Abstract
Today, combination antiviral therapy with pegylated interferon-alpha and ribavirin (RBV) allows many patients infected with hepatitis C virus (HCV) to achieve a sustained virological response, which is equivalent to cure. Data also support the clinical benefit of combination antiviral therapy in patients coinfected with HCV and HIV, and in patients who have received a liver transplant.
Antiviral therapy with pegylated interferon-alpha and RBV is, however, associated with a high incidence and significant magnitude of anemia. This anemia may have several mechanisms, including bone marrow suppression and hemolysis. In addition, patients coinfected with HIV may have both pre-existing and RBV-associated anemia. Management of anemia in patients with HCV through RBV dose reduction or treatment discontinuation may compromise the effectiveness of treatment, because studies have demonstrated that treatment adherence or maintenance of antiviral therapy dose is an important predictor of sustained virological response.
Anemia associated with combination antiviral therapy in patients with HCV is frequently associated with an inadequate or blunted endogenous erythropoietin response. Accumulating evidence now supports the use of recombinant human erythropoietin (rHuEpo) to manage anemia in these patients, with the objective of maintaining the RBV dose, but clinical standards are lacking. The present article reviews the data relevant to the use of rHuEpo in this patient population and proposes a set of clinical practice standards to assist clinicians in selecting patients for rHuEpo and in implementing rHuEpo therapy effectively.
Keywords: Anemia, Hepatitis C virus, Recombinant human erythropoietin, Ribavirin
Abstract
De nos jours, la polythérapie antivirale à l’interféron pégylé alpha et à la ribavirine (RBV) permet à de nombreux patients infectés par le virus de l’hépatite C (VHC) de profiter d’une réaction virologique soutenue, équivalant à la guérison. Les données appuient également le bienfait clinique de la polythérapie antivirale chez les patients co-infectés par le VHC et le VIH et chez les patients greffés du foie.
La thérapie antivirale à l’interféron pégylé alpha et à la RBV s’associe toutefois à une forte incidence d’anémie de grande envergure. Cette anémie peut se manifester par divers mécanismes, y compris la suppression médullaire et l’hémolyse. De plus, les patients co-infectés par le VIH peuvent souffrir à la fois d’anémie préexistante et d’anémie causée par la RBV. La prise en charge de l’anémie chez les patients atteints du VHC par une réduction de la dose de RBV ou l’abandon du traitement peut compromettre l’efficacité du traitement. En effet, les études ont démontré que l’adhésion au traitement ou le maintien de la dose antivirale constituent des prédicteurs importants d’une réaction virologique soutenue.
L’anémie associée à la polythérapie antivirale chez les patients atteints du VHC s’associe souvent à l’insuffisance ou à l’émoussement de la réaction de l’érythropoiétine endogène. Les données probantes s’accumulent pour soutenir le recours à l’érythropoiétine humaine recombinante (rHuEpo) afin de prendre en charge l’anémie chez ces patients en vue de maintenir la dose de RBV, mais il n’existe pas de normes cliniques à cet effet. Le présent article analyse les données reliées à l’utilisation de rHuEpo au sein de cette population de patients et inclut une série de normes de pratique clinique pour aider les cliniciens à sélectionner les patients qui prendront de la rHuEpo et à implanter une thérapie efficace à la rHuEpo.
Antiviral therapy now allows many patients infected with hepatitis C virus (HCV) to achieve a sustained virological response (SVR), which is equivalent to cure (1). The highest response rates are achieved with a combination of pegylated interferon-alpha (PEG IFN-α) and ribavirin (RBV). The recent publication Management of Viral Hepatitis: A Canadian Consensus Conference 2004, states that based on the improvements achieved in SVR with PEG IFN-α/RBV combination therapy, all HCV-infected patients should be evaluated for suitability for treatment (1). Combination antiviral therapy (PEG IFN-αplus RBV) is, however, associated with a significant incidence of anemia that may require RBV dose reduction or treatment discontinuation, thus compromising the effectiveness of treatment.
A growing body of evidence and increasing clinical experience now exist concerning the use of recombinant human erythropoietin (rHuEpo) to manage anemia in HCV patients treated with PEG IFN-α/RBV. However, practice standards do not yet exist. Therefore, a group of clinicians with expertise in managing patients with HCV have reviewed the available data and have proposed consensus clinical guidelines for the use of rHuEpo. Because all published data concerning the management of RBV-induced anemia in this population have been generated in clinical trials of epoetin alpha, the discussion focuses on that agent. No data are available for the use of darbepoetin alpha.
RESPONSE TO COMBINATION ANTIVIRAL THERAPY FOR HCV
The main predictors of response to combination antiviral therapy with PEG IFN-α/RBV are viral genotype, viral load and treatment adherence. SVR rates of 78% to 82% have been achieved in patients infected with HCV genotypes 2 and 3, whereas the SVR rate for other genotypes (mainly genotype 1) is approximately 44% (2,3). Viral loads below 800,000 IU/mL are associated with higher SVRs than higher viral loads. Finally, treatment adherence is an important factor affecting outcome. Dose reductions may be used to manage treatment side effects such as anemia. Minor reductions in total dose, which still allow for more than 80% of the total dose to be administered, reduce SVR slightly, whereas greater reductions reduce SVR to a greater degree (4). Premature treatment discontinuation is associated with very low SVR rates. The extent to which timing of dose reduction and discontinuation affects SVR has, however, not yet been fully explored.
In patients infected with HCV genotype 1, the lack of early virological response (EVR), measured after 12 weeks of therapy, reliably predicts lack of SVR (4,5). Patients who achieve EVR have a 65% to 72% chance of achieving SVR, whereas the chance of SVR in those who do not achieve EVR is less than 3%. Measurement of EVR is not helpful in patients infected with HCV genotype 2 or 3 because 97% of these patients achieve EVR.
Clinical studies have identified effective PEG IFN-α/RBV regimens for patients infected with different HCV genotypes. The Canadian Consensus Conference also recommended that patients who have not responded or who have relapsed after successful non-PEG IFN-αantiviral treatment should receive a standard dose and duration of PEG IFN-α/RBV (1). Patients coinfected with HCV and HIV should receive full-dose combination therapy with aggressive supportive therapy to treat drug-induced side effects and prevent reductions in SVR.
IMPORTANCE OF TREATMENT ADHERENCE
Several studies have demonstrated the importance of treatment adherence to overall treatment success. McHutchison et al (6) reviewed data from previous trials of combination therapy with IFN-α2b or PEG IFN-αplus RBV in 1521 patients infected with HCV genotype 1. Treatment was verified from drug records and patient diaries. Patients were divided into two groups: those who took at least 80% of the total dose of both drugs for at least 80% of the expected treatment duration, and those who did not. Patients who achieved 80% adherence had SVR rates of 52% for IFN-α/RBV and 63% for PEG IFN-α/RBV, whereas patients who did not achieve 80% adherence had SVR rates of 18% and 25%, respectively. The authors concluded that adherence enhances the likelihood of EVR.
Davis et al (5) examined degrees of viral inhibition during the initial weeks of treatment among patients with HCV treated with PEG IFN-α/RBV combination therapy with the objective of identifying factors associated with eventual lack of response. The authors found that the best predictor of SVR was an EVR comprising a 2 log drop in HCV RNA after 12 weeks of therapy. Between 69% and 76% of patients achieved EVR, and 67% to 80% of patients achieving EVR went on to achieve SVR. Patients not achieving EVR as defined in the present analysis do not respond to additional drug therapy.
These studies demonstrate the importance of maintenance of antiviral dose or treatment adherence to EVR and to SVR, especially for patients infected with HCV genotype 1.
CONSIDERATIONS IN TREATING LIVER TRANSPLANT PATIENTS WITH HCV
Hepatitis C after liver transplantation presents a treatment challenge. First, the disease progresses more rapidly than in the nontransplant setting. Second, although precise response rates are not known, it is clear that response rates are lower than the nontransplant setting.
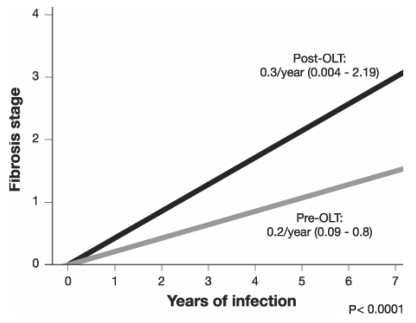
Berenguer et al (7) evaluated the natural history of HCV disease progression in the orthotopic liver transplant (OLT) population by means of biopsies of 284 patients and found that fibrosis scores increased linearly with time from transplantation (Figure 1).
Figure 1).
Progression of fibrosis in patients with hepatitis C virus infection pre-orthotopic liver transplantation (pre-OLT) and post-OLT according to duration of infection. Adapted from reference 7
Fibrosis also progresses much more rapidly in patients after OLT than in patients who have not received a liver transplant (7). The time interval from infection to cirrhosis, usually between 20 and 40 years in non-OLT patients, is decreased to between nine and 12 years in OLT recipients. In an attempt to identify factors affecting the progression of fibrosis, Chopra et al (8) studied 58 patients with HCV who had undergone OLT. The study found that genotype was a factor, with genotype 1a being associated with an increased risk of fibrosis, and that cytomegalovirus infection also increased the rate of fibrosis.
Dumortier et al (9) reported on a pilot study involving 12 months of combination therapy with PEG IFN-αand RBV in 20 patients transplanted for HCV. The time between transplantation and antiviral therapy was 28 months and 80% of the patients were infected with genotype 1. Overall, 75% of patients had a biochemical response and 55% a virological response. In nine patients, the virological response persisted six months after treatment. The mean METAVIR score for activity and fibrosis decreased from A1.8 F2.2 before treatment to A0.3 F1.6 at the end of treatment. The RBV dose was reduced in 16 patients, 13 of whom experienced dose reductions for anemia. The PEG IFN-αdose was reduced in six patients. The authors concluded that combination antiviral therapy was beneficial for HCV recurrence in patients transplanted for HCV but anemia limited RBV dosing.
Other studies have also shown that treatment is limited by toxicity, mainly bone marrow suppression and anemia (10–12). Given that patients with recurrent HCV after transplantation may not have a second chance at therapy, it is important to maximize the likelihood of a response, including maintaining maximal doses of both IFN and RBV. Appropriate management of anemia and bone marrow suppression may improve the ability to deliver maximal doses of therapy.
CONSIDERATIONS IN TREATING HCV/HIV COINFECTED PATIENTS
Similar considerations apply to patients with HIV/HCV coinfection. Liver disease tends to progress more rapidly (13,14) and response rates to treatment are decreased compared with those without HIV infection (15,16).
Estimates of the prevalence of HCV/HIV coinfection vary. Globally, nearly one-third of patients infected with HIV may be coinfected with HCV (10). Since the advent of highly active antiretroviral therapy and longer survival of patients infected with HIV, HCV has become a major cause of morbidity and mortality in patients infected with HIV (17). Coinfected individuals tend to have higher viral loads and relatively more severe HCV disease (13,14); they also experience more rapid progression of hepatic fibrosis and an increased incidence of cirrhosis and death due to liver disease.
Given that good response rates are more difficult to achieve, the use of supportive treatment to maintain maximal doses of IFN and RBV is important.
COMBINATION ANTIVIRAL THERAPY AND ANEMIA
Anemia is a common side effect that can jeopardize treatment adherence and EVR, thus potentially compromising the effectiveness of treatment. Gaeta et al (18) performed a multicentre retrospective study to compare the ‘real-world’ discontinuation rate for combination HCV therapy with the 27% seen in randomized controlled trials. They enrolled 441 consecutive patients scheduled to receive IFN-α/RBV combination therapy at five centres. The authors found that adverse events resulted in discontinuation in 108 (24.5%) patients, discontinuation was higher in the first six months and the reason for discontinuation was anemia in approximately one-third of patients.
The anemia seen in patients receiving combination antiviral therapy may have several mechanisms (19). IFN-αsuppresses bone marrow, reducing red blood cell production, whereas RBV causes a dose-dependent hemolytic anemia. Thus, combination therapy not only decreases red cell mass but reduces the compensatory reticulocytosis expected with hemolysis.
The magnitude of decrease of hemoglobin (Hb) has been evaluated in a retrospective analysis of data from two studies of combination therapy (20). Among the 594 patients for whom data were available, initial Hb levels exceeded 120 g/L among women (n=208) and 130 g/L among men (n=386). Hb decreased by at least 30 g/L in 54% of the patient population and by more than 25% from baseline in approximately 28% of patients. Almost 10% of men and 7% of women experienced an Hb decrease of at least 50 g/L. These trials indicate both a high incidence and significant magnitude of anemia in patients treated with combination antiviral therapy.
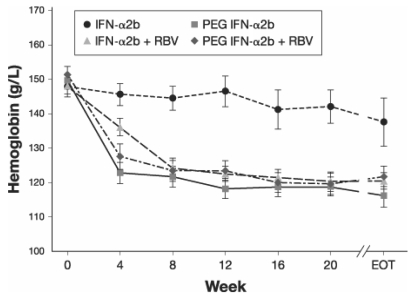
A study in 133 patients receiving antiviral treatment for HCV with IFN-αor PEG IFN-αalone or in combination with RBV evaluated the impact of treatment on hematopoiesis (21). In the groups receiving combination therapy, Hb decreased by 20 g/L or 30 g/L by four weeks and remained low throughout therapy, whereas in the group receiving IFN-αmonotherapy, Hb decreased only slightly by eight weeks but also remained decreased throughout therapy (Figure 2). Hb decreased substantially more with PEG IFN-αmonotherapy than with IFN-αmonotherapy. As Hb decreased in the groups receiving combination therapy, the concentration of endogenous erythropoietin increased.
Figure 2).
Impact of four hepatitis C antiviral treatment regimens on hemoglobin levels during 24 to 48 weeks of therapy. EOT End of therapy; PEG IFN-α2b Pegylated interferon-alpha2b; RBV Ribavirin. Adapted from reference 21
However, the increase in endogenous erythropoietin may be inadequate. Balan et al (22) performed a multicentre observational study that included 97 evaluable patients. The authors found the endogenous erythropoietin response to anemia to be decreased in this patient population compared with patients with iron deficiency anemia.
ANEMIA IN HCV/HIV COINFECTED PATIENTS
Combination therapy for HCV in coinfected individuals is associated with a more profound anemia than seen in monoinfected patients (20). This situation is compounded by a high prevalence of pretreatment anemia and by the potential need for treatment with other medications causing anemia. Sulkowski et al (23) have shown that rHuEpo use will effectively correct anemia associated with HCV treatment and allows maintenance of RBV dosing.
rHuEpo AND ANEMIA MANAGEMENT IN HCV PATIENTS RECEIVING COMBINATION THERAPY
Clinical studies have now demonstrated that treatment with rHuEpo can maintain Hb levels in patients treated with combination antiviral therapy for HCV. In the past, low Hb disqualified patients from receiving antiviral therapy for HCV due to the potential for worsening anemia. Today, however, patients with pre-existing anemia and those who develop anemia as a result of treatment can be treated with rHuEpo. This may obviate the need for RBV dose reduction, improve treatment adherence and improve the likelihood of SVR.
Dieterich et al (24) performed an open-label, parallel-group study at seven centres to determine the efficacy of rHuEpo in treating anemia and minimizing RBV dose reduction in patients with HCV receiving IFN-α/RBV combination therapy. The study enrolled 64 patients with a maximum Hb level of 120 g/L during the first 24 weeks of antiviral therapy and randomly assigned them to receive rHuEpo 40,000 IU subcutaneously weekly or standard of care (SOC) for anemia management. SOC consisted of an RBV dose reduction to 600 mg/day when Hb dropped to less than 100 g/L and later RBV discontinuation at an Hb level of 85 g/L with transfusion (24). The primary end point was the change in Hb level and the secondary end point was the RBV dosage, both measured from baseline to week 16 of treatment (Figure 3).
Figure 3).
Mean hemoglobin (Hb) levels (A) and mean ribavirin dose (B) with recombinant human erythropoietin (rHuEpo) and standard of care in patients with hepatitis C virus being treated with combination therapy with ribavirin plus interferon-alpha. Adapted from reference 24
The mean increase in Hb was 28 g/L for rHuEpo versus 4 g/L for SOC (P<0.0001) and the mean change in RBV dose was −34 mg/day for rHuEpo compared with −146 mg/day for SOC (P=0.060). The mean Hb in the rHuEpo group at week 16 (138 g/L) was significantly higher (P<0.0001) than in the SOC group (114 g/L). From week 4 onward, significantly more patients in the rHuEpo group than in the SOC group had no RBV dose reductions (P<0.011). By the conclusion of the study, 83% of patients treated with rHuEpo had maintained an RBV dose of at least 800 mg/day compared with 54% of patients in the SOC group (P=0.022). This result may be clinically beneficial because evidence indicates that RBV doses below 800 mg/day may be associated with a decreased likelihood of SVR.
The authors also found rHuEpo to be well-tolerated in this population (24). The study was neither designed nor powered to assess quality of life (QoL); however, QoL data were collected for descriptive purposes. Baseline values for both groups indicated comparable levels of existing impairment, and QoL improvement at 16 weeks was greater for the rHuEpo group than the SOC group. The QoL changes were both qualitatively and quantitatively similar to those seen in anemic cancer patients and HIV patients treated with rHuEpo.
Afdhal et al (25) performed a prospective, double-blind randomized controlled trial of rHuEpo 40,000 IU once weekly versus placebo in patients receiving IFN-α/RBV or PEG IFN-α/RBV combination therapy. The objectives of the study were to determine whether rHuEpo could maintain RBV dose, improve QoL and increase Hb in anemic HCV-infected patients. The trial was designed with an eight-week double-blind phase (DBP) followed by an eight-week open-label phase (OLP) during which placebo patients were crossed over to rHuEpo. The study enrolled 185 patients who were receiving combination therapy and who had a maximum Hb level of 120 g/L. The mean Hb at randomization was 108 g/L in both groups. During the trial, the RBV dose was increased or decreased based on Hb levels. During the DBP, 4% of the rHuEpo group and 3% of the placebo group discontinued HCV treatment, and during the OLP, 3% of the rHuEpo group and 6% of the original placebo group discontinued HCV treatment.
At the end of the DBP, 88% of rHuEpo patients had maintained their RBV dose compared with 60% of placebo patients (P<0.0001) (25). At the end of the OLP, 78% of the original rHuEpo group were receiving an RBV dose that was at least equivalent to the starting dose compared with 64% of patients in the original placebo group. Patients in the original placebo group also had a significant increase in RBV dose (P<0.001) during the OLP.
Mean QoL scores improved significantly in the rHuEpo group compared with the placebo group from randomization to the end of the DBP (25). Scores increased similarly for patients who crossed over from placebo to the OLP. Mean Hb increased by 22 g/L in the rHuEpo group to a mean of 130 g/L and by 1 g/L in the placebo group to a mean of 109 g/L. A similar improvement was seen during the OLP for the placebo group, and the Hb was maintained in the epoetin group. The study was not designed to assess the effect of rHuEpo treatment on SVR. However, more patients in the rHuEpo group were able to maintain the RBV dose.
Pockros et al (26) performed a detailed analysis of the Afdhal et al (25) QoL data. The objectives of the study were to compare health-related quality of life (HRQL) of patients in the study with that of the general population and patients with other chronic conditions, including untreated HCV, congestive heart failure, clinical depression and type II diabetes, and to evaluate retrospectively the relationship between HRQL and Hb levels. HRQL is now known to be impaired in patients with HCV to a similar extent to that seen in cancer patients undergoing chemotherapy (27,28). In cancer patients, HRQL directly correlates with Hb levels, and rHuEpo treatment not only increases Hb but improves HRQL domains such as energy, activity and overall QoL.
In this study, HRQL was assessed at randomization, at the end of the DBP and at the end of the OLP using two instruments: the Short Form-36 (SF-36) and the linear analogue self-assessment scale. The SF-36 is a validated and widely used HRQL instrument and the linear analogue self-assessment scale has been extensively used to measure HRQL in anemic patients. Mean SF-36 scores of anemic HCV-infected patients receiving combination therapy at study randomization were significantly lower than those of both the general population and patients with untreated HCV infection. In addition, scores on several functional domains were lower than for all comparison populations. Cirrhosis was evaluated as a contributing factor, but values were not different for patients with or without cirrhosis. Patients with the greatest increases in Hb from randomization to the end of the DBP also had the greatest improvements in HRQL, and Hb increases of at least 20 g/L were associated with significant improvements on both scales. In contrast, patients whose Hb decreased during the DBP generally experienced a decrease in HRQL. Hb was a significant independent predictor of changes in HRQL and the most consistent predictor for HRQL improvement. The study was not designed to evaluate SVR, and patients were not stratified by genotype or other factors known to influence SVR. Nevertheless, at six-month follow-up, 53% of the rHuEpo group had achieved SVR compared with 45% of the placebo group.
In addition to the above studies, that did not evaluate the effect of rHuEpo on SVR, there are now preliminary data that indicate that the use of rHuEpo to treat RBV-induced anemia improves SVR rates as well as maintaining Hb and RBV dose levels (29). Furthermore, at least one cost-efficacy analysis has demonstrated that this is a cost-effective form of treatment (30).
INADEQUATE RESPONSE TO rHuEpo
An inadequate response to rHuEpo has been noted in some dialysis patients in the chronic renal failure population (31). The most common cause is absolute or functional iron deficiency, but chronic inflammation may also reduce response, leading to a need for higher rHuEpo dose levels. Chronic inflammation may also be a cause of reduced response to rHuEpo in patients with HCV. These causes of inadequate response to rHuEpo must be differentiated from antibody-mediated pure red cell aplasia (PRCA), a rare occurrence. Stravitz et al (32) reported on a case of PRCA with resulting transfusion dependence, which resolved 16 weeks after discontinuing rHuEpo. Analysis of stored serum samples identified a decrease in erythropoietin concentrations from supraphysiological levels at week 12 to undetectable levels at week 24 of therapy, along with a parallel rise in the titre of neutralizing antibodies. Antierythropoietin antibodies cross-react with endogenous erythropoietin and with recombinant preparations. As a result, erythropoietin treatment should be discontinued rather than switched to another product.
PRCA tends to occur late after initiation of erythropoietin treatment, whereas other causes of inadequate epoetin response are usually operative from treatment initiation. A paradoxical Hb decrease after a response to erythropoietin may indicate the development of PRCA and should prompt discontinuation of rHuEpo and antiviral therapy.
CONSENSUS RECOMMENDATIONS: CLINICAL STANDARDS FOR THE USE OF rHuEpo
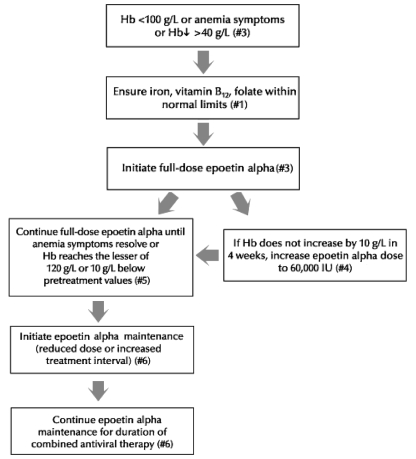
The use of rHuEpo is medically appropriate in certain clinical situations in patients infected with HCV and treated with combination antiviral therapy. The following recommendations reflect a group consensus on clinical standards for use of rHuEpo. These recommendations are based on the available evidence and on clinical experience of the discussion group. Figure 4 summarizes the recommended approach to the management of anemia related to combination antiviral therapy in this patient population.
Figure 4).
Approach to the use of epoetin alpha in the management of anemia associated with combination antiviral therapy in patients infected with hepatitis C virus. Hb Hemoglobin
Clinical considerations in deciding on rHuEpo treatment
In all patients, the decision to initiate rHuEpo treatment requires consideration of the risks of treatment failure with reduction of RBV dose, the cost of rHuEpo and the possible complications resulting from the use of rHuEpo. Patients with a high likelihood of response, such as those infected with HCV genotype 2 or 3, may tolerate RBV dose reduction without much decrease in the likelihood of achieving SVR. In contrast, dose reduction should be avoided, if possible, in patients in whom the response rate is lower and in whom a maximal effort is required to increase the individual’s chance of response. This group includes patients who are HIV-positive and those who have received a liver transplant. Similarly, dose reduction should also be avoided in patients for whom achieving response is more urgent, such as individuals with cirrhosis or advanced age combined with stage 3 fibrosis, because these patients may not have a second opportunity for treatment with new agents.
Reduction in RBV dose appears to be more critical to sustained response rates if it occurs in the first 12 weeks of therapy. As a result, maintenance of Hb levels during the first 12 weeks of therapy is more important than at later stages during treatment. However, RBV dose reductions after 12 weeks may also reduce the overall response rate.
Recommendation 1: Deficiencies possibly contributing to anemia
All HCV patients treated with RBV should be assessed for other causes of anemia, including iron, folate and vitamin B12 deficiencies, and any deficiencies identified should be corrected. Folic acid supplementation (5 mg/day) is appropriate because RBV-induced hemolysis may contribute to folate deficiency.
Recommendation 2: Assessment
All patients who receive RBV should have a baseline assessment for clinical risks associated with anemia, such as poor exertional tolerance, which may indicate underlying cardiovascular or pulmonary disease. Clinical parameters and symptoms related to anemia, such as fatigue and increased heart rate, should be documented before treatment. During treatment, patients should be routinely assessed for the development of new anemia-related symptoms.
Recommendation 3: Indications for treatment with epoetin alpha
There are two forms of erythropoietin available in Canada, epoetin alpha and darbepoetin, which is a modified form of human erythropoietin. In the absence of data supporting the use of darbepoetin alpha, these recommendations apply to only the use of epoetin alpha. The following are recommended indications for treatment with epoetin alpha:
a decline in Hb from baseline of more than 40 g/L,
Hb concentration of 100 g/L or less; or
symptomatic anemia, which may occur at any Hb concentration following a rapid fall in Hb concentration.
Recommendation 4: Epoetin alpha regimens
The following are recommended possible initial epoetin alpha regimens:
dose of 40,000 IU subcutaneously per week; or
initiation of treatment at 20,000 IU subcutaneously per week with dose increase based on response.
Epoetin alpha dose may be increased to a maximum of 60,000 IU subcutaneously per week if an inadequate response is seen after four weeks of treatment with 40,000 IU subcutaneously per week.
Recommendation 5: Epoetin alpha treatment duration
Full-dose epoetin alpha treatment should be maintained until either of the following:
resolution of anemia-related symptoms or
recovery of Hb concentration to the lesser of 120 g/L or 10 g/L below pretreatment values.
It is not necessary to treat until Hb returns to baseline levels. Hb usually decreases again if epoetin alpha is discontinued during combined antiviral treatment. Therefore, consideration should be given to epoetin alpha maintenance therapy.
Recommendation 6: Epoetin alpha maintenance treatment
Once the Hb has recovered or the symptoms of anemia have resolved, it is recommended that the dose of epoetin alpha be reduced or the treatment interval lengthened to maintain Hb recovery during continued combination antiviral treatment.
Recommendation 7: Retreatment of previous antiviral treatment failures
Patients who developed symptomatic anemia with a prior course of antiviral treatment should be started on epoetin alpha when treatment with IFN and RBV is initiated. Approval for reimbursement should be sought before starting treatment.
Recommendation 8: Patients with coronary artery disease
Patients with cardiovascular problems may be less tolerant of anemia, may risk poorer outcomes with RBV dose reductions and may experience more adverse events with epoetin alpha treatment. These patients may be poor candidates for antiviral therapy. However, if treatment with PEG IFN-α/RBV is initiated, the decision to treat these patients with epoetin alpha should be individualized and if the benefits of epoetin alpha treatment are thought to outweigh the risks, epoetin alpha treatment should be initiated with caution.
Recommendation 9: PRCA
The magnitude of the risk of PRCA is not known currently but the potential for PRCA should be borne in mind when considering epoetin alpha treatment and patients should be informed of the risks. Patients in whom Hb continues to fall despite administration are at risk for PRCA; these patients should have epoetin alpha and RBV withdrawn and should have the effects of drug withdrawal on Hb monitored.
These recommendations were achieved by consensus among physicians from Toronto and London, Ontario, who are experienced in the management of patients with chronic HCV, including those coinfected with HIV and those who have received a liver transplant. Although these recommendations have been developed in Ontario, they are likely to be applicable elsewhere in Canada.
No attempt was made to evaluate the cost-effectiveness of epoetin alpha use.
This document represents the opinions of the authors and is not a consensus statement.
Footnotes
DISCLOSURES: Dr Sherman is a speaker for and a member of the advisory boards of Hoffmann-La Roche Ltd, Bristol-Myers Squibb Canada and Gilead Canada. He is also a member of the advisory board of Novartis Pharmaceuticals Canada Inc. Dr Heathcote is a member of the medical advisory board of Hoffmann-La Roche Ltd, Schering Canada Inc and Axcan Pharma. She is also supported by unrestricted grants from Hoffmann-La Roche Ltd, Schering Canada Inc and Axcan Pharma, as well as a restricted grant from Schering Canada Inc. Dr Wong is a consultant and speaker for Hoffmann-La Roche Ltd and a speaker for Axcan Pharma, Gilead Sciences, Ortho Biotech and Schering Canada Inc. Dr Feinman has received consulting fees from the advisory boards of Gilead Canada, Ortho Biotech and Johnson & Johnson Inc, as well as Schering Canada Inc. Dr Yim is a member of the nurses advisory board for Hoffmann-La Roche Ltd and Ortho Biotech. Dr Levstik has participated in clinical trials and advisory boards for Schering Canada Inc, Hoffmann-La Roche Ltd and Wyeth Pharmaceuticals. He has also participated on the advisory board for Ortho Biotech.
REFERENCES
- 1.Sherman M, Bain V, Villeneuve JP, et al. Management of Viral Hepatitis: A Canadian Consensus Conference 2004. The Public Health Agency of Canada<http://www.phac-aspc.gc.ca> (Version current at September 21, 2005).
- 2.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 3.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 4.Ferenci P, Fried MW, Shiffman ML, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–33. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–52. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 6.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–9. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer M, Ferrell L, Watson J, et al. HCV-related fibrosis progression following liver transplantation: Increase in recent years. J Hepatol. 2000;32:673–84. doi: 10.1016/s0168-8278(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 8.Chopra KB, Demetris AJ, Blakolmer K, et al. Progression of fibrosis in patients with chronic hepatitis C after orthotopic liver transplantation. Transplantation. 2003;76:1487–91. doi: 10.1097/01.TP.0000088668.28950.7C. [DOI] [PubMed] [Google Scholar]
- 9.Dumortier J, Scoazec JY, Chevallier P, Boillot O. Treatment of recurrent hepatitis C after liver transplantation: A pilot study of peginterferon alfa-2b and ribavirin combination. J Hepatol. 2004;40:669–74. doi: 10.1016/j.jhep.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Babatin M, Schindel L, Burak KW. Pegylated-interferon alpha 2b and ribavirin for recurrent hepatitis C after liver transplantation: From a Canadian experience to recommendations for therapy. Can J Gastroenterol. 2005;19:359–65. doi: 10.1155/2005/745197. [DOI] [PubMed] [Google Scholar]
- 11.Toniutto P, Fabris C, Fumo E, et al. Pegylated versus standard interferon-alpha in antiviral regimens for post-transplant recurrent hepatitis C: Comparison of tolerability and efficacy. J Gastroenterol Hepatol. 2005;20:577–82. doi: 10.1111/j.1440-1746.2005.03795.x. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Luna H, Khatib A, Sharma P, et al. Treatment of recurrent hepatitis C infection after liver transplantation with combination of pegylated interferon alpha2b and ribavirin: An open-label series. Transplantation. 2004;77:190–4. doi: 10.1097/01.TP.0000100481.14514.BB. [DOI] [PubMed] [Google Scholar]
- 13.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 14.Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100:56–63. doi: 10.1111/j.1572-0241.2005.40670.x. [DOI] [PubMed] [Google Scholar]
- 15.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 16.Carrat F, Bani-Sadr F, Pol S, et al. ANRS HCO2 RIBAVIC Study Team. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: A randomized controlled trial. JAMA. 2004;292:2839–48. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 17.Salmon-Ceron D, Lewden C, Morlat P, et al. The Mortality 2000 study group. Liver disease as a major cause of death among HIV infected patients: Role of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42:799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Gaeta GB, Precone DF, Felaco FM, et al. Premature discontinuation of interferon plus ribavirin for adverse effects: A multicentre survey in ‘real world’ patients with chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1633–9. doi: 10.1046/j.1365-2036.2002.01331.x. [DOI] [PubMed] [Google Scholar]
- 19.Sulkowski MS. Anemia in the treatment of hepatitis C virus infection. Clin Infect Dis. 2003;37(Suppl 4):315–22. doi: 10.1086/376911. [DOI] [PubMed] [Google Scholar]
- 20.Sulkowski MS, Wasserman R, Brooks L, Ball L, Gish R. Changes in haemoglobin during interferon alpha-2b plus ribavirin combination therapy for chronic hepatitis C virus infection. J Viral Hepat. 2004;11:243–50. doi: 10.1111/j.1365-2893.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 121.Schmid M, Kreil A, Jessner W, et al. Suppression of haematopoiesis during therapy of chronic hepatitis C with different interferon alpha mono and combination therapy regimens. Gut. 2005;54:1014–20. doi: 10.1136/gut.2004.057893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balan V, Schwartz D, Wu GY, et al. Erythropoietic response to anemia in chronic hepatitis C patients receiving combination pegylated interferon/ribavirin. Am J Gastroenterol. 2005;100:299–307. doi: 10.1111/j.1572-0241.2005.40757.x. [DOI] [PubMed] [Google Scholar]
- 23.Sulkowski MS, Dieterich DT, Bini EJ, et al. For the HIV/HCV Coinfection Study Group. Epoetin alfa once weekly improves anemia in HIV/hepatitis C virus-coinfected patients treated with interferon/ribavirin: A randomized controlled trial. J Acquir Immune Defic Syndr. 2005;39:504–6. doi: 10.1097/01.qai.0000167158.90722.73. [DOI] [PubMed] [Google Scholar]
- 24.Dieterich DT, Wasserman R, Bräu N, et al. Once-weekly epoetin alfa improves anemia and facilitates maintenance of ribavirin dosing in hepatitis C virus-infected patients receiving ribavirin plus interferon alfa. Am J Gastroenterol. 2003;98:2491–9. doi: 10.1111/j.1572-0241.2003.08700.x. [DOI] [PubMed] [Google Scholar]
- 25.Afdhal NH, Dieterich DT, Pockros PJ, et al. Epoetin alfa maintains ribavirin dose in HCV-infected patients: A prospective, double-blind, randomized controlled study. Gastroenterology. 2004;126:1302–11. doi: 10.1053/j.gastro.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Pockros PJ, Schiffman ML, Schiff ER, et al. Epoetin alfa improves quality of life in anemic HCV-infected patients receiving combination therapy. Hepatology. 2004;40:1450–8. doi: 10.1002/hep.20482. [DOI] [PubMed] [Google Scholar]
- 27.Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn H. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: Improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol. 2001;19:2875–82. doi: 10.1200/JCO.2001.19.11.2875. [DOI] [PubMed] [Google Scholar]
- 28.Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic patients receiving epoetin alfa therapy. Cancer. 2002;95:888–95. doi: 10.1002/cncr.10763. [DOI] [PubMed] [Google Scholar]
- 29.Shiffman ML, Price A, Hubbard S, et al. Treatment of hepatitis C virus (HCV) genotype 1 with peginterferon alfa-2b (PEGIFN) high weight based dose ribavirin (RVN) and epoetin alfa (EPO) enhances sustained virologic response (SVR) Hepatology 200542217A(Abst) [Google Scholar]
- 30.Spiegel BM, Chen K, Chiou CF, Robbins S, Younossi ZM. Erythropoietic growth factors for treatment-induced anemia in hepatitis C: A cost-effectiveness analysis. Clin Gastroenterol Hepatol. 2005;3:1034–42. doi: 10.1016/s1542-3565(05)00695-6. [DOI] [PubMed] [Google Scholar]
- 31.Horl WH, Jacobs C, Macdougall I, et al. European best practice guidelines 14–16: Inadequate response to epoetin. Nephrol Dial Transplant. 2000;15(Suppl 4):43–50. [PubMed] [Google Scholar]
- 32.Stravitz RT, Chung H, Sterling RK, et al. Antibody-mediated pure red cell aplasia due to epoetin alfa during antiviral therapy of chronic hepatitis C. Am J Gastroenterol. 2005;100:1415–9. doi: 10.1111/j.1572-0241.2005.41910.x. [DOI] [PubMed] [Google Scholar]