Abstract
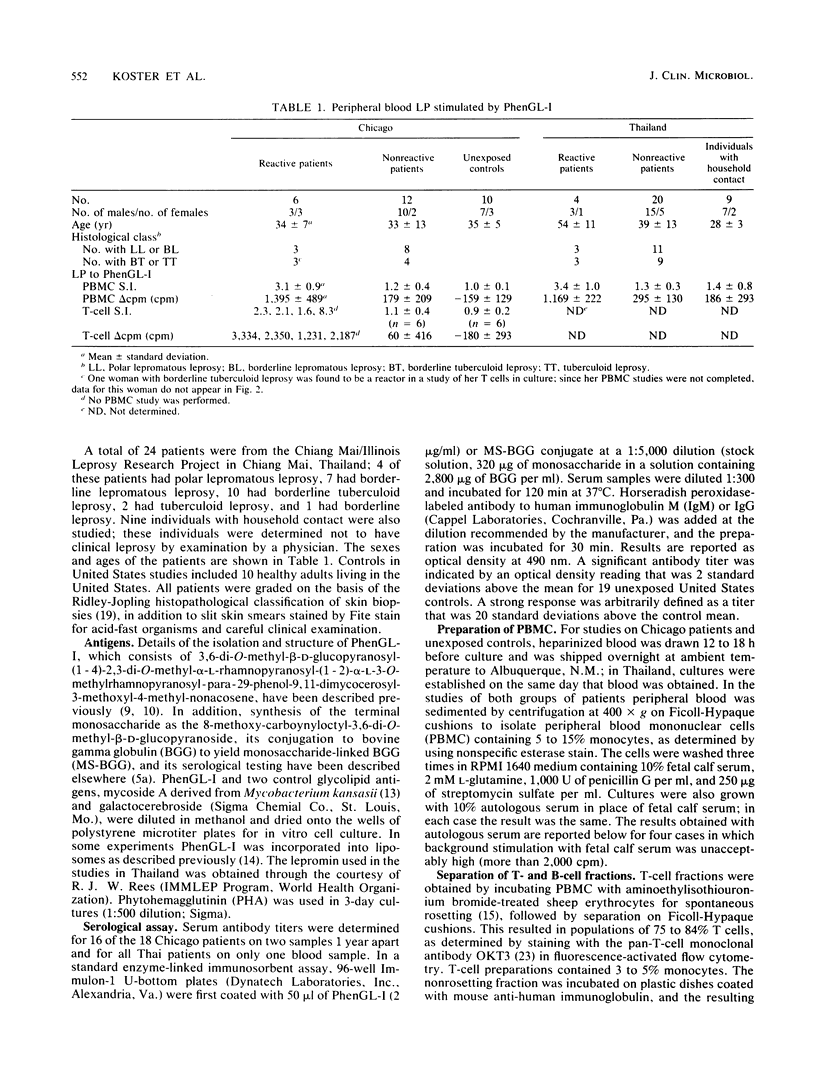
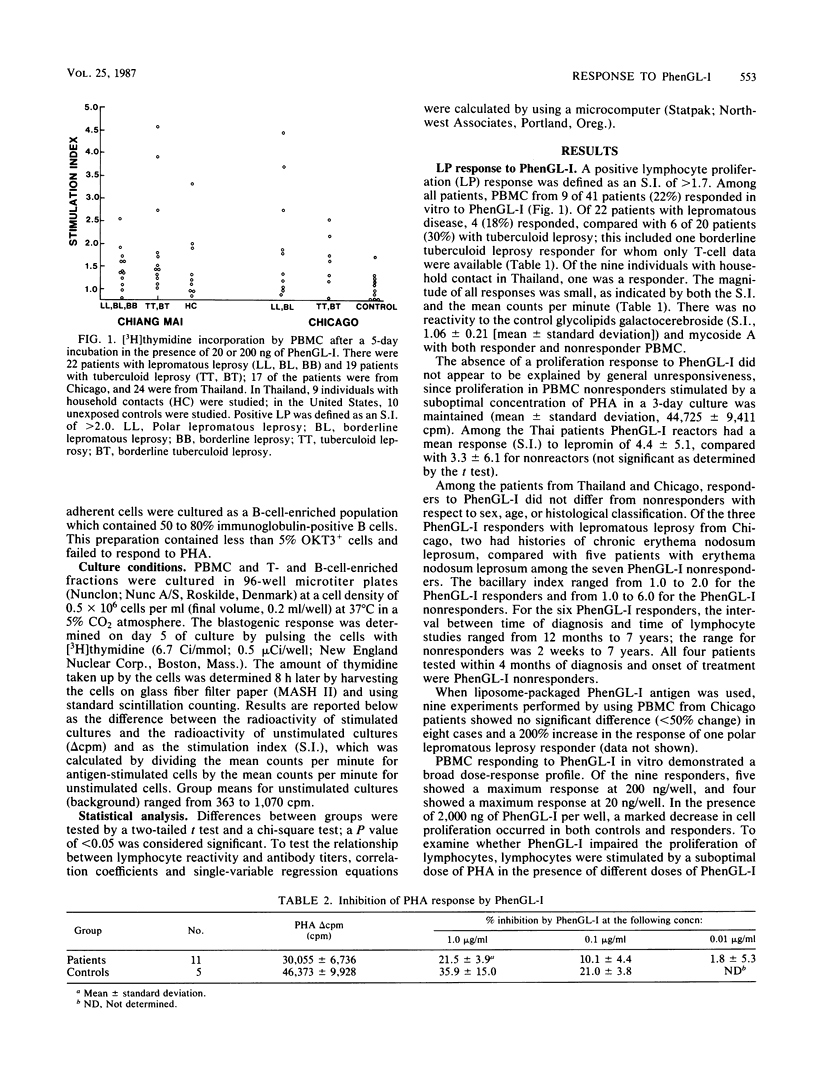
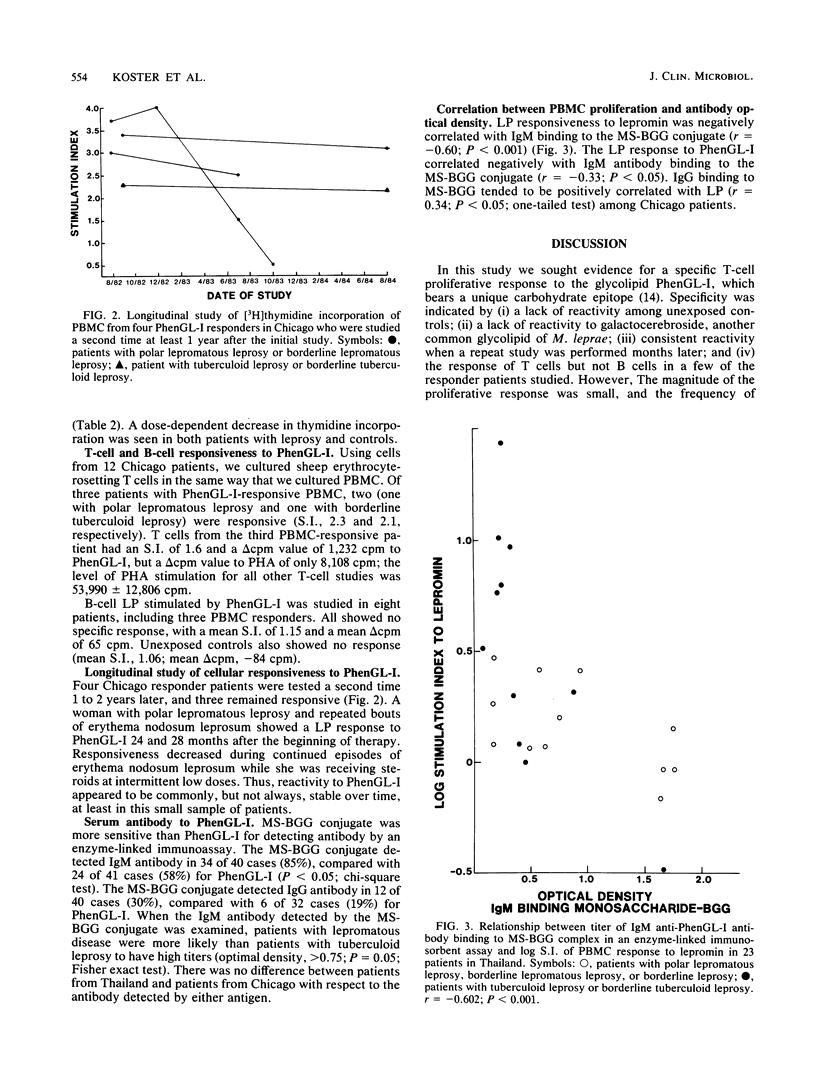
The ability of phenolic glycolipid I (PhenGL-I) of Mycobacterium leprae to stimulate in vitro lymphocyte proliferation (LP) was tested in cultures of peripheral blood cells from 42 patients with leprosy in Chicago and Thailand, 9 individuals with household contact in Thailand, and 10 unexposed North American controls. Only 10 responders (24%) were found among the patients, and the degree of LP was small. Responders were found among patients with lepromatous (18%) or tuberculoid (30%) leprosy without relation to age, complications, duration of treatment, or lepromin responsiveness. The specificity of the response was supported by a lack of response to two other glycolipids, by responses by T cells but not B cells, and by the observation that three of four responders tested maintained their responses to PhenGL-I for at least 1 year. Serum immunoglobulin M (IgM) and IgG antibodies were measured in the same patients by using PhenGL-I or its terminal monosaccharide conjugated to a bovine serum albumin carrier in an enzyme-linked immunosorbent assay. The presence of IgM antibody correlated negatively with LP to lepromin and to PhenGL-I in patients with tuberculoid leprosy. We conclude that circulating T cells from some leprosy patients proliferate in the presence of PhenGL-I in vitro, but the response is weak, possibly due to concomitant suppression or inhibition. The predominance of IgM antibody to PhenGL-I may be related to a lack of a T-helper-cell-mediated switch to IgG antibody response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984 Aug;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H. Regulation of IgG memory responses by helper and suppressor T cells activated by the type 2 antigen, polyvinylpyrrolidone. J Exp Med. 1985 Jun 1;161(6):1357–1367. doi: 10.1084/jem.161.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett S. J., Lowe C., Payne S. N., Draper P. Phenolic glycolipid 1 of Mycobacterium leprae causes nonspecific inflammation but has no effect on cell-mediated responses in mice. Infect Immun. 1984 Dec;46(3):802–808. doi: 10.1128/iai.46.3.802-808.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett S. J., Payne S. N., Draper P., Gigg R. Analysis of the major antigenic determinants of the characteristic phenolic glycolipid from Mycobacterium leprae. Clin Exp Immunol. 1984 Apr;56(1):89–96. [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Komisar J. L., Schweitzer P. A. CH isotype 'switching' during normal B-lymphocyte development. Annu Rev Immunol. 1984;2:493–548. doi: 10.1146/annurev.iy.02.040184.002425. [DOI] [PubMed] [Google Scholar]
- Cho S. N., Fujiwara T., Hunter S. W., Rea T. H., Gelber R. H., Brennan P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-beta-D-glucopyranosyl epitope for the serodiagnosis of leprosy. J Infect Dis. 1984 Sep;150(3):311–322. doi: 10.1093/infdis/150.3.311. [DOI] [PubMed] [Google Scholar]
- Cho S. N., Yanagihara D. L., Hunter S. W., Gelber R. H., Brennan P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983 Sep;41(3):1077–1083. doi: 10.1128/iai.41.3.1077-1083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Hunter S. W., Cho S. N., Aspinall G. O., Brennan P. J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharide protein conjugate for serodiagnosis of leprosy. Infect Immun. 1984 Jan;43(1):245–252. doi: 10.1128/iai.43.1.245-252.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Miller R. A., Young D. B., Khanolkar S. R., Buchanan T. M. Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect Immun. 1985 Aug;49(2):371–377. doi: 10.1128/iai.49.2.371-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Goodman J. W., Sercarz E. E. The complexity of structures involved in T-cell activation. Annu Rev Immunol. 1983;1:465–498. doi: 10.1146/annurev.iy.01.040183.002341. [DOI] [PubMed] [Google Scholar]
- Harboe M., Closs O., Bjune G., Kronvall G., Axelsen N. H. Mycobacterium leprae specific antibodies detected by radioimmunoassay. Scand J Immunol. 1978;7(2):111–120. doi: 10.1111/j.1365-3083.1978.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981 Sep;147(3):728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Koster F. T., Teuscher C., Matzner P., Umland E., Yanagihara D., Brennan P. J., Tung K. S. Strain variations in the murine cellular immune response to the phenolic glycolipid I antigen of Mycobacterium leprae. Infect Immun. 1986 Feb;51(2):495–500. doi: 10.1128/iai.51.2.495-500.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Robertsson J. A., Fossum C., Svenson S. B., Lindberg A. A. Salmonella typhimurium infection in calves: specific immune reactivity against O-antigenic polysaccharide detectable in in vitro assays. Infect Immun. 1982 Aug;37(2):728–736. doi: 10.1128/iai.37.2.728-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsson J. A., Svenson S. B., Lindberg A. A. Salmonella typhimurium infection in calves: delayed specific skin reactions directed against the O-antigenic polysaccharide chain. Infect Immun. 1982 Aug;37(2):737–748. doi: 10.1128/iai.37.2.737-748.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. E., Onderdonk A. B., Kasper D. L., Finberg R. W. Cellular immunity to Bacteroides fragilis capsular polysaccharide. J Exp Med. 1982 Apr 1;155(4):1188–1197. doi: 10.1084/jem.155.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Y., Sosman J., Irigoyen O., Friedman S. M., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. I. Collaborative T-T interactions in the immunoregulation of B cell differentiation. J Immunol. 1980 Dec;125(6):2402–2408. [PubMed] [Google Scholar]
- Young D. B., Buchanan T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science. 1983 Sep 9;221(4615):1057–1059. doi: 10.1126/science.6348948. [DOI] [PubMed] [Google Scholar]
- Young D. B., Dissanayake S., Miller R. A., Khanolkar S. R., Buchanan T. M. Humans respond predominantly with IgM immunoglobulin to the species-specific glycolipid of Mycobacterium leprae. J Infect Dis. 1984 Jun;149(6):870–873. doi: 10.1093/infdis/149.6.870. [DOI] [PubMed] [Google Scholar]
- Young D. B., Khanolkar S. R., Barg L. L., Buchanan T. M. Generation and characterization of monoclonal antibodies to the phenolic glycolipid of Mycobacterium leprae. Infect Immun. 1984 Jan;43(1):183–188. doi: 10.1128/iai.43.1.183-188.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


