Summary
Desmocollin 3 (DSC3) belongs to a subfamily of cadherins and is a major component of desmosomes in keratinocytes of stratified epithelia, such as the epidermis. Based on its amino acid sequence homology to classical cadherins, such as E-cadherin, it has been postulated that DSC3 functions as a cell-adhesion molecule. To test this hypothesis, we assessed the function of DSC3 in the development and maintenance of stratified epithelia, in particular the epidermis and hair follicles. Using a conditional null allele, we show that loss of Dsc3 function in the epidermis causes impaired cell–cell adhesion, leading to intra-epidermal blistering and telogen hair loss. Furthermore, the lesions in Dsc3-null skin resemble those observed in individuals with pemphigus vulgaris (PV), indicating that impaired Dsc3 function could be a potential cause of PV-like inherited or acquired skin blistering diseases.
Keywords: Desmosomes, Desmocollin 3, Skin fragility, Hair loss, Dsc3 null mice
Introduction
Desmosomes are cell–cell adhesion structures (junctions) that are particularly abundant in tissues and organs that have to withstand significant mechanical stress, such as skin and heart. The functions of desmosomes are not restricted to maintaining tissue integrity. There is also indirect evidence that that these junctions have an important function in cell sorting and tissue formation during mammalian embryonic development (e.g. Runswick et al., 2001) (reviewed by Schmidt and Koch, 2007).
The basic building blocks of desmosomes belong to three protein families: the desmosomal cadherins (desmocollins, DSC1-DSC3; desmogleins, DSG1-DSG4 in humans), plakins (desmoplakin, DSP) and armadillo proteins (plakoglobin, JUP; plakophilins, PKP1-PKP3). The desmosomal cadherins (Holthofer et al., 2007; Schmidt and Koch, 2007), together with PERP (Ihrie and Attardi, 2005), form the transmembrane core of the desmosome. Within the core, direct heterophilic interactions between DSG and DSC proteins are believed to contribute to cell–cell adhesion (Chitaev and Troyanovsky, 1997). DSC and DSG proteins are linked via JUP, at least one member of the PKP family, and DSP to the intermediate filament (IF) cytoskeleton.
The notion that desmosomal function is required for development and tissue homeostasis is underscored by the finding that impaired desmosomal adhesion causes various acquired and inherited tissue fragility disorders. DSGs are targeted by auto-antibodies in the autoimmune disease pemphigus vulgaris (PV) and pemphigus foliaceus (PF) (Amagai et al., 2006), and mutations in the human DSP, JUP, PKP1, PKP2, DSG1, DSG2 and DSG4 genes cause skin and/or heart disorders (e.g. arrhythmogenic right ventricular dysplasia/cardiomyopathy) (Schmidt and Koch, 2007).
Little is known about the biological function of the three DSC genes (DSC1-DSC3). All three genes encode N-glycosylated type 1 transmembrane proteins that belong to the cadherin family of ‘calcium-dependent cell-adhesion molecules’. Each gene encodes two proteins (a- and b-form) that differ only with respect to their C-terminal cytoplasmic amino acid sequences.
All three DSC genes are expressed in the epidermis. In mouse and human epidermis, Dsc2/DSC2 is very weakly expressed in the basal cell layer of the epidermis in certain body regions, Dsc3/DSC3 is expressed mainly in the basal and first suprabasal cell layers, whereas Dsc1/DSC1 expression is restricted to the uppermost portion of the epidermis (Chidgey et al., 1997; King et al., 1997; Nuber et al., 1995; Nuber et al., 1996; Theis et al., 1993). As predicted from its expression pattern, studies using knockout mice indicated that Dsc1 is required to maintain cell adhesion in the upper epidermis (Chidgey et al., 2001). A comparison of conventional Dsc1-null mice and mice that express a C-terminal truncated DSC1 variant (Cheng et al., 2004) suggested that the DSC1a- and DSC1b-specific C-terminal sequences are not required for cell adhesion, but might be involved in cell signaling. The roles of DSC2 and DSC3 in the epidermis are currently not known.
It has been speculated that the ratio of DSC3 to DSC1 might be a crucial factor in controlling epidermal differentiation. This speculation is based on the observation that keratinocytes shift from Dsc3 to Dsc1 expression during terminal differentiation in the suprabasal cell layers of the interfollicular epidermis (e.g. Chidgey et al., 1997). Furthermore, overexpression of Dsc3 in differentiating (suprabasal) keratinocytes of transgenic mice has been shown to affect epidermal differentiation (Hardman et al., 2005). As DSC3 is the main DSC isoform synthesized in the basal and first suprabasal cell layers of the interfollicular epidermis and the outer root sheath of hair follicles, we hypothesized that DSC3 might be required for cell–cell adhesion in these tissues.
We have previously shown that a conventional Dsc3-null mutation results in embryonic lethality, with most mutant embryos dying before they can implant into the uterus (Den et al., 2006). To study the role of DSC3 in the epidermis, we generated mouse lines with a conditional Dsc3-null mutation. Our results demonstrate that DSC3 is required to maintain cell adhesion and hair follicle anchorage in the epidermis. Furthermore, mutant mice develop skin blisters with histology similar to that found in individuals with the autoimmune disease pemphigus vulgaris [PV; (Amagai et al., 2006)]. We therefore speculate that loss of DSC3 function in humans, either due to a mutation or due to pathogenic auto-antibodies against this protein, could occur in a subset of individuals with PV.
Results
Generation of mice with a Dsc3-null mutation in the skin
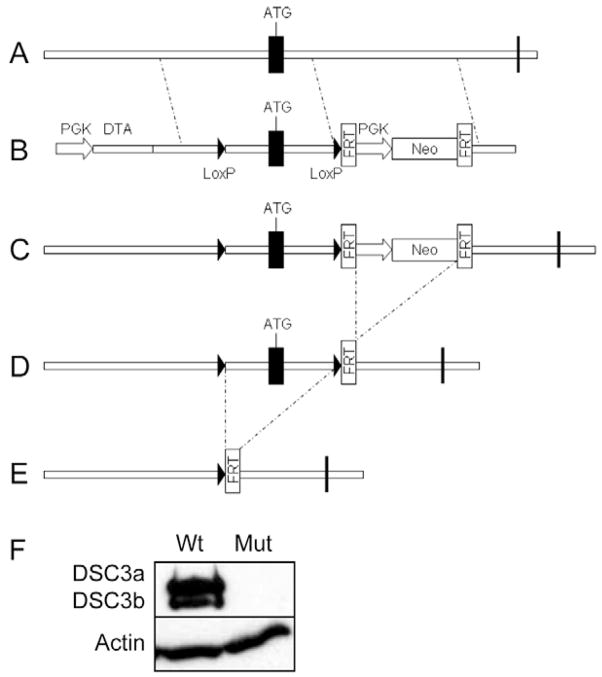
As a conventional Dsc3-null mutation results in embryonic lethality, we generated a conditional Dsc3-null allele (Dsc3tm2PKo; referred to as Dsc3fl in the following sections; Fig. 1). LoxP sequences were introduced in the promoter and intron 1 of the Dsc3 gene. Cre-mediated recombination between these LoxP sites was predicted to delete exon 1 as well as a region of the Dsc3 promoter, thus ablating gene expression (Fig. 1E). In order to ensure gene inactivation in stratified epithelia, we used a K14-Cre transgene, which is constitutively active in the basal layer of the epidermis beginning at embryonic day 14 (E14; see Fig. S1 in the supplementary material). This transgene is thus active at around the time when the first hair follicles are formed in the back skin of mouse embryos (E13.5–E14.5).
Fig. 1.
Schematic representation of the targeting strategy used to generate Dsc3fl (Dsc3tm2Ko) mice. (A) Exon 1 (ATG) of the Dsc3 gene was targeted with the vector shown in B. In the targeting construct, loxP sites were inserted in the promoter and intron 1. A neomycin minigene (PGK-Neo), flanked by FRT sites, was also inserted into intron 1. (C) The neo cassette was removed from recombinant ES cell clones via transient expression of FLPe recombinase, leaving a single FRT site in intron 1. (D) The resulting Dsc3fl/+ ES cells were used to generate mutant mice. (E) Cre-mediated recombination in bigenic mice (Dsc3fl/fl/K14-Cre) will lead to the deletion of exon 1, as well as part of the Dsc3 promoter and intron 1. (F) Newborn wild-type keratinocytes (Wt) synthesize both DSC3a and DSC3b, whereas Dsc3fl/fl/K14-Cre keratinocytes (Mut) do not express these proteins. The western blot shown was probed with a DSC3 antibody, stripped and re-probed with a β-actin antibody (loading control). Vertical bars indicate exons.
Mice that were homozygous for the floxed allele (Dsc3fl/fl) developed normally, excluding a hypomorphic effect of the loxP sites on Dsc3 gene expression. Next, we generated bigenic mice that carried the floxed Dsc3 allele and the K14-Cre transgene (Dsc3fl/fl/K14-Cre; referred to as mutant mice below). Keratinocytes isolated from newborn mutant mice were analyzed by western blotting for the expression of DSC3. As predicted, wild-type (wt) keratinocytes expressed both DSC3a and DSC3b, whereas mutant keratinocytes lacked expression of both proteins (Fig. 1F). Moreover, DSC3 was absent from the epidermis of newborn Dsc3 mutant mice, as judged by immunofluorescence microscopy using mouse DSC3-specific antibodies (Fig. 2D).
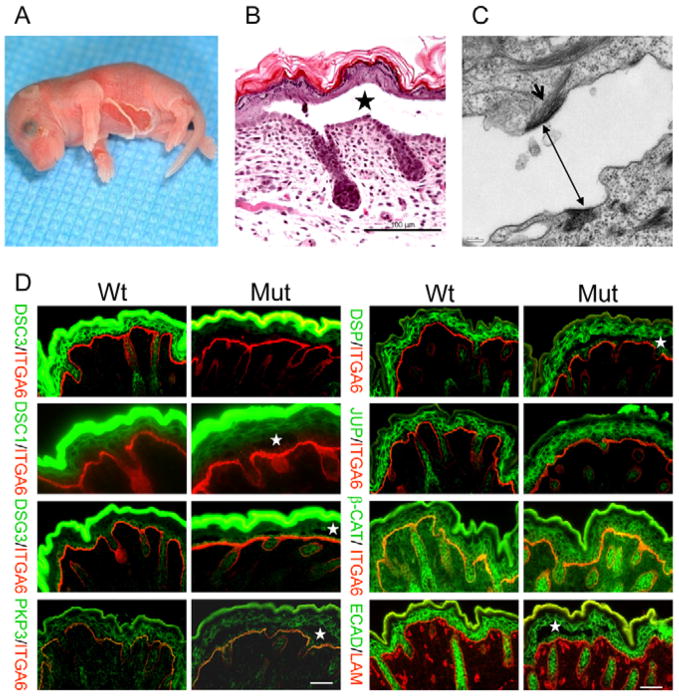
Fig. 2.
Characterization of newborn epidermis in mutant mice. (A) Newborn pup with severe ventral skin blistering. (B) Histology of an intra-epidermal blister in newborn mutant mice. Acantholysis is present immediately above the basal cell layer (star indicates blister cavity; bar, 100 μm). (C) Electron micrograph of a blister. Two half desmosomes are present in the plasma membranes of the acantholytic cells (double-headed arrow). The arrow indicates intermediate filaments that are attached to the half desmosome (bar, 0.2 μm). (D) Immunofluorescence microscopy using newborn skin of mutant mice (Mut) and wild-type (Wt) controls. The antibodies used are indicated. White stars indicate blister cavities. Junctional proteins are similarly distributed in both genotypes. ITGA6 (α6 integrin) or laminin staining (red) demarcates the basement membrane zone. The strong green fluorescence (DSC1, DSC3, DSG3 staining) is due to unspecific binding of the secondary antibody to the stratum corneum. Bars, 50 μm.
Intra-epidermal blistering in newborn Dsc3fl/fl/K14-Cre mice
Our breeding studies indicated that mutant mice were born with a frequency consistent with Mendelian inheritance, i.e. we did not find evidence for embryonic lethality. This was expected, as even mice that completely lack stratified epithelia, such as p63-null mice, develop to term (reviewed by Koster and Roop, 2007).
Approximately 10% of newborn mutant mice developed severe epidermal blistering within hours of birth (Fig. 2A). Even careful handling of newborn pups often induced skin blistering, indicating that skin lesion were induced by mild mechanical stress.
Lesions were restricted to the skin, i.e. lesions did not develop in internal stratifying epithelia, such as the palate, the tongue and the forestomach (data not shown).
Pups that showed macroscopic skin blistering usually died within a few hours of birth. Histological examinations of the lesions revealed intra-epidermal blistering with acantholysis (cell–cell separation) just above the basal cell layer (Fig. 2B). Later stages of acantholysis also showed lateral separation of basal keratinocytes. Electron microscopy analysis of affected skin showed that half-desmosomes were present in the plasma membranes of keratinocytes at the bottom and the roof of the blister (Fig. 2C; see Fig. S3 in the supplementary material), suggesting impaired desmosome function as the cause of the defect.
In order to determine whether the Dsc3-null mutation affected keratinocyte differentiation, we stained newborn mutant and control skin with antibodies against desmosomal proteins (DSC3, DSC1, DSG3, PKP3, DSP, JUP), adherens junction proteins (β-Catenin, E-cadherin), cytoskeletal proteins (K5, K14, K10) and cornified envelope proteins (loricrin, filaggrin) (Fig. 2D, and data not shown). We did not find significant differences in the expression patterns of these proteins between mutant and control skins, suggesting that Dsc3 is not required for keratinocyte differentiation.
Weak cell adhesion between mutant keratinocytes
In order to determine whether the primary defect underlying intra-epidermal blistering in mutant skin was weakened cell–cell adhesion, we tested the mechanical stress resistance of cell sheets generated in vitro from newborn mutant and control keratinocytes (Fig. 3A,B). These cell sheets were generated under conditions that allow for the formation of desmosomes. In wild-type keratinocytes, these desmosomes contain DSC3 (Fig. 1F, and data not shown). Cell sheets generated from mutant keratinocytes showed significantly less stress resistance than those generated from wild-type keratinocytes. Furthermore, fragmentation of mutant keratinocyte cell sheets did not increase the release of cytoplasmic enzymes (Fig. 3C), indicating that the fragility was caused by loss of cell–cell adhesion and not by cell lysis. We did not observe any effects of the Dsc3-null mutation on proliferation or migration of cultured newborn keratinocytes (data not shown).
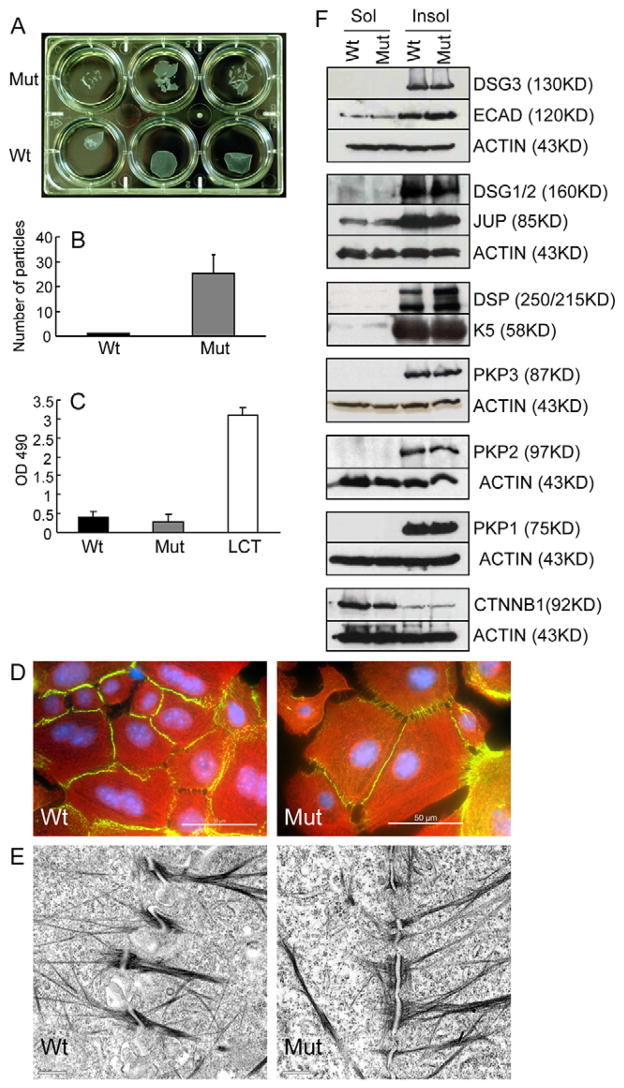
Fig. 3.
Weak cell adhesion between mutant keratinocytes. (A–C) Newborn mutant (Mut) and wild-type control (Wt) keratinocytes were grown in vitro to confluency, and then cultured in high Ca2+ medium for 24 hours to induce desmosome formation. The cell sheets were then lifted from the plate and exposed to mechanical stress (Huen et al., 2002). (A) Mutant cell sheets showed increased susceptibility to stress. Each well represents cells from a single pup, i.e. three mutant and three wild-type pups were used. (B) Quantitative evaluation of particles generated in the experiment shown above (average number of particles; n=3; error bar, standard deviation). (C) LDH release into the culture medium of stressed cell sheets. Mutant samples did not show increased cell lysis (LCT, lysis control; wild-type cells were treated with a detergent to release LDH; positive control). (D) Immunofluorescence staining of wild-type and mutant keratinocytes with antibodies against DSP (green) and K14 (red) (DAPI staining of the nucleus, blue). Bars, 50 μm. (E) Electron micrograph of desmosomes formed in newborn wild-type and mutant keratinocytes that were cultured in high Ca2+ medium for 24 hours. Morphologically normal desmosomes were formed in mutant keratinocytes. Bar, 0.5 μm. (F) Western blot analysis of newborn keratinocytes cultured as described in A. Detergent-insoluble (junction associated) and detergent-soluble (non-junctional) protein fractions were isolated as described (Cheng et al., 2004) and probed with the antibodies listed. β-Actin or keratin 5 (K5) were used as loading controls.
Immunofluorescence staining of mutant and control keratinocytes with antibodies against K14 and DSP did not reveal obvious differences in the density of desmosomes, or their coupling to the IF cytoskeleton (Fig. 3D). Next we performed electron microscopy on newborn mutant and wild-type keratinocytes that had been cultured in high Ca2+ medium for 24 hours to induce desmosome assembly (Fig. 3E). Mutant desmosomes appeared morphologically normal. In order to determine whether loss of Dsc3 expression affected desmosome size, we compared the lengths of mutant and wild-type plaques. Using randomly selected electron micrographs of newborn cultured keratinocytes form both genotypes, we measured 94 wild-type and 90 mutant desmosomes. The average diameter of the wild-type plaque was 0.404±0.143 μm and that of the mutant plaque was 0.369±0.143 μm (no significant difference, P=0.104). We thus concluded that loss of Dsc3 expression had no significant effect on the size of the desmosomal plaque in cultured keratinocytes. The findings summarized above demonstrate that, at least in cell culture and in the absence of mechanical stress, mutant cells can form morphologically normal desmosomes.
Western blot analysis using protein extracts from cultured keratinocytes with antibodies against desmosomal (DSG3, DSG1/2, JUP, DSP, PKP3, PKP2, PKP1) and adherens (E-cadherin, β-catenin) junction proteins did not reveal any change in expression levels due to Dsc3 gene ablation (data not shown). We also compared the relative solubility of these proteins in extraction buffers containing the non-ionic detergent Triton X100. Triton X100 insolubility is a characteristic feature of proteins integrated into cell junctions (Pasdar and Nelson, 1988; Pasdar and Nelson, 1989). Again, we did not find any changes between mutant and control samples (Fig. 3F).
Given that loss of Dsg3 function has been shown to induce suprabasal skin blisters (Koch et al., 1997) that are histologically identical to the ones we observed in our mice, it was important to determine whether our conditional Dsc3-null mutation caused skin blistering indirectly by affecting Dsg3 expression. This was not the case as DSG3 levels were not changed in Dsc3 mutant keratinocytes. Interestingly, the expression levels of the DSC3-binding proteins JUP and PKP3 (Bonne et al., 2003) were not changed either, suggesting that the steady state levels of these proteins are independent of their interaction with DSC3.
We were not able to assess DSC2 protein levels in this experiment because currently there is no antibody available that recognizes mouse DSC2. Nevertheless, we assessed Dsc2 expression levels by quantitative RT-PCR and found a small increase in mRNA levels in response to Dsc3 ablation (1.8 fold increase; P=0.0397; data not shown).
With the exception of the mild increase in Dsc2 expression, these data indicate that loss of Dsc3 function fails to induce a compensatory upregulation of other desmosomal cadherins in cultured keratinocytes.
Several recent publications point to a crucial role of cell signaling pathways, in particular p38/MAPK, JUP, β-catenin and NFκB signaling, in mediating the cellular response to impaired cadherin function (see Schmidt and Koch, 2007). We therefore compared the expression levels of key components of these pathways [JUP, β-Catenin, ERK, JNK, p65 (Rel A), p38] in mutant and wild-type keratinocytes by western blotting. No differences were found with respect to the expression levels or the subcellular distributions (cytoplasmic versus nuclear) of these proteins in cultured mutant keratinocytes (supplementary material Fig. S2).
Skin erosions in adult Dsc3fl/fl/K14-Cre mice
All newborn mutant pups examined thus far developed histologically detectable blisters in the epidermis. However, not all pups showed macroscopic skin blistering. Those that did not show obvious skin lesions usually developed to adulthood. We do not know which factors control the severity of the phenotype in newborn mice. However environmental factors, such as the behavior of the mother, and genetic modifier genes in the mixed genetic background of these mice (C57BL/6J, 129Sv), might be contributing factors. Despite the absence of macroscopic skin blistering in some newborn mutant mice, severe skin lesions were observed in all mutant mice that reached adulthood (≥3 months of age). As illustrated in Fig. 4, these lesions ranged from severe epidermal hyperplasia due to an increase in basal cell proliferation to a complete loss of the epidermis in large sections of the skin. The latter lesions showed massive inflammation and scar formation. Attempts to re-epithelialize the wound bed were visible; however, keratinocytes within the epithelial tongue that penetrated the wound bed showed typical suprabasal acantholysis (arrows in Fig. 4B). This continued blistering explained the persistence of severe skin wounds in older mutant mice.
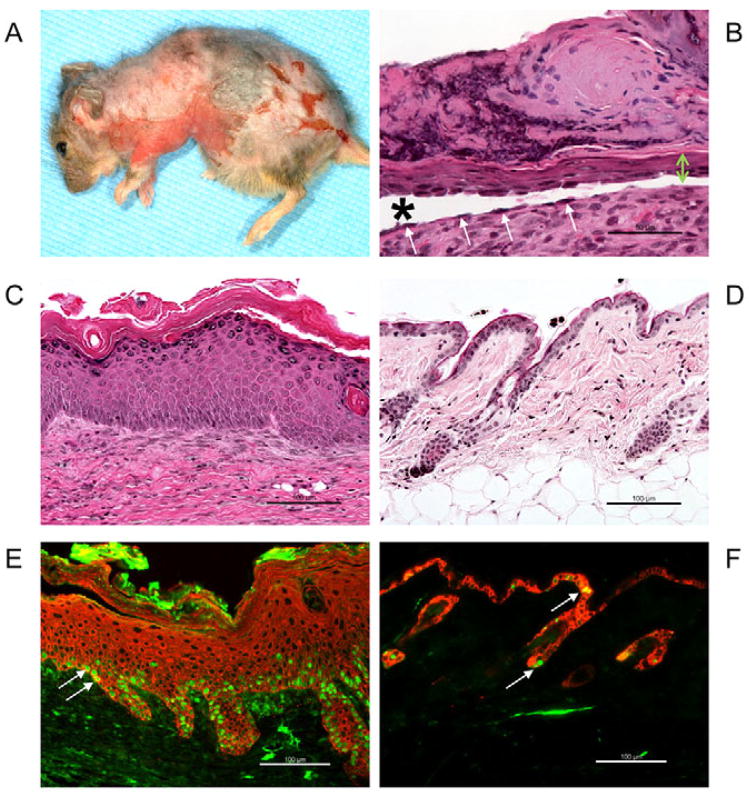
Fig. 4.
Skin phenotype of adult Dsc3fl/fl/K14-Cre mice. (A) A 160-day-old mutant mouse showing severe skin lesions, including a partial loss of the epidermis and hair follicles. (B,C) Histological analysis of the mouse shown in A. (B) Massive inflammation, scarring and re-epithelialization in an area where the epidermis was completely lost. A thin tongue of keratinocytes is migrating underneath the scab in an attempt to re-epithelialize the wound bed (the tongue is marked by a green double-headed arrow). The cells of this epithelial tongue undergo acantholysis (loss of cell–cell adhesion leading to blistering), a phenotype that might be the cause of wound healing defect and persistent skin blistering in older mice. Arrows indicate basal keratinocytes that have lost their adhesion to suprabasal cells, but that are still attached to the basement membrane. Owing to the severity of this phenotype, we now do not maintain these mice beyond 3 months of age. Star indicates blister cavity. (C) Hyperplastic area of mutant epidermis. (D) Age-matched normal skin of a wild-type mouse. The epidermis is only approximately two cell layers thick. (E) Immunofluorescence staining of hyperplastic mutant epidermis with K14 (red) and Ki67 (green) antibodies. The number of proliferating Ki67-positive nuclei (arrows) is dramatically increased compared with the wild-type control shown in F. (F) Age-matched wild-type epidermis stained with K14 (red) and Ki67 (green). Only a few keratinocytes in the interfollicular epidermis are Ki67 positive (arrows). Bars, 100 μm.
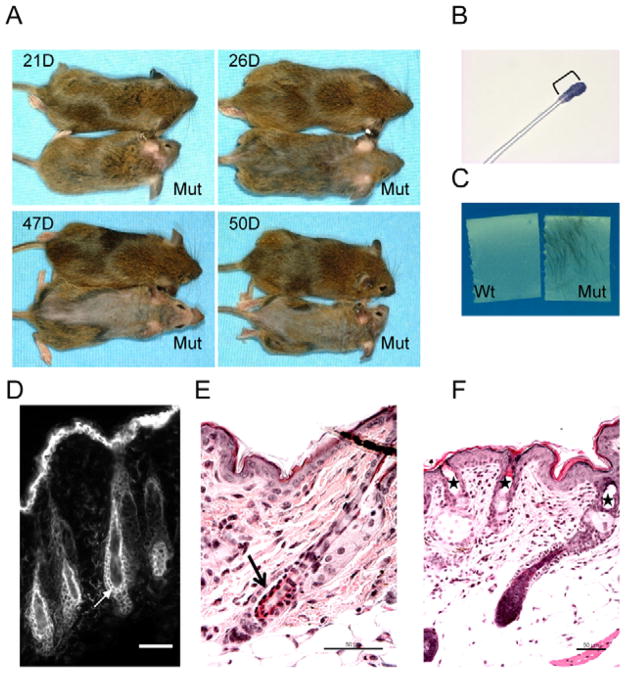
Telogen hair loss in Dsc3fl/fl/K14-Cre mice
Mutant mice showed hair loss on their backs at weaning age (around 21 days after birth). This process began on the head and progressed towards the tails of the animals (Fig. 5A). The hair eventually grew back, but patches of hair loss occurred in older mice, and some mice completely lost their hair (see, for example, mouse in Fig. 4A). The timing and pattern of the initial hair loss suggested a defect in the anchorage of telogen follicles. Telogen is the resting phase of the hair growth cycle, which is initiated on the head and neck of mice at around the time of weaning, i.e. where hair loss occurred first in mutant mice (Koch et al., 1998). Initially, we performed a hair tape-stripping experiment on mice of weaning age (Fig. 5B,C). A short piece of sticky tape was placed on the coat of mice and gently pulled off. Hair fibers attached to the tape were washed off, stained with a histological dye and analyzed microscopically (Fig. 5B). This analysis indicated that mutant mice lose telogen hair.
Fig. 5.
Hair-loss phenotype in Dsc3fl/fl/K14-Cre mice. (A) Beginning around the time of weaning, mutant mice developed hair loss that started at the head and proceeded towards the tail. (B,C) Tape-stripping experiments indicated telogen hair loss in mutant (Mut) but not wild-type (Wt) mice. A single cell layer of epithelial cells (stained blue with a histological dye in B) was still attached to the telogen hair club of loosely anchored mutant hair (bracket in B). (D). Staining of wild-type telogen skin with a DSC3 antibody. The two epithelial cell layers surrounding the club (arrow) express DSC3. (E) Acantholysis (arrow) between the two cell layers anchoring the telogen club hair in mutant skin. (F) Cyst formation (stars) in mutant skin resulting from telogen hair loss. Bars, 50 μm.
A telogen hair is anchored in the skin by two epithelial cell layers that surround the lower region of the hair shaft (the telogen club). In wild-type mice, these two cell layers express DSC3 (Fig. 5D). We therefore hypothesized that loss of cell adhesion (acantholysis) between these two cell sheets could account for the hair loss. This hypothesis was confirmed by histological analysis (the arrow in Fig. 5E indicates an area with acantholysis). Furthermore, hairless mutant skin showed empty cysts in the epidermis, which are most probably remnants of the lost telogen club hairs (Fig. 5F).
Discussion
The role of Dsc3 in epidermal homeostasis and skin disorders
To our knowledge, the skin phenotype of Dsc3fl/fl/K14-Cre mice is more severe than that of any other desmosomal cadherin-null mouse model reported so far. Our results indicate that DSC3 is essential for maintaining structural integrity of the interfollicular epidermis and for anchorage of telogen hair shafts. We did not find evidence for an effect of the Dsc3-null mutation on desmosome number or morphology. However, our in vitro adhesion assays unequivocally demonstrated weakened cell–cell adhesion in mutant keratinocytes, indicating impaired desmosome function.
Interestingly, the histopathology of lesions in Dsc3 mutant mice is indistinguishable from that found in lesions of individuals with PV. Therefore, it is tempting to speculate that mutations in the human DSC3 gene or auto-antibodies against the DSC3 protein could cause PV-like skin disorders. Although mutations in DSC3 have not been found in any human disorder, Bolling and colleagues recently reported an individual with palmoplantar keratoderma (thickening of the skin of palms and soles) and intra-epidermal blistering that histologically resembled PV. Interestingly, this individual synthesized DSC3 auto-antibodies but not DSG auto-antibodies (Bolling et al., 2007). The authors thus concluded that these DSC3 antibodies are likely to be the cause of the PV-like lesions. Together with the data presented in this manuscript, these findings suggest the possibility that impaired DSC3 function could cause PV-like skin disease.
Similarities and differences in the epidermal phenotypes of conditional Dsc3-null mice and conventional Dsg3-null mice
The hair phenotype observed in Dsc3-null and Dsg3-null skin was virtually identical in terms of the gross appearance and the cause of hair loss [acantholysis between the two cell layers surrounding the telogen club; this study and Koch et al. (Koch et al., 1998)]. These findings demonstrate that DSC3 and DSG3 are both required to anchor hair in the resting phase of the hair growth cycle. Furthermore, these data are consistent with the hypothesis that heterophilic molecular interactions between DSC3 and DSG3 are required for keratinocyte adhesion in the two epithelial cell layers that anchor the telogen hair club in the skin.
Nevertheless, the Dsc3- and Dsg3-null mutations have different effects on the interfollicular epidermis and internal stratified epithelia, such as palate, tongue, esophagus and forestomach. Whereas Dsg3-null mice develop spontaneous mucous membrane lesions and rarely overt skin lesions (Koch et al., 1997), we observed only skin lesions in Dsc3-null mice. These lesions, however, were lethal in ~10% of newborn mice.
The skin specificity of lesions in Dsc3 mice might be due to a lack of compensatory DSC isoforms expressed in this organ. In the skin, Dsc2 is expressed in the basal layer of the epidermis and thus partially overlaps in expression pattern with Dsc3 (e.g. King et al., 1997; Lorimer et al., 1994; Theis et al., 1993). The expression level of Dsc2, however, is very low in the interfollicular epidermis (see references above). Furthermore, our gene expression studies indicate that Dsc2 mRNA expression, at least in cell culture, is only mildly increased in Dsc3-null keratinocytes. Together, these observations suggest that Dsc2 is not present in sufficient amounts to fully compensate for the loss of Dsc3. The only other DSC isoform synthesized in the epidermis is DSC1. As this protein is only detected in the superficial epidermis, it cannot compensate for the loss of DSC3 in the basal and first suprabasal cell layer.
In contrast to the epidermis, Dsc2 is expressed at significant levels in mucous membranes and other internal stratified epithelia (e.g. King et al., 1997; Koch et al., 1992; Lorimer et al., 1994; Theis et al., 1993). Therefore, DSC2 may compensate for the Dsc3-null mutation in these tissues, thus explaining the lack of a phenotype.
Taken together, our results demonstrate for the first time that DSC3 is absolutely required for normal desmosome function and maintenance of tissue integrity in the interfollicular epidermis. Furthermore, our conditional Dsc3-null mouse model will be ideally suited to investigate the role of this gene in other biological processes, such as epithelial tumor development and metastasis.
Materials and Methods
Animal experimentation
Animal experiments were conducted in compliance with all applicable local and federal requirements, and were approved by the Institutional Animal Care and Use Committees (IACUC) of Baylor College of Medicine and the University of Colorado Denver.
Generation of a conditional Dsc3-null allele
The targeting vector shown in Fig. 1B was constructed by BAC recombineering technology using a 129/Sv BAC clone containing the Dsc3 gene using the reagents and protocols provided by the ‘Counter Selection BAC Modification Kit’ (Gene Bridges, Dresden, Germany). We introduced loxP sites in the promoter (position −1499) and intron 1 (position +1718) of the Dsc3 gene. A neomycin-resistance minigene (PGK-Neo), which was flanked by FRT sites, was used as a negative selection marker. A DTA cassette was used as a counter selection marker. The targeting vector was introduced into W4/129S6 mouse ES cells (Taconic) and clones that had undergone homologous recombination at the Dsc3 gene locus were identified by Southern blot analysis using external probes (data not shown). A single recombination event was confirmed by Southern blot analysis using a neomycin minigene hybridization probe. Transient transfection of recombinant clones with an FLPe expression plasmid (pCAGGS-FLPe, Gene Bridges) resulted in the deletion of the neomycin cassette (which was confirmed by Southern blots; data not shown). We generated two genetically independent conditional Dsc3-null lines. Dsc3fl/fl/K14-Cre mice (K14-Cre, Jackson Laboratories) from both lines showed the same phenotype.
Cell adhesion, migration and proliferation assays
Keratinocyte cultures, cell migration, proliferation and cell adhesion assays were carried out essentially as described by Chen et al. and Huen et al. (Chen et al., 2006; Huen et al., 2002).
Immunofluorescence microscopy and western blotting
Protein extraction and western blotting were carried out following previously published protocols (Cheng et al., 2004). The following antibodies were used: DSC1 and DSC3 (Cheng et al., 2004); DSG3 (Koch et al., 1998); PKP1, PKP2 and PKP3 (generous gifts from Dr Werner W. Franke, German Cancer Center, Heidelberg, Germany); DSG1/2 (DG3.10, Research Diagnostics); DSP (Research Diagnostics); JUP (clone PG 5.1, Research Diagnostics); β-catenin (Santa Cruz Biotechnology); ABC β-catenin (Upstate Biotechnologies), E-cadherin (Sigma), K5, K10, K14, loricrin and filaggrin (generously provided by Dr Dennis Roop, UCD); β-actin (clone AC-15, Abcam); tubulin (Sigma); lamin B (Santa Cruz Biotechnology); integrin α6 (Chemicon); NF-κB p65 (Cell Signaling); phospho-NF-κB p65 (Cell Signaling); SAPK/JNK (56G8, Cell Signaling); p38 MAPK (Cell Signaling); phospho-p38 MAKP (Cell Signaling); and Erk1/2 (p44/42 MAPK, Cell Signaling). Alexa Fluor- (Alexa Fluor 488 and 594) or HRP-coupled secondary antibodies were purchased from Invitrogen and Vector Laboratories, respectively. Two microscopes were used to document our experiments: a Nikon Eclipse 90I microscope equipped with a Coolsnap HQ2 and a DS-Fi1 camera (NIS-Elements AR software from Nikon was used to capture images); and a Nikon Eclipse E600 microscope using a Photometrics Cool Snap FX camera in conjunction with the Metavue v6.1r5 imaging software package (Universal Imaging Corporation).
Electron microscopy
EM samples were processed by the Microscopy Core at UCD. Photographs were taken with a TECNAI G1 12 BioTwin microscope. Tissue processing was carried out essentially as described (Koch et al., 1998).
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/121/17/2844/DC1
Acknowledgments
We thank Maria Merched-Sauvage, Daniel Young and Marvin Coughenour for their expert technical assistance. Special thanks to Drs Werner W. Franke and Dennis R. Roop for providing antibodies, and to Dr James Fitzpatrick (UCD) for his expert advice regarding skin histopathology. We are also grateful to Dr Maranke I. Koster (UCD) for critical reading of this manuscript and for helpful suggestions. This work has been supported by a grant from the National Institutes of Health (NIH/NIAMS) to P.J.K. (RO1 AR050439).
References
- Amagai M, Ahmed AR, Kitajima Y, Bystryn JC, Milner Y, Gniadecki R, Hertl M, Pincelli C, Kurzen H, Fridkis-Hareli M, et al. Are desmoglein autoantibodies essential for the immunopathogenesis of pemphigus vulgaris, or just “witnesses of disease”? Exp Dermatol. 2006;15:815–831. doi: 10.1111/j.1600-0625.2006.00499_1.x. [DOI] [PubMed] [Google Scholar]
- Bolling MC, Mekkes JR, Goldschmidt WF, van Noesel CJ, Jonkman MF, Pas HH. Acquired palmoplantar keratoderma and immunobullous disease associated with antibodies to desmocollin 3. Br J Dermatol. 2007;157:168–173. doi: 10.1111/j.1365-2133.2007.07920.x. [DOI] [PubMed] [Google Scholar]
- Bonne S, Gilbert B, Hatzfeld M, Chen X, Green KJ, van Roy F. Defining desmosomal plakophilin-3 interactions. J Cell Biol. 2003;161:403–416. doi: 10.1083/jcb.200303036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cheng X, Merched-Sauvage M, Caulin C, Roop DR, Koch PJ. An unexpected role for keratin 10 end domains in susceptibility to skin cancer. J Cell Sci. 2006;119:5067–5076. doi: 10.1242/jcs.03298. [DOI] [PubMed] [Google Scholar]
- Cheng X, Mihindukulasuriya K, Den Z, Kowalczyk AP, Calkins CC, Ishiko A, Shimizu A, Koch PJ. Assessment of splice variant-specific functions of desmocollin 1 in the skin. Mol Cell Biol. 2004;24:154–163. doi: 10.1128/MCB.24.1.154-163.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidgey M, Brakebusch C, Gustafsson E, Cruchley A, Hail C, Kirk S, Merritt A, North A, Tselepis C, Hewitt J, et al. Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation. J Cell Biol. 2001;155:821–832. doi: 10.1083/jcb.200105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidgey MA, Yue KK, Gould S, Byrne C, Garrod DR. Changing pattern of desmocollin 3 expression accompanies epidermal organisation during skin development. Dev Dyn. 1997;210:315–327. doi: 10.1002/(SICI)1097-0177(199711)210:3<315::AID-AJA11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Chitaev NA, Troyanovsky SM. Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell-cell adhesion. J Cell Biol. 1997;138:193–201. doi: 10.1083/jcb.138.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Z, Cheng X, Merched-Sauvage M, Koch PJ. Desmocollin 3 is required for pre-implantation development of the mouse embryo. J Cell Sci. 2006;119:482–489. doi: 10.1242/jcs.02769. [DOI] [PubMed] [Google Scholar]
- Hardman MJ, Liu K, Avilion AA, Merritt A, Brennan K, Garrod DR, Byrne C. Desmosomal cadherin misexpression alters {beta}-catenin stability and epidermal differentiation. Mol Cell Biol. 2005;25:969–978. doi: 10.1128/MCB.25.3.969-978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthofer B, Windoffer R, Troyanovsky S, Leube RE. Structure and function of desmosomes. Int Rev Cytol. 2007;264:65–163. doi: 10.1016/S0074-7696(07)64003-0. [DOI] [PubMed] [Google Scholar]
- Huen AC, Park JK, Godsel LM, Chen X, Bannon LJ, Amargo EV, Hudson TY, Mongiu AK, Leigh IM, Kelsell DP, et al. Intermediate filament-membrane attachments function synergistically with actin-dependent contacts to regulate intercellular adhesive strength. J Cell Biol. 2002;159:1005–1017. doi: 10.1083/jcb.200206098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Attardi LD. A new Perp in the lineup: linking p63 and desmosomal adhesion. Cell Cycle. 2005;4:873–876. doi: 10.4161/cc.4.7.1836. [DOI] [PubMed] [Google Scholar]
- King IA, Angst BD, Hunt DM, Kruger M, Arnemann J, Buxton RS. Hierarchical expression of desmosomal cadherins during stratified epithelial morphogenesis in the mouse. Differentiation. 1997;62:83–96. doi: 10.1046/j.1432-0436.1997.6220083.x. [DOI] [PubMed] [Google Scholar]
- Koch PJ, Goldschmidt MD, Zimbelmann R, Troyanovsky R, Franke WW. Complexity and expression patterns of the desmosomal cadherins. Proc Natl Acad Sci USA. 1992;89:353–357. doi: 10.1073/pnas.89.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, Mahoney MG, Ishikawa H, Pulkkinen L, Uitto J, Shultz L, Murphy GF, Whitaker-Menezes D, Stanley JR. Targeted disruption of the pemphigus vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to pemphigus vulgaris. J Cell Biol. 1997;137:1091–1102. doi: 10.1083/jcb.137.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, Mahoney MG, Cotsarelis G, Rothenberger K, Lavker RM, Stanley JR. Desmoglein 3 anchors telogen hair in the follicle. J Cell Sci. 1998;111:2529–2537. doi: 10.1242/jcs.111.17.2529. [DOI] [PubMed] [Google Scholar]
- Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- Lorimer JE, Hall LS, Clarke JP, Collins JE, Fleming TP, Garrod DR. Cloning, sequence analysis and expression pattern of mouse desmocollin 2 (DSC2), a cadherin-like adhesion molecule. Mol Membr Biol. 1994;11:229–236. doi: 10.3109/09687689409160432. [DOI] [PubMed] [Google Scholar]
- Nuber UA, Schafer S, Schmidt A, Koch PJ, Franke WW. The widespread human desmocollin Dsc2 and tissue-specific patPterns of synthesis of various desmocollin subtypes. Eur J Cell Biol. 1995;66:69–74. [PubMed] [Google Scholar]
- Nuber UA, Schafer S, Stehr S, Rackwitz HR, Franke WW. Patterns of desmocollin synthesis in human epithelia: immunolocalization of desmocollins 1 and 3 in special epithelia and in cultured cells. Eur J Cell Biol. 1996;71:1–13. [PubMed] [Google Scholar]
- Pasdar M, Nelson WJ. Kinetics of desmosome assembly in Madin-Darby canine kidney epithelial cells: temporal and spatial regulation of desmoplakin organization and stabilization upon cell-cell contact. I Biochemical analysis. J Cell Biol. 1988;106:677–685. doi: 10.1083/jcb.106.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasdar M, Nelson WJ. Regulation of desmosome assembly in epithelial cells: kinetics of synthesis, transport, and stabilization of desmoglein I, a major protein of the membrane core domain. J Cell Biol. 1989;109:163–177. doi: 10.1083/jcb.109.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runswick SK, O’Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001;3:823–830. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Koch PJ. Desmosomes: Just cell adhesion or is there more? Cell Adhesion & Migration. 2007;1:28–32. doi: 10.4161/cam.1.1.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Theis DG, Koch PJ, Franke WW. Differential synthesis of type 1 and type 2 desmocollin mRNAs in human stratified epithelia. Int J Dev Biol. 1993;37:101–110. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/121/17/2844/DC1