Abstract
BACKGROUND AND AIM:
Peptic ulcer disease (PUD) affects 10% of the world population. Helicobacter pylori infection and the use of a nonsteroidal anti-inflammatory drug (NSAID) are the principal factors associated with PUD. The aim of the present study was to evaluate a cohort of patients with PUD and determine the association between H pylori infection and NSAID use.
PATIENTS AND METHODS:
The medical charts of patients with endoscopic diagnosis of PUD were retrospectively reviewed from September 2002 to August 2003. Patients were divided into three groups according to ulcer etiology: H pylori infection (group 1); NSAID use (group 2); and combined H pylori infection and NSAID use (group 3).
RESULTS:
One hundred two patients were evaluated: 36 men (35.3%) and 66 women (64.7%). Forty patients had H pylori infection, 43 had used NSAIDs and 15 had combined H pylori infection and NSAID use; four patients with ulcers secondary to malignancy were excluded. The frequency of women was significantly higher in group 2 (P=0.01). The mean age of patients in group 1 was significantly lower than in the other two groups (P=0.003). PUD developed earlier in group 3 than in group 2 (5.0±4.7 months versus 1.4±2.1 months, respectively, P=0.018). Thirty-two patients (32.7%) had bleeding peptic ulcer. Group 2 had a higher risk of bleeding peptic ulcer than the other two groups (P=0.001).
CONCLUSIONS:
The development of PUD was observed earlier in the combined H pylori and NSAID group than in patients with only NSAID use. This suggests a synergic effect between the two risks factors in the development of PUD.
Keywords: Bleeding peptic ulcer, Helicobacter pylori, Nonsteroidal anti-inflammatory drugs, Peptic ulcer disease
Abstract
HISTORIQUE ET OBJECTIFS :
Les ulcères gastroduodénaux (UG) touchent 10 % de la population mondiale. L’infection à Helicobacter pylori et le recours aux anti-inflammatoires non stéroïdiens (AINS) sont les principaux facteurs associés aux UG. La présente étude vise à évaluer une cohorte de patients atteints d’UG et à déterminer l’association entre les infections à H pylori et l’utilisation d’AINS.
PATIENTS ET MÉTHODOLOGIE :
Le dossier médical de patients recevant un diagnostic endoscopique d’UG a fait l’objet d’une analyse rétrospective entre septembre 2002 et août 2003. Les patients ont été séparés en trois groupes d’après l’étiologie de l’ulcère : infection à H pylori (groupe 1), utilisation d’AINS (groupe 2) et association d’infection à H pylori et d’utilisation d’AINS (groupe 3).
RÉSULTATS :
Cent deux patients ont été évalués, soit 36 hommes (35,3 %) et 66 femmes (64,7 %). Quarante patients étaient atteints d’une infection à H pylori, 43 avaient utilisé des AINS, 15 associaient une infection à H pylori à l’utilisation d’AINS et quatre patients atteints d’un ulcère secondaire à une tumeur maligne ont été exclus. La fréquence de femmes était considérablement plus élevée au sein du groupe 2 (P=0,01). L’âge moyen des patients du groupe 1 était considérablement moins élevé qu’au sein des deux autres groupes (P=0,003). L’UG s’était développé plus rapidement au sein du groupe 3 que du groupe 2 (5,0±4,7 mois par rapport à 1,4±2,1 mois, respectivement, P=0,018). Trente-deux patients (32,7 %) souffraient d’un UG hémorragique. Les patients du groupe 2 présentaient des risques d’UG hémorragique plus élevés que ceux des autres groupes (P=0,001).
CONCLUSIONS :
L’apparition de l’UG s’observait plus rapidement au sein du groupe associant une infection à H pylori et l’AINS que chez ceux qui ne prenaient que des AINS. Cette constatation laisse supposer un effet synergique entre les deux facteurs de risque dans l’apparition de l’UG.
Helicobacter pylori infection and the use of nonsteroidal anti-inflammatory drugs (NSAIDs) are the most important risk factors in the pathogenesis of peptic ulcer disease (PUD) (1,2). Other established risk factors for PUD are: 60 years or older; previous episode of PUD (ulcer or hemorrhage); systemic disease (rheumatoid arthritis, cardiovascular disease and osteoarthritis); use of oral anticoagulants or steroids; alcohol consumption; and tobacco smoking.
H pylori infection affects approximately 60% of the world population and is associated with 90% of duodenal ulcers (DU) and with 80% of gastric ulcers (GU) (3), with a prevalence that varies depending on the socioeconomic conditions of the population (4). In most cases, H pylori-infected people remain asymptomatic and only 10% develop complications such as PUD. H pylori produces chronic and persistent inflammation mediated by host and bacterial genetic factors (5).
NSAIDs, including acetylsalicylic acid (ASA), are among the most used medications in the world (6). PUD among NSAID users occurs in 15% to 30% of patients. NSAID use is higher in patients with advanced age, who also represent the group with a higher risk of H pylori infection (7,8). Gastrointestinal symptoms secondary to NSAID use vary greatly. PUD secondary to NSAID use is asymptomatic in up to 40% of consumers. Bleeding peptic ulcer (BPU) occurs in 3% to 4.5% of the cases (9).
The association between H pylori infection and use of NSAIDs in the pathogenesis of PUD has been controversial (10,11). Both are recognized as independent risk factors for its occurrence; however, studies on this issue have not been consistent. Some studies show a synergistic effect between both (12–14), while others cannot ascertain this association (15–18).
The aim of the present study was to evaluate PUD characteristics in a cohort of patients, and the association between H pylori infection and NSAID use.
PATIENTS AND METHODS
The medical charts of patients evaluated in the endoscopy department at the Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán (Mexico City, Mexico) with endoscopic diagnosis of GU or DU were retrospectively reviewed from September 2002 to August 2003. Patients were divided into groups according to ulcer etiology: H pylori infection (group 1); use of NSAIDs (group 2); and combined H pylori infection and use of NSAIDs (group 3).
Patients’ demographic characteristics, time from starting NSAID therapy to development of PUD symptoms and to endoscopy, associated disease diagnoses and clinical presentation were determined. During endoscopic evaluation, biopsies are routinely taken from the antrum (two), body (one) and fundus (one) in patients with the presence of a GU or DU; thus, the H pylori diagnosis was made by histological evaluation in all cases.
An NSAID-associated ulcer was considered to be a lesion at least 3 mm in diameter, found during endoscopic evaluation, with history of continuous NSAID use, for at least one week in the month before the examination, and H pylori-negative by histopathology. An H pylori-associated ulcer was considered to be a lesion at least 3 mm in diameter, found during endoscopic evaluation, positive for H pylori infection by histopathology and without history of NSAID use. A combined ulcer was considered to be a lesion at least 3 mm in diameter, found during endoscopic evaluation, positive for H pylori infection by histopathology and with a history of continuous NSAID use for at least one week in the month before the endoscopy.
All patients who were found to have GU or DU at endoscopy were asked about their NSAID consumption status including the type of NSAID used and the dose and duration of use, which was then documented in the respective chart.
Statistical analysis
The categorical variables were analyzed with Pearson’s χ2, with Yates correction or Fisher’s exact test for frequencies (n) less than five in the charts. Dimensional variables were evaluated with ANOVA. Results were expressed as absolute and relative frequencies in nominal variables or as means and SDs for quantitative variables. The ORs were calculated with the equation (a × d) ÷ (b × c) with a 95% CI. Statistical significance was defined as P≤0.05. The data were analyzed with the SPSS 10.0 program (SPSS Inc, USA).
RESULTS
One hundred two patients were identified, 36 men (35.3%) and 66 women (64.7%). General patient characteristics and ulcer location are summarized in Table 1. Forty patients had ulcers secondary to H pylori infection, 43 secondary to NSAID use and 15 had combined H pylori and NSAID use; four patients with neoplastic ulcers were excluded from the analysis (one with lymphoma and three with adenocarcinomas).
TABLE 1.
Characteristics of patients with gastric and duodenal ulcer
| Characteristic | Helicobacter pylori (n=40) | NSAID use (n=43) | Combined Helicobacter pylori and NSAID use (n=15) | P* |
|---|---|---|---|---|
| Sex (M/F) | 19/21 | 8/35 | 7/8 | 0.01 |
| Age ± SD (years) | 54.6±14.2 | 64.3±15.6 | 67.8±15.5 | 0.003 |
| Ulcer location | ||||
| Gastric | 20 (50%) | 34 (79%) | 9 (60%) | 0.02 |
| Duodenal | 20 (50%) | 9 (21%) | 6 (40%) | |
| Bleeding ulcers | 5 (13%) | 21 (49%) | 6 (40%) | 0.001 |
χ2 and ANOVA. F Female; M Male; NSAID Nonsteroidal anti-inflammatory drug
The frequency of women was significantly higher in group 2 than in the other two groups (P=0.01). The mean age of patients in group 1 was significantly lower than that in the other two groups (P=0.003). The frequency of DU secondary to H pylori infection was 50%. GU was significantly higher in group 2 in comparison with the rate in the other groups (P=0.02, Table 1).
Thirty-two patients (32.7%) had BPU, two presenting with hematemesis and 30 with melena. BPU occurred in 19 of 44 patients (43%) with DU and in 13 of 22 patients (59%) with GU, with no statistical difference. Group 2 had a higher risk of BPU than that in the other two groups (OR 3.81; 95% CI 1.56 to 9.3; P=0.001). The presence of H pylori infection alone or in combination with NSAID consumption was not a risk factor for BPU (OR 0.164; 95% CI 0.056 to 0.478; and OR 1.46; 95% CI 0.47 to 4.53, respectively, Table 1).
In group 3, PUD developed earlier than in group 2 (5.0±4.7 months versus 1.4±2.1 months, respectively, P=0.018, Figure 1). Group 2 and group 3 had a higher frequency of osteoarthritis than group 1. There were no differences in the Forrest classification, smoking and biochemical markers among the three groups (Table 2).
Figure 1).
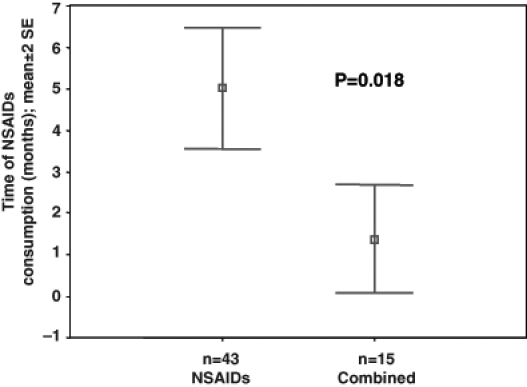
Time of peptic ulcer disease development in nonsteroidal anti-inflammatory drugs (NSAIDs) group and combined Helicobacter pylori and NSAID use group. SE Standard error
TABLE 2.
Comorbidities, Forrest classification, smoking and biochemical parameters
| H pylori (n=40) | NSAID use (n=43) | Combined H pylori and NSAID use (n=15) | P | |
|---|---|---|---|---|
| Associated diseases, n (%) | ||||
| None | 14 (35) | 5 (12) | 4 (27) | 0.02 |
| Diabetes mellitus II | 6 (15) | 3 (7) | – | |
| Hypertension | 3 (8) | 7 (16) | 3 (20) | |
| Osteoarthritis | 1 (3) | 8 (19) | 3 (20) | |
| Lupus | 3 (8) | 3 (7) | – | |
| Rheumatoid arthritis | – | 3 (7) | 1 (7) | |
| Others | 13 (33) | 14 (33) | 2 (13) | |
| Forrest classification (n) | ||||
| IA | 1 | – | – | NS |
| IB | 1 | 1 | 1 | |
| IIA | 1 | 2 | 3 | |
| IIB | 3 | 5 | 1 | |
| IIC | 7 | 3 | 2 | |
| III | 27 | 32 | 8 | |
| Smoking, n (%) | 8 (20) | 12 (28) | 3 (20) | NS |
| Hemoglobin (g/L) ± SD | 122±26 | 112±31 | 125±28 | NS |
| Platelets (×103/mL) ± SD | 211±130 | 262±183 | 257±156 | NS |
| Prothrombin time (s) ± SD | 14±9 | 12±3 | 11±2 | NS |
| Creatinine (μmol/L) ± SD | 97.24±61.88 | 106.08±123.76 | 75.2±35.36 | NS |
H pylori Helicobacter pylori; NS Not significant; NSAIDs Nonsteroidal anti-inflammatory drugs
DISCUSSION
PUD characterization has changed over decades and varies according to the population studied. PUD secondary to H pylori infection occurred in 50% of patients in the present study group and more often in patients with advanced age, which is in concordance with the recent knowledge of the disease (1). Similarly, H pylori infection occurred more often in patients with advanced age (1). Patients with chronic diseases that require treatment with NSAIDs, especially women, are more prone to develop PUD. NSAID use and combined ulcers were significantly higher in groups 2 and 3.
It is estimated that 22% to 63% of people with chronic NSAID use have H pylori infection (19). In the present study, we found that 15% had NSAID use with concomitant H pylori infection. This reduced frequency may be due to false-negatives, antibiotic use or proton pump inhibitor use, and underreporting of NSAID use among patients.
A meta-analysis (12) that included 463 studies evaluating the association between H pylori infection and NSAID use found a higher frequency of uncomplicated PUD among NSAID users who were H pylori-positive (41.7%) in comparison with users who were H pylori-negative (25.9%). PUD was 3.5 times more frequent in an NSAID user when infection with H pylori was present. NSAID use and H pylori infection increased the risk of gastrointestinal hemorrhage 1.79- and 4.85-fold, respectively, and 6.13-fold when found simultaneously.
In our study, BPUs were found more frequently in group 2 (NSAID use) than the other groups. H pylori infection was not a risk factor for BPU (OR 0.164; 95% CI 0.056 to 0.478; P=0.001). The combination of NSAID use and H pylori infection was not associated with an increased risk of BPU (OR 1.46; 95% CI 0.47 to 4.53). PUD developed earlier in NSAID users with H pylori infection than those with NSAID consumption alone (5.0±4.7 months versus 1.4±2.1 months, respectively, P=0.018), which suggests a synergic effect promoting PUD.
Chan et al (20) evaluated 92 patients with documented H pylori infection who started therapy with NSAIDs, and then randomly assigned the patients to H pylori eradication treatment or placebo. After eight weeks of follow-up, PUD was found in 7% of the treated group and in 28% of those not treated (P=0.01). However, this study was questioned due to a short follow-up and the use of bismuth in some patients. In another study by Chan et al (21), similar results were observed in patients with NSAID use, and PUD developed at six months in 4% of the eradication treatment group versus in 27% of those without treatment (P<0.005).
The decision to search for and treat H pylori must rest on the identification of risk factors and whether the use of NSAIDs is recent or chronic (22). In patients with risk factors who are starting NSAIDs, the identification and treatment of H pylori infection reduce PUD occurrence. There is no indication for screening and treating H pylori patients with chronic NSAID use without gastrointestinal symptoms. Hawkey et al (H pylori Eradication in generaL Practice [HELP] study) (23) randomly assigned H pylori eradication treatment or no treatment at all to 285 patients with chronic NSAID use and found no difference in PUD occurrence.
In patients with ASA use for cardiovascular protection, eradication therapy is equally effective as proton pump inhibitors to prevent PUD. In this group of patients, H pylori infection must intentionally be investigated and treated (24). In our study, there were no significant differences in PUD occurrence among the different types of NSAIDs, including ASA. The Maastricht consensus results recommend H pylori eradication among NSAID users, but clearly state that eradication will not prevent recurrences of BPU in high-risk patients or improve the healing process in PUD (25).
It is important to mention that in our study, because of the small size of the population, certain potential associations (ie, NSAIDs and H pylori together lead to more bleeding, OR=1.46) can neither be demonstrated nor disproven.
In summary, PUD secondary to NSAID use is strongly associated with female sex and older age. Bleeding PUD was also more frequent in the two groups with consumption of NSAIDs.
The development of PUD was observed earlier in the combined H pylori and NSAID group than in patients with only NSAID use, which may suggest a synergic effect between the two risks factors in the development of PUD.
REFERENCES
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–99. doi: 10.1056/NEJM199906173402407. (Erratum 1999;341:548). [DOI] [PubMed] [Google Scholar]
- 3.Walsh JH, Peterson WL. The treatment of Helicobacter pylori infection in the management of peptic ulcer disease. N Engl J Med. 1995;333:984–91. doi: 10.1056/NEJM199510123331508. [DOI] [PubMed] [Google Scholar]
- 4.Malaty HM, Graham DY. Importance of childhood socioeconomic status on the current prevalence of Helicobacter pylori infection. Gut. 1994;35:742–5. doi: 10.1136/gut.35.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran AP, Wadstrom T. Pathogenesis of Helicobacter pylori. Curr Opin Gastroenterol. 1998;14(Suppl):S9–14. [Google Scholar]
- 6.Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: The second hundred years. Gastroenterology. 1997;112:1000–16. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- 7.Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120:594–606. doi: 10.1053/gast.2001.21907. [DOI] [PubMed] [Google Scholar]
- 8.Dooley CP, Cohen H, Fitzgibbons PL, et al. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562–6. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 9.Lanza FL. A guideline for the treatment and prevention of NSAID-induced ulcers. Members of the Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2037–46. doi: 10.1111/j.1572-0241.1998.00588.x. [DOI] [PubMed] [Google Scholar]
- 10.Barr M, Buckley M, O’Morain C. Review article: Non-steroidal anti-inflammatory drugs and Helicobacter pylori. Aliment Pharmacol Ther. 2000;14(Suppl 3):43–7. doi: 10.1046/j.1365-2036.2000.00399.x. [DOI] [PubMed] [Google Scholar]
- 11.Ballesteros-Amozurrutia [Peptic ulcer and Helicobacter pylori. Results and consequences of its eradication] Rev Gastroenterol Mex. 2000;65(Suppl 2):41–9. [PubMed] [Google Scholar]
- 12.Loeb DS, Talley NJ, Ahlquist DA, Carpenter HA, Zinsmeister AR. Long-term nonsteroidal anti-inflammatory drug use and gastroduodenal injury: The role of Helicobacter pylori. Gastroenterology. 1992;102:1899–905. doi: 10.1016/0016-5085(92)90311-l. [DOI] [PubMed] [Google Scholar]
- 13.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: A meta-analysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 14.Aalykke C, Lauritsen J, Hallas J, Reinholdt S, Krogfelt K, Lauritsen K. Helicobacter pylori and risk of ulcer bleeding among users of nonsteroidal anti-inflammatory drugs: A case-control study. Gastroenterology. 1999;116:1305–9. doi: 10.1016/s0016-5085(99)70494-4. [DOI] [PubMed] [Google Scholar]
- 15.Laine L, Marin-Sorensen M, Weinstein WM. Nonsteroidal antiinflammatory drug-associated gastric ulcers do not require Helicobacter pylori for their development. Am J Gastroenterol. 1992;87:1398–402. [PubMed] [Google Scholar]
- 16.Pilotto A, Leandro G, Di Mario F, Franceschi M, Bozzola L, Varerio G. Role of Helicobacter pylori infection on upper gastrointestinal bleeding in the elderly: A case-control study. Dig Dis Sci. 1997;42:586–91. doi: 10.1023/a:1018807412030. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Hernandez H, Jacobo-Karam JS, Jaquez-Quintana JO, et al. [Gastropathy caused by non-steroidal anti-inflammatory agents and its association with Helicobacter pylori] Rev Invest Clin. 2003;55:254–9. [PubMed] [Google Scholar]
- 18.Santolaria S, Lanas A, Benito R, Perez-Aisa M, Montoro M, Sainz R. Helicobacter pylori infection is a protective factor for bleeding gastric ulcers but not for bleeding duodenal ulcers in NSAID users. Aliment Pharmacol Ther. 1999;13:1511–8. doi: 10.1046/j.1365-2036.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 19.Heresbach D, Raoul JL, Bretagne JF, et al. Helicobacter pylori: A risk and severity factor of non-steroidal anti-inflammatory drug induced gastropathy. Gut. 1992;33:1608–11. doi: 10.1136/gut.33.12.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan FK, Sung JJ, Chung SC, et al. Randomised trial of eradication of Helicobacter pylori before non-steroidal anti-inflammatory drug therapy to prevent peptic ulcers. Lancet. 1997;350:975–9. doi: 10.1016/s0140-6736(97)04523-6. [DOI] [PubMed] [Google Scholar]
- 21.Chan FK, To KF, Wu JC, et al. Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: A randomized trial. Lancet. 2002;359:9–13. doi: 10.1016/s0140-6736(02)07272-0. [DOI] [PubMed] [Google Scholar]
- 22.Hunt RH, Bazzoli F. Review article: Should NSAID/low-dose aspirin takers be tested routinely for H pylori infection and treated if positive? Implications for primary risk of ulcer and ulcer relapse after initial healing. Aliment Pharmacol Ther. 2004;19(Suppl 1):9–16. doi: 10.1111/j.0953-0673.2004.01830.x. [DOI] [PubMed] [Google Scholar]
- 23.Hawkey CJ, Tulassay Z, Szczepanski L, et al. Randomised controlled trial of Helicobacter pylori eradication in patients on non-steroidal anti-inflammatory drugs: HELP NSAIDs study. Helicobacter eradication for lesion prevention. Lancet. 1998;352:1016–21. doi: 10.1016/s0140-6736(98)04206-8. (Erratum 1998;352:1634). [DOI] [PubMed] [Google Scholar]
- 24.Lai KC, Lam SK, Chu KM, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346:2033–8. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 25.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection – the Maastricht 2-2000 consensus report. Aliment Pharmacol Ther. 2002;16:167–80. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]


