Abstract
BACKGROUND:
The aim of the present study was to examine the diversity of liver diseases in outpatients referred because of elevated serum ferritin.
METHODS:
A retrospective review was performed of outpatient referrals for serum ferritin elevations made to a tertiary care centre liver clinic between 1999 and 2005. Information regarding serum ferritin, transferrin saturation, liver biopsy, liver iron concentration and final diagnosis was extracted. Patients were stratified into two groups based on ferritin concentration: ferritin concentration 300 μg/L to 1000 μg/L, and ferritin concentration greater than 1000 μg/L.
RESULTS:
A total of 482 charts were reviewed, of which 119 (25%) had ferritin concentrations greater than 1000 μg/L. HFE-linked hemochromatosis, nonalcoholic steatohepatitis and alcohol-related liver disease were the top three diagnoses. HFE-linked hemochromatosis accounted for 28% to 42% of the diagnoses in all subgroups. The percentage of patients diagnosed with HFE-linked hemochromatosis was similar in the ferritin 300 μg/L to 1000 μg/L and the ferritin greater than 1000 μg/L groups (P=0.067). Among patients with ferritin greater than 1000 μg/L, 63% underwent a liver biopsy. Of those with an elevated liver iron concentration (greater than 35 μmol/g dry weight), 71% had a transferrin saturation greater than 50% (88% of C282Y homozygotes and 43% of non-C282Y homozygotes). In non-C282Y homozygotes with an elevated serum ferritin concentration greater than 1000 μg/L, 64% did not have iron overload on liver biopsy.
CONCLUSION:
HFE-linked hemochromatosis accounted for less than one-half of the diagnoses in an outpatient population referred for elevated ferritin, suggesting a need to search further for an alternate cause.
Keywords: Hemochromatosis, HFE, Iron overload
Abstract
CONTEXTE :
La présente étude avait pour but d’examiner la diversité des maladies du foie diagnostiquées chez des patients externes, dirigés vers un centre spécialisé pour de l’hyperferritinémie.
METHODE :
Nous avons procédé à un examen rétrospectif des dossiers de patients externes, dirigés, entre 1999 et 2005, vers un centre de soins tertiaires, spécialisé en hépatologie pour une ferritinémie élevée. Nous avons recueilli des données sur le taux de ferritine sérique, la saturation en transferrine, les biopsies du foie, les concentrations de fer dans le foie et le diagnostic définitif. Les patients ont été divisés en deux groupes : ceux ayant une ferritinémie variant entre 300 μg/l et 1000 μg/l et ceux ayant une ferritinémie supérieure à 1000 μg/l.
RÉSULATS :
Nous avons examiné 482 dossiers au total; sur ce nombre, 119 (25 %) faisaient état d’une ferritinémie supérieure à 1000 μg/l. Les trois principaux diagnostics étaient l’hémochromatose liée au gène HFE, la stéatose hépatique non alcoolique et les maladies hépatiques alcooliques. L’hémochromatose liée au gène HFE constituait de 28 à 42 % des diagnostics posés dans tous les sous-groupes. Le pourcentage de patients atteints d’hémochromatose liée au gène HFE était comparable dans les deux groupes de ferritinémie : valeurs variant entre 300 μg/l et 1000 μg/l et valeurs supérieures à 1000 μg/l (P=0,067). Parmi les patients qui avaient une ferritinémie supérieure à 1000 μg/l, 63 % avaient subi une biopsie du foie. Par ailleurs, 71 % des patients qui avaient une forte concentration de fer dans le foie (valeur supérieure à 35 μmol/g en poids sec) présentaient une saturation en transferrine supérieure à 50 % (88 % des patients homozygotes pour la mutation C282Y et 43 % des patients non homozygotes pour cette même mutation). Enfin, chez les porteurs non homozygotes de la mutation C282Y qui avaient une ferritinémie supérieure à 1000 μg/l, 64 % ne présentaient pas de surcharge en fer à la biopsie du foie.
CONCLUSION :
L’hémochromatose liée au gène HFE constituait moins de la moitié des diagnostics posés chez les patients externes, dirigés vers un centre spécialisé pour un taux élevé de ferritine, ce qui justifierait une recherche approfondie sur une autre cause possible d’hyperferritinémie.
Elevation of serum ferritin is a common finding on routine bloodwork in patients presenting with fatigue. These individuals are often referred for assessment of hemochromatosis. Although iron overload is one cause for an elevated serum ferritin concentration, many other disorders can result in varying degrees of hyperferritinemia. Mild elevations of serum ferritin are commonly seen with daily alcohol consumption, obesity and chronic inflammation. Most liver diseases can have an elevated serum ferritin secondary to inflammation, and transferrin saturation can also be elevated in other liver diseases (1,2). Extreme elevations in ferritin concentration (greater than 10,000 μg/L) can be seen in systemic diseases such as adult-onset juvenile rheumatoid arthritis, disseminated histiocytosis, Still’s disease and other inflammatory diseases (3–5). The aim of the present study was to review the diversity of diseases associated with elevated serum ferritin in a tertiary referral clinic for hemochromatosis and liver diseases.
METHODS
This was a retrospective study of outpatient referrals for ferritin elevation to the hemochromatosis and liver disease clinic at University Hospital in London, Ontario, between 1999 and 2005. All patients reviewed had ferritin concentrations greater than 300 μg/L (normal lab reference range 15 μg/L to 200 μg/L for women, and 30 μg/L to 300 μg/L for men). Patients were stratified into two groups based on their ferritin concentration at their first clinic visit: ferritin 300 μg/L to 1000 μg/L, and ferritin greater than 1000 μg/L. Those who were referred for reasons other than hyper-ferritinemia (eg, known hepatitis) with a coincident high ferritin concentration were excluded. The ferritin concentration, transferrin saturation, aspartate aminotransferase levels, alanine amino-transferase (ALT) levels, HFE mutations (C282Y and H63D), liver biopsy results, liver iron concentration values and final clinical diagnosis were collected. Individuals in the present study with potentially more than one factor contributing to a high ferritin concentration were placed into the ‘uncertain diagnosis’ group.
Liver biopsy reports were reviewed. The paraffin blocks of those patients with excess stainable iron were submitted for biochemical quantification of liver iron concentration. Liver iron concentration was also determined if requested by the ordering physician. Liver iron concentration was measured by atomic absorption spectrophotometry. Liver tissue was removed from paraffin-embedded blocks by washing in xylene and then drying to a constant weight before analysis (6).
Mean ferritin concentrations and liver iron concentrations were compared using Student’s t test. A χ2 test was used to compare proportions. Statistics were analyzed using Winstat 3.0 (Kalmia Co, USA).
RESULTS
A total of 482 charts were reviewed, of which 119 (25%) had serum ferritin concentrations greater than 1000 μg/L. There was a male predominance of approximately 3:1. The mean age among all groups was approximately 50 years, with a range of 49.5 to 55.3 years (Table 1). All patients with HFE-linked hemochromatosis (ie, C282Y or H63D mutations of the HFE gene) were Caucasian. The group of patients with iron overload from other causes included patients with Asian, East Indian, Caribbean and Arab ancestry.
TABLE 1.
Clinical and biochemical profiles of patients among subgroups
| Patient profile | Ferritin concentration (μg/L)
|
||||
|---|---|---|---|---|---|
| 300 to 400 | 401 to 500 | 501 to 600 | 601 to 1000 | Greater than 1000 | |
| Men, % | 77 | 75 | 88 | 75 | 77 |
| Women, % | 23 | 25 | 12 | 25 | 23 |
| Mean age, years | 49.5 | 52.3 | 51.6 | 55.3 | 53.5 |
| Liver biopsy, n | 12 | 17 | 7 | 35 | 75 |
| Iron overload, % (ratio) | 8 (1/12) | 12 (2/17) | 43 (3/7) | 34 (12/35) | 45 (34/75) |
| Mean transferrin saturation, % | 36 | 42 | 39 | 49 | 59 |
| Hemochromatosis, % (ratio) | 28 (23/83) | 33 (29/89) | 29 (16/56) | 38 (51/135) | 42 (50/119) |
| C282Y homozygote, % (ratio) | 35 (8/23) | 57 (17/29) | 62.5 (10/16) | 69 (35/51) | 86 (43/50) |
| H63D homozygote, % (ratio) | 35 (8/23) | 21 (6/29) | 6 (1/16) | 12 (5/51) | 2 (1/50) |
| Compound heterozygote (C282Y/H63D), % (ratio) | 30 (7/23) | 21 (6/29) | 31 (5/16) | 20 (10/51) | 12 (6/50) |
Iron overload is defined as a liver iron concentration greater than 35 μmol/g (normal range 0 μmol/g to 35 μmol/g)
In patients with a ferritin concentration greater than 1000 μg/L, 42% (50 of 119) had HFE-linked hemochromatosis, of which 86% (43 of 50) were C282Y homozygotes, 12% (six of 50) were C282Y heterozygotes and 2% (one of 50) were H63D homozygotes. Other diagnoses included chronic alcohol consumption, alcoholic liver disease, nonalcoholic steatohepatitis, other causes of iron overload and newly diagnosed chronic viral hepatitis (Figure 1). In 9% (11 of 119) of the cases, the cause of the iron overload remained uncertain despite a thorough workup including liver biopsy. Of those with other causes for iron overload, diagnoses included multiple, red cell transfusions, intravenous iron therapy, aceruloplasminemia and juvenile hemochromatosis. There were six patients with iron overload, confirmed by liver biopsy, of unknown etiology. Three patients had hereditary hyperferritinemia cataract syndrome (7). This diagnosis was made based on a family history of bilateral, early-onset cataracts, elevated ferritin concentrations with no biochemical or tissue evidence of iron overload, and a mutation in the iron-responsive element of the L-ferritin gene. One per cent had miscellaneous diagnoses, including autoimmune hepatitis, hepatitis B and lymphoma (Figure 1). Overall, 66.3% (79 of 119) of these patients underwent a liver biopsy as part of their workup.
Figure 1).
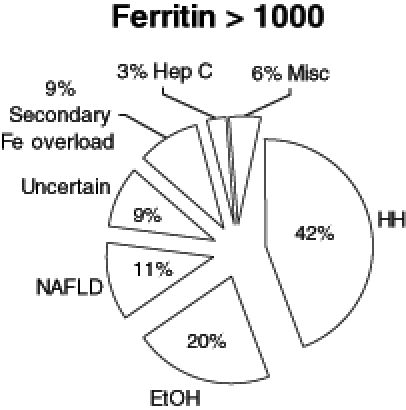
Diagnoses in patients (n=119) with elevated ferritin (Fe) (greater than 1000 μg/L). EtOH Alcoholic liver disease; Hep C Hepatitis C; HH HFE-related hemochromatosis; Misc Miscellaneous; NAFLD Nonalcoholic fatty liver disease
In the group with serum ferritin greater than 1000 μg/L, 71% (27 of 38) of those who had documented iron overload by biopsy (liver iron concentration greater than 35 μmol/g dry weight) had a transferrin saturation greater than 50% (normal range 20% to 55%). Of the C282Y homozygotes who had an elevated liver iron concentration, 88% (21 of 24) had an elevated transferrin saturation compared with 43% (six of 14) of the non-C282Y homozygotes. Liver iron concentration was measured only if there was evidence of significant stainable iron on liver biopsy, as determined by the pathologist or by request of the ordering physician. Forty-four of the patients with ferritin concentrations greater than 1000 μg/L had liver iron concentrations determined, of which 34 (77%) were elevated above 35 μmol/g. Among those with iron overload, the liver iron concentrations were higher in the C282Y homozygotes than in non-C282Y homozygotes, ie, all other patients including compound heterozygotes and H63D homozygotes, (mean 297 μmol/g, range 74.6 μmol/g to 632 μmol/g versus 134 μmol/g, range 36.1 μmol/g to 514 μmol/g, respectively; P=0.0028 [reference range 0 μmol/g to 35 μmol/g]). Thirty-two of 50 (64%) of the non-C282Y homozygotes and six of 25 (24%) of the C282Y homozygotes with a serum ferritin greater than 1000 μg/L did not have iron overload on liver biopsy.
In patients with ferritin 300 μg/L to 1000 μg/L, 33% (119 of 363) had HFE-related hemochromatosis, of which 59% (70 of 119) were C282Y homozygotes, 24% (28 of 119) were compound heterozygotes (C282Y/H63D) and 17% (20 of 119) were H63D homozygotes. Eighty-six per cent (313 of 363) of the patients in this group had genetic testing for HFE mutations. These patients were further stratified into four subgroups based on ferritin concentration (Table 1). HFE-linked hemochromatosis, and alcoholic and nonalcoholic steatohepatitis made up the majority of the diagnoses. Other causes of iron overload included porphyria cutanea tarda and iron-loading anemias. Miscellaneous diagnoses included hepatitis B and C, autoimmune hepatitis, methotrexate-induced liver injury, granulomatous hepatitis and primary biliary cirrhosis (Figure 2). The number of patients who underwent biopsies in this group was significantly less at 18.5% (67 of 363). Among the patients tested for HFE mutations with a ferritin concentration between 300 μg/L to 1000 μg/L, the mean (± SD) ferritin concentrations in C282Y homozygotes and in non-C282Y homozygotes were not statistically different (633±184 μg/L and 575±223 μg/L, respectively; P=0.057). There was no statistical difference in the percentage of patients diagnosed with hemochromatosis in the ferritin 300 μg/L to 1000 μg/L group versus the ferritin greater than 1000 μg/L group (P=0.067).
Figure 2).
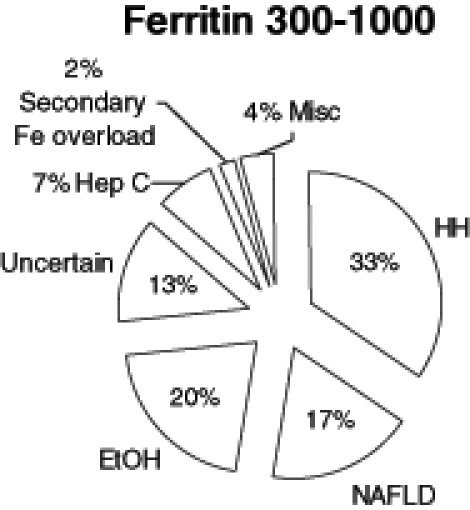
Diagnoses in patients (n=363) with ferritin (Fe) concentrations between 300 μg/L and 1000 μg/L. EtOH Alcoholic liver disease; Hep C Hepatitis C; HH HFE-related hemochromatosis; Misc Miscellaneous; NAFLD Nonalcoholic fatty liver disease
Transaminases were higher in non-C282Y homozygotes than in the C282Y homozygotes (ALT 72.6 IU/L and 41 IU/L, respectively; P=0.046; aspartate aminotransferase 66.2 IU/L and 36.4, respectively; P=0.034). The mean transferrin saturation and mean ferritin concentration was also significantly higher in C282Y homozygotes than in nonhomozygotes (74.3% versus 38.2%, respectively; P<0.0001; and 1167 μg/L versus 801 μg/L, respectively; P=0.0001).
DISCUSSION
Although the diagnosis of HFE-linked hemochromatosis was a common diagnosis in outpatient referrals for elevated serum ferritin, more than 50% of these referred patients had an alternate explanation for their elevated ferritin. The clinic at the University of Western Ontario is known for the study of iron overload and this likely results in a referral bias, which may increase the percentage of patients with HFE-linked hemochromatosis. HFE-linked hemochromatosis, alcoholic liver disease and nonalcoholic fatty liver disease made up the majority of the diagnoses among all subgroups of patients (60% to 80%). The percentage of patients diagnosed with HFE-linked hemochromatosis was similar, regardless of ferritin concentration (P=0.067). The only previous study that has examined diagnoses of high ferritin concentrations focused on inpatients with extreme elevations (greater than 1000 μg/L) of serum ferritin (8). In that study (8), the diagnoses, in order of decreasing frequency, included liver disease, renal disease, malignancy, HIV infection, non-HIV infection, sickle cell disease and red blood cell transfusions. Those with liver disease had a mean (± SD) serum ferritin concentration of 2753.1±656.8 μg/L. That study differs from the current study in its selection of patients, and this may explain the predominance of infectious and inflammatory etiologies in those patients.
A serum ferritin concentration between 300 μg/L and 1000 μg/L is common. The Hemochromatosis and Iron Overload Screening (HEIRS) (9) study (a recent, large, multi-centre screening study) demonstrated that 5.9% of 43,453 Caucasian subjects had elevated ferritin in a primary care population, and 19% of Asian subjects had elevated ferritin. One of the limitations of the present study is the small percentage of liver biopsies performed on this group of patients. It will likely be difficult to circumvent this issue, given the invasive nature of liver biopsies and the risks outweighing the benefits in these patients. Previous studies have shown that patients with hemochromatosis and a ferritin concentration less than 1000 μg/L are unlikely to have significant fibrosis, and therefore, a liver biopsy is rarely recommended (10,11). Other noninvasive testing, such as magnetic resonance imaging studies, may help, although sensitivity is lower at low iron concentrations (12). In the present study, some patients had abdominal imaging demonstrating fatty liver. Even when liver biopsies are performed, such as in the group with serum ferritin concentrations greater than 1000 μg/L, 9% of patients still had an unknown diagnosis, which is comparable with the other subgroups. A biopsy is particularly useful in non-C282Y homozygotes with a ferritin concentration greater than 1000 μg/L; in many of these, iron overload was not found (64%). Another diagnostic challenge is the lack of specificity of an elevated ferritin concentration as demonstrated by the variety of diagnoses. Genetic testing can be helpful in the diagnosis of HFE-linked hemochromatosis. Genetic testing for new iron genes (ferroportin, hemojuvelin, hepcidin and transferrin receptor 2) may never become commercially available because of the rarity of these conditions (13). Because obesity, diabetes mellitus and daily alcohol consumption are relatively common, it is difficult to determine the contribution of one or more of these conditions to the serum ferritin concentration. Even in patients who have an HFE mutation, it may be difficult to tease out the expressing phenotype with iron overload from the nonexpressing phenotype with coexistent fatty liver disease. Transferrin saturation is frequently used to screen for hemochromatosis, with the assumption that it reflects iron overload. In the HEIRS study (9), transferrin saturation was found to be greater than 50% in 84% of men and greater than 45% in 73% of women who were C282Y homozygotes. This is similar to our finding that 88% of C282Y homozygotes with biopsy-proven iron overload had an elevated transferrin saturation. In contrast, two other screening studies have found a low sensitivity (50% to 60%) of a transferrin saturation of 50% or more (14,15). These latter studies can be explained by the high prevalence of nonexpressing C282Y homozygotes, and elevated transferrin saturation remains a useful test in the detection of an iron-loaded C282Y homozygote. In the present study, only 43% of non-C282Y homozygotes with biopsy-proven iron overload had a transferrin saturation of greater than 50%. Therefore, a normal transferrin saturation result in a non-C282Y homozygote with an elevated ferritin level does not exclude iron overload.
The mean transaminase concentrations were significantly higher in non-C282Y homozygotes than in C282Y homozygotes. None of the homozygotes had a transaminase elevation more than three times the upper limit of normal, regardless of ferritin concentration. A previous study (16) from our centre demonstrated that 69% of typical patients had an elevated ALT concentration with the mean (± SD) ALT concentrations being 43.8±28.5 U/L in noncirrhotic patients and 84.4±99.3 U/L in cirrhotic patients.
The overall prevalences of C282Y heterozygotes and H63D heterozygotes was 10.3% and 18.8%, respectively. These values are consistent with that observed in large screening studies of the general population (9), so they are not over-represented in this referred population. These mutations are usually associated with normal iron studies, and therefore, we would not expect an increase in prevalence of heterozygotes in patients with hyperferritinemia.
Because the majority of patients referred for hyperferritinemia do not have HFE-linked hemochromatosis, it is prudent to search for an alternate diagnosis in such individuals. Obesity, alcohol and viral hepatitis (B and C) are the most common diagnoses. Transferrin saturation testing may be helpful, because a high percentage of C282Y homozygotes with iron overload have a transferrin saturation of greater than 50%. Transferrin saturation, however, may be less helpful in predicting iron overload in non-C282Y homozygotes. Mild ferritin elevations (300 μg/L to 1000 μg/L) may be more difficult to diagnose definitively in the absence of useful, noninvasive testing. Elevated liver enzymes appear to decrease the probability of a diagnosis of HFE-linked hemochromatosis. The clinical management of patients with mild hyperferritinemia has not been well studied. Observation over time or a trial as a voluntary blood donor are both useful approaches in this group.
REFERENCES
- 1.Bacon BR, Olynyk JK, Brunt EM, Britton RS, Wolff RK. HFE genotype in patients with hemochromatosis and other liver diseases. Ann Intern Med. 1999;130:953–62. doi: 10.7326/0003-4819-130-12-199906150-00002. [DOI] [PubMed] [Google Scholar]
- 2.Alla V, Bonkovsky H. Iron in non-hemochromatotic liver disorders. Semin Liver Disease. 2005;25:461–72. doi: 10.1055/s-2005-923317. [DOI] [PubMed] [Google Scholar]
- 3.Bell H, Skinningsrud A, Raknerud N, Try K. Serum ferritin and transferrin saturation in patients with chronic alcoholic and non-alcoholic liver diseases. J Intern Med. 1994;236:315–22. doi: 10.1111/j.1365-2796.1994.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 4.Giler S, Moroz C. The significance of ferritin in malignant diseases. Biomedicine. 1978;28:203–6. [PubMed] [Google Scholar]
- 5.Esumi N, Ikushima S, Hibi S, Todo S, Imashuku S. High serum ferritin level as a marker of malignant histiocytosis and virus-associated hemophagocytic syndrome. Cancer. 1988;61:2071–6. doi: 10.1002/1097-0142(19880515)61:10<2071::aid-cncr2820611023>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Beilby JP, Prins AW, Swanson NR. Determination of hepatic iron concentration in fresh and paraffin-embedded tissue. Clin Chem. 1999;45:573–4. [PubMed] [Google Scholar]
- 7.Wong K, Barbin Y, Chakrabarti S, Adams PC. A point mutation in the iron-responsive element of the L-ferritin in a family with hereditary hyperferritinemia cataract syndrome. Can J Gastroenterol. 2005;19:253–5. doi: 10.1155/2005/796963. [DOI] [PubMed] [Google Scholar]
- 8.Lee MH, Means RT., Jr Extremely elevated serum ferritin levels in a university hospital: Associated diseases and clinical significance. Am J Med. 2005;98:566–71. doi: 10.1016/s0002-9343(99)80015-1. [DOI] [PubMed] [Google Scholar]
- 9.Adams PC, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–78. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 10.Guyader D, Jacquelinet C, Moirand R, et al. Noninvasive prediction of fibrosis in C282Y homozygous hemochromatosis. Gastroenterology. 1998;115:929–36. doi: 10.1016/s0016-5085(98)70265-3. [DOI] [PubMed] [Google Scholar]
- 11.Beaton M, Guyader D, Deugnier Y, Moirand R, Chakrabarti S, Adams P. Noninvasive prediction of cirrhosis in C282Y-linked hemochromatosis. Hepatology. 2002;36:673–8. doi: 10.1053/jhep.2002.35343. [DOI] [PubMed] [Google Scholar]
- 12.Gandon Y, Olivie D, Guyader D, et al. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363:357–62. doi: 10.1016/S0140-6736(04)15436-6. [DOI] [PubMed] [Google Scholar]
- 13.Pietrangelo A. Non-HFE hemochromatosis. Semin Liver Dis. 2005;25:450–60. doi: 10.1055/s-2005-923316. [DOI] [PubMed] [Google Scholar]
- 14.Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T. Penetrance of 845G –> A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359:211–8. doi: 10.1016/S0140-6736(02)07447-0. [DOI] [PubMed] [Google Scholar]
- 15.Adams PC, Kertesz AE, McLaren CE, Barr R, Bamford A, Chakrabarti S. Population screening for hemochromatosis: A comparison of unbound iron-binding capacity, transferrin saturation, and C282Y genotyping in 5211 voluntary blood donors. Hepatology. 2000;31:1160–4. doi: 10.1053/he.2000.6984. [DOI] [PubMed] [Google Scholar]
- 16.Lin E, Adams PC. Biochemical liver profile in hemochromatosis. A survey of 100 patients. J Clin Gastroenterol. 1991;13:316–20. doi: 10.1097/00004836-199106000-00013. [DOI] [PubMed] [Google Scholar]


