Abstract
Transforming growth factor-β (TGF-β) binds to and signals via two serine-threonine kinase receptors, type I (TβRI) and type II (TβRII). The oligomerization of TGF-β receptors modulates ligand binding and receptor trafficking and may contribute to signal diversification. However, numerous features of the molecular domains that determine the homo- and hetero-oligomerization of full-length receptors at the cell surface and the mode of these interactions remain unclear. Here, we address these questions through computerized immunofluorescence co-patching and patch/fluorescence recovery after photobleaching measurements of different combinations of epitope-tagged receptors and their mutants in live cells. We show that TβRI and TβRII are present on the plasma membrane both as monomers and homo- and hetero-oligomers. The homodimerization of TβRII depends on a cytoplasmic juxtamembrane region (amino acid residues 200–220). In contrast, the cytoplasmic domain of TβRI is dispensable for its homodimerization. TβRI·TβRII hetero-oligomerization depends on the cytoplasmic domain of TβRI and on a C-terminal region of TβRII (residues 419–565). TGF-β1 elevates TβRII homodimerization to some degree and strongly enhances TβRI·TβRII heteromeric complex formation. Both ligand-induced effects depend on the region encompassed between residues 200–242 of TβRII. Furthermore, the kinase activity of TβRI is also necessary for the latter effect. All forms of the homo- and hetero-oligomers, whether constitutively present on the membrane or formed upon TGF-β1 stimulation, were stable in the time-scale of our patch/FRAP measurements. We suggest that the different forms of receptor oligomerization may serve as a basis for the heterogeneity of TGF-β signaling responses.
Transforming growth factor-β (TGF-β)3 comprises a large superfamily of cysteine knot growth factors which regulate diverse biological processes including cell proliferation, differentiation, migration, and death (1–4). They were implicated in embryonic development, immune responses, hematopoiesis, and cancer (1, 2, 4–6). TGF-β signals via two receptor Ser/Thr kinases, type I and type II (TβRI and TβRII) (3, 4, 7–9). TβRII can bind ligands but requires TβRI for signaling, whereas TβRI alone is incapable of ligand binding (8, 10–13). TβRII is a constitutively active kinase regulated by autophosphorylation (14, 15). In the presence of ligand, TβRII phosphorylates specific Ser residues in TβRI, mediating its activation (13, 16). In turn, TβRI phosphorylates Smad2/3 proteins, mediating their translocation together with Smad4 to the nucleus, where they regulate transcription of target genes (2–4, 17).
TβRI and TβRII can physically associate (13, 18–21). Earlier we demonstrated in live cells that both TβRI (22) and TβRII (23) can form homodimers even in the absence of ligand, and biological evidence supports the homo-oligomerization of both receptors (12, 15, 24, 25). On the other hand, the tendency of TβRI and TβRII to form heterotetramers is strongly elevated by TGF-β (18–21).
Recent studies have shown that the extracellular (EC) domains of TβRI and TβRII form ternary complexes in the presence of ligand and in the absence of the transmembrane (TM) and cytoplasmic (CY) domains (19, 20). Similarly, the EC domain of TβRII was shown to be monomeric and to form homodimers upon ligand binding (22, 26). However, this does not preclude interactions between the CY domains of the receptors. Indeed, TβRI and TβRII constructs devoid of the EC domain demonstrated a tendency to form heteromeric complexes (27), and crystallographic data on the CY domain of TβRI suggest potential homodimerization (28). Moreover, the fact that TGF-β2 (unlike TGF-β1 and TGF-β3) requires both TβRI and TβRII for efficient binding suggests that at least a fraction of the cell-surface receptors forms heterocomplexes before ligand binding (14, 29, 30).
Although the EC domains of TβRII and TβRI form ligand-mediated ternary complexes (19, 20), it is not known whether the CY domains contribute to the interactions in the hetero-complexes or alter them in the full-length receptors situated in the cell membrane, and the domains regulating heteromeric and homomeric interactions between the receptor subunits remain unclear. Here we report a detailed investigation of these questions in live cells using epitope-tagged TGF-β receptor mutants. Employing computerized immunofluorescence co-patching and patch/FRAP studies, we show the existence of a heterogeneous receptor population at the cell surface comprised of monomers and homo and hetero-oligomers. All the complexes formed were stable on the time-scale of the FRAP studies. Importantly, the homomeric (TβRII) and heteromeric (TβRI·TβRII) interactions depended on distinct CY regions in each receptor, whereas the CY domain of TβRI was dispensable for TβRI homodimerization. These results have implications for the potential role of different TGF-β receptor oligomers in the multitude of TGF-β signaling outputs.
EXPERIMENTAL PROCEDURES
Materials—Human Recombinant human TGF-β1 was from PeproTech (Rocky Hill, NJ). Fatty acid-free bovine serum albumin (BSA), fraction V) was from Sigma. 9E10 mouse IgG against the myc epitope tag (α-myc), HA.11 rabbit antiserum and 12CA5 mouse IgG against the influenza hemagglutinin (HA) epitope tag (α-HA) were from Covance Research Products (Princeton, NJ). Rabbit IgG against the HA protein of the X:31 influenza virus strain (α-X:31 HA) was donated by J. M. White (University of Virginia, Charlottesville, VA) (31). IgG of Fc125 mouse monoclonal anti-Japan HA (α-Japan HA; directed against the HA protein of the Japan influenza virus strain) was a gift from T. J. Braciale (University of Virginia, Charlottesville, VA). IgG fractions and Fab′ fragments were prepared as described (23). Normal goat IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA). Alexa 546-GαR IgG, Alexa 546-goat anti-mouse (GαM) F(ab′)2, Alexa 488-GαM IgG, and Alexa 488-GαM F(ab′)2 were from Invitrogen. Fluorescent F(ab′)2 were converted to Fab′ as described (22). All other reagents were from Sigma.
Cell Culture and Expression Plasmids—COS7 cells were grown in media supplemented with 10% fetal calf serum and antibiotics as described (22, 32). All media and tissue culture reagents were from Biological Industries (Beit Haemek, Israel). Truncation, deletion, and alanine substitution mutants of extracellularly myc- or HA-tagged TβRII or TβRI in pcDNA3 or pcDNA1 (Invitrogen) employed in the current study are depicted in Fig. 1. Construction of the TβRII mutants (except TβRII-Val-419; Fig. 1A) was described (33), and the Val-419 truncation mutant was constructed similarly by insertion of a stop codon after Val-419. TβRI truncation mutants were generated by the same PCR-based protocol, except that myc- or HA-tagged TβRI cDNAs (22) in pcDNA3 were used as a template instead of tagged TβRII cDNAs. In addition, kinase-dead point mutants of TβRII (K277R mutation) or TβRI (K232R) were generated using HA- or myc-tagged TβRII or TβRI in pcDNA1 as templates for the PCR site-directed mutagenesis. For some control experiments, we have also employed expression vectors for the extracellularly myc-tagged α subunit of the human interleukin-9 receptor cloned in the pMX-IRES-GFP1.1 retroviral vector (34) (donated by S. N. Constantinescu, Ludwig Institute for Cancer Research, Brussels, Belgium) or for influenza HA protein variants Japan HA(2A520) (containing a GS to AA mutation at positions 520 and 521 in the transmembrane region; see Refs. 35, 36) in pCB6 (a gift from M. G. Roth, University of Texas Southwestern Medical School, Dallas, TX) and X:31 BHA-PI, comprised of the ectodomain of HA from the X:31 strain fused to the glycosylphosphatidylinositol anchor addition signal of decay accelerating factor, in the pEE14 vector; the latter (donated by J. M. White) is the BHA-PI (K/S) chimera described earlier (37).
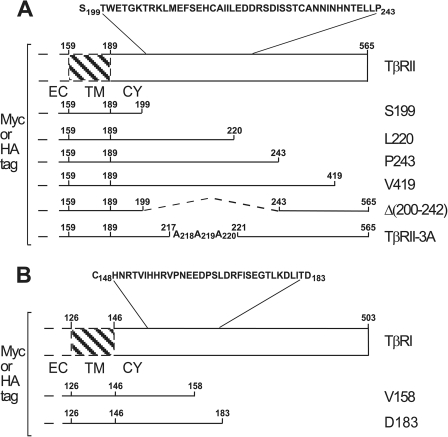
FIGURE 1.
Schematic representation of TβRII, TβRI, and their mutants. Myc- or HA-tagged TβRII or TβRI in pcDNA3 or pcDNA1 were prepared as described (Ref. 33 and “Experimental Procedures”). The numbers to the right of the truncation mutants indicate the last amino acid residue after which the stop codon was inserted. Dashed lines indicate deletions. The numbers after the Ala substitutions are those of the residues replaced by Ala. A, human TβRII (for sequence, see Ref. 9) consists of 565 amino acids. The EC domain ends at position 159, where the putative TM domain starts (up to residue 189). The CY domain is 375 residues long. The membrane-proximal region between amino acids 199 and 243 was deleted in the mutant TβRII Δ(200–242). B, human TβRI (ALK5; for sequence, see Ref. 8) consists of 503 amino acids. The last residue of the EC domain is at position 126, and the putative TM domain ends at residue 146. The sequence of the membrane-proximal region present in whole or in part in the TβRI truncation mutants is shown.
Immunofluorescence Co-patching—COS7 cells were plated on glass coverslips in 35-mm dishes. After 24 h they were transfected by FuGENE 6 (Roche Applied Science) with 1 μg of DNA each of expression vectors for differently tagged (HA- or myc-tagged) TβRI or TβRII (or control vectors)/dish; in cases of single transfections, the DNA was completed to 2 μg by empty vector. At 44–48 h post-transfection they were subjected to antibody-mediated immunofluorescence co-patching to measure their oligomerization directly at the cell surface (22, 38). Cells expressing pairs of receptors carrying different epitope tags (e.g. HA-TβRII together with myc-TβRII or myc-TβRI) were washed twice with serum-free medium and incubated (30 min, 37 °C) to allow digestion of serum-derived ligands. After washing twice with cold Hank's balanced salt solution (HBSS) supplemented with 20 mm Hepes (pH 7.4) and 2% BSA (HBSS/Hepes/BSA), the cells were incubated in the same buffer with normal goat IgG (200 μg/ml, 30 min, 4 °C) to block nonspecific binding. This was followed by successive incubation in the cold (to avoid internalization and enable exclusive cell surface labeling) in HBSS/Hepes/BSA with primary anti-tag IgGs (20 μg/ml each, 45 min): rabbit HA.11 (α-HA) together with 9E10 mouse α-myc followed by labeling/patching with Alexa 594-GαR and Alexa 488-GαM IgG (20 μg/ml each, 30 min, 4 °C). The cells were washed, fixed in methanol (5 min, -20 °C) and acetone (3 min, -20 °C), and mounted in Prolong Antifade (Invitrogen). In experiments where ligand (250 pm TGF-β1) was present, it was added at 4 °C 30 min before labeling with IgGs and was included at the same concentration during all subsequent incubations.
Fluorescence digital images were recorded using a CCD camera (CoolSnap HQ, Photometrics) coupled to an Axioimager D.1 fluorescence microscope (Carl Zeiss Microimaging) with a 100×/1.4 NA oil-immersion objective as described (39). The Alexa 488 (green) and Alexa 594 (red) TIFF images were exported to Image-Pro Plus (Media Cybernetics, Bethesda, MD) and subjected to quantitative analysis of the extent of co-patching using an algorithm we have recently developed (39). Briefly, the program segments the patches in a user-defined region of interest, subtracts the background, and identifies the center of mass of each object in the green and red images. This is followed by nearest-neighbor analysis, calculating the distances from each green patch to the nearest red patch (and vice versa). Patches whose nearest neighbor is within one optical resolution unit (up to 0.2 μm) are considered colocalized (39). The % co-patching of e.g. green with red patches is given by dividing the number of the green patches colocalized with red patches by the total number of green patches. 20–25 cells were analyzed in each case.
FRAP and Patch/FRAP—Cells were grown and transfected with pairs of differently tagged TGF-β receptors as for the co-patching experiments. 44–48 h post-transfection the cells were washed with cold HBSS/Hepes/BSA, blocked with normal goat IgG, and labeled successively at 4 °C (45 min incubations) with (i) monovalent Fab′ fragments against one tag (e.g. mouse α-myc Fab′;30 μg/ml) together with whole IgG against the second tag (rabbit α-HA IgG, 20 μg/ml) or (ii) Alexa 488-GαM Fab′ (30 μg/ml) together with Alexa 594-GαR IgG (20 μg/ml). This protocol results in one receptor cross-linked and immobilized by IgGs, whereas the second receptor is labeled exclusively by monovalent fluorescent Fab′. This enables FRAP measurements of the effects of cross-linking/immobilizing one receptor on the lateral diffusion of the coexpressed receptor, enabling the identification of complex formation between them and the distinction between transient and stable interactions (31, 40). To minimize internalization during the experiment, measurements were at 15 °C, replacing samples within 30 min. FRAP experiments were conducted as described earlier (41, 42) using a monitoring argon ion laser beam (1 microwatt) at 488 nm, focused to a Gaussian radius of 0.85 ± 0.02 μm (63×/1.4 NA oil immersion objective). A 5-milliwatt pulse (10–20 ms) bleached 60–75% of the fluorescence in the illuminated region, and fluorescence recovery was followed by the attenuated monitoring beam. The lateral diffusion coefficient (D) and the mobile fraction (RF) were extracted from the fluorescence recovery curves by nonlinear regression analysis, fitting to the lateral diffusion equation (43).
RESULTS
Differential Dependence of TβRII and TβRI Homo-oligomerization on the Cytoplasmic Domains Revealed by Computerized Immunofluorescence Co-patching—To investigate whether determinants in the CY domain of TβRII are involved in its homodimerization, we generated several truncation and deletion mutants of epitope-tagged TβRII (Fig. 1A) and explored the effects of these mutations on the ability to form homo-oligomers. To detect the formation of homomeric complexes between the receptor mutants in the plasma membrane of live cells, we employed a recently developed computerized digital analysis version of immunofluorescence co-patching (Ref. 39; see “Experimental Procedures”). This method is based on the expression of two receptors carrying two different epitope tags at their extracellular termini at the surface of live cells. One receptor is forced into micropatches by a double layer of IgGs using a fluorescent (e.g. green) secondary IgG. The second receptor is patched and labeled by anti-tag primary IgG from another species and secondary IgG conjugated to another fluorophore (red). Receptors residing in oligomers are swept into mutual micropatches, which appear yellow when the two images are overlapped. To measure the homo-oligomerization of TβRII, we co-expressed myc- and HA-tagged versions of each TβRII mutant in COS7 cells and subjected them to immunofluorescence co-patching studies in the absence or presence of ligand. The extent of co-patching was determined by algorithms written for Image-Pro Plus, defining green and red patches as overlapping if their intensity peaks were separated by less than 0.2 μm (39). To evaluate the level of random co-patching (uncorrelated overlap of patches) in the same image, which depends on the density of patches at the cell surface, a “randomized” region of interest was created by overlaying the green image from one region of interest on the red image of an identically sized neighboring region (see Fig. 2). Co-localization in the randomized image is due to random overlap of patches, because the patches in the two overlaid images are unrelated. The randomized values can then be subtracted to obtain the actual co-patching percentage (% co-patching), a procedure that was not possible before the development of the computerized approach (38, 44). Typical images of a co-patching experiment are shown in Fig. 2, A–C, and the total and randomized % co-patching of these images is depicted in Fig. 2D. The average data from multiple experiments after subtraction of the contribution of randomized co-patching are given in Fig. 2E. In accord with previous studies (23), HA-and myc-tagged wild-type (wt) TβRII exhibited a significant level of homomeric co-patching already in the absence of ligand (25%), with a minor increase in the presence of 250 pm TGF-β1 (to 32%). It should be noted that the level of homodimerization is higher by a factor of 3/2 than the % co-patching (discussed in Ref. 38). This occurs because of statistical considerations; for a system where two identical receptors carrying different epitope tags form dimers, dimers containing two receptors with the same tag may also form but would not be swept into mutual patches with receptors carrying the other tag. The fraction of “same tag” complexes expected for a dimer is ⅓, leaving ⅔ of the dimers containing two different tags and capable of forming two-color patches. Thus, the 25% co-patching of wt TβRII before ligand binding (Fig. 2E) represents 38% homo-dimerization.
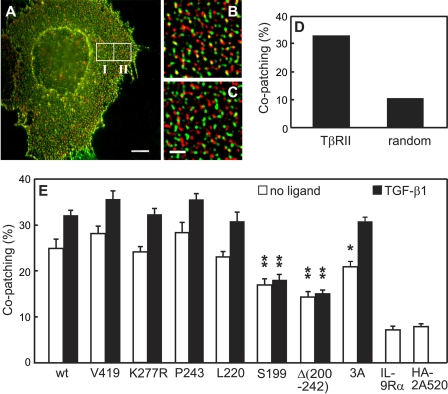
FIGURE 2.
The membrane-proximal cytoplasmic region (amino acids 200–220) is involved in TβRII homomeric complex formation. COS7 cells were co-transfected by pairs of vectors encoding HA- and myc-tagged versions of the same TβRII construct (Fig. 1A). In control experiments, myc-TβRII was replaced by myc-tagged IL-9 receptor α subunit (myc-IL-9Rα) or Japan HA-2A520. At 44–48 h post-transfection, live cells were labeled consecutively in the cold (to eliminate endocytosis) by a series of antibodies to mediate patching and fluorescent labeling (see “Experimental Procedures”). In control experiments with Japan HA-2A520, the primary α-myc IgG was replaced by Fc125 mouse α-Japan HA. In experiments with ligand (250 pm TGF-β1), it was added at 4 °C 30 min before labeling with IgGs and maintained during subsequent incubations. The patching protocol results in HA-tagged receptors labeled by Alexa 594 (red) and myc-tagged receptors by Alexa 488 (green). Bars, 10 μm(A) and 2 μm(C). A, a typical image of a cell transfected with wt myc-TβRII and wt HA-TβRII and subjected to co-patching in the absence of ligand. White borders depict regions of interest (10 × 10 μm) analyzed for co-patching and for randomized co-localization (panels B and C, respectively). B, a zoomed image of region I in panel A. Using an algorithm written for Image-Pro Plus, green and red patches were defined as overlapping if their centers were separated by less than 0.2 μm (39). C, a zoomed randomized image to evaluate random overlap. The image was generated by merging the red channel of region I with the unrelated green channel of region II. D, computer analysis results of the specific images shown in panels B and C. E, averaged data (20–35 cells in each case) quantifying homomeric complex formation between TβRII constructs. The numbers of red (R), green (G), and overlapping (yellow, Y) patches were determined by computerized analysis as described (39), and the randomized control value was subtracted. The % co-patching (% of one tagged receptor in mutual patches with receptor carrying the other tag) is given by 100 × (Y/(Y + R)) for the red-labeled receptors and by 100 × (Y/(Y + G)) for the green-labeled receptors. Because these values were very close, a single mean ± S.E. value is depicted for each receptor pair. The homomeric co-patching of wt TβRII (∼25%) was mildly but significantly increased upon the addition of TGF-β1(p < 10-4, Student's t test) and so was the case for all mutants except Ser-199 and Δ(200–242) (p > 0.05). Comparison of the % co-patching of each mutant without and with ligand to the levels measured similarly for wt TβRII shows a highly significant reduction for Ser-199 and Δ(200–242); **, p < 10-6. Mutation of the single TβRII endocytosis signal (TβRII-3A) had only a minor effect on the co-patching in the absence of ligand (*, p < 0.05) and no significant effect in the presence of ligand (p > 0.4).
Progressive truncation of the CY domain of TβRII at Val-419, Pro-243, and Leu-220 had no significant effects on its homomeric co-patching level (Fig. 2E) either in the absence or presence of ligand, suggesting that the C-terminal part of the CY domain (from residue 220 and on) is dispensable for TβRII homo-oligomerization. In addition, the kinase activity of TβRII is not required for its homo-oligomerization, as suggested by the similar co-patching levels observed for the kinase-dead K277R mutant; this notion is further supported by the lack of effect of truncations in the middle (Val-419) or start (Pro-243 and Leu-220) of the kinase domain. However, further truncation of the CY domain (at Ser-199) strongly reduced the % co-patching both without and with ligand (16 and 18%, respectively). These low levels are only slightly above the control co-patching levels measured between TβRII and unrelated membrane proteins, epitope-tagged IL-9 receptor or the HA-2A520 non-raft (35, 36) HA mutant (Fig. 2E). These findings suggest that the 200–220 segment of TβRII plays a role in the homomeric interactions. This conclusion is supported by the similar reduction in the co-patching level measured for the deletion mutant Δ(200–242), which has the entire CY domain except for the specific segment deleted. The 200–220 segment appears to be crucial also for the ligand-induced enhancement in the homomeric co-patching of TβRII, as this enhancement disappears in the mutants Ser-199 and Δ(200–242) but not in Leu-220 (Fig. 2E).
It should be noted that this segment includes the single clathrin-dependent internalization signal of TβRII, 218IIL220 (33). To eliminate the possibility that the co-patching measured is due to clustering of the receptors in coated pits, we measured the co-patching of TβRII-3A, an endocytosis-defective TβRII mutant (33). This mutant showed high co-patching levels, only slightly lower than those of wt TβRII, suggesting that colocalization in coated pits may only have a minor contribution to the measured co-patching.
Taken together, these results demonstrate that the TβRII population at the cell surface is heterogeneous, comprised of significant fractions of both homodimers (38 and 48% without and with ligand, respectively) and monomers. The membrane proximal cytoplasmic region between amino acids 200 and 220 is involved in TβRII homo-dimerization in the absence of ligand and also affects the ligand-mediated enhancement of homomeric complex formation.
To explore the potential role of the TβRI CY domain in homomeric interactions, we conducted analogous co-patching studies on wt TβRI and its truncation mutants, Asp-183 and Val-158 (Fig. 1B). In this set of experiments the effect of TGF-β1 was not measured because TβRI does not bind ligand in the absence of TβRII. As shown in Fig. 3, wt TβRI exhibited a higher level of homomeric co-patching than TβRII (38%, suggesting 38 × 3/2 = 57% homo-dimerization), in accord with a former report (22). In view of the report that TβRI is partially located in raft domains (45), which may putatively contribute to the high level of co-patching, we examined the co-patching of TβRI with a raft-resident unrelated protein (BHA-PI) and with a non-raft HA mutant (HA-2A520); both yielded low co-patching levels (Fig. 3), eliminating the above possibility. Asp-183, a TβRI truncation mutant lacking the kinase domain, exhibited co-patching levels resembling wt TβRI (Fig. 3). Further truncation of TβRI at Val-158 not only did not reduce the co-patching but actually increased it significantly to ∼50%, suggesting 75% homo-dimerization. These results indicate that unlike TβRII, the CY domain of TβRI is not required for its homo-oligomerization.
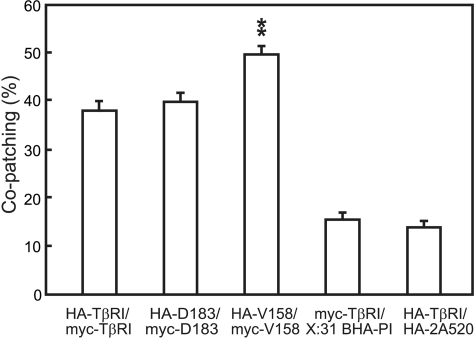
FIGURE 3.
Immunofluorescence co-patching demonstrates that TβRI homomeric complex formation does not require the cytoplasmic domain. COS7 cells were co-transfected with pairs of HA-TβRI and myc-TβRI or their truncation mutants. Control experiments employed co-transfection by a vector encoding wt HA- or myc-tagged TβRI together with either an unrelated raft-resident protein (X:31 BHA-PI) or an unrelated non-raft HA mutant (Japan HA-2A520), which were then patched using rabbit α-X:31 HA or mouse α-Japan HA as primary antibodies. Immunofluorescence co-patching experiments were performed and analyzed as detailed in Fig. 2. Bars are the mean ± S.E. of 20–40 cells in each case. The average level of co-patching in the randomized images (∼10%) was subtracted. TβRI displayed a high level of co-patching, suggesting extensive homo-oligomerization. Truncation of nearly all the CY domain (the Val-158 mutant) resulted in a significant further increase in co-patching as compared with full-length TβRI (**, p < 10-7).
The C-terminal Cytoplasmic Domains of TβRII and TβRI Are Essential for Efficient Heteromeric Complex Formation—To investigate the roles of determinants in the CY domains of TβRII and TβRI in the formation of heteromeric complexes, we employed co-patching studies between TβRI and TβRII constructs. The full-length receptors exhibited a significant level of co-patching (35%) already in the absence of ligand (Fig. 4). For hetero-oligomerization, the co-patching experiment measures complex formation between different receptors, so there is no statistical correction involved, and the % co-patching directly gives the % of receptors in hetero-oligomers (18). In accord with previous results (18), the addition of TGF-β1 markedly increased TβRI·TβRII co-patching (to 55%). To explore the dependence of the heteromeric interactions on the TβRII CY domain, we measured the ability of various TβRII mutants to interact with full-length myc-TβRI (Fig. 4A). TβRI·TβRII hetero-oligomerization was sensitive to relatively short truncations in the TβRII CY domain; truncation at Val-419 was sufficient to reduce the co-patching to 22%, very close to the reduction mediated by larger truncations (Pro-243, Leu-220, and Ser-199) (Fig. 4A). This effect is not due to the loss of TβRII kinase activity due to truncation of part of the kinase domain, because the kinase-dead K277R TβRII mutant showed a high degree of co-patching with wt TβRI. Moreover, the Δ(200–242) TβRII mutant, which lacks the 200–242 CY segment that was important for TβRII homo-oligomerization, showed a high co-patching level in the absence of ligand (Fig. 4A), suggesting that homo-oligomerization of TβRII is not a prerequisite for heteromeric complex formation with TβRI in the absence of ligand. However, the TβRII CY segment between residues 221 and 242 appears to be required for the ligand-mediated incremental increase in heterocomplex formation, which is detected for TβRII truncations up to Pro-243 but absent in the Leu-220, Ser-199, or Δ(200–242) TβRII mutants (Fig. 4A). As in the case of heteromeric co-patching in the absence of ligand, the ligand-mediated increase in TβRI·TβRII co-patching did not depend on TβRII kinase activity, as TβRII-K277R and wt TβRII exhibited similar co-patching levels with wt TβRI also in the presence of ligand (Fig. 4A).
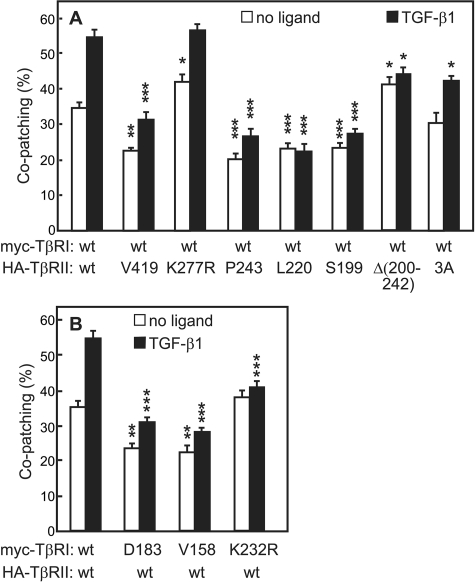
FIGURE 4.
Immunofluorescence co-patching reveals involvement of the CY domains of TβRII and TβRI in heteromeric complex formation. COS7 cells were co-transfected transiently with pairs of receptors carrying different epitope tags as indicated in panels A and B. Co-patching experiments were performed and analyzed as in Fig. 2. For experiments in the presence of ligand, TGF-β1 was added at 250 pm. Bars are the mean ± S.E. of experiments on 20–40 cells. A, heteromeric co-patching between wt myc-TβRI and HA-TβRII mutants. The co-patching measured for randomized images (∼10%) was subtracted. TGF-β1 mediated a highly significant increase (p < 10-8) in the co-patching of TβRI with full-length TβRII and either wt, kinase-dead (K277R) or endocytosis-defective (the 3A mutant), and a significant TGF-β-mediated increase (p < 10-4) was retained for TβRII truncations down to Pro-243 (the Val-419 and Pro-243 mutants). However, further truncations (at Leu-220 or Ser-199) or deletion of the 200–242 segment of TβRII resulted in a loss of the ligand-induced effect (p > 0.05). For each set of conditions (without and with ligand), the co-patching of wt myc-TβRI with each HA-TβRII mutant was compared with the basic co-patching level between the two wt receptors under similar conditions. Asterisks indicate significant differences from these basic levels (***, p < 10-13; **, p < 10-8;*, p < 10-3). B, co-patching between wt HA-TβRII and myc-TβRI mutants. Randomized co-patching (∼10%) was subtracted. TGF-β1 strongly enhanced the co-patching between the wt receptors (p < 10-7), and a lower but significant ligand effect was retained upon truncation of TβRI (p < 10-3). The heteromeric co-patching of TβRII with kinase-dead TβRI (K232R mutation) was insensitive to the ligand (p > 0.05), although its ability to hetero-oligomerize with TβRII was not compromised. The co-patching of wt HA-TβRII with each myc-TβRI mutant was compared with its co-patching level with wt myc-TβRI under similar conditions (without or with ligand); asterisks indicate significant differences from these basic levels (***, p < 10-13; **, p < 10-8).
We next studied the dependence of TβRI·TβRII hetero-oligomerization on the TβRI CY domain by measuring the co-patching of full-length HA-TβRII with the myc-TβRI truncation mutants Asp-183 and Val-158 (Fig. 4B). Both truncations resulted in decreased co-patching with TβRII (from 35 to 24% in the absence of ligand and from 55 to 33% in the presence of ligand; Fig. 4B). The TGF-β1-mediated increase in the heteromeric co-patching was retained in the truncation mutants of TβRI, although it became less pronounced (Fig. 4B). Simultaneous truncation of the CY domains of both receptors (HA-TβRII-Ser-199/myc-TβRI-Val-158) did not have additional effects on their hetero-oligomerization (data not shown). However, unlike the case of heteromeric co-patching in the absence of ligand, the ligand-mediated increase in TβRI·TβRII co-patching was dependent on TβRI kinase activity (Fig. 4B). These results indicate that determinants in the CY domain of TβRI contribute to its hetero-oligomerization with TβRII, unlike the situation for TβRI homodimerization.
Patch/FRAP Studies Demonstrate Stable Interactions between TβRII and TβRI Homomeric Complexes—The immunofluorescence co-patching studies can only reveal relatively stable interactions between receptors, whereas labile complexes may dissociate during the patching step (31, 46). The mode of interactions (stable association versus transient interactions on the FRAP timescale) can be determined by patch/FRAP (31, 47). In these studies one receptor is patched/immobilized by cross-linking with a double layer of IgGs, and the effect on the lateral diffusion of the coexpressed receptor (carrying a different extracellular epitope tag and labeled exclusively by monovalent Fab′ fragments) is measured by FRAP (see “Experimental Procedures”). Complex formation between the receptors may lead to either a reduction in RF or in D, depending on the FRAP timescale relative to the dissociation/association rates of the complex. Complex lifetimes longer than the characteristic FRAP times led to a reduction in RF, as bleached molecules would not undergo appreciable dissociation from the immobile clusters during the FRAP measurement. On the other hand, short complex lifetimes result in several association/dissociation cycles for each fluorescent molecule during the recovery phase, leading to a reduction in D (31, 47).
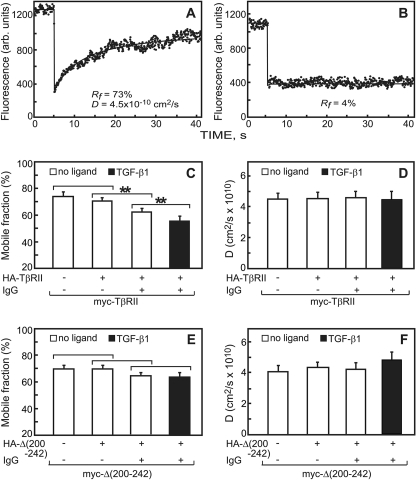
We first employed patch/FRAP to investigate the interaction mode between TβRII subunits in homomeric complexes (Fig. 5). Typical curves of the lateral diffusion of myc-TβRII in the absence of IgG and its immobilization due to IgG-mediated patching are shown in Fig. 5, A and B. The average results of many such experiments are summarized in Fig. 5, C and D. Immobilization of HA-TβRII mediated a significant reduction in RF of the coexpressed myc-TβRII compared with cells coexpressing these receptors without IgG cross-linking (Fig. 5C, second bar from the left), without affecting the D value (Fig. 5D). Reduction in RF with no change in D is typical for stable interactions between receptors on the FRAP time scale.
FIGURE 5.
Patch/FRAP studies demonstrate stable TβRII homomeric complexes which are lost in the Δ(200–242) mutant. COS7 cells were co-transfected with pairs of expression vectors encoding myc- and HA-tagged TβRII or Δ(200–242). In control experiments with singly expressed myc-tagged receptors, the HA-tagged construct was replaced by empty vector (left-most bars). 44–48 h after transfection live cells were subjected to the patching/labeling protocol described under “Experimental Procedures,” resulting in HA-tagged receptors patched and labeled by Cy3-GαR IgG, whereas the myc-tagged counterparts are labeled exclusively by Alexa 488-coupled non-cross-linking Fab′. FRAP experiments were conducted at 15 °C to avoid internalization. Where indicated, TGF-β1 (250 pm) was added at 4 °C 30 min before incubation with the antibodies and maintained at all the following stages. Controls experiments (no cross-linking) were conducted similarly, except that exclusive Fab′ labeling was employed. A, a representative FRAP curve of the lateral diffusion of myc-TβRII in a cell co-expressing HA-TβRI (no IgG cross-linking; control). B, a representative FRAP curve showing immobilization of IgG-cross-linked HA-TβRII. Only the RF value is shown, as the recovery was too low to allow determination of D. C and E, average RF values derived from multiple experiments. D and F, average D values derived from the patch/FRAP measurements. Bars are the mean ± S.E. of 30–40 measurements in each case. Asterisks indicate significant differences between the RF values of the pairs indicated by brackets (**, p < 10-3; Student's t test). No significant differences were found in the D values as a result of IgG-mediated cross-linking, comparing each bar to the co-expressed uncross-linked control (p > 0.4).
To examine whether ligand binding affects the mode of TβRII homomeric interactions, we performed analogous experiments in the presence of TGF-β1. The addition of the ligand mediated a further decrease in RF of the coexpressed Fab′-labeled receptors but had no effect on D (Fig. 5, C and D). We conclude that TGF-β1 binding does not alter the mode of the interactions but further shifts the balance in favor of the homomeric complexes. Similar observations were made for homomeric interactions of TβRII-Leu-220 (data not shown), the shortest TβRII truncation mutant that exhibited efficient homo-oligomerization in co-patching studies (Fig. 2).
The patch/FRAP studies on homomeric complex formation of the TβRII deletion mutant Δ(200–242) presented a different picture than that observed for wt TβRII. This mutant is of interest because in the co-patching studies it was defective in both basal and ligand-mediated homo-oligomerization. Immobilization of HA-Δ(200–242) had no significant effect on either D or RF of myc-Δ(200–242) as compared with the values obtained for coexpression without IgG cross-linking (Fig. 5, E and F). The results of all these patch/FRAP experiments are in good correlation with the data of the co-patching studies, including the detection of ligand-mediated increase in homomeric complex formation and the loss of such interactions upon deletion of the 201–241 segment of TβRII.
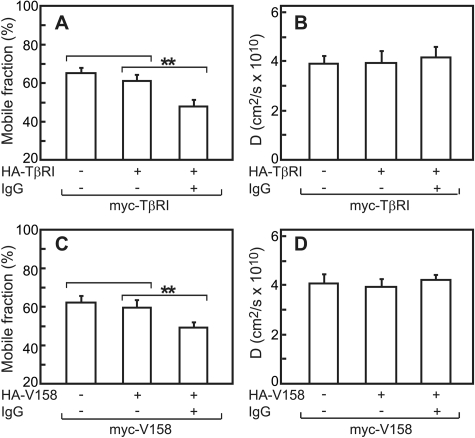
We next employed patch/FRAP to explore the mode of homomeric interactions among TβRI subunits. IgG-mediated immobilization of HA-TβRI mediated a significant reduction in RF of coexpressed myc-TβRI, as compared with the values without cross-linking (Fig. 6A). At the same time, D was unaffected (Fig. 6B). Analogous results were obtained for the Val-158 truncation mutant of TβRI (Fig. 6, C and D). These experiments were carried out without ligand, as TβRI does not bind TGF-β in the absence of TβRII. The above findings suggest that both TβRI and TβRII display stable homomeric interactions on the FRAP time scale (around 1 min). Furthermore, there is an excellent correlation between the patch/FRAP results and the co-patching measurements.
FIGURE 6.
Patch/FRAP studies demonstrate stable homomeric TβRI and TβRI-Val-158 complexes. COS7 cells were co-transfected with pairs of differently tagged (myc and HA) TβRI or TβRI-Val-158 (see “Experimental Procedures”). Patching/labeling and patch/FRAP experiments were performed without ligand, as TβRI does not bind ligand on its own. A and C, average RF values. B and D, average D values. Bars are the mean ± S.E. of 30–40 measurements in each case. Asterisks indicate differences between the RF values of the pairs indicated by brackets (**, p < 10-5; Student's t test). No significant differences were observed between the D values relative to the control lacking IgG cross-linking (p > 0.5).
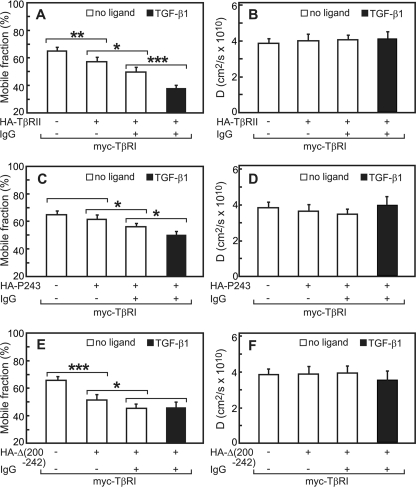
Patch/FRAP Studies Demonstrate Stable Interactions in TβRII/TβRI Heteromeric Complexes—To explore the dynamics of the heteromeric interactions between TβRI and TβRII, we conducted patch/FRAP studies on differently tagged TβRI·TβRII pairs without and with ligand. Coexpression of full-length myc-TβRI with HA-TβRII (no IgG cross-linking) already led to a mild but significant reduction in RF of myc-TβRI, suggesting that TβRI·TβRII complexes, which form to some degree in the absence of ligand (see Fig. 4), display a subpopulation that is laterally immobile. This indicates that heterocomplex formation results in the recruitment of a fraction of the TβRI·TβRII complexes into immobile structures at the cell surface. IgG cross-linking of HA-TβRII resulted in a further significant reduction in RF of myc-TβRI. In both cases, the D value was not significantly altered (Fig. 7B). Importantly, the effect of immobilizing HA-TβRII by IgG cross-linking on RF of myc-TβRI was strongly augmented by the addition of TGF-β1; the reduction in RF was highly significant not only relative to the RF level in uncross-linked coexpressing cells but also relative to the IgG-cross-linked cells in the absence of ligand. Again, D was not altered significantly. These results suggest stable interactions among the receptors in the heteromeric complexes, which are enhanced after TGF-β1 binding. They are in good correlation with the co-patching experiments (Fig. 4), which show heteromeric complex formation in the absence of ligand as well as a significant ligand-mediated increase in the co-patching level.
FIGURE 7.
Patch/FRAP studies demonstrate stable heteromeric interactions between TβRI and TβRII and distinguish between mutants based on the modulation of their interactions by TGF-β1. COS7 cells were co-transfected by myc-TβRI and an HA-tagged TβRII construct. Patching/labeling and patch/FRAP experiments were performed as in Fig. 5. Similar results (not shown) were obtained when the IgG/Fab′ labeling was swapped (IgG patching of myc and Fab′ labeling of the HA-tagged receptors). A, C, and E, average RF values. B, D, and F, average D values. Bars are mean ± S.E. of 30–40 measurements in each case. Asterisks indicate significant differences between the RF values of the pairs indicated by brackets (*, p < 0.05; **, p < 10-3; ***, p < 10-5).
We next investigated the heteromeric interactions of HA-TβRII-Pro-243 with myc-TβRI. The Pro-243 mutant was chosen because in the co-patching experiments it showed high homo-oligomerization but was defective in basal hetero-oligomerization with TβRI (Figs. 2 and 4). In accord with the reduced basal (ligand-independent) heteromeric interactions of this mutant (Fig. 4A), its expression with myc-TβRI in the absence of IgG cross-linking had no significant effect on the RF value of myc-TβRI (Fig. 7C). IgG cross-linking of this mutant induced a minor but significant reduction in RF of myc-TβRI, and TGF-β1 induced an additional significant reduction in RF (Fig. 7C); under all conditions there were no significant changes in D (Fig. 7D).
The interactions of the TβRII mutant Δ(200–242) with TβRI are also of interest, as the co-patching experiments (Fig. 4A) suggested that its basal interactions with TβRI are normal, but they become non-responsive to ligand binding. Coexpression with HA-TβRII-Δ(200–242) was sufficient to reduce significantly RF of myc-TβRI even without IgG cross-linking, in accord with the persistence of the basal heteromeric interactions. The reduction was even stronger than in the case of full-length HA-TβRII, in accord with the higher level of co-patching between Δ(200–242) and myc-TβRI (Fig. 4A). IgG-mediated immobilization of Δ(200–242) further reduced RF of myc-TβRI (Fig. 7E). Importantly, the addition of TGF-β1 did not cause further changes in RF of myc-TβRI (compare bars 3 and 4 in Fig. 7E), in line with the loss of the ligand-mediated increase in heterocomplex formation as detected by co-patching (Fig. 4A). In all cases there were no significant effects on the D values (Fig. 7F). The high level of immobilization (low RF) of TβRI in the presence of the Δ(200–242) TβRII mutant and the lack of effect of TGF-β1 on the interactions (Fig. 7E) are in excellent agreement with the co-patching results between this pair of receptors in the absence and presence of ligand (Fig. 4A).
DISCUSSION
In the present study we have employed biophysical methods to analyze the oligomerization of TGF-β receptors on the surface of live, intact cells. Our major findings are (i) in both resting and ligand-stimulated cells, the population of TβRII and TβRI at the cell surface is heterogeneous, comprised of monomers, homo- and heterodimers, (ii) once established, the homo- and heterodimers are stable at least for several minutes, (iii) the juxtamembrane region (residues 200–220) of TβRII is a determinant of both its basal and ligand-induced homodimerization, (iv) different regions of the TβRII cytoplasmic domain, namely the regions that precede and follow the kinase domain, contribute to its heterodimerization with TβRI, and (v) even though the EC and TM domains are sufficient for maximal homodimerization of TβRI, its CY domain contributes to its heterodimerization capacity. Specifically, the kinase activity of TβRI is needed for the TGF-β1-mediated increase in heterocomplex formation.
These findings expand those of recent studies which employed different experimental techniques. Thus, a recent study on the crystal structure of a ternary heterocomplex comprised of the EC domains of TβRI, TβRII, and the TGF-β3 ligand (20) reported the complex to be a hetero-tetramer of the receptors in association with the dimeric ligand. This study showed that the EC domains are sufficient for TβRI·TβRII heterocomplex formation in the presence of ligand. Moreover, the ability to isolate and crystallize the ligand-bound complex attests to the stability of the complexes formed, in accord with our findings in live cells (Fig. 7). However, the crystallographic analysis does not address the nature and extent of homodimers of TβRI and TβRII in live cells as well as the potential contribution of the CY and TM domains (absent in the crystal) to the formation of TβRI and TβRII homo-and hetero-oligomers even before ligand binding. In the current manuscript we addressed the issues of both the extent and stability of the various complexes formed by the TGF-β receptors in the presence and absence of TGF-β1 using biophysical studies that provide quantitative information on these parameters for the receptors in their native environment.
Former studies by us and by others have detected the existence of both homomeric and heteromeric complexes of the TGF-β receptors already before ligand binding (18, 22, 23, 27, 48). Such complexes are likely to be induced by the CY and/or TM domains, as the soluble EC domains did not form heteromeric complexes in the absence of ligand (19). To quantify homo- and hetero-complex formation among TGF-β receptors in live cells, we conducted immunofluorescence co-patching experiments (Figs. 2, 3, 4) and patch/FRAP studies (Figs. 5, 6, 7) with co-expressed (differently tagged) pairs of TβRI and TβRII. These studies demonstrated the presence of a mixed oligomeric population of TβRI and TβRII at the cell surface both in the absence or presence of ligand. Thus, both homomeric and dimeric populations of each receptor type were observed, whether homomeric (e.g. expression of HA-TβRI/myc-TβRI) or heteromeric (myc-TβRI/HA-TβRII) (Figs. 2, 3, 4). Importantly, the patch/FRAP studies demonstrated that all these complexes are stable at least for several minutes (Fig. 5, 6, 7), indicating that there is no significant exchange between the monomeric and dimeric receptor subpopulations on this time scale. Interestingly, the addition of ligand (TGF-β1) did not alter the mode of interactions but was able to increase the extent of oligomerization of the receptor complexes capable of binding ligand (TβRII homodimerization, Figs. 2 and 5; TβRI·TβRII hetero-oligomerization, Figs. 4 and 7). The detection of homomeric TβRI and TβRII complexes is in accord with reports on the importance of the homomeric interactions in TGF-β signaling (12, 15, 24). It should be noted that although these experiments employed cells overexpressing the transfected receptors, there several indications that the oligomerization patterns observed hold also for low expression levels and for the endogenous receptors. First, in all cases where oligomerization of endogenous receptors can be studied (i.e. homomeric complex formation of wt TβRII or wt TβRI and hetero-oligomerization of wt TβRI with wt TβRII; the receptor mutants employed to dissect the interaction domains are not encountered endogenously), we have validated the oligomerization results by ultracentrifugation-based size determination of the endogenous receptor complexes from untransfected cells (18, 22). These studies demonstrated major fractions of homodimers for wt TβRI and TβRII already in the absence of ligand and a very high level of a complex size consistent with ligand-bound TβRI·TβRII hetero-tetramer in the presence of TGF-β1 (18, 22). Second, the co-patching and patch/FRAP experiments enable the selection of single cells under the microscope according to their expression level. The high sensitivity of our system (Zeiss AxioImager D.1 microscope with 100×/1.4 NA objective for the co-patching studies or 63×/1.4 NA objective for FRAP together with a specially selected high sensitivity photomultiplier tube) enabled us to analyze cells expressing as low as 4000–6000 surface receptors (evaluated by photomultiplier-based measurement of the fluorescence intensity on cells expressing known levels of epitope-tagged receptors labeled extracellular by fluorescent antibodies, as described by us earlier; Refs. 18 and 23). Cells with these expression levels, which are somewhat higher but still in the same order of magnitude as endogenous receptors, yielded co-patching and patch/FRAP results similar to those obtained on cells with 10-fold higher expression levels.
To explore the contribution of different regions in the CY domains of TβRII and TβRI to homomeric and heteromeric complex formation, we coexpressed differently tagged pairs of a variety of TβRI and TβRII mutants and subjected the cells to co-patching and patch/FRAP studies. The results of the co-patching (Fig. 2E) and patch/FRAP (Fig. 5, C and E) experiments identify a short juxtamembrane segment in the CY domain of TβRII (residues 200–220) as critical for efficient and stable TβRII homodimerization. On the other hand, truncation of nearly the entire CY domain of TβRI did not reduce its homodimerization and even slightly augmented it (Figs. 3 and 6C). Interestingly, the 200–220 segment of TβRII, which encompasses Ser-213, is a major site for intramolecular TβRII autophosphorylation (15). Furthermore, phosphorylation of Ser-213 was shown to be required for TGF-β signaling (15). This raised the possibility that in the absence of Ser-213 phosphorylation, TβRII homo-oligomerization may be impaired. However, two lines of evidence show that this is not the case; as shown in Fig. 2E, kinase-dead TβRII-K277R exhibited the same co-patching level as wt TβRII both in the absence or presence of TGF-β1, and this was also the case for several TβRII truncation mutants (Val-419, Pro-243, and Leu-220) that lack part or all of the kinase domain. Together with the studies on the role of both intra- and intermolecular autophosphorylation of TβRII in signaling (15), these results suggest that TβRII homo-oligomerization and signaling are independently regulated.
Analogous studies on TβRI·TβRII heteromeric complex formation revealed the involvement of different determinants in the hetero-oligomerization. Here, a different CY region of TβRII (starting at Val-419) was central for heterocomplex formation already in the absence of ligand (Fig. 4A). Notably, the TGF-β1-mediated increase in TβRI·TβRII oligomerization depended on the TβRII segment between amino acids 221–242 (Fig. 4A), close to but not overlapping with the segment required for homomeric interactions of TβRII. A different pattern emerges regarding the role of the TβRI CY domain in heterocomplex formation; although truncations of TβRI down to Asp-183 or Val-158 did not reduce its homodimerization, they strongly reduced its ability to form heterocomplexes with TβRII already in the absence of ligand (Fig. 4B). Interestingly, these truncations also reduced markedly (although not fully) the ligand-mediated increase in heterocomplex formation (Fig. 4B), in accord with the proposed role of the cytoplasmic domain of TβRI in heterocomplex formation (Fig. 4B). Because there are no reports on the potential phosphorylation of either TβRII or TβRI itself by TβRI kinase activity, it is possible that the dependence of the modulatory effect of TGF-β1 on TβRI·TβRII heterocomplex formation involves TβRI interaction with and phosphorylation of additional protein targets.
Some of the receptor mutants employed in the current studies contain relatively large truncations or deletions; it is, therefore, possible that part of the reduced oligomerization in such mutants is due to effects on receptor stability and surface expression levels. Several lines of evidence suggest that this is not the case. First, all the receptor mutants employed in the current studies arrive at the plasma membrane and are labeled by antibodies to their extracellular epitope tags, showing that they are not grossly misfolded and are not retained in the endoplasmic reticulum. Second, all the receptors exhibited rather uniform surface distribution before IgG-mediated patching and were laterally mobile to similar extents (Figs. 5, 6, 7), suggesting that they are not appreciably misfolded or aggregated at the plasma membrane. Third, the fluorescence intensity values measured at the plasma membrane by the FRAP confocal setup under nonbleaching conditions provide a quantitative comparison between the surface expression levels of the receptors (33, 47), providing that they are labeled by similar antibodies (anti-myc and Alexa 488-GαM Fab′ to label the myc-tagged versions of the receptor mutants in the current studies). The values obtained were all in the same range, suggesting no gross differences in surface expression levels. Moreover, many of the mutations did not markedly reduce complex formation among the receptors, suggesting that the basic features required for their oligomerization were retained. Thus, all the TβRI truncation mutants exhibited high homodimerization, and their hetero-oligomerization with TβRII increased in response to ligand (Figs. 3, 4 and 6C); similarly, truncations of TβRII up to Leu-220 did not interfere with its homo-oligomerization without or with ligand (Fig. 2), and deletion of the 200–242 segment of TβRII did not reduce its ligand-independent dimerization (Figs. 4A and 7E).
Our demonstration that the TGF-β receptor population at the cell surface is heterogeneous and contains monomers, homodimers, and heterocomplexes both in the absence or presence of ligand has important implications for the diversity of TGF-β signaling. At the level of homomeric receptor complexes, a monomeric TβRII would not be susceptible to either stimulatory (Ser-409) or inhibitory (Ser-416) regulation by interchain autophosphorylation (15). In view of the evidence that homomeric TβRII and TβRI complexes are involved in the activation of specific TGF-β signaling pathways (12, 15, 24), the monomeric receptor subpopulation could serve either to activate different pathways or to attenuate the canonical pathways that require homodimers. At the level of heteromeric complexes, the presence of a subpopulation of such complexes before ligand binding (preformed complexes) may further regulate TGF-β signaling. One such example is provided by TGF-β2, which unlike TGF-β1 does not bind effectively to either TβRII or TβRI and requires their co-expression for binding and signaling, suggesting that it binds preferentially to preformed complexes (30). This notion gains further support from the related bone morphogenetic protein receptor system, where preformed complexes between the type I and type II receptors were shown to activate distinct signaling pathways (38, 49).
Acknowledgments
We thank S. N. Constantinescu, M. G. Roth, and J. M. White for plasmid constructs and T. J. Braciale and J. M. White for antibodies.
This work was supported in part by Israel Science Foundation Grant 185/05 and by The Israel Cancer Research Fund and The Israel Cancer Association (to Y. I. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TGF-β, transforming growth factor-β;TβRI, TβRII, types I and II TGF-β receptors; BSA, bovine serum albumin; CY, cytoplasmic; D, lateral diffusion coefficient; EC, extracellular; GαM, goat anti-mouse IgG; GαR, goat anti-rabbit IgG; HA, influenza hemagglutinin; HBSS, Hank's balanced salt solution; RF, mobile fraction; TM, transmembrane; wt, wild-type; FRAP, fluorescence recovery after photobleaching.
References
- 1.Roberts, A. B., and Sporn, M. B. (1990) in Peptide Growth Factors and Their Receptors (Sporn, M. B., and Roberts, A. B., eds) pp. 419-472, Springer-Verlag, Heidelberg, Germany
- 2.Alexandrow, M. G., and Moses, H. L. (1995) Cancer Res. 55 1452-1457 [PubMed] [Google Scholar]
- 3.Attisano, L., and Wrana, J. L. (2002) Science 296 1646-1647 [DOI] [PubMed] [Google Scholar]
- 4.Shi, Y., and Massague, J. (2003) Cell 113 685-700 [DOI] [PubMed] [Google Scholar]
- 5.Markowitz, S., Wang, J., Myeroff, L., Parsons, R., Sun, L., Lutterbaugh, J., Fan, R. S., Zborowska, E., Kinzler, K. W., Vogelstein, B., Brattain, M., and Willson, J. K. V. (1995) Science 268 1336-1338 [DOI] [PubMed] [Google Scholar]
- 6.Hu, P. P., Datto, M. B., and Wang, X. F. (1998) Endocr. Rev. 19 349-363 [DOI] [PubMed] [Google Scholar]
- 7.Heldin, C. H., Miyazono, K., and ten Dijke, P. (1997) Nature 390 465-471 [DOI] [PubMed] [Google Scholar]
- 8.Franzen, P., ten Dijke, P., Ichijo, H., Yamashita, H., Schulz, P., Heldin, C. H., and Miyazono, K. (1993) Cell 75 681-692 [DOI] [PubMed] [Google Scholar]
- 9.Lin, H. Y., Wang, X.-F., Ng-Eaton, E., Weinberg, R. A., and Lodish, H. F. (1992) Cell 68 775-785 [DOI] [PubMed] [Google Scholar]
- 10.Inagaki, M., Moustakas, A., Lin, H. Y., Lodish, H. F., and Carr, B. I. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 5359-5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laiho, M., Weis, F. M., Boyd, F. T., Ignotz, R. A., and Massague, J. (1991) J. Biol. Chem. 266 9108-9112 [PubMed] [Google Scholar]
- 12.Luo, K., and Lodish, H. F. (1996) EMBO J. 15 4485-4496 [PMC free article] [PubMed] [Google Scholar]
- 13.Wrana, J. L., Attisano, L., Carcamo, J., Zentella, A., Doodey, J., Laiho, M., Wang, X.-F., and Massague, J. (1992) Cell 71 1003-1014 [DOI] [PubMed] [Google Scholar]
- 14.Lin, H. Y., Moustakas, A., Knaus, P., Wells, R. G., Henis, Y. I., and Lodish, H. F. (1995) J. Biol. Chem. 270 2747-2754 [DOI] [PubMed] [Google Scholar]
- 15.Luo, K., and Lodish, H. F. (1997) EMBO J. 16 1970-1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moustakas, A., Lin, H. Y., Henis, Y. I., Plamondon, J., O'Connor McCourt, M. D., and Lodish, H. F. (1993) J. Biol. Chem. 268 22215-22218 [PubMed] [Google Scholar]
- 17.Massague, J. (1998) Annu. Rev. Biochem. 67 753-791 [DOI] [PubMed] [Google Scholar]
- 18.Wells, R. G., Gilboa, L., Sun, Y., Liu, X., Henis, Y. I., and Lodish, H. F. (1999) J. Biol. Chem. 274 5716-5722 [DOI] [PubMed] [Google Scholar]
- 19.Zuniga, J. E., Groppe, J. C., Cui, Y., Hinck, C. S., Contreras-Shannon, V., Pakhomova, O. N., Yang, J., Tang, Y., Mendoza, V., Lopez-Casillas, F., Sun, L., and Hinck, A. P. (2005) J. Mol. Biol. 354 1052-1068 [DOI] [PubMed] [Google Scholar]
- 20.Groppe, J., Hinck, C. S., Samavarchi-Tehrani, P., Zubieta, C., Schuermann, J. P., Taylor, A. B., Schwarz, P. M., Wrana, J. L., and Hinck, A. P. (2008) Mol. Cell 29 157-168 [DOI] [PubMed] [Google Scholar]
- 21.Yamashita, H., ten Dijke, P., Franzen, P., Miyazono, K., and Heldin, C. H. (1994) J. Biol. Chem. 269 20172-20178 [PubMed] [Google Scholar]
- 22.Gilboa, L., Wells, R. G., Lodish, H. F., and Henis, Y. I. (1998) J. Cell Biol. 140 767-777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henis, Y. I., Moustakas, A., Lin, H. Y., and Lodish, H. F. (1994) J. Cell Biol. 126 139-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weis-Garcia, F., and Massague, J. (1996) EMBO J. 15 276-289 [PMC free article] [PubMed] [Google Scholar]
- 25.Stockwell, B. R., and Schreiber, S. L. (1998) Curr. Biol. 8 761-770 [DOI] [PubMed] [Google Scholar]
- 26.Letourneur, O., Goetschy, J. F., Horisberger, M., and Grutter, M. G. (1996) Biochem. Biophys. Res. Commun. 224 709-716 [DOI] [PubMed] [Google Scholar]
- 27.Zhu, H. J., and Sizeland, A. M. (1999) J. Biol. Chem. 274 29220-29227 [DOI] [PubMed] [Google Scholar]
- 28.Huse, M., Chen, Y. G., Massague, J., and Kuriyan, J. (1999) Cell 96 425-436 [DOI] [PubMed] [Google Scholar]
- 29.Wrana, J. L., Attisano, L., Wieser, R., Ventura, F., and Massague, J. (1994) Nature 370 341-347 [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez, C., Chen, F., Weinberg, R. A., and Lodish, H. F. (1995) J. Biol. Chem. 270 15919-15922 [DOI] [PubMed] [Google Scholar]
- 31.Shvartsman, D. E., Kotler, M., Tall, R. D., Roth, M. G., and Henis, Y. I. (2003) J. Cell Biol. 163 879-888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, X., Sun, Y., Ehrlich, M., Lu, T., Kloog, Y., Weinberg, R. A., Lodish, H. F., and Henis, Y. I. (2000) Oncogene 19 5926-5935 [DOI] [PubMed] [Google Scholar]
- 33.Ehrlich, M., Shmuely, A., and Henis, Y. I. (2001) J. Cell Sci. 114 1777-1786 [DOI] [PubMed] [Google Scholar]
- 34.Liu, X., Constantinescu, S. N., Sun, Y., Bogan, J. S., Hirsch, D., Weinberg, R. A., and Lodish, H. F. (2000) Anal. Biochem. 280 20-28 [DOI] [PubMed] [Google Scholar]
- 35.Scheiffele, P., Roth, M. G., and Simons, K. (1997) EMBO J. 16 5501-5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin, S., Naim, H. Y., Rodriguez, A. C., and Roth, M. G. (1998) J. Cell Biol. 142 51-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemble, G. W., Danieli, T., and White, J. M. (1994) Cell 76 383-391 [DOI] [PubMed] [Google Scholar]
- 38.Gilboa, L., Nohe, A., Geissendorfer, T., Sebald, W., Henis, Y. I., and Knaus, P. (2000) Mol. Biol. Cell 11 1023-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lachmanovich, E., Shvartsman, D. E., Malka, Y., Botvin, C., Henis, Y. I., and Weiss, A. M. (2003) J. Microsc. 212 122-131 [DOI] [PubMed] [Google Scholar]
- 40.Henis, Y. I., Katzir, Z., Shia, M. A., and Lodish, H. F. (1990) J. Cell Biol. 111 1409-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Axelrod, D., Koppel, D. E., Schlessinger, J., Elson, E. L., and Webb, W. W. (1976) Biophys. J. 16 1055-1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koppel, D. E., Axelrod, D., Schlessinger, J., Elson, E. L., and Webb, W. W. (1976) Biophys. J. 16 1315-1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen, N. O., Felder, S., and Elson, E. L. (1986) in Handbook of Experimental Immunology (Weir, D. M., Herzenberg, L. A., Blackwell, C. C., and Herzenberg, L. A., eds) pp. 24.21-24.23, Blackwell Scientific Publications, Edinburgh, UK
- 44.Constantinescu, S. N., Keren, T., Socolovsky, M., Nam, H. S., Henis, Y. I., and Lodish, H. F. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 4379-4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Razani, B., Zhang, X. L., Bitzer, M., von Gersdorff, G., Bottinger, E. P., and Lisanti, M. P. (2001) J. Biol. Chem. 276 6727-6738 [DOI] [PubMed] [Google Scholar]
- 46.Keren, T., Roth, M. G., and Henis, Y. I. (2001) J. Biol. Chem. 276 26356-26363 [DOI] [PubMed] [Google Scholar]
- 47.Eisenberg, S., Shvartsman, D. E., Ehrlich, M., and Henis, Y. I. (2006) Mol. Cell. Biol. 26 7190-7200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen, R. H., Moses, H. L., Maruoka, E. M., Derynck, R., and Kawabata, M. (1995) J. Biol. Chem. 270 12235-12241 [DOI] [PubMed] [Google Scholar]
- 49.Nohe, A., Hassel, S., Ehrlich, M., Neubauer, F., Sebald, W., Henis, Y. I., and Knaus, P. (2002) J. Biol. Chem. 277 5330-5338 [DOI] [PubMed] [Google Scholar]