Abstract
The immediate early gene c-fos, and its protein product c-Fos, are known to be induced in neurons of mammals and fish as a result of neuronal stimulation. The purpose of this study was to quantitatively examine CNS alterations in killifish, Fundulus heteroclitus, in relation to harmful algal bloom (HAB) toxin exposure. c-Fos expression was visualized using immunocytochemistry in the brains of killifish exposed to the excitatory neurotoxins domoic acid (DA) and brevetoxin (PbTx-2), and a paralytic neurotoxin, saxitoxin (STX), released from HABs. In addition, a simulated transport stress experiment was conducted to investigate effects of physical stress on c-Fos induction. Groups of fish were exposed to the different stress agents, brain sections were processed for c-Fos staining, and expression was quantified by brain region. Fish exposed to DA, STX, and transport stress displayed significant alterations in neuronal c-Fos expression when compared to control fish (p ≤ 0.05). DA, PbTx-2, and transport stress increased c-Fos expression in the optic tecta regions of the brain, whereas STX significantly decreased expression. This is the first study to quantify c-Fos protein expression in fish exposed to HAB toxins. General alterations in brain activity, as well as knowledge of specific regions within the brain activated in association with HABs or other stressors, provides valuable insights into the neural control of fish behavior as well as sublethal effects of specific stressors in the CNS.
Keywords: Fish, c-Fos, Brevetoxin, Saxitoxin, Domoic acid, Fundulus heteroclitus
1. Introduction
Behavioral changes in organisms can result from complex alterations at the biochemical and physiological levels of organization. Knowledge of these underlying processes is crucial to understanding the control of behavior and response to stimuli. Organisms can induce cellular change by adapting to stimuli, and mediating repair and regeneration processes through alterations in gene expression. Adaptation to environmental conditions is only possible due to the rapidity and flexibility of neuronal signal transduction, and downstream changes in gene expression (Rybnikova et al., 2003).
Immediate early genes (IEGs), localized within the nuclei of neurons, are induced as a result of electrical or second messenger stimulation. Induction requires no synthesis of transcription factors, and IEGs can be induced rapidly with activation within 15–30 min of stimulus reception (Herdegen and Leah, 1998). IEG activation then codes for new proteins such as cytokines, enzymes, HSP70, as well as transcription factors leading to neuronal phenotypic changes (Herrera and Robertson, 1996). These phenotypic changes alter gene expression through the dimerization of c-Fos and AP-1 complex binding (Herdegen and Leah, 1998; Bakin and Curran, 1999; Zhang et al., 2002; Hansson et al., 2003). As a result, c-Fos induction can be used as a biomarker of neuronal and regional brain activity when animals are exposed to different types of stressful stimuli (Martinez et al., 2002; Rybnikova et al., 2003).
Studies have been conducted in rats and birds to investigate the role of the c-fos gene in on physiological and behavioral responses (Hansson et al., 2003). In rats, it has been demonstrated that c-Fos expression mediates neuronal excitation and enhances survival (Zhang et al., 2002). c-fos can be induced in rats through glutamate receptor agonists, ion channel flux, dioxins, and the mind altering drugs haloperidol and clozapine (Morgan and Curran, 1986; Sonnenberg et al., 1989; Murphy and Feldon, 2001; Cheng et al., 2002). Further, it appears that drug-induced patterns of Fos expression are dependent on the animals' concurrent behavioral status (Murphy and Feldon, 2001). c-Fos expression is also associated with long term memory and walking behaviors (rats), and imprinting and courting behaviors (zebra finches, Taeniopygia guttat) (Sadananda and Bischof, 2002; Espana et al., 2003).
In fish, the mechanisms and outcome of c-Fos protein expression is not well understood. Fish are exposed to similar stressors as mammals and birds, i.e., xenobiotics, neurotoxins, and environmental estrogens, and c-Fos expression may serve as an important measure of exposure and response. It has been demonstrated that the c-fos gene is highly conserved between vertebrate species (Kindy and Verma, 1990; Schreiberagus et al., 1993; Bosch et al., 1995). Previous studies using rainbow trout have described increased c-Fos expression in the brainstem following startle responses, indicating that Fos expression can be utilized as a anatomical marker of neuronal activity to investigate higher order behaviors (Bosch et al., 1995, 2001; Matsuoka et al., 1998). However, no studies to date have investigated alterations in c-Fos expression in fish exposed to ecologically relevant chemical stressors.
Harmful algal blooms (HABs) in the middle Atlantic states, and HAB-associated fish morbidity and mortality, have increased in frequency and severity over the past several decades. The ability to predict and characterize environmental effects of different HAB species are essential to HAB remediation and control. Domoic acid (DA), brevetoxin (PbTx-2), and saxitoxin (STX) are neurotoxins released by Pseudo-nitzschia, Karenia, and Alexandrium spp., respectively. These species have been demonstrated harmful to fish through behavioral alterations and mortality (Steidinger et al., 1973; White, 1977; Lefebvre et al., 2001; Landsberg, 2002). This study examined alterations in c-Fos expression in six regions of the brain from killifish exposed to DA, PbTx-2, or STX.
The goal of this work was to develop and test the utility of c-Fos expression, visualized through immunocytochemistry, as a measure of exposure and effect in killifish, Fundulus heteroclitus, exposed to harmful algal bloom (HAB) neurotoxins. The effect of a physical stressor, simulated transport stress, was also examined. c-Fos expression was evaluated through the examination of brains from stressed and control fish using anti c-Fos polyclonal antibody staining. In addition to general changes in brain activity, knowledge of the specific regions within the brain activated during stress provides valuable insight into the neural control of fish behavior.
2. Methods
2.1. Exposures
Fish were exposed for 60 min in 4 L glass beakers containing 3 L of static artificial seawater (6 PSU, pH 8, 25 °C) to DA (IP injection, 0 and 5 mg/kg, Sigma–Aldrich), PbTx-2, (aqueous, 0 and 40 ppb, nominal concentration with an emulsifying vehicle (EL-620, 0.001%)), and STX (aqueous, 0, 75, and 150 ppb, nominal concentrations). Nominal toxin exposure concentrations and duration were based on preliminary LC50 experiments (data not shown) and other studies investigating HAB exposure on fish (Lefebvre et al., 2001, 2004). Actual exposure concentrations were typically within 10% of nominal concentrations, based on repeated radioimmunoassay and receptor binding assay results from preliminary experiments with PbTx-2 and STX, respectively (data not shown). Three sets of controls were conducted: a sham IP injection control of phosphate buffered saline (DA), an emulsifying vehicle control in artificial seawater (EL-620, 0.001%, PbTx-2), and an artificial seawater control (STX). To simulate transport stress, fish were placed in a 10-L covered transport bucket, and the bucket was vertically dropped 0.5 cm to the floor at a rate of 15 times per minute for 60 min. This was reproducibly accomplished with aid of a vertically mounted reciprocating apparatus. Control fish were placed in the same bucket and affixed to the apparatus, but not dropped. Sample sizes were four fish per treatment in the DA and PbTx-2 exposures, and six fish per treatment in the STX and simulated transport stress exposures. Behavioral observations were conducted every 20 min during the exposures and any alterations were recorded.
2.2. Tissue preservation and processing
After exposure, fish were deeply anesthetized with buffered MS-222 and opened on the left flank to expose the heart. Fish were then perfused by inserting a syringe needle through the ventricle and injecting ice cold heparinized PBS. The atrium was then cut to allow bleed-out. The perfusion concluded after the gills blanched, indicating the perfusate had reached the brain. After perfusion, the dorsal aspect of the cranium was dissected away leaving the brain exposed. The brain and cranium of the fish were then preserved in 10% neutral buffered formalin containing 2.5% acrolein for 3 h. Brains were then removed from the crania and transferred to a solution of 30% sucrose, where they remained until embedding.
Intact brains were then embedded in egg gel molds and post-fixed in 4% paraformaldehyde overnight. Brains were then stored in 30% sucrose, frozen with dry ice, and sectioned on a cryomicrotome at a thickness of 25 μm. Section were collected and placed in a cryoprotectant solution and stored at −20 °C until processed for immunocytochemistry (Watson et al., 1986).
2.3. c-Fos immunocytochemistry
Every third brain section was selected, rinsed in potassium phosphate buffered saline (KPBS), and incubated in sodium borohydride for 20 min to remove any residual acrolein. To enhance signal resolution and reduce background staining, an antigen retrieval procedure was followed using a sodium citrate buffer (pH 6.0). Sections were rinsed with KPBS and the citrate buffer (5:1 solution of 0.01 sodium citrate and 0.01 M citric acid), and microwaved for 30 s. Sections were then immediately rinsed with KPBS, immersed into the polyclonal primary antibody (sheep anti-c-Fos, 1:1000 in KPBS containing 0.4% Triton-X, Chemicon, Temecula, CA), incubated at room temperature for 60 min, and then at 4 °C for 48 h.
After incubation in the primary antibody, sections were rinsed in KPBS, immersed in the secondary antibody (biotinylated rabbit–anti-sheep, IgG 1:600 in KPBS with 0.4% Triton-X, Vector, Burlingame, CA), and incubated for 60 min at room temperature. Sections were then rinsed again in KPBS, immersed into avidin–biotin complex (Vector Stain ABC kit, 45 μL of avidin and 45 μL of biotin per 10 ml of KPBS with 0.4% Triton-X), and incubated at 25 °C for 60 min. Sections were then rinsed in KPBS and 0.175 M sodium acetate. For visualization of c-Fos expression, sections were incubated in nickel-DAB chromogen (0.002 g 3,3 diaminobenzidine, Sigma–Aldrich, 0.25 g nickel sulfate, and 8.31 μL of H2O2/10 ml of sodium acetate) for 20 min and rinsed with the sodium acetate and KPBS. After staining, sections were stored in KPBS at 4 °C until mounting.
Sections of each fish brain were then floated in saline and mounted in order, anterior to posterior, onto gelatin-subbed microscope slides, and allowed to dry overnight. Once dry, slides were immersed through a series of ethanol concentrations and histoclear. Finally, slides were cover slipped using histomount (National Diagnostics, Atlanta, GA), cleaned, and observed for differences in c-Fos expression between treatments using standard light microscopy.
Data were recorded from six regions of the killifish brain were selected based on consistent c-Fos expression observed in pilot experiments: the anterior telencephalon (area ventralis telencephali pars ventralis (Vv) and dorsalis (Vd)), the posterior telencephalon (diencephalic ventricle (DiV) and anterior parvocellular preoptic nucleus (PPa)), two regions in the optic tectum (anterior and posterior periventricular grey zone, L1 and L2), the midbrain tegmentum (ventrolateral nucleus of the torus semicircularis (TSvl), nucleus lateralis valvulae (NLV)), and the rhombencephalon (medial longitudinal fascicle (MLF)) (Fig. 1) (Peter et al., 1975; Wulliman et al., 1996).
Fig. 1.
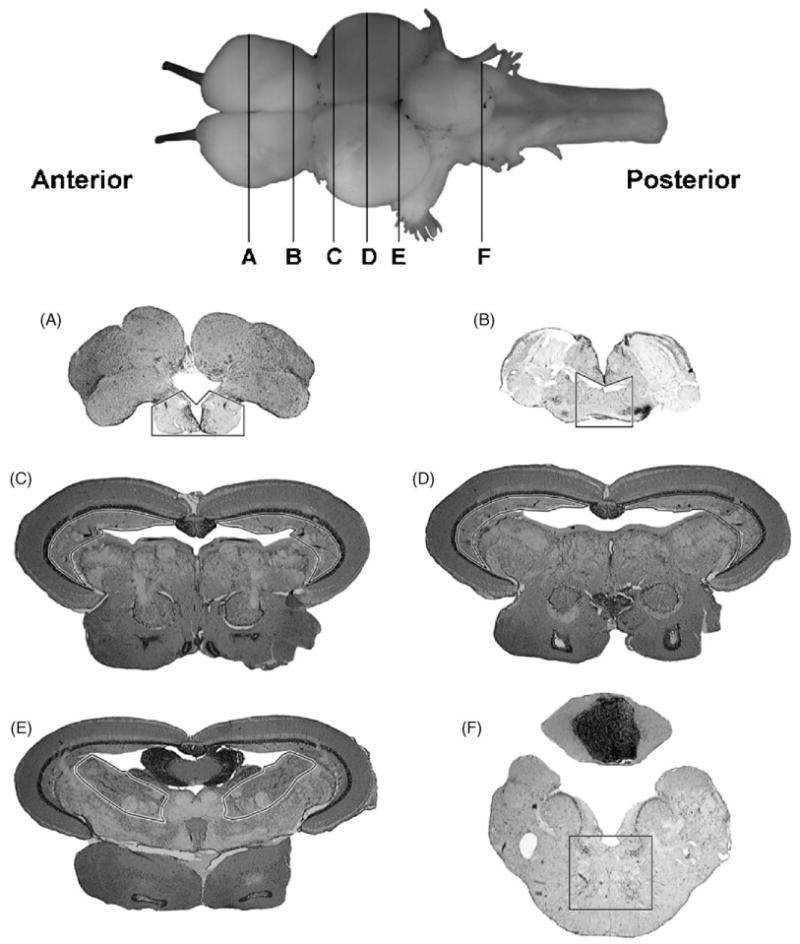
Regions (section level) of the killifish brain investigated for alteration in c-Fos expression. Anterior telencephalon (A), posterior telencephalon (B), anterior optic lobe (C), posterior optic lobe (D), midbrain tegmentum (E), rhombencephalon (brainstem, F).
2.4. Data collection and analysis
Images from each brain section were viewed using a Nikon Eclipse 800 microscope through a 10× objective lens with consistent illumination. Digital images were captured through the microscope using a Photmetrics Sensys digital camera (Biovision Tecnologies, Exton, PA) connected to a Macintosh G4 computer. Regions of interest from digitized brain section images (Fig. 1) were outlined and imported into NIH-Image for total section area calculation. For c-Fos stain area calculation, the images were consistently processed by “black” level-adjustment thresholding set to 64% in Adobe Photoshop™ in order to limit the images to contain only areas of DAB staining. This level of thresholding was determined to be optimal to consistently visualize areas of staining, based on preliminary experiments. Black areas of stain were subsequently analyzed in NIH-Image and normalized by the total section area.
The percentage of stain area to total region area was then calculated and the percentages were compared between treatments. DA, PbTx-2, and transport data were log transformed to meet the assumptions of the ANOVA procedure prior to analysis. A one-tailed, two-way ANOVA was used to analyze the effects of concentration (stress verses control) and region of the brain (Fig. 1) on c-Fos expression, with the a priori hypothesis that DA, PbTx-2, and transport stress fish would increase expression (PROC MIXED, SAS, vs. 8.1, Cary, NC). In order to investigate dose response effects of the STX exposure, concentration (0, 75 and 150 ppb) was treated as a continuous variable for the two-tailed ANOVA procedure. For all experiments, a Tukey–Kramer post hoc mean comparison test was used to evaluate differences (α ≤ 0.05) between control and exposed fish.
3. Results
No mortality occurred in any of the exposure treatments, and the immunocytochemistry method consistently revealed reproducible c-Fos expression in the nucleus of killifish neurons. Strong, punctuate nuclear staining was visualized in neurons of the telencephalon (area ventralis telencephali), mesencephalon (optic tectum), and metencephalon (torus semicircularis), combined with cytoplasmic staining in the mylencephalon and rhombencephalon. Although nuclear staining of c-Fos in the killifish was found throughout the brain, expression was greatest in the anterior and posterior optic tecta (Fig. 2).
Fig. 2.
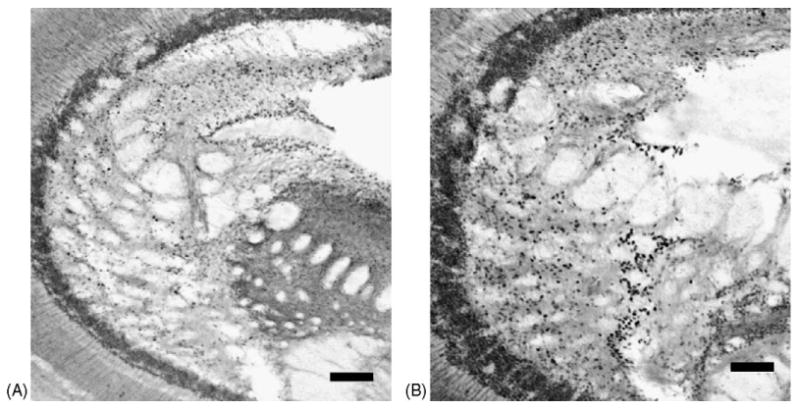
Representative posterior optic tecta from control (sham control, A), and stressed (DA-exposed, B) killifish brains (100× magnification). Note the increase in c-Fos expression represented by black punctuate nuclei in the stressed brain (B). The STX exposure displayed a decrease in c-Fos expression when compared to controls. Scale bar = 100 μm.
The regional brain data had relatively high variances within all treatments, with control fish expressing low levels of c-Fos protein in all regions investigated (Figs. 3–6). As a result of the variability in c-Fos expression, no significant differences were found when comparing whole brain data from control to exposed fish (p > 0.05). However, statistically significant alterations in c-Fos expression between treatments were observed within specific regions of exposed fish brains.
Fig. 3.
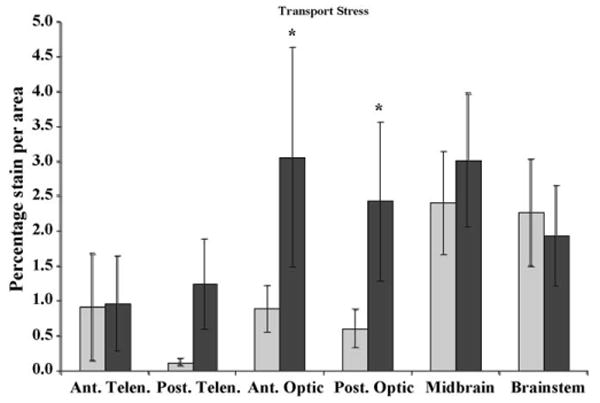
c-Fos expression in control (light gray) and transport stressed (dark gray) killifish brains (N = 6, data are means ± S.E.). Ant. Telen., anterior telencephalon; Post. Telen., posterior telencephalon; Ant. Optic, anterior optic lobe; Post. Optic, posterior optic lobe; Midbrain, midbrain tegmentum; and Brainstem, rhombencephalon. The y-axis is the percentage of c-Fos expression per area (mm2). Significant increases in c-Fos expression occurred in the Ant. and Post. Optic regions (*p = 0.05).
Fig. 6.
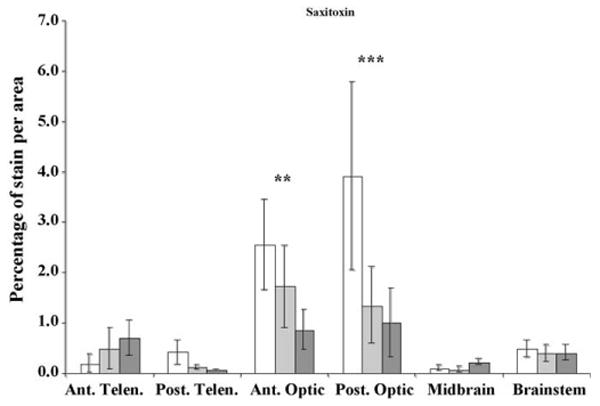
c-Fos expression in seawater control (0 ppb, white bars), low (75 ppb, light gray), and high (150 ppb, dark gray) saxitoxin (STX) exposed killifish brains (N = 6, data are means ± S.E.). Ant. Telen., anterior telencephalon; Post. Telen., posterior telencephalon; Ant. Optic, anterior optic lobe; Post. Optic, posterior optic lobe; Midbrain, midbrain tegmentum; and Brainstem, rhombencephalon. The y-axis is percentage of c-Fos expression per area (mm2). Dose dependant significant decreases in c-Fos expression occurred in the Ant. Optic and Post. Optic regions (**p = 0.01 and ***p = 0.001, respectively).
3.1. Simulated transport stress
c-Fos expression in fish exposed to simulated transport was significantly increased in the anterior and posterior optic tecta (p = 0.038 and 0.052, respectively; Fig. 3). The posterior telencephalon (area ventralis telencephali, pars dorsalis, lateralis, and ventralis) and dorsal midbrain tegmentum also exhibited increased c-Fos expression (p = 0.173 and 0.335, respectively; Fig. 3).
3.2. DA exposure
c-Fos expression significantly increased in the anterior optic tectum of DA-exposed fish compared to controls (p = 0.055, Fig. 4). Increased c-Fos expression was also observed in the anterior telencephalon, and the midbrain tegmentum of DA-exposed fish (p = 0.165 and 0.245, respectively; Fig. 4). Behavioral alterations in DA-exposed killifish included hyperactivity, minimal to moderate corkscrew swimming, and occasional listing.
Fig. 4.
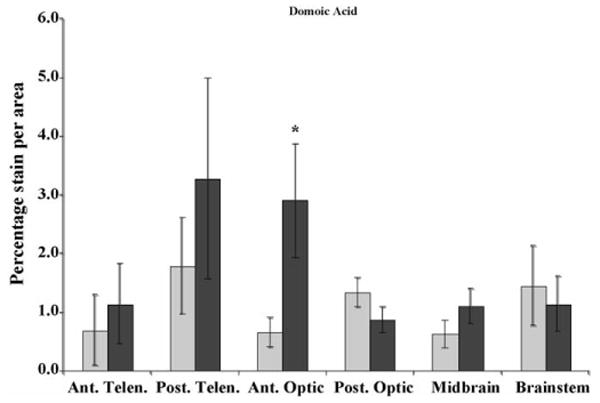
c-Fos expression in sham control (light gray) and domoic acid exposed (dark gray) killifish brains (N = 4, data are means ± S.E.). Ant. Telen., anterior telencephalon; Post. Telen., posterior telencephalon; Ant. Optic, anterior optic lobe; Post. Optic, posterior optic lobe; Midbrain, midbrain tegmentum; and Brainstem, rhombencephalon. The y-axis is the percentage of c-Fos expression per area (mm2). Significant increases in c-Fos expression occurred in the Ant. Optic region (*p = 0.05).
3.3. PbTx-2 exposure
c-Fos expression did not significantly increase in PbTx-2-exposed fish (Fig. 5). However, increased c-Fos labeling in PbTx-2-exposed fish was observed in the anterior telencephalon, posterior optic tectum and midbrain tegmentum (p = 0.231, 0.312, and 0.134, respectively; Fig. 5). No behavioral alterations PbTx-2-exposed killifish were observed.
Fig. 5.
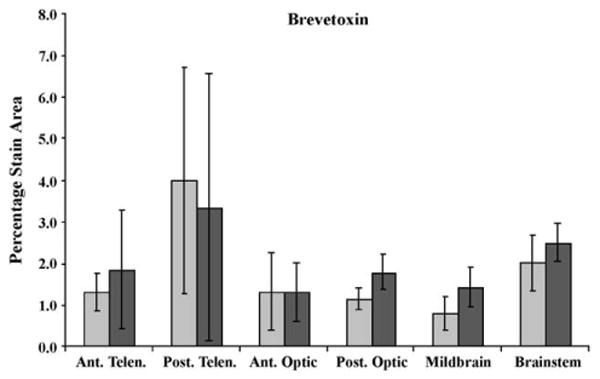
c-Fos expression in vehicle control (light gray) and PbTx-2-exposed (brevetoxin) (dark gray) killifish brains (N = 4, data are means ± S.E.). Ant. Telen., anterior telencephalon; Post. Telen., posterior telencephalon; Ant. Optic, anterior optic lobe; Post. Optic, posterior optic lobe; Midbrain, midbrain tegmentum; and Brainstem, rhombencephalon. The y-axis is the percentage of c-Fos expression per area (mm2).
3.4. STX exposure
In contrast to the other stressors, fish exposed to increasing concentrations of STX displayed significant decreases in c-Fos expression in the anterior and posterior optic lobes (p = 0.007 and 0.001, respectively, Fig. 6). Behavioral alterations in STX-exposed killifish included mild paralysis, a decrease in general activity, occasional floating at the surface and lying on the bottom.
4. Discussion
This study examined c-Fos protein expression in fish exposed to physical stress and HAB neurotoxins in killifish. Stressor-induced alterations in c-Fos expression were found in killifish brains, primarily in the periventricular gray zone of the optic tecta. In addition, we observed neuronal nuclear staining in the fore- and mid-brain in the killifish in response to stress. Nuclear staining of c-Fos protein in fish neurons has not been previously reported. Bosch et al. (1995, 2001) described cytoplasmic staining of Fos-like protein without evidence of nuclear staining in rainbow trout, Oncorhynchus mykiss. This is the first report we know of describing neuronal nuclear staining of c-Fos protein in a teleost brain using immunocytochemistry, which is reasonable considering c-Fos is a nuclear transcription protein. However, similar to descriptions by Bosch et al. (1995), cytoplasmic staining was observed in the rhombencephalon and anterior brainstem of killifish.
Alterations in c-Fos expression in this study were observed from killifish exposed to simulated transport stress (increased expression), domoic acid (increased expression), and saxitoxin (decreased expression) after sixty minutes. The largest difference between stressed and control killifish in this study occurred in the optic tecta, a region where Bosch et al. (1995, 2001) found no staining in rainbow trout. Increased expression of Fos-like protein in rainbow trout, exposed to vibratory stress, has been previously quantified by in the preoptic nucleus, mesencephalon (medial longitudinal fasiculus), oculomotor nerve, torus semicircularis, and nucleus rubber (Bosch et al., 2001). Bosch et al. also located staining throughout the brain resulting from startle response stress, including areas not previously known to participate in startle response behavior; significant differences were located only in the rhombencephalon. In addition, Fos-like expression was located in both control and treated brains, with significant increases in startled fish, and controls expressing far less than stressed fish (Bosch et al., 1995, 2001). c-Fos expression was observed in control killifish as well, but in most cases, quantative expression was altered compared to exposed fish.
The simulated transport stress experiment in the present study comprised a combination of stimuli including visual, lateral line, and auditory cues. This overall stress is confirmed by the significant increase in c-Fos expression in the anterior and posterior optic tecta, as well as increases in all other regions investigated. Future work should investigate neuronal activation of c-Fos to simply visual or lateral line stimuli, in an attempt to better locate regional neuronal activation for each stimulus.
DA-exposed killifish displayed significant increases in c-Fos expression in the anterior optic lobe, and non-significant, but notable, increases in the telencephalon and the midbrain tegmentum. In addition, behavioral alterations were observed in DA-exposed fish, including disorientation and loss of equilibrium in the water column. DA binds to neuronal NMDA/glutamate channels, AMPA/kinate receptors, and the L-type voltage sensitive calcium channels (VSCC). DA increases intracellular calcium concentrations, reversing the operation of the Na/Ca exchanger, creating alterations in neuronal signaling and survival (Berman et al., 2002). In the wild, DA can bioaccumulate in fish, exposing predatory fish, marine mammals, birds, and humans to DA (Scholin et al., 2000; Lefebvre et al., 2002). Anchovies exposed to DA through IP injection in the laboratory display alterations in swimming behavior, with an ED50 of 3.2 mg/kg (Lefebvre et al., 2001). Our study confirms alterations, at the neuronal level in killifish, resulting from DA exposure in the laboratory. c-Fos labeling may additionally be used to investigate the effects of DA on neural activity in fish.
PbTx-2-exposed fish had non-significant increased c-Fos expression compared with control fish. This finding, although not statistically significant, is consistent with data from DA exposed fish, where alterations in neuronal signaling should be similar. PbTx-2 has a specific binding affinity for VSSCs in excitable membranes, holding VSSCs open, increasing the influx of sodium ions, and increasing Ca2+ influx through the voltage-gated calcium channel (VGCC) (Deshpande et al., 1993; Berman and Murray, 1999). This cascade of events, leading to Ca2+ influx and neuronal depolarization, is similar to the effects of DA exposure, albeit through VSSCs instead of NMDA receptors.
There are several possible explanations for the less notable c-Fos expression observed in PbTx-2 exposures compared to DA-exposed fish. Different routes of exposure for the two neurotoxins (DA were administered by IP injection, while PbTx was aqueous), may, in part, account for some of the differences in outcome. Behavioral alterations observed in the DA exposure were not seen in the PbTx exposed fish, suggesting the dose was not equivalently high to illicit a behavioral effect. Finally, effects of brevetoxin observed from this experiment are based on relatively low, environmental concentrations, and a small sample size. Higher, but still environmentally relevant brevetoxin concentrations may elicit greater c-Fos expression. Future studies are required, at higher concentrations with a larger sample size, to investigate any significant effect of brevetoxin on c-Fos expression.
In STX-exposed fish, we found a significant depression in optic lobe c-Fos expression, which is not surprising considering that the mechanism of action is to block Na+ channel conductance. STX mediates toxicity by obstructing the ion pore of the VSSC, thereby stopping flow of sodium into excitable cells, resulting in nerve dysfunction (Anderson, 1997). Behavioral alterations observed in STX-exposed fish included paralysis and a decrease in general activity, with occasional floating at the surface and lying on the bottom. In zebrafish, Danio rerio, STX-induced significant effects on physiology, growth, and survival of larvae including a rapid loss of sensory function (Lefebvre et al., 2004). Tetrodotoxin (TTX), a neurotoxin with the same mechanism of action as STX, has been demonstrated to inhibit c-Fos expression in the rat cortex, visual systems, and striatum (LaHoste et al., 2000; Hausmann et al., 2001; Lu et al., 2001). STX depression of c-Fos in fish further demonstrates that teleost neurons respond similarly to mammalian neurons when exposed to similar stressors. STX could potentially affect neurons that control swimming and other motor functions, which in turn may lead to a decrease in predator avoidance and prey capture behaviors.
Regional alterations in c-Fos expression in the brain of killifish can have higher-level effects on behavior. Schooling and motor behaviors are controlled by the telencephalon, diencephalon, mesencephalon (optic tectum), and medulla (Smith, 1982), regions in which increased c-Fos expression was observed in killifish. Our study confirms that alterations in behavior observed in DA- and STX-exposed killifish may be related to neuronal stress in the telencephalon and optic tecta. Future studies should investigate and quantify the relationship between c-Fos expression and behavior in an attempt to correlate changes in regional brain activity with behavioral alterations. This is the first study to investigate alterations in c-Fos expression resulting from sub-lethal HAB toxin exposure in an ecologically relevant fish species. These resulting alterations may serve to compromise or enhance the survival of exposed animals (Zhang et al., 2002), including exposed killifish and other estuarine species. Ultimately, this study shows that alterations in c-Fos expression provides a sensitive measure of low-level HAB stress exposure in fish.
Acknowledgments
Portions of the project were supported by the U.S. Environmental Protection Agency, Science to Achieve Results (STAR) program (#R82-8224). We thank Gloria Hoffman for her support and insights, and Mohamed Ali and Tracey David for their assistance with the data collection and processing. All experiments within this manuscript complied with the current regulations set by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland (Protocol #R-00-36B).
References
- Anderson DM. Bloom dynamics of toxic Alexandrium species in the northeastern US. Limnol Oceanogr. 1997;42:1009–1022. [Google Scholar]
- Bakin AV, Curran T. Role of DNA 5-methylcytosine transferase in cell transformation by fos. Science. 1999;283:387–390. doi: 10.1126/science.283.5400.387. [DOI] [PubMed] [Google Scholar]
- Berman FW, Lepage KT, Murray TF. Domoic acid neurotoxicity in cultured cerebellar granule neurons is controlled preferentially by the NMDA receptor Ca2+ influx pathway. Brain Res. 2002;924:20–29. doi: 10.1016/s0006-8993(01)03221-8. [DOI] [PubMed] [Google Scholar]
- Berman FW, Murray TF. Brevetoxins cause acute excitotoxicity in primary cultures of rat cerebellar granule neurons. J Pharmacol Exp Ther. 1999;290:439–444. [PubMed] [Google Scholar]
- Bosch TJ, Maslam S, Roberts BL. Fos-like immunohistochemical identification of neurons active during the startle response of the rainbow trout. J Comp Neurol. 2001;439:306–314. doi: 10.1002/cne.1352. [DOI] [PubMed] [Google Scholar]
- Bosch TJ, Maslam S, Roberts BL. A polyclonal antibody against mammalian Fos can be used as a cytoplasmic neuronal-activity marker in a teleost fish. J Neurosci Methods. 1995;58:173–179. doi: 10.1016/0165-0270(94)00174-f. [DOI] [PubMed] [Google Scholar]
- Cheng SB, Kuchiiwa S, Nagatomo I, Akasaki Y, Uchida M, Tominaga M, Hashiguchi W, Kuchiiwa T, Nakagawa S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin treatment induces c-Fos expression in the forebrain of the Long-Evans rat. Brain Res. 2002;931:176–180. doi: 10.1016/s0006-8993(02)02257-6. [DOI] [PubMed] [Google Scholar]
- Deshpande SS, Adler M, Sheridan RE. Differential actions of brevetoxin on phrenic-nerve and diaphragm muscle in the rat. Toxicon. 1993;31:459–470. doi: 10.1016/0041-0101(93)90181-h. [DOI] [PubMed] [Google Scholar]
- Espana RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Sommer W, Rimondini R, Andbjer B, Stromberg L, Fuxe K. c-fos reduces corticosterone-mediated effects on neurotrophic factor expression in the rat hippocampal CA1 region. J Neurosci. 2003;23:6013–6022. doi: 10.1523/JNEUROSCI.23-14-06013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann A, Marksteiner J, Hinterhuber H, Humpel C. Magnetic stimulation induces neuronal c-fos via tetrodotoxin-sensitive sodium channels in organotypic cortex brain slices of the rat. Neurosci Lett. 2001;310:105–108. doi: 10.1016/s0304-3940(01)02073-0. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Kindy MS, Verma IM. Developmental expression of the Xenopus-Laevis Fos protooncogene. Cell Growth Differ. 1990;1:27–37. [PubMed] [Google Scholar]
- LaHoste GJ, Henry BL, Marshall JF. Dopamine D-l receptors synergize with D-2, but not D-3 or D-4, receptors in the striatum without the involvement of action potentials. J Neurosci. 2000;20:6666–6671. doi: 10.1523/JNEUROSCI.20-17-06666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg JH. The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci. 2002;10:113–390. [Google Scholar]
- Lefebvre KA, Bargu S, Kieckhefer T, Silver MW. From sanddabs to blue whales: the pervasiveness of domoic acid. Toxicon. 2002;40:971–977. doi: 10.1016/s0041-0101(02)00093-4. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Dovel SL, Silver MW. Tissue distribution and neurotoxic effects of domoic acid in a prominent vector species, the northern anchovy Engraulis mordax. Mar Biol. 2001;138:693–700. [Google Scholar]
- Lefebvre KA, Trainer VL, Scholz NL. Morphological abnormalities and sensorimotor deficits in larval fish exposed to dissolved saxitoxin. Aquat Toxicol. 2004;66:159–170. doi: 10.1016/j.aquatox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Lu B, Lund RD, Coffey PJ. Basal increase in c-Fos-like expression in superior colliculus of royal college of surgeons dystrophic rats can be abolished by intraocular injection of tetrodotoxin. Neuroscience. 2001;107:109–115. doi: 10.1016/s0306-4522(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- Matsuoka L, Fuyuki K, Shoji T, Kurihara K. Identification of c-fos related genes and their induction by neural activation in rainbow trout brain. Bba-Gene Struct Expr. 1998;1395:220–227. doi: 10.1016/s0167-4781(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Role of ion flux in the control of C-Fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Feldon J. Interactions between environmental stimulation and antipsychotic drug effects on forebrain C-FOS activation. Neuroscience. 2001;104:717–730. doi: 10.1016/s0306-4522(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Peter RE, Macey MJ, Gill VE. Stereotaxic atlas and technique for forebrain nuclei of killifish, Fundulus heteroclitus. J Comp Neurol. 1975;159:103–127. doi: 10.1002/cne.901590107. [DOI] [PubMed] [Google Scholar]
- Rybnikova EA, Pelto-Huikko M, Shalyapina VG. Expression of early genes in the rat brain after administration of corticoliberin into the neostriatum. Neurosci Behav Physiol. 2003;33:81–84. doi: 10.1023/a:1021135516562. [DOI] [PubMed] [Google Scholar]
- Sadananda M, Bischof HJ. Enhanced fos expression in the zebra finch (Taeniopygia guttata) brain following first courtship. J Comp Neurol. 2002;448:150–164. doi: 10.1002/cne.10232. [DOI] [PubMed] [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, Cordaro J, DeLong R, De Vogelaere A, Harvey J, Haulena M, Lefebvre K, Lipscomb T, Loscutoff S, Lowenstine LJ, Marin R, Miller PE, McLellan WA, Moeller PDR, Powell CL, Rowles T, Silvagni P, Silver M, Spraker T, Trainer V, Van Dolah FM. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature. 2000;403:80–84. doi: 10.1038/47481. [DOI] [PubMed] [Google Scholar]
- Schreiberagus N, Horner J, Torres R, Chiu FC, Depinho RA. Zebra fish myc family and max genes—differential expression and oncogenic activity throughout vertebrate evolution. Mol Cell Biol. 1993;13:2765–2775. doi: 10.1128/mcb.13.5.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR. Fish Neurotoxicology. In: Weber LJ, editor. Fish Neurotoxicology. Raven Press; New York: 1982. pp. 107–151. [Google Scholar]
- Sonnenberg JL, Mitchelmore C, Macgregorleon PF, Hempstead J, Morgan JI, Curran T. Glutamate receptor agonists increase the expression of Fos, Fra, and Ap-1 DNA-binding activity in the mammalian brain. J Neurosci Res. 1989;24:72–80. doi: 10.1002/jnr.490240111. [DOI] [PubMed] [Google Scholar]
- Steidinger KA, Burklew M, Ingle RM. The Effects of Gymnodinium Breve Toxin on Estuarine Animals. Academic Press; New York: 1973. [Google Scholar]
- Watson RE, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- White AW. Dinoflagellate toxins as a probable cause of an Atlantic herring (Clupea harengus harengus) kill and pteropods as apparent vector. J Fisheries Res Board, Can. 1977;34:2421–2424. [Google Scholar]
- Wulliman MF, Rupp B, Reichert H. Neuroanatomy of the Zebrafish Brain: A Topological Atlas. Birkhauser Verlag; Basel: 1996. [Google Scholar]
- Zhang JH, Zhang DS, McQuade JS, Behbehani M, Tsien JZ, Xu M. c-fos regulates neuronal excitability and survival. Nat Genet. 2002;30:416–420. doi: 10.1038/ng859. [DOI] [PubMed] [Google Scholar]


