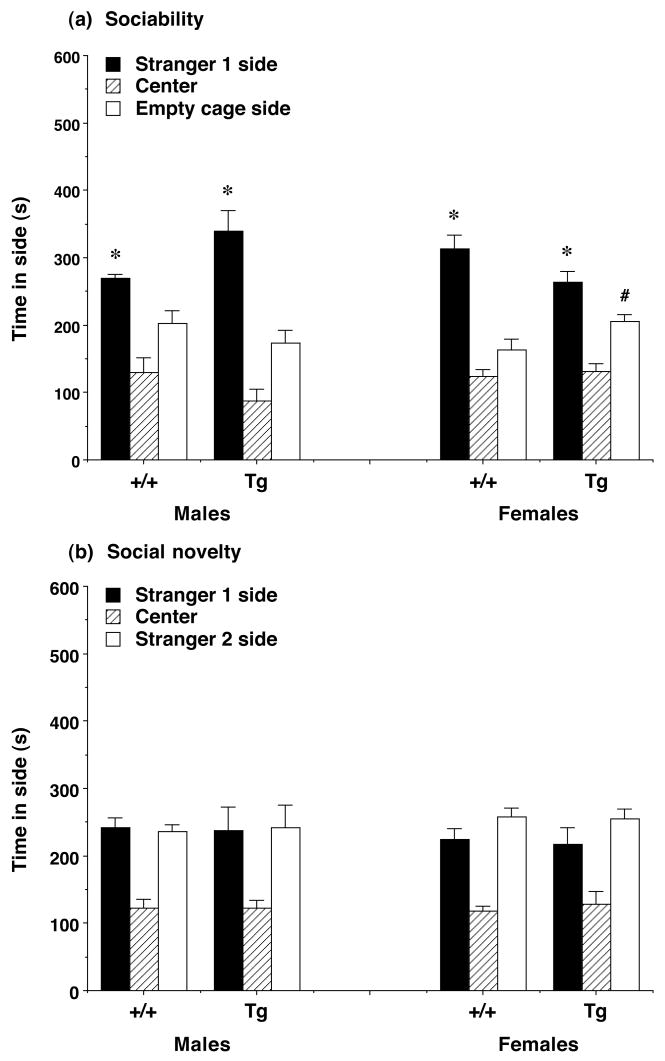
Social approach in Igf-1 mice
During the test for sociability, both the male and female Igf-1 lines had a significant preference for spending time in the side with the stranger mouse (Figure 6a) [post-hoc tests following repeated measures ANOVA, main effect of side for males, F(1,11)=23.87, p=0.0005; and females, F(1,20)=24.65, p<0.0001]. In contrast, none of the groups demonstrated a significant side preference when a new unfamiliar mouse (stranger 2) was introduced during the social novelty test (Figure 6b). Overexpression of Igf-1 had no significant effects in the male experimental groups for any of the measures taken in the social approach tests. In the female groups, the Igf-1 transgenic mice spent significantly more time in the side containing the empty cage than the wildtype mice during the test for sociability [post-hoc tests following repeated measures ANOVA, main effect of Igf-1 genotype, F(1,20)=4.9, p=0.0386]. No other significant effects of Igf-1 genotype were found in the female mice.
Figure 6. Time spent in each side by Igf-1 mice during the tests for (a) sociability and (b) preference for social novelty.
All groups had a significant preference for proximity to stranger 1 in the test for sociability. Data shown are mean + SEM for each group. * p<0.05, within-group comparison, stranger 1 side different from empty cage side. # p<0.05, comparison with same measure in +/+ female mice.
Low exploration in En2−/− and Dhcr7+/− mice
None of the En2 or Dhcr7 experimental groups, either wildtype or mutant, showed social preference in the choice tests (data not shown). Examination of the data suggests that low numbers of entries (Table 3), especially in the En2+/+ and −/− mice, confounded findings from the choice task. Overall, 74% of the mice from the En2 groups, and 25% of the mice from the Dhcr7 groups, had zero entries for one or two of the side chambers. In contrast, no mice from the Slc6a4 or Igf-1 experimental groups had a zero entry score. Findings from a 5-min activity test in a novel open field confirm the intrinsic low levels of exploration in the En2 and Dhcr7 mice (Table 3). In particular, these mouse lines had markedly deficient rearing movements and time spent in the center region of the open field.
Discussion
In addition to profound deficits in social interaction, the core symptoms of autism include aberrant repetitive behavior and restricted interests (American Psychiatric Association, 2000). The impairments in social function may involve a different set of genes than symptoms related to the repetitive behavior domain (Ronald et al. 2006; see also Ronald et al. 2005). Complex neuropsychiatric disorders with this type of genetic heterogeneity and phenotypic diversity present difficulties for large-scale genome linkage and candidate gene association studies. Recently, investigators have focused on endophenotyping approaches for genetic analysis of clinical syndromes such as autism or schizophrenia, measuring social or neurocognitive traits (Duvall et al. 2007, Gur et al. 2007, Horan et al. 2008). The present studies used genetically-engineered mouse lines to evaluate a quantifiable social trait as a heritable marker for impaired social function relevant to autism.
One challenge for the development of mouse models for autism is that the fundamental mechanisms underlying symptomatology are unknown. However, several of the candidate genes implicated in autism play a role in synaptic function, suggesting that disruption of synaptic mechanisms may be a common factor across ASDs (Abrahams & Geschwind 2008). In the present studies, the Fmr1- and Slc6a4- null mouse lines provided models of dysregulated synaptic function associated with specific candidate genes for autism. Fmr1 silencing can lead to abnormal synaptic plasticity, which has been linked to prolonged glutamatergic signaling (Hou et al. 2006, Huber et al. 2002, Nakamoto et al. 2007, Nosyreva & Huber 2006). Synaptic disruption in Fmr1-null mice includes aberrant dendritic morphology, characterized by longer, thinner spines and a higher spine density, comparable to abnormalities observed in fragile X syndrome (Comery et al. 1997, Irwin et al. 2002, McKinney et al. 2005). Similarly, activation of serotonergic pathways is regulated by the serotonin transporter. Loss of Slc6a4 results in prolonged signaling, which may have a profound impact on normal brain development and function (Murphy & Lesch 2008). Regionally-specific alterations in dendritic morphology and increased spine density have been reported in Slc6a4-null mice (Wellman et al. 2007).
Our results show that deficits in social approach are found with the targeted disruption of either Fmr1 or Slc6a4. The mice null for Fmr1 on an FVB/129 background failed to demonstrate significant preference for spending time in the social-partner side in the choice task, in contrast to Fmr1−/y mice on a C57BL/6J background. The lack of preference could not be attributed to low exploration, low activity, or higher levels of anxiety-like behavior in the mutant mice. In line with these findings, male Slc6a4−/− mice (on a C57BL/6J background) spent significantly less time than wildtype controls in the proximity of the unfamiliar social partner. As with the fragile X model, lack of social preference was not associated with low total number of entries during the test, nor with changes in anxiety-like behavior. A similar link between genetic changes leading to altered synaptic function and deficient social approach has been reported for Gabrb3 (GABAA receptor subunit β3) -null mice (DeLorey et al. 2008), Mecp2 (methyl-CpG-binding protein-2) -mutant mice (Moretti et al. 2005), and Nlgn3 (neuroligin-3) R451C knockin mice (Tabuchi et al. 2007). It is notable that GABRB3, MECP2, and NLGN3, as well as FMR1 and SLC6A4, are found on chromosomal loci associated with susceptibility for ASDs (Abrahams & Geschwind 2008).
In addition to specific behavioral characteristics, age-dependent brain overgrowth has been observed in autism (Aylward et al. 2002, Courchesne et al. 2003, Hazlett et al. 2005) and fragile X syndrome (Chiu et al. 2007). Igf-1 transgenic mice were used to model this neuroanatomical abnormality. A previous study with Igf-1 null mice provided evidence that this gene is important for dendritic growth and synaptogenesis (Cheng et al. 2003). Interestingly, loss of Igf-1 led to significant decreases in dendritic spine length and density, which are opposite to the alterations observed with Fmr1 deficiency (Comery et al. 1997, Irwin et al. 2002, McKinney et al. 2005). These findings suggest that Igf-1 overexpression might induce abnormal growth of dendritic spines, and therefore, have detrimental effects on synapse function similar to targeted disruption of Fmr1. However, in contrast to the fragile X-model mice, the Igf-1 transgenic mice did not show deficits in social approach or in any other behavioral measure. The unchanged phenotype of the Igf-1 mutants demonstrates that even overt alterations in normal brain development do not necessarily lead to social endophenotypes.
En2-null mice served as a model of altered cerebellar morphology observed in autism (Kuemerle et al. 2007, Murcia et al. 2005). Cheh et al. (2006) found that En2−/− mice have higher levels of serotonin than wildtype controls in cerebellum, but not frontal cortex, hippocampus, or striatum. Thus, the En2−/− mice could provide information on the behavioral effects of a regionally-specific enhancement of serotonin signaling. The Dhcr7+/− mice were investigated as another interesting mutant with dysregulation of serotonin signaling. These mice reflect the disrupted cholesterol biosynthesis observed in Smith-Lemli-Opitz syndrome (Fitzky et al. 2001). There is evidence that reductions in cholesterol lead to decreased activity of the serotonin transporter (Magnani et al. 2004, Nomura et al. 2008, Scanlon et al. 2001), which could underlie increases in hindbrain serotonin observed in Dhcr7-null mice during prenatal development (Waage-Baudet et al. 2003). Unfortunately, the behavioral phenotypes of the En2 and Dhcr7 lines, both wildtype and mutant, included markedly low exploration. Other researchers have reported general hypoactivity in mutant mouse lines on a 129S2/SvPas (Gerlai et al. 1996) or a 129S6/SvEvTac (Holmes et al. 2003) background. Inbred strain distributions of anxiety-like behavior (Bouwknecht & Paylor 2002, Brooks et al. 2005, Rodgers et al. 2002; see also Cook et al. 2002) confirm low exploration in specific 129 substrains. In the present studies, the lack of exploration in the social approach task precluded the detection of social endophenotypes in the En2 and Dhcr7 mutant mice.
Our findings with the Fmr1-null mice illustrate the importance of background strain in determining the effects of genetic alteration. Recently, Fmr1-null mice on a C57BL/6J × FVB/NJ hybrid background were reported to have normal social preference in a three-chambered choice task (McNaughton et al. 2008). However, depending upon the behavioral assay, Fmr1−/y mice on a C57BL/6J background can exhibit altered social responses. Fmr1-null C57BL/6J mice have been found to have deficits in social interaction with repeated presentations of an ovariectomized female during a habituation procedure (Mineur et al. 2006). Spencer et al. (2005) evaluated Fmr1-null C57BL/6J mice across several domains of social behavior. In a repeated partition test, the mutant mice had decreased social interest for the unfamiliar stranger mouse at the beginning of the twenty-minute procedure, and increased social interest by the end of the testing period. We observed a similar lack of significant preference in the Fmr1−/y FVB/129 mice during the first ten-minute assay (the sociability test), but not the following ten-minute assay (the social novelty test). Spencer et al. (2005) have suggested that decreased social interest at the beginning of a test may reflect increased social anxiety in Fmr1 mutants. However, changes in anxiety-like behavior may be dependent upon the particular assay. In line with previous reports (Mineur et al. 2002, Nielsen et al. 2002), our study on elevated plus maze performance did not indicate a general increase in anxiety-like behavior in Fmr1−/y mice on either background strain.
Other researchers have found changes in social behavior in Slc6a4−/− mice on a C57BL/6J background. Holmes et al. (2002) noted decreased aggression in male Slc6a4-null mice during a resident-intruder test, without any changes in investigatory social interest. The mutant mice were also hypoactive in the home cages and in an open field. Kalueff et al. (2007a) found that female Slc6a4−/− mice had less initiation of sniffing directed toward the social partner in a free interaction test. The Slc6a4−/− mice also had decreased exploration in an open field test, as well as reduced approaches in a novel object test. Therefore, results from these social interaction tests may have reflected hypoactivity and higher levels of neophobia in the Slc6a4-null mice, rather than an intrinsic deficit in social interest. The issue of hypoactivity and low exploration is also problematic for the evaluation of depression-like behavior in Slc6a4-null mice (Kalueff et al. 2006). In the present study, reduced social approach was observed in Slc6a4−/− mice without decreases in approach toward a non-social novel object (the empty wire cage) or fewer entries during the test. The low percent time (ranging from 5% to 9%) spent in the open arms of the elevated plus maze by the Slc6a4 line may have prevented the detection of increases in anxiety-like behavior in the mutant mice.
The dependence of social preference on background strain in the Fmr1−/− mice suggests that modifier genes can attenuate or exacerbate the consequences of Fmr1 loss. One important conclusion from the findings in the Slc6a4 line is that the C57BL/6J background does not necessarily confer protection from the effects of genetic alteration on social approach in the three-chambered choice task. In the Slc6a4-null mice, modifier genes present in the C57BL/6J background did not prevent the changes induced by disrupted transporter function, which may indicate a stronger association of the serotonin signaling pathway, rather than Fmr1-mediated events, with fundamental alterations in social motivation. However, many other factors could have affected social behavior in the mutant lines, including altered learning ability, deficits in sustained attention, subtle olfactory dysfunction, or other traits not assessed in these experiments.
The social approach test used in the present studies included an assay for social novelty preference to provide a secondary measure of social approach, based on discrimination between two partners (stranger 1 and the more-novel stranger 2). Previous work has shown that high sociability does not predict subsequent preference for social novelty in inbred mouse strains, suggesting that the two assays are measuring different components of social behavior (Moy et al. 2007, 2008). A similar dissociation between sociability and social novelty preference was evident in the mutant mouse lines of the present studies. In particular, neither the Slc6a4 nor the Igf-1 wildtype groups had significant preference for the stranger 2 mouse, even though both groups had significant sociability, and were on a background characterized by positive social novelty preference (C57BL/6J; Moy et al. 2007, 2008). The lack of social novelty preference suggests that, across multiple generations, the Slc6a4 and Igf-1 mouse lines have diverged from the original C57BL/6J background.
In the Slc6a4 groups, only the heterozygous mice showed a significant preference for social novelty. Previous work has shown that Slc6a4+/− mice retain about 50% of normal serotonin transporter binding (Bengel et al. 1998, Montanez et al. 2003). Behavior in Slc6a4+/− mice is usually not different from wildtype mice, or else parallels, to a lesser extent, changes observed in Slc6a4−/− mice, supporting a gene dose-dependent function for some behavioral domains (Holmes et al. 2002, 2003, Kalueff et al. 2007a). However, one study found that serotonin levels in the frontal cortex were significantly increased in Slc6a4+/− mice, but decreased in null mutant mice, in comparison to controls (Bengel et al. 1998). It is possible that a reduction, versus a loss, of transporter function could lead to qualitatively different alterations in specific brain regions, and to different profiles of social behavior.
Our findings, together with published reports in other mutant mouse lines (DeLorey et al. 2008, Moretti et al. 2005, Tabuchi et al. 2007), provide evidence that synaptic dysfunction through various mechanisms can lead to similar deficits in social approach. The results are in line with human genetic analyses that have identified disruption of synaptic function as a possible cellular mechanism underlying symptoms in ASDs (Abrahams & Geschwind 2008). In addition, recent work has shown that genetic alterations thought to restore normal synaptic function can reverse, either fully or partially, abnormalities in dendritic morphology, plasticity, and behavior in Fmr1-null double transgenic mice (Dolen et al. 2007, Hayashi et al. 2007). Overall, these studies support the utility of mouse models tolink specific genes and signaling pathways to heritable social endophenotypes, and to examine possible underlying mechanisms relevant to autism.
Acknowledgments
This work was supported by the Fragile X Research Foundation (FRAXA; PI: Dr. J.M.L.), NIH STAART grant U54 MH66418 (project PI: Dr. T.R.M.), NICHD grants P30 HD03110 (PI: Dr. Joseph Piven) and HD008299 (Dr. A.J.D.), NIMH grant MH035321 (PI: Dr. William Greenough), and by the NIMH Intramural Research Program (Drs. J.N.C. and D.L.M.). We thank Dr. William Greenough for providing Fmr1 mice, Dr. Karl Herrup and Dr. Barbara Kuemerle for providing En2 breeding pairs, Dr. Kathleen Sulik and Dr. Heather Baudet for providing Dhcr7 breeding pairs, and Dr. Ping Ye for providing Igf-1 mice for these studies.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature Reviews, Genetics. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Ann N Y Acad Sci. 1990;600:331–340. doi: 10.1111/j.1749-6632.1990.tb16893.x. discussion 341–332. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeven AT, Oostra BA, Reyniers E, de Boulle K, D’Hooge R, Cras P, van Velzen D, Nagels G, Martin J-J, de Deyn PP, Darby JK, Willems PJ. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Benayed R, Gharani N, Rossman I, Mancuso V, Lazar G, Kamdar S, Bruse SE, Tischfield S, Smith BJ, Zimmerman RA, Dicicco-Bloom E, Brzustowicz LM, Millonig JH. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. Am J Hum Genet. 2005;77:851–868. doi: 10.1086/497705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Boonstra AM, Kooij JJ, Buitelaar JK, Oosterlaan J, Sergeant JA, Heister JG, Franke B. An exploratory study of the relationship between four candidate genes and neurocognitive performance in adult ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147:397–402. doi: 10.1002/ajmg.b.30595. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Greenwood TA, Swerdlow NR, Light GA, Schork NJ. Advances in endophenotyping schizophrenia. World Psychiatry. 2008;7:11–18. doi: 10.1002/j.2051-5545.2008.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Pask T, Jones L, Dunnett SB. Behavioural profiles of inbred mouse strains used as transgenic backgrounds. II: cognitive tests. Genes Brain Behav. 2005;4:307–317. doi: 10.1111/j.1601-183X.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr 5-HTTLPR Genotype-Specific Phenotype in Children and Adolescents With Autism. Am J Psychiatry. 2006;163:2148–2156. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Brune CW, Korvatska E, Allen-Brady K, Cook EH, Jr, Dawson G, Devlin B, Estes A, Hennelly M, Hyman SL, McMahon WM, Munson J, Rodier PM, Schellenberg GD, Stodgell CJ, Coon H. Heterogeneous association between engrailed-2 and autism in the CPEA network. Am J Med Genet B Neuropsychiatr Genet. 2008;147:187–193. doi: 10.1002/ajmg.b.30585. [DOI] [PubMed] [Google Scholar]
- Bukelis I, Porter FD, Zimmerman AW, Tierney E. Smith-Lemli-Opitz syndrome and autism spectrum disorder. Am J Psychiatry. 2007;164:1655–1661. doi: 10.1176/appi.ajp.2007.07020315. [DOI] [PubMed] [Google Scholar]
- Cheh MA, Millonig JH, Roselli LM, Ming X, Jacobsen E, Kamdar S, Wagner GC. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Res. 2006;1116:166–176. doi: 10.1016/j.brainres.2006.07.086. [DOI] [PubMed] [Google Scholar]
- Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103:1043–1050. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Mervis RF, Niu SL, Salem N, Jr, Witters LA, Tseng V, Reinhardt R, Bondy CA. Insulin-like growth factor 1 is essential for normal dendritic growth. J Neurosci Res. 2003;73:1–9. doi: 10.1002/jnr.10634. [DOI] [PubMed] [Google Scholar]
- Chiu S, Wegelin JA, Blank J, Jenkins M, Day J, Hessl D, Tassone F, Hagerman R. Early acceleration of head circumference in children with fragile × syndrome and autism. J Dev Behav Pediatr. 2007;28:31–35. doi: 10.1097/01.DBP.0000257518.60083.2d. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MN, Bolivar VJ, McFadyen MP, Flaherty L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav Neurosci. 2002;116:600–611. [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. Jama. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- da Rocha FF, Malloy-Diniz L, Lage NV, Romano-Silva MA, de Marco LA, Correa H. Decision-making impairment is related to serotonin transporter promoter polymorphism in a sample of patients with obsessive-compulsive disorder. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.05.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin B, Cook EH, Jr, Coon H, Dawson G, Grigorenko EL, McMahon W, Minshew N, Pauls D, Smith M, Spence MA, Rodier PM, Stodgell C, Schellenberg GD. Autism and the serotonin transporter: the long and short of it. Mol Psychiatry. 2005;10:1110–1116. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience. 2000;100:423–429. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Duvall JA, Lu A, Cantor RM, Todd RD, Constantino JN, Geschwind DH. A quantitative trait locus analysis of social responsiveness in multiplex autism families. Am J Psychiatry. 2007;164:656–662. doi: 10.1176/ajp.2007.164.4.656. [DOI] [PubMed] [Google Scholar]
- Errijgers V, Van Dam D, Gantois I, Van Ginneken CJ, Grossman AW, D’Hooge R, De Deyn PP, Kooy RF. FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes Brain Behav. 2007;6:552–557. doi: 10.1111/j.1601-183X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Fitzky BU, Moebius FF, Asaoka H, Waage-Baudet H, Xu L, Xu G, Maeda N, Kluckman K, Hiller S, Yu H, Batta AK, Shefer S, Chen T, Salen G, Sulik K, Simoni RD, Ness GC, Glossmann H, Patel SB, Tint GS. 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Opitz/RSH syndrome. J Clin Invest. 2001;108:905–915. doi: 10.1172/JCI12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Millen KJ, Herrup K, Fabien K, Joyner AL, Roder J. Impaired motor learning performance in cerebellar En-2 mutant mice. Behav Neurosci. 1996;110:126–133. doi: 10.1037//0735-7044.110.1.126. [DOI] [PubMed] [Google Scholar]
- Gharani N, Benayed R, Mancuso V, Brzustowicz LM, Millonig JH. Association of the homeobox transcription factor, ENGRAILED 2, 3, with autism spectrum disorder. Mol Psychiatry. 2004;9:474–484. doi: 10.1038/sj.mp.4001498. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Elisseou NM, McKinney BC, Greenough WT. Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 2006;1084:158–164. doi: 10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Jackson AW, 3rd, Levitas A, Rimland B, Braden M. An analysis of autism in fifty males with the fragile X syndrome. Am J Med Genet. 1986;23:359–374. doi: 10.1002/ajmg.1320230128. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berl) 2002;161:160–167. doi: 10.1007/s00213-002-1024-3. [DOI] [PubMed] [Google Scholar]
- Horan WP, Braff DL, Nuechterlein KH, Sugar CA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Green MF. Verbal working memory impairments in individuals with schizophrenia and their first-degree relatives: Findings from the Consortium on the Genetics of Schizophrenia. Schizophr Res. 2008;103:218–228. doi: 10.1016/j.schres.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Bucan M. Social behavior as an endophenotype for psychiatric disorders: development of mouse models. Current Genomics. 2001;2:41–54. [Google Scholar]
- Hranilovic D, Bujas-Petkovic Z, Vragovic R, Vuk T, Hock K, Jernej B. Hyperserotonemia in adults with autistic disorder. J Autism Dev Disord. 2007;37:1934–1940. doi: 10.1007/s10803-006-0324-6. [DOI] [PubMed] [Google Scholar]
- Hu VW, Frank BC, Heine S, Lee NH, Quackenbush J. Gene expression profiling of lymphoblastoid cell lines from monozygotic twins discordant in severity of autism reveals differential regulation of neurologically relevant genes. BMC Genomics. 2006;7:118. doi: 10.1186/1471-2164-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Idupulapati M, Gilbert ME, Harris JB, Chakravarti AB, Rogers EJ, Crisostomo RA, Larsen BP, Mehta A, Alcantara CJ, Patel B, Swain RA, Weiler IJ, Oostra BA, Greenough WT. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am J Med Genet. 2002;111:140–146. doi: 10.1002/ajmg.10500. [DOI] [PubMed] [Google Scholar]
- Ivanco TL, Greenough WT. Altered mossy fiber distributions in adult Fmr1 (FVB) knockout mice. Hippocampus. 2002;12:47–54. doi: 10.1002/hipo.10004. [DOI] [PubMed] [Google Scholar]
- Johns JM, Nelson CJ, Meter KE, Lubin DA, Couch CD, Ayers A, Walker CH. Dose-dependent effects of multiple acute cocaine injections on maternal behavior and aggression in Sprague-Dawley rats. Dev Neurosci. 1998;20:525–532. doi: 10.1159/000017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL, Herrup K, Auerbach BA, Davis CA, Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science. 1991;251:1239–1243. doi: 10.1126/science.1672471. [DOI] [PubMed] [Google Scholar]
- Joyner AL, Skarnes WC, Rossant J. Production of a mutation in mouse En-2 gene by homologous recombination in embryonic stem cells. Nature. 1989;338:153–156. doi: 10.1038/338153a0. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007a;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Gallagher PS, Murphy DL. Are serotonin transporter knockout mice ‘depressed’?: hypoactivity but no anhedonia. Neuroreport. 2006;17:1347–1351. doi: 10.1097/01.wnr.0000230514.08962.76. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Jensen CL, Murphy DL. Locomotory patterns, spatiotemporal organization of exploration and spatial memory in serotonin transporter knockout mice. Brain Res. 2007b;1169:87–97. doi: 10.1016/j.brainres.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kooy RF, D’Hooge R, Reyniers E, Bakker CE, Nagels G, De Boulle K, Storm K, Clincke G, De Deyn PP, Oostra BA, Willems PJ. Transgenic mouse model for the fragile X syndrome. Am J Med Genet. 1996;64:241–245. doi: 10.1002/(SICI)1096-8628(19960809)64:2<241::AID-AJMG1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Kuemerle B, Gulden F, Cherosky N, Williams E, Herrup K. The mouse Engrailed genes: A window into autism. Behav Brain Res. 2007;176:121–132. doi: 10.1016/j.bbr.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani F, Tate CG, Wynne S, Williams C, Haase J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279:38770–38778. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- McKinney BC, Grossman AW, Elisseou NM, Greenough WT. Dendritic spine abnormalities in the occipital cortex of C57BL/6 Fmr1 knockout mice. Am J Med Genet B Neuropsychiatr Genet. 2005;136:98–102. doi: 10.1002/ajmg.b.30183. [DOI] [PubMed] [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav Neurosci. 2008;122:293–300. doi: 10.1037/0735-7044.122.2.293. [DOI] [PubMed] [Google Scholar]
- Millen KJ, Wurst W, Herrup K, Joyner AL. Abnormal embryonic cerebellar development and patterning of postnatal foliation in two mouse Engrailed-2 mutants. Development. 1994;120:695–706. doi: 10.1242/dev.120.3.695. [DOI] [PubMed] [Google Scholar]
- Mills JL, Hediger ML, Molloy CA, Chrousos GP, Manning-Courtney P, Yu KF, Brasington M, England LJ. Elevated levels of growth-related hormones in autism and autism spectrum disorder. Clin Endocrinol (Oxf) 2007;67:230–237. doi: 10.1111/j.1365-2265.2007.02868.x. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Sluyter F, de Wit S, Oostra BA, Crusio WE. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus. 2002;12:39–46. doi: 10.1002/hipo.10005. [DOI] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Montanez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem. 2003;86:210–219. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Moon J, Beaudin AE, Verosky S, Driscoll LL, Weiskopf M, Levitsky DA, Crnic LS, Strupp BJ. Attentional dysfunction, impulsivity, and resistance to change in a mouse model of fragile X syndrome. Behav Neurosci. 2006;120:1367–1379. doi: 10.1037/0735-7044.120.6.1367. [DOI] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 2004;43:491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Murcia CL, Gulden F, Herrup K. A question of balance: a proposal for new mouse models of autism. Int J Dev Neurosci. 2005;23:265–275. doi: 10.1016/j.ijdevneu.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for rapid quantitation of autism-like social deficits in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2007;104:15537–15542. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DM, Derber WJ, McClellan DA, Crnic LS. Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile X syndrome. Brain Res. 2002;927:8–17. doi: 10.1016/s0006-8993(01)03309-1. [DOI] [PubMed] [Google Scholar]
- Nomura K, Castanon-Cervantes O, Davidson A, Fukuhara C. Selective serotonin reuptake inhibitors and raft inhibitors shorten the period of Period1-driven circadian bioluminescence rhythms in rat-1 fibroblasts. Life Sci. 2008;82:1169–1174. doi: 10.1016/j.lfs.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Piven J, Tsai GC, Nehme E, Coyle JT, Chase GA, Folstein SE. Platelet serotonin, a possible marker for familial autism. J Autism Dev Disord. 1991;21:51–59. doi: 10.1007/BF02206997. [DOI] [PubMed] [Google Scholar]
- Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment Retard Dev Disabil Res Rev. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Hodge RD, Ye P, Zhang J, Ng W, O’Kusky JR, D’Ercole AJ. In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. Eur J Neurosci. 2004;19:2056–2068. doi: 10.1111/j.0953-816X.2004.03320.x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A. Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour. Physiol Behav. 2002;77:301–310. doi: 10.1016/s0031-9384(02)00856-9. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happe F, Bolton P, Butcher LM, Price TS, Wheelwright S, Baron-Cohen S, Plomin R. Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry. 2006;45:691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happe F, Plomin R. The genetic relationship between individual differences in social and nonsocial behaviours characteristic of autism. Dev Sci. 2005;8:444–458. doi: 10.1111/j.1467-7687.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40:10507–10513. doi: 10.1021/bi010730z. [DOI] [PubMed] [Google Scholar]
- Sikora DM, Pettit-Kekel K, Penfield J, Merkens LS, Steiner RD. The near universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz syndrome. Am J Med Genet A. 2006;140:1511–1518. doi: 10.1002/ajmg.a.31294. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A Neuroligin-3 Mutation Implicated in Autism Increases Inhibitory Synaptic Transmission in Mice. Science. 2007;318(5847):71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney E, Nwokoro NA, Porter FD, Freund LS, Ghuman JK, Kelley RI. Behavior phenotype in the RSH/Smith-Lemli-Opitz syndrome. Am J Med Genet. 2001;98:191–200. doi: 10.1002/1096-8628(20010115)98:2<191::aid-ajmg1030>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Gutknecht L, Carlier M, Spitz E, Antoine C, Slama F, Carsalade V, Cohen DJ, Ferrari P, Roubertoux PL, Anderson GM. Role of the serotonin transporter gene in the behavioral expression of autism. Mol Psychiatry. 2001;6:434–439. doi: 10.1038/sj.mp.4000873. [DOI] [PubMed] [Google Scholar]
- Waage-Baudet H, Lauder JM, Dehart DB, Kluckman K, Hiller S, Tint GS, Sulik KK. Abnormal serotonergic development in a mouse model for the Smith-Lemli-Opitz syndrome: implications for autism. Int J Dev Neurosci. 2003;21:451–459. doi: 10.1016/j.ijdevneu.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Wang L, Jia M, Yue W, Tang F, Qu M, Ruan Y, Lu T, Zhang H, Yan H, Liu J, Guo Y, Zhang J, Yang X, Zhang D. Association of the ENGRAILED 2 (EN2) gene with autism in Chinese Han population. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:434–438. doi: 10.1002/ajmg.b.30623. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Zhong H, Serajee FJ, Nabi R, Huq AH. No association between the EN2 gene and autistic disorder. J Med Genet. 2003;40:1–4. doi: 10.1136/jmg.40.1.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]