Preface
Acute pain and emotion are processed in two forebrain networks and cingulate cortex is in both. Although Brodmann’s cingulate gyrus had two divisions and was not based on any functional criteria, functional imaging reports the location of activity by this model. Recent cingulate cytoarchitectural studies support a four-region model with subregions based on connections and qualitatively unique functions. Although pain and emotion activity have been widely reported, some view these as emergent products of the brain rather than small aggregates of neurons. Here we assess pain and emotion in each cingulate subregion and assess whether pain is co-localized with negative affect. Amazingly, these activation patterns do not simply overlap.
Keywords: Nociception, affect, limbic cortex, neurocytology, midline thalamus, visceral pain, anterior cingulate cortex, midcingulate cortex
Pain is evoked during noxious body stimulation or through negative emotional events and memories. To understand pain we need to consider how and where in the brain it hurts. In previous decades there has been an emphasis on pain “sensation”, which involves assessing the location and intensity of noxious stimuli. Somatosensory localization and intensity coding, however, are not necessarily linked with emotional responses, if they are processed in different parts of the brain. Moreover, linkage of pain and its affective (autonomic) substrates in the brain was not a viable research target until the conscious reports of human subjects during noxious stimulation could be related to changes in the brain with functional imaging. Imaging psychophysics allows one to correlate brain changes with sensory stimulation parameters. In terms of pain, this meant that modulating the level of unpleasantness might provide insight into the substrate of affect. Just as important and in parallel over the past decade, there has been a significant series of studies on emotional modulation of brain circuits that are assessed with scripts, faces, or films with emotional or non-emotional content. This provides control conditions and subject reports that were not previously possible in experimental animals and methods of relating emotion to specific brain circuits. The value of human functional imaging is apparent in studies of the amygdala during fear conditioning. An integrated study of the nociceptive connections, emotional activation and behavioural conditioning has provided important insights into the sensory inputs to the amygdala and its projections to parts of what are generally termed the emotional motor systems and this has been pivotal to driving new research paradigms.1,2 In spite of the wealth of information about the amygdalar substrates of emotion, this mechanistic approach must be broadened to the great expanse of the limbic cortex that is presumed to subserve many painful and emotional functions and diseases.
One of two views guide investigators in their analysis of the cerebral mechanisms of pain and emotion. The global model posits that most of the cerebral cortex is involved in some way in emotion and that perceptions that are associated with emotional experiences are an emergent property of the brain. Although many parts of the brain contribute to emotion; each area does not necessarily make an equal contribution. The alternative view is that some areas store memories with positive or negative valences and drive associated autonomic outputs, while other areas provide sensory and short-term memory substrates not specific to emotion and they cannot access autonomic outputs. Systems involved in all short-term memory including emotional memories, for example, are not emotion-specific processors. Although the circuits that engage the cingulate cortex in pain processing have long been known, we are only now in a position to link specific aspects of pain perception with its localized emotional substrates that was not possible without human functional imaging.
Two-domain pain model and the cingulate gyrus
Traditionally, pain processing is viewed according to two cognitive domains.3 The sensory-discriminative domain involves stimulus localization and intensity and is assessed in a number of ways including the visual analogue scale, while the affective-motivational domain involves the affective component of pain and is measured with ratings of unpleasantness. Before human functional imaging came into general use, the emphasis had been on sensory-discriminative processing in the somatosensory system that includes the primary and secondary somatosensory cortices and posterior parietal cortex. With the introduction of positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), it has become clear that other telencephalic regions are also engaged during acute noxious stimulation. These include motor cortex and premotor areas (supplementary and premotor cortices), cerebellar cortex, and the striatum that are active during acute noxious stimulation4,5 but do not easily fit into the two domains of pain processing based on sensation.
Most important in the present context is the fact that several limbic structures are activated during noxious stimulation of the body and these medially located structures are together referred to as the medial pain system. These include the midline and intralaminar thalamic nuclei (MITN) which project to limbic cortex, the periaqueductal gray, the amygdala, and the anterior cingulate cortex (ACC).6 In addition, it is possible that the anterior insula lies between the two pain systems and is involved in aspects of processing associated with each including sensory coding, body state assessment, and autonomic regulation. Although the two-domain model might have general utility, noxious stimuli can activate 8–10 areas in the brain, which indicates there might be more than two domains of pain processing. Indeed, studies of cingulate cortex suggest this region might be involved in three aspects of pain processing which does not fit the two-domain model.
Human functional imaging studies indicate that the ACC might mediate affective responses to noxious stimuli. The extensive studies of Paul MacLean7 and others support the general notion that the cingulate cortex is a pivotal region for emotion. Interestingly, there are several caveats to the simple proposition that pain and emotion are linked in the cingulate gyrus. Most studies of cerebral activation during acute noxious stimulation do not consider the fact that the ACC is involved in other functions that are unrelated to pain and include coding for the reward properties of particular behaviours8,9 and activation during romantic love.10 The “pain-centered” view seeks to identify pain-specific processing functions and raises many interesting paradoxes about the organization and functions of the cingulate cortex. First, the effort to identify pain-specific, cingulate processing derives from nociceptive-specific lamina I neurons in the spinal cord where labelled-line theories trace pain-specific processing through the mediodorsal thalamic nucleus.11 Unfortunately, these connections have not been experimentally demonstrated and their specificity to any part of cingulate cortex never shown. That is, many thalamic nuclei project to any one part of the cingulate cortex rather than a single, nociceptive nucleus. Moreover, there are many thalamic nuclei that provide nociceptive input to cingulate cortex (see below), and they are not limited to one nucleus as labeled-line theorists assume. Second, only part of ACC is involved in emotion. The subgenual part of ACC (sACC) is involved in autonomic and classical conditioning functions according to electrical stimulation and neuron recording studies,12,13 while emotion associated activity is not part of the most frequently pain activated region in posterior ACC also known as the midcingulate region. The question remains, does the entire ACC contribute equally to the affective responses associated with pain? Third, no part of the cingulate cortex is activated only by noxious stimulation, although there could be small aggregates of purely nociceptive neurons14 that might respond under other, yet untested, cognitive conditions. Additionally, it does not appear that a cingulate region, subregion or area is engaged only by noxious stimulation; that is, no cingulate region or area appears to be nociceptive specific. Thus, what is the relation between pain and affect vis-à-vis autonomic regulation and pain in relation to premotor planning and motor output? The answers to these questions derive from the four-region model of the cingulate gyrus.
New approach to cingulate pain and emotion
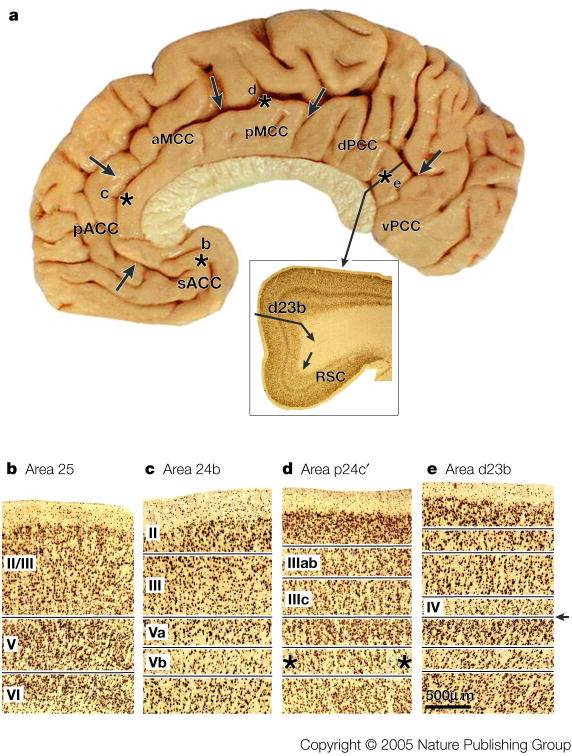
The logic to study the role of the cingulate cortex in nociception should not be based on labelled-line theories; particularly since no pain-only processing inputs or areas have yet been identified. Rather than asking where pain processing occurs, we apply a new logic by considering what the structural and functional organization of the cingulate gyrus is and how nociceptive signals are used in cingulate cortex to accomplish behavioural goals such as avoiding noxious stimuli. This approach requires a multidisciplinary view of the functions of cingulate cortex for the monkey and human brains.15, 16 Figure 1 summarizes the four regions and their subregions that emerge from integrated, neurobiological assessments. A region is an aggregate of areas that have a similar underlying cytoarchitectural motif, common circuitry, and functions. These regions and their subdivisions are ACC (s, subgenual; p, pregenual), midcingulate cortex (MCC; a, anterior; p, posterior), posterior cingulate cortex (PCC; d, dorsal; v, ventral), and retrosplenial cortex (RSC) on the ventral bank of the posterior cingulate gyrus that is not exposed on the gyral surface. The borders of each region were defined with cytoarchitectural analyses of postmortem cases and then coregistered to Talairach and Tournoux’s stereotaxic atlas in the human17 and the VCA in monkey.18 The cellular organization of each area implies differential functions that are still not understood. For example, the presence of a layer IV provides for an intracortical stage of processing in areas 23 and 31 that is not present in areas 25, 24, or 24′. Box #1 demonstrates key neuron and laminar differences for each region with an antibody for neurons-specific nuclear binding protein (NeuN) and this documents the cytological basis of the four-region model.
Figure 1. Distribution of the Four Cingulate Regions & Subregions.
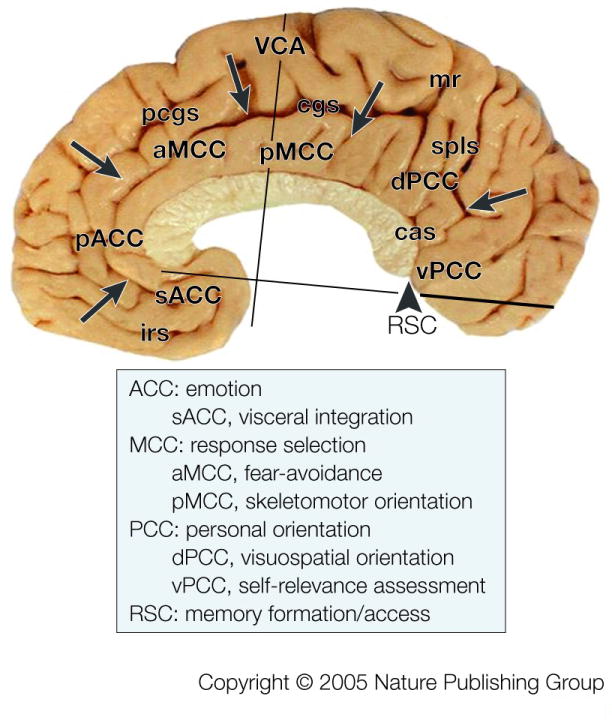
Borders are marked with arrows and were determined in this and six other postmortem cases that were coregistered to a stereotaxic atlas with the vertical plane at the anterior commissure (VCA) and the anterior-posterior commissural line. A functional overview derived from many literature analyses is provided with general regional function along with subregional specializations where known. Abbreviations: cas, callosal sulcus; cgs, cingulate sulcus; irs, inferior rostral sulcus; mr, marginal ramus of cgs; pcgs, paracingulate sulcus.
Cingulate gyrus organization that is viewed in terms of a neurobiological model does not simply reflect places in a three-dimensional coordinate system. These regions reflect circuitry and functional organization as discussed previously and summarized here.15–17 Indeed, the four-region model predicts the outcomes of information processing in the cingulate gyrus and the following facts are of particular relevance. The ACC is involved in autonomic control and storage of emotional memories. However, each region is not uniform because subfunctions within them can result from particular connections that alter processing. Although a large literature documents the role of ACC in autonomic regulation and emotion,12, 13 one of the first human imaging studies on this subject showed that the sACC subregion is involved in negatively valenced affect in healthy women.19 A review of the human imaging in the context of the four-region model showed a prominent activation both during sad events in sACC and during happiness in a rostral position in ACC17 in a subregion we term pregenual ACC (pACC). In terms of pain processing, a response in pACC has been demonstrated with magnetoencephalography and may be associated with C-fiber activation and the suffering component of pain.20 Finally, subgenual area 25 has many autonomic projections including those to the central nucleus of the amygdala, parabrachial nucleus, periaqueductal gray, and light projections to the nucleus of the solitary tract and dorsal motor nucleus have been reported.12 These autonomic projections and emotion functions assure this region is qualitatively distinct from cortex dorsal to the corpus callosum.
The MCC is involved in response selection, has two separate cingulate motor areas that project to the spinal cord and motor cortices21, 22 and this region, including cortex dorsal to it, can be engaged in cognitive tasks that do not necessarily require movement and decisions that are based on the reward value of particular behavioural outcomes.23, 8 In terms of premotor functions, parts of MCC might have little or nothing to do with pain sensation per se. Büchel et al.24 used high resolution fMRI to show that caudal parts of the aMCC are separately activated by innocuous and noxious activity and might be related to a third site for cognitive processing rather than nociception and intensity ratings as such. Finally, the PCC is involved in visuospatial orientation that is mediated through its extensive parietal lobe connections and assessment of self-relevant sensation. The dorsal part of PCC (dPCC) may be involved in orienting the body toward innocuous and noxious somatosensory stimuli and may share some functions with pMCC. Although RSC is poorly understood, it seems to have a role in memory access particularly for valenced information and likely contributes to the functions of PCC via its substantial connections through area 23.
Nociceptive neurons and large aggregate responses
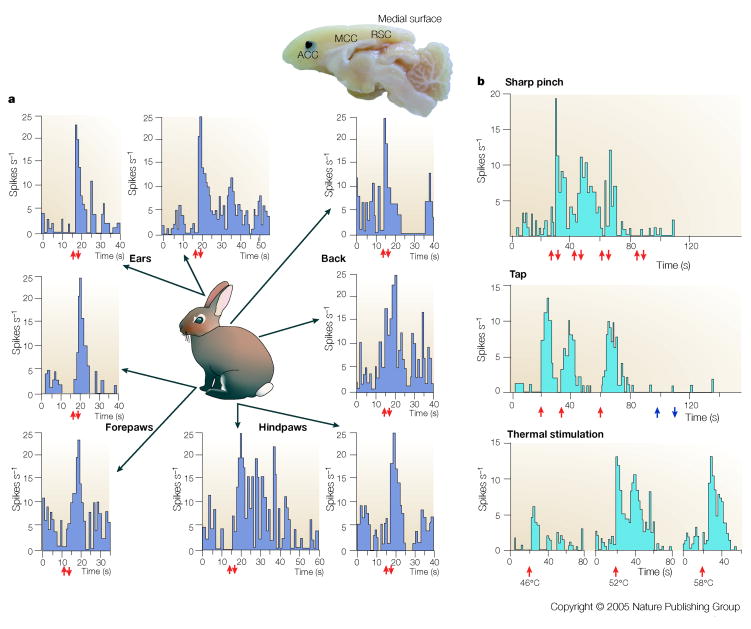
Noxious somatic stimuli provoke pain and avoidance behaviours and these are impaired with lesions of the cingulate gyrus in humans25 and experimental animals26. Moreover, destruction of somatosensory cortex greatly impairs stimulus localization without altering pain affect; presumably because the medial pain system, including cingulate cortex, is intact.27 Based on neurosurgical outcomes and connections with the MITN28, we studied neuron discharge properties in relation to noxious stimulation in the halothane anesthetized rabbit preparation.14 These findings are summarized in FIG. 2 and demonstrate the following essential facts. First, neurons in ACC do not recognize where the noxious stimulus is on the body surface because stimulation anywhere on their body can evoke a discharge. Notice that responses for the rabbit in the left panel of the figure are from a single neuron. Second, neurons respond mainly to noxious stimuli including pressure and temperatures over 46°C. Taping of the skin is the only innocuous stimulus to drive these neurons. Third, there is a major aggregate of nociceptive neurons just dorsal and rostral to the genu of the corpus callosum. Interestingly, these responses reflect in many ways the properties of neurons in the MITN that have projections to cingulate cortex and have large and bilateral receptive fields that are mainly nociceptive but with some tap responses, and a large percentage of nociceptive multimodal responses.29, 30
Figure 2. Nociceptive Cingulate Neurons.
Medial surface of the rabbit brain showing its cingulate regions (ACC, MCC, RSC) and the location of a high density of nociceptive neurons (black) and a low-moderate density of such neurons (grey). (The rabbit does not have PCC areas 23 and 31 like primates.) The rasters display neuron spike discharges for two neurons and the arrows show stimulus onset (↑) and offset (↓). Noxious mechanical stimulation with a serrated forceps evoked a response of the neuron on the left regardless of where the stimulus was applied to the skin and demonstrates that no one neuron in ACC can determine where on the body a noxious stimulus is located. The neuron on the right is a multimodal nociceptive neuron because it responds briskly to both noxious mechanical and heat stimulation. Although the unit did respond to tap stimulation, light brushing of the skin (open arrows) failed to evoke a response. These multimodal nociceptive units provide little information about the characteristics of particular noxious stimuli.
Acute nociceptive responses in human include those that are mediated by cingulate cortex, which is one of the most frequently activated regions in the pain neuromatrix.4, 5 Lenz et al.31 used subdural recording electrodes to show that laser-evoked, nociceptive potentials could be evoked directly from the MCC with latencies of 211–242 milliseconds for negative and 325–352 milliseconds for positive potentials. A short-latency response has also been evoked in pACC by Ploner et al. using magnetoencephalography20. The latency of this latter response was 0.5–1.5 sec which indicates that it is associated with the perception of second pain which is characterized by greater unpleasantness and the sensation of burning. Validation of the role of pACC in unpleasantness comes from a recent study in which the intensity and unpleasantness of noxious cutaneous thermal, laser-evoked responses were independently manipulated and pACC activity was significantly elevated32. Interestingly, the sACC is a site of negatively valenced memory storage (below) but was not involved in either of the latter studies.
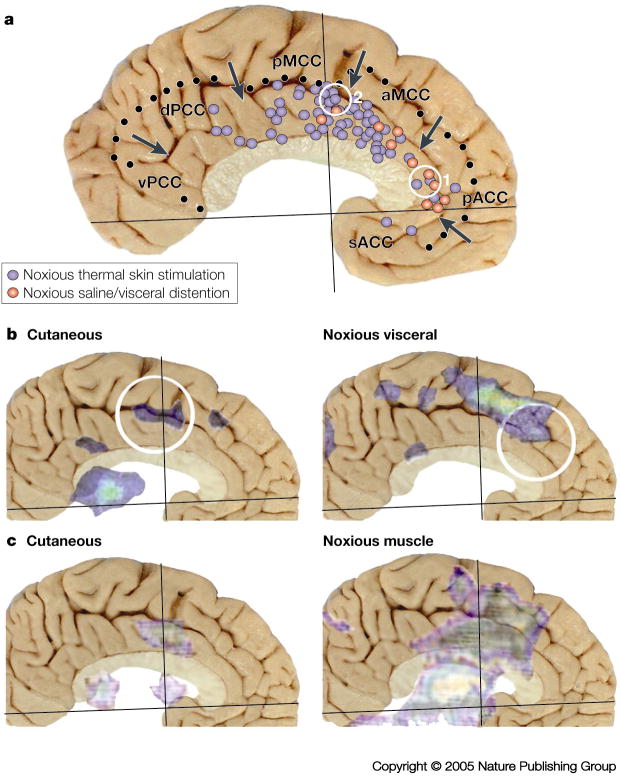
A summary of peak voxel activation during acute nociceptive responses in the cingulate cortex is shown in FIG. 3. We studied nociceptive responses in the context of the four-region model using PET and showed that both pACC and MCC had elevated cerebral blood flow when noxious heat was applied to the back of the hand when controlled for innocuous heating to the same skin33. FIG. 3A shows that most cutaneous activity is evoked in MCC with almost no preference for its anterior or posterior divisions, while there were fewer activation sites in pACC and almost none in sACC or dPCC. Human imaging studies also show coding for the intensity of noxious stimulation in pACC and MCC as occurs in other components of the pain neuromatrix.34,35 Finally, although there are relatively few studies of nociceptive visceral responses, they show a preference for pACC and to a lesser extent aMCC. These responses were evoked with hypertonic saline (applied either on the tongue or administered intravenously) or with noxious distention of the esophagus, colon or rectum.
Figure 3. Human imaging during acute nociceptive stimulation.
A. Summary of peak activation sites in 40 studies during noxious thermal stimulation of the skin and noxious hypertonic saline or visceral distention (References provided online). Cutaneous activations were almost homogeneous throughout MCC with somewhat fewer in the rostral part of aMCC and most in the dorsal and rostral part of pMCC, while visceral activity was greatest in pACC and some in rostral aMCC. The two yellow circles are for activations associated with the opioid placebo (1.55) and the acupuncture placebo (2.57). B./C. Activation sites for two studies were coregistered to the postmortem control case because two different noxious stimulation paradigms were applied to the same subjects providing a more accurate differentiation of the topography of activation sites. As a rule, noxious cutaneous stimulation evoked small and more caudal activity in pMCC, while noxious esophageal distention (B.36) or electrical stimulation of muscle (C.37) evoked larger and more rostral activity in aMCC. Differential activation of parts of MCC likely reflects the differential recruitment of the caudal and rostral cingulate motor areas and their associated cognitive functions.
Plotting peak voxel activations must be viewed critically because they are generated in many separate subject populations and this type of analysis does not take into consideration the full distribution of an activation site. These facts together may lead to misinterpreting the actual size of the activations and make comparison among conditions difficult. Two studies provide important evidence that cutaneous and deep tissue/visceral noxious stimulation activates a larger and different part of the cingulate cortex because both modalities of stimulation were employed in the same subjects. Strigo et al.36 tried to balance noxious stimulation to a similar intensity in visceral and cutaneous sites and observed activation of pMCC by cutaneous thoracic stimulation, while esophageal distention evoked activity more dorsal and anterior in aMCC (FIG. 3B). Svensson et al.37 showed a similarly sized laser-evoked, noxious cutaneous activation of pMCC compared to that of Strigo et al.,36 while intramuscular electrical stimulation evoked a substantially larger and more anterior site in aMCC and pMCC. Thus, deep tissue stimulation activates aMCC and this could be related to generation of emotion and/or avoidance behaviours. It also appears that deep tissue/visceral activations enhance activation of the rostral cingulate motor area in the rostral part of the cingulate cortex, whereas cutaneous nociceptive stimuli will activate primarily the caudal cingulate motor area in pMCC. These activations do not simply differ in size; they activate qualitatively unique cortical subregions.
Sources of nociceptive inputs to cingulate cortex
The well documented responses of the cingulate cortex during nociceptive stimulation require a source by which such information can access this cortex. Our hypothesis that the MITN provide the primary source of nociceptive information is based on several observations. Nociceptive responses are short latency (see above) within 200 milliseconds of stimulus onset and this does not favor prior processing through other cortical sites. As also noted above, some MITN share nociceptive response properties with cingulate neurons and this indicates a functional linkage. Ablation of most cortical input to the rabbit nociceptive region does not block the nociceptive responses, whereas lidocaine block of the MITN activity abolishes such activity14.
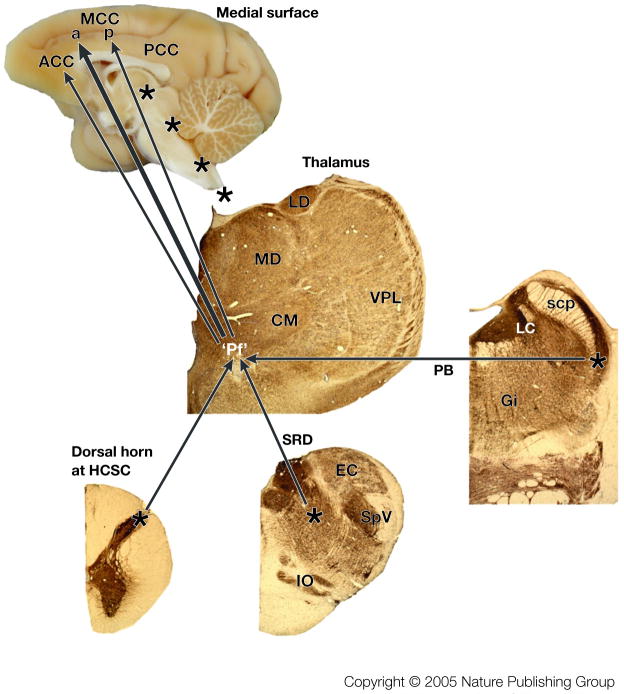
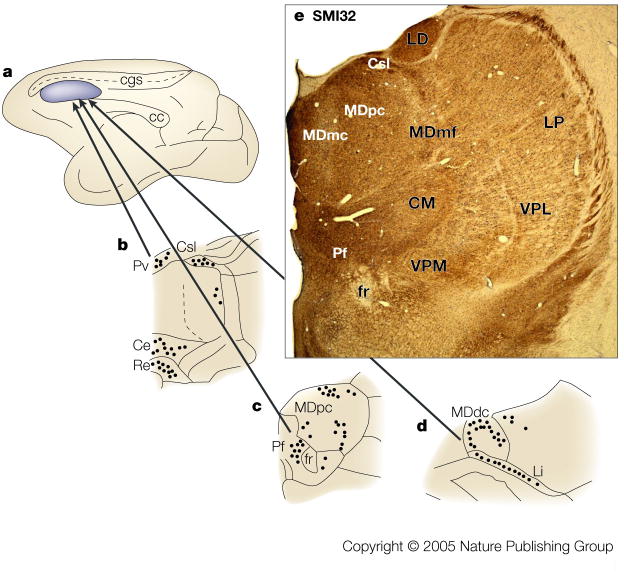
Figure 4 summarizes the primary sources of nociceptive inputs to the cingulate cortex. The “Pf” refers to the parafascicular nucleus as representative of as many as ten MITN that receive differing amounts of spinothalamic input and project to the cingulate cortex. A discussion of MITN organization and projections is provided in BOX 2. The three main nociceptive inputs to the MITN arise from the spinothalamic tract (reviewed for cingulate cortex by Vogt et al., 1993), the pronociceptive subnucleus reticularis dorsalis,38,39 and the parabrachial nucleus.40,41 Each of these inputs to the parafascicular nucleus might transmit cutaneous, muscle, and visceral nociceptive signals and the net consequence of these inputs is that neurons in the cingulate cortex have almost full body receptive fields for cutaneous, muscle, and visceral noxious stimuli.
Figure 4. Nociceptive afferents to cingulate cortex.
Three sources of nociceptive inputs to “Pf” arise from lamina I of the spinal cord, subnucleus reticularis dorsalis (SRD) and the parabrachial nucleus (PB). The four asterisks along the medial surface of the monkey brain show the levels at which the thalamic, PB, SRD, and high cervical spinal cord sections were photographed. These are immunohistochemical sections for an antibody to microtubule associated protein 2 (MAP2) which label neuron dendrites and they were counterstained with thionin because some neurons do not express this antigen. The Pf nucleus is enclosed in quotation marks because it is a representative of the MITN of which there are many nuclei that receive nociceptive spinothalamic inputs and project in turn to cingulate cortex (see BOX #2). We believe the greatest density of nociceptive inputs is to aMCC as shown with the large arrow, while more modest projections are to ACC and pMCC. Abbreviations: CM, centre medianum thalamic nucleus; EC, external cuneate nucleus; Gi, gigantocellular reticular nucleus; IO, inferior olive; LC, locus coeruleus; LD, laterodorsal thalamic nucleus; MD, mediodorsal thalamic nucleus; scp, superior cerebellar peduncle; SpV, spinal nucleus and tract of the trigeminal nerve; VPL, ventral posterolateral thalamic nucleus.
Figure 4 also indicates that a more dense projection from the MITN terminates in aMCC than that in ACC and pMCC. This view is supported by a study in which tracers were injected into the rostral cingulate motor area in aMCC and the caudal cingulate motor area in pMCC.42 Thirty-nine percent of thalamic neurons in the centrolateral, CM, and Pf nuclei were labelled after aMCC injections, and 14.6% were labelled in the same nuclei after pMCC. Since these nuclei transmit nociceptive information, this substantial difference suggests a higher level of nociceptive activation in aMCC than in pMCC and differential involvement of the cingulate motor areas in pain processing.
Role of pMCC and dPCC in nociception
Most studies of nociceptive processing in the cingulate cortex emphasize ACC and MCC because it is thought that these parts of cingulate cortex mediate the affective component of pain. FIG. 3 shows that the PCC is rarely activated during acute noxious stimulation of either cutaneous or visceral tissues. Activation of pMCC, however, could certainly be associated with a different type of nociceptive response, particularly when it is considered that nociceptive MITN inputs to this region are less than to aMCC. Indeed, the cingulate subregion model requires that the composition of the pMCC activation be considered in a different light than more rostral activations. For example, the cingulate motor areas have neurons with different properties; neurons in the caudal area have shorter latencies to movement and weaker links to reward contingencies than those in the rostral cingulate motor area.23
Reports of very early responses using electrophysiological techniques implicate pMCC in sensory orientation rather than affect per se. Nociceptive stimulus discrimination has peak latency at 172 milliseconds after noxious stimulation in dorsal PCC43 and Bentley et al.44 show that both pMCC and dPCC have short-latency, nociceptive responses using cranial surface, electroencephalographic recording. Furthermore, Niddam et al.45 used evoked potentials to show that painful and non-painful electrical stimulation of muscle activated the caudal cingulate motor area and dPCC and Huang et al.46 evaluated movement-associated activity during simple finger movements and showed sites in the caudal cingulate motor area and dPCC with magnetoencephalography. These observations indicate that pMCC and dPCC are involved in orienting the body to sensory stimuli including nociceptive ones and it is unlikely that pMCC nociceptive activations are specific for noxious stimuli. The extent to which pain activation of pMCC and dPCC requires emotion depends upon the role of both regions in emotion.
Cingulate Emotion Processing
Although emotion, like pain, is consciously perceived as a uniform experience, it is not equally engaged throughout the brain or the cingulate gyrus. Our view is that, rather than an emergent property of the whole brain, emotion is processed in different areas according to the memory valence, autonomic associations, and sensory driving that are necessary for the internal content and behavioural output relevant for each class of emotion. This hypothesis can be applied to the entire brain and the regionalized cingulate gyrus. Consider a meta-analysis of simple emotions by Phan et al.47 who plotted functional response peaks in the brain during happiness, sadness, anger and fear generated with word pairs, scripts, or faces with emotional valence. At first glance, it appears that the cingulate gyrus is more or less completely engaged during emotion; a reassuring conclusion for those who view the cingulate cortex as a unitary component of the limbic system that subserves emotion. Indeed, the notion of limbic cortex requires that emotion be a primary function of a region/subregion/area for it to be part of this system. Are all parts of the cingulate cortex equally involved in emotion? The answer to this question can be found in the cingulate regions and subregions and this will have profound implications for how the cingulate cortex processes nociceptive information.
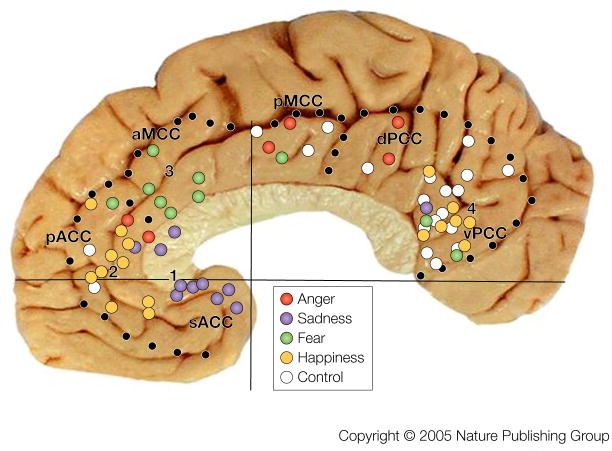
An assessment of responses during emotion considered the proposition that cingulate emotion processing is not equally distributed in the gyrus because the four-region model based on cytoarchitectures, circuits, and functions predicts specialized contributions from each region and subregion.17 The goal of this study was to link activations during simple emotion to particular cytoarchitectural entities. Emotion involves events, objects and memories with a positive or negative valence and these can be associated with affective (autonomic) changes. Simple emotions, such as happiness and sadness, are those that easily valence objects or events, while complex emotions, such as anxiety and guilt, require subtle interpretations of events and cues that often depend on context. For example, “slashing knife” is usually an unambiguous and negatively valenced object, “carrying a knife” could cause anxiety if one is observing another person on a dark street or it might be a positive event if one is preparing for an evening dinner. Thus, the former is a simple emotion, while the latter is a complex one that depends on context. As shown in FIG. 5, most of MCC was not active during simple emotions, while cortex around the genu and splenium of the corpus callosum (perigenual and perisplenial cortex, respectively) were highly active. Only the rostral part of aMCC was active during fear. This distribution supports the prediction that the cingulate cortex is not uniformly involved in emotion.
Figure 5. Cingulate Emotion Processing.
Peak activation sites from 23 studies during simple emotions in the context of the regionalized cingulate gyrus17 (references provided online). Four groups of active sites are numbered and control conditions with non-emotional scripts and faces are coded with white dots. Each numbered aggregate of sites is located in a different subregion and this suggests a different role in processing of emotional information; i.e., a different relevance to autonomic integration, skeletomotor output, and personal orientation as predicted by the four-region, neurobiological model.
Figure 5 can be taken much further in terms of the “submodal” processing of emotion in the cingulate gyrus. Emotion submodalities refer to direct autonomic regulation, valenced memories, and skeletomotor responses showing valence such as facial expressions and crying. Indeed, there are four aggregates of emotion-generated activity numbered in FIG. 5 and we conclude the following from each. First, activity during sadness is greatest in sACC and this location was first reported by George et al.19 Importantly, this site of memory storage for negatively valenced events is comprised mainly of area 25 which has many direct projections to subcortical autonomic centers, and we generally refer to its function as autonomic integration (FIG. 1). No other region has these specific connections.12, 15 Second, activity during happiness occurs more rostrally and dorsally in pACC. Differentiation of these emotions into subregions of ACC emphasizes their specific role in mediating internal responses to different emotional states. Third, fear is associated with activity mainly in aMCC. This part of MCC receives amygdala input47 and the amygdala has been implicated in fear49 and nociception50. No other cingulate region has high and direct amygdala input as well as a significant role in fear. Fourth, the vPCC has a high level of activity during happiness and this might be construed as equivalent to pACC. However, the four-region model and consideration of control condition activity prevents this spurious conclusion. Indeed, vPCC is active during both emotion and non-emotion conditions and this is not true for pACC. The vPCC does not have autonomic projections to subcortical autonomic motor nuclei nor does electrical stimulation evoke autonomic changes. The role of vPCC is better characterized in terms of assessing the self-relevance of emotional events and stimuli; more as an emotional pre-processor, so that emotional information can gain access to the cingulate emotion subregions. Indeed, the vPCC has reciprocal connections with sACC48 that might assist in establishing the personal relevance of sensory information coming into the cingulate gyrus.
Thus, there are four levels of emotion-relevant activity in the cingulate cortex and they sort according to subregions in the four-region neurobiological model. Having plotted pain and emotion-evoked activations into the same coordinate system as the postmortem histological analyses, we are in a position to consider direct relationships between pain and emotion in subregions of the cingulate gyrus.
Pain and emotion linkages
A new perspective on the literature about pain and emotion processing in the cingulate gyrus is provided by the four-region neurobiological model and its associated subregions. Indeed, linkages can be made directly on the basis of numerous studies rather than attempting a single group analysis and this provides added confidence in the conclusions. Furthermore, both matches and mismatches with expected parallels between processing in these two distributed networks become more apparent in this context. Amazingly, the most common acute pain and simple emotion plots suggest there are complex relationships between these cortical functions rather than a simple overlap of negative emotions and pain affect as predicted from the dual-cognitive model of pain processing. Of course, it needs to be reiterated that these plots are of peak voxel activity from many studies, they do not represent the full extent of activation, and the studies employed were only those reporting cingulate activations. In spite of these caveats, the following observations appear to be justified.
First, the fear and pain sites overlap in aMCC and validate the general conclusion of this region in avoidance behaviours. This match between the two systems occurs in the context of heavy MITN inputs to it.
Second, it is surprising that pMCC has no consistent emotion activations yet robust nociceptive responses. Assuming that nociceptive responses are generally short-latency, it seems reasonable to conclude that these evoke skeletomotor, body orientation to the noxious stimulus without affective (autonomic) or emotional (valenced) content. This would likely be mediated via the caudal cingulate motor area that appears to operate more as a skeletomotor integrator rather than in the assessment of behavioural outcomes using valence-coded information.
Third, visceral nociceptive activity is associated mainly with the pACC yet this is not an autonomic integrative center like sACC. Indeed, although this region is most often associated with happiness in studies of simple emotion, amplification of unpleasantness during noxious stimulation enhances the activity in the caudal part of pACC and not sACC32. The four-region model would have predicted preferential sACC activation during noxious stimulation of skin and viscera and this is one of the most striking incongruities (mismatches) in these observations and requires further explanation. Although sACC is sensitive to susceptibility artifacts with high field strength magnets, many studies of acute pain were done with PET in which this was not an issue. It is also true that pain anticipation can reduce cerebral blood flow in sACC51, 52 and this could contribute to a general lack of signal in this region during acute pain. Finally, sad events that evoked sACC activity tended to be associated with personally relevant events and not a simple, external, noxious stimulus. It is possible that pain could engage sACC is a person-specific manner and the currently used stimulation paradigms are not relevant to the internal states of individual subjects. Another way of expressing this concern is to say that the unpleasantness of a noxious thermal stimulus does not generate an adequately negative emotional event to drive sACC.
Fourth, acute nociceptive stimulation does not activate vPCC as part of a generalized self-relevance assessment. It appears that the MITN-mediated nociceptive signal bypasses processing in vPCC and this latter system is primarily involved in visual stimulus assessment. Indeed, nociceptive stimulation actually shuts off much of PCC activity.33, 35 Thus, emotion activations of vPCC have little or nothing to do with pain affect because this subregion does not receive MITN inputs and there may be a cognitively mediated mechanism whereby activity in this area is inactivated during noxious stimulation. Inactivation of vPCC could be one mechanism of reducing the overall perception of noxious stimulation and reduce suffering.
Fifth, the dPCC does not appear to have a specific role in pain processing because it can be activated with noxious and innocuous stimulation. Importantly, this region and adjacent pMCC likely dominate activity in the caudal cingulate motor area and mediate rapid, body orientation to somatic stimuli and they both have little or nothing to do with emotion.
It appears that the four-region neurobiological model of the cingulate gyrus and its subregions are a productive way to assess interchanges between pain and emotion networks in the cingulate gyrus. We predict that this will be true for many of its other essential functions of this region. Surprisingly, the cingulate cortex is not uniformly involved in emotion and not all pain-activation sites are associated with affect or emotion – facts that should lead to a better understanding of how each is processed. Although the MITN provide direct circuits to each cingulate region, this projection differs in density to each subregion and each subregion employs this information differently for pain processing. In conclusion, the cingulate gyrus mediates three main aspects of pain processing: fear-avoidance in aMCC, unpleasantness in pACC, and skeletomotor orientation of the body to the noxious stimulus in pMCC and dPCC. The MCC and dPCC are generally engaged in premotor planning and may have little to do with sensation. Even the fear signal in aMCC might be more closely associated with predicting behavioural outcomes than sensory affect per se.
Hypnoanalgesia and opiate and acupuncture placebos
Pain regulation and associated negative affect might eventually be resolved in the context of the cingulate subregions. Hypnotic induction of analgesia targets aMCC53 which is consistent with the notion that fear-reduction is part of the analgesic effect that is mediated by the cerebral cortex. The mechanisms of opiate analgesia are less clear but seem to be in line with the distribution of subregions. The highest level of opioid receptor binding is in pACC54, which is the site of the opioid placebo effect55 as shown in FIG. 3A-1. This is also the same region of elevated activity during attention to the unpleasantness of nociceptive cutaneous stimulation32 and the point at which negative affect displaces binding of the mu-opioid agonist carfentanil in pACC56. Thus, hypnoanalgesia, opioid drugs and the opioid placebo target different subregions that have emotional functions and regulate autonomic outputs.
Interestingly, different placebo effects are mediated by different cingulate subregions. Acupuncture and the acupuncture placebo in patients with painful osteoarthritis activate the dorsal part of pMCC.57 It is striking that the somatic acupuncture placebo is located in the region of most dense acute somatic pain sites (FIG. 3A-2.), while the opioid placebo is located in the region of highest visceral activation and opioid receptor density (FIG. 3A.-1.). Thus, there may be a map of placebos that are organized according to the distribution of the cingulate subregions. Moreover, hypnoanalgesia and opiate analgesia could provide important and independent tests of the subregion model and suggest strategies for searching for the etiologies and treatments of chronic pain and stress syndromes as well as mood, motor, and thought disorders.
Model evolution and applications
The four-region model is a theoretical construct. That is to say, MCC is more than a location on the cingulate gyrus in the sense of posterior ACC, caudal ACC, dorsal ACC and the many other terms used to identify activity in this region. The MCC is a region and a concept that represents a circuitry with a limited number of functional outputs. Although less apparent in the nomenclature when we use the terms, ACC, PCC, and RSC also represent theoretical constructs as well as structural entities. The value of the regional model derives from its ability to make specific, a priori predictions about experimental outcomes. Interestingly, it is not expected to be “correct” in all instances and some of the more profound outcomes arise when an observation cannot be predicted and forces a new functional perspective as occurred when colocalizing pain and emotion.
Since the four-region model was first proposed in 1993 (Chapter 16), our main concern was assuring the accuracy of anatomical and functional criteria. Further observations required changes. For example, amygdala connections, differential properties of the cingulate motor areas and studies of simple emotions indicated that subregions existed for MCC and recently the PCC.18 It appears that defining the cingulate regions and subregions is essentially complete and their cytoarchitectures are well understood. Is research into the structure and functions of the cingulate gyrus complete? There are three major areas of investigation now opening and these will keep us occupied for the 21st century.
First, we know very little of the distribution of particular transmitter receptor systems and their transduction mechanisms in each area of the primate brain. New neuronal phenotypes will certainly become apparent as the molecular biology of cingulate cortex evolves over the coming decades.
Second, the functions of each subregion are still poorly understood. Iteractions of reward and punishment systems are not understood because these dynamics are usually evaluated in the contexts of different theoretical and disciplinary contexts.
Third, the relations of each neuronal phenotype to the etiology and progression of many diseases is not understood but will be uncovered in the context of the regional and subregional organization. Alzheimer’s disease, obsessive-compulsive disorder, posttraumatic stress disorder, depression, chronic and functional pain disorders and many others may be linked to cingulate cortex via their primary etiology.
The theoretical model of cingulate cortex will continue to evolve as our understanding of its neuronal features is explored. Most importantly, the initiation and progression of numerous diseases across the cingulate gyrus will continually push evolutionary changes in how we model this part of the brain.
Glossary
Labelled-line theories: These theories predict that a line or projection from lamina I of the spinal cord is specific for nociceptive stimulation and this line is maintained throughout the central nervous system; i.e., through the thalamus and directly to parts of the cerebral cortex. There is no evidence for a labeled-line into the cingulate gyrus.
Box 1.
Box 2.
Acknowledgments
This work was supported by NIH NINDS grant #RO1NS44222.
References
- 1.Aggleton JP. The Amygdala. Oxford University Press; New York: 2001. [Google Scholar]
- 2.Davidson RJ, Scherer KR, Goldsmith HH. Handbook of Affective Sciences. Oxford University Press; New York: 2003. [Google Scholar]
- 3.Melzack R, Casey KL. Sensory, motivational and central control determinants of pain. A new conceptual model. In: Kenshalo DR, editor. The Skin Senses. Springfield, IL: Thomas; 1968. pp. 423–439. [Google Scholar]
- 4.Derbyshire SWG. Exploring the pain neuromatrix. Curr Rev Pain. 2000;6:467–477. doi: 10.1007/s11916-000-0071-x. [DOI] [PubMed] [Google Scholar]
- 5.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain: A review and meta-analysis. Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 6.Vogt BA, Sikes RW, Vogt LJ. Anterior cingulate cortex and the medial pain system. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus. Boston: Birkhäuser Boston, Inc; 1993. pp. 313–344. This review provides the first definition of the medial pain system from nociceptor to the cerebral cortex via the MITN including circuits, neurophysiology, and opioid receptor binding. Previous efforts emphasized the thalamus for the two systems because the projections of nociceptive thalamic nuclei were not appreciated. Chapter 1 of this book is the first report of the logical and factual basis for the midcingulate region in monkey and rabbit brains. [Google Scholar]
- 7.MacLean P. The Triune Brain in Evolution; Role in Paleocerebral Functions. Plenum Publishing; New York: 1990. [DOI] [PubMed] [Google Scholar]
- 8.Bush G, Vogt BA, Homes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision-making. Proc Natl Acad Sci. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolls ET, O’Doherty J, Kringlebach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13:309–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- 10.Bartels A, Zeki S. The neural basis of romantic love. NeuroReport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- 11.Craig AD. Pain mechanisms: Labeled lines versus convergence in central processing. Ann Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 12.Neafsey EJ, Terreberry RR, Hurley KM, Ruit KG, Frysztak RJ. Anterior cingulate cortex in rodents: Connections, visceral control functions, and implications for emotion. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus. Burkhaser; Boston: 1993. pp. 206–223. Evidence is reviewed for the concept that area 25 is a visceromotor control region based on electrical stimulation and connection studies mainly in rodents. Autonomic regulation by sACC is pivotal to understanding the subdivisionas of ACC into its subgenual and pregenual parts. [Google Scholar]
- 13.Buchanan, Powell . Cingulothalamic and prefrontal control of autonomic function. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus. Burkhaser; Boston: 1993. pp. 206–223. [Google Scholar]
- 14.Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophsyiol. 1992;68:1720–1731. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- 15.Vogt BA, Vogt LJ, Nimchinsky EA, Hof PR. Primate cingulate cortex chemoarchitecture and its disruption in Alzheimer’s disease. In: Bloom FE, Björkund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy. 1997. pp. 455–528. [Google Scholar]
- 16.Vogt BA, Hof PR, Vogt LJ. Cingulate Gyrus. In: Paxinos G, Mai JK, editors. The Human Nervous System. 2. Academic Press; 2004. pp. 915–949. [Google Scholar]
- 17.Vogt BA, Berger GR, Derbyshire SWJ. Structural and Functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogt BA, Vogt L, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol. 2005;485:218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. One of the first demonstrations of where in the cingulate gyrus memories with negative valences are stored in sACC. This subregion provides a substrate for vulnerability to major depression as discussed by Helen Mayberg and her colleagues (2005, Neuron 45:651–660) [DOI] [PubMed] [Google Scholar]
- 20.Ploner M, Gross J, Timmermann L, Schnitzler A. Cortical representation of first and second pain sensation in humans. Proc Natl Acad Sci. 2002;99:12444–12448. doi: 10.1073/pnas.182272899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morecraft RJ, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J Comp Neurol. 1992;322:471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- 23.Shima K, Aya K, Mushiake H, Inase M, Aizawa H, Tanji J. Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J Neurophysiol. 1991;65:188–202. doi: 10.1152/jn.1991.65.2.188. This study provides the neurophysiological characterization of the two cingulate motor areas. These motor areas are key outputs from the cingulate gyrus for mediating skeletomotor functions, they are pivotal to the subregional differentiation of MCC into two parts, and they suggest different mechanisms for somatic pain responses. The functions of the cingulate gyrus cannot be understood outside the context of these two motor areas. [DOI] [PubMed] [Google Scholar]
- 24.Büchel C, Bornhövd K, Quante M, Glauche V, Bromm B, Weiller C. Dissociabel neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: A parametric single-trial laser functional magnetic resonance imaging study. J Neurosci. 2002;22:970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballantine HT, Cassidy WL, Flanagan NB, Marino R., Jr Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg. 1967;26:488–495. doi: 10.3171/jns.1967.26.5.0488. [DOI] [PubMed] [Google Scholar]
- 26.Gabriel M. Discriminative avoidance learning: A model system. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus. Boston: Birkhäuser Boston, Inc; 1993. pp. 478–523. [Google Scholar]
- 27.Ploner M, Freund H-J, Schnitzler A. Pain affect without pain sensation in a patient with a postcentral lesion. Pain. 1999;81:211–214. doi: 10.1016/s0304-3959(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 28.Vogt BA, Rosene DL, Pandya DN. Science. Vol. 204. 1979. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey; pp. 205–207. The first demonstration of MITN projections to the cingulate cortex. These nuclei were later shown to project to many limbic areas in the primate cerebral cortex including the anterior insula and orbitofrontal cortex as well as the amygdala and this projection system may be a network integrator for the limbic/medial parts of the pain neuromatrix. [DOI] [PubMed] [Google Scholar]
- 29.Casey KL. Unit analysis of nociceptive mechanisms in the thalamus of the awake squirrel monkey. J Neurophysiol. 1966;29:727–750. doi: 10.1152/jn.1966.29.4.727. [DOI] [PubMed] [Google Scholar]
- 30.Dong WK, Ryu H, Wagman IH. Nociceptive responses of neurons in medial thalamus and their relationship to spinothalamic pathways. J Neurophysiol. 1978;41:1592–1613. doi: 10.1152/jn.1978.41.6.1592. [DOI] [PubMed] [Google Scholar]
- 31.Lenz FA, Rios M, Zirh A, Chau D, Krauus G, Lesser RP. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J Neurophysiol. 1998;79:2231–2234. doi: 10.1152/jn.1998.79.4.2231. [DOI] [PubMed] [Google Scholar]
- 32.Kulkarni B, Bentley DE, Elliot R, Youell P, Watson A, Derbyshire SWG, Frackowiak RSJ, Friston KJ, Jones AKP. Attention to pain localization and unpleasantness discriminate the functions of the medial and lateral pain systems. Eur J Neurosci. 2005 doi: 10.1111/j.1460-9568.2005.04098.x. in press. [DOI] [PubMed] [Google Scholar]
- 33.Vogt BA, Derbyshire SWJ, Jones AKP. Pain processing in four regions of human cingulate cortex localized with coregistered PET and MR imaging. Eur J Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 34.Derbyshire SWG, Jones AKP, Gyulai F. Pain processing during three levels of noxious stimulation produces differential patterns of cerebral activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 35.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: A bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 36.Strigo IA, Duncan GH, Boivin M, Bushness MC. Differentiation of visceral and cutaneous pain in the human brain. J Neurophysiol. 2003;89:3294–3303. doi: 10.1152/jn.01048.2002. [DOI] [PubMed] [Google Scholar]
- 37.Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL. Cerebral proecessing of acute skin and muscle pain in humans. J Neurophysiol. 1997;78:450–460. doi: 10.1152/jn.1997.78.1.450. [DOI] [PubMed] [Google Scholar]
- 38.Villanueva L, Cliffer KD, Sorkin LS, Le Bars D, Willis WD., Jr Convergence of heterotopic nociceptive information onto neurons of caudal medullary reticular formation in monkey (Macacca fascicularis) J Neurophysiol. 1990;63:1118–1127. doi: 10.1152/jn.1990.63.5.1118. The first demonstration of the functional properties of neurons in the pronociceptive SRD nucleus in the monkey. Since this nucleus projects to the parafascicular nucleus in the thalamus, it is a pivotal source of nociceptive input to the cingulate gyrus and partially explains the large receptive fields of nociceptive neurons in ACC. [DOI] [PubMed] [Google Scholar]
- 39.Villanueva L, Debois C, Le Bars D, Bernard J-F. Organization of diencephalic projections from the medullary subnucleus reticularis dorsalis: A retrograde and anterograde tracer study in the rat. J Comp Neurol. 1998;390:133–160. [PubMed] [Google Scholar]
- 40.Bester H, Bourgeais L, Villanueva L, Besson J-M, Bernard J-F. Differential projections to the intralaminar and gustatory thalamus from the parabrachial area: A PHA-L study in the rat. 1999 [PubMed] [Google Scholar]
- 41.Saper CB. Pain as a visceral sensation. Prog Brain Res. 2000;122:237–243. doi: 10.1016/s0079-6123(08)62142-1. [DOI] [PubMed] [Google Scholar]
- 42.Hatanka N, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Nambu A, Takada M. Thalamocortical and intracortical connections of monkey cingulate motor areas. J Comp Neurol. 2003;462:121–138. doi: 10.1002/cne.10720. [DOI] [PubMed] [Google Scholar]
- 43.Schlereth T, Baumgärtner U, Magerl W, Stoeter P, Treede R-D. Left-hemisphere dominance in early nociceptive processing in the human parasylvian cortex. Neuroimage. 2003;20:441–454. doi: 10.1016/s1053-8119(03)00345-8. [DOI] [PubMed] [Google Scholar]
- 44.Bentley DE, Derbyshire SWG, Youell PD, Jones AKP. Caudal cingulate cortex involvement in pain processing: an inter-individual laser evoked potential source localization study using realistic head models. Pain. 2003;102:265–271. doi: 10.1016/S0304-3959(02)00405-0. [DOI] [PubMed] [Google Scholar]
- 45.Niddam DM, Chen L-F, Yu-Te W, Hsieh J-C. Spatiotemporal brain dynamics in response to muscle stimulation. NeuroImage. 2005;25:942–951. doi: 10.1016/j.neuroimage.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Huang M-X, Harrington DL, Paulson KM, Weisend MP, Lee RR. Temporal dynamics of ipsilateral and contralateral motor activity during voluntary finger movement. Human Brain Mapping. 2004;23:26–39. doi: 10.1002/hbm.20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 48.Vogt BA, Pandya DN. Cingulate cortex of rhesus monkey. II. Cortical afferents. J Comp Neural. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- 49.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: Electrophysiological evidence for an involvement in prai processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- 51.Simpson JR, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P. Does anticipation of pain affect cortical nociceptive systems ? J Neurosci. 2002;22:3206–3214. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faymonville M-E, Laureys S, Deguelde C, Del Fiore C, Luxen A, Franck G, Lamy M, Maquet P. Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology. 2000;92:1257–1267. doi: 10.1097/00000542-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Vogt BA, Watanabe H, Grootoonk S, Jones AKP. Topography of diprenorphine binding in human cingulate gyrus and adjacent cortex derived from PET and MR images. Human Brain Mapping. 1995;3:1–12. [Google Scholar]
- 55.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia- Imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. The first study to demonstrate colocalization of opioid binding and the opioid placebo in cingulate cortex. The exact location of this opioid placebo site is shown in FIG. 3A.-1. in pACC. [DOI] [PubMed] [Google Scholar]
- 56.Zubieta J-K, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA. Regulation of human affective responses by anterior cingulate and limbic μ-opioid neurotransmission. Arch Gen Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- 57.Pariente J, White P, Frackowiak RSJ, Lewith G. Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture. NeuroImage. 2005;25:1161–1167. doi: 10.1016/j.neuroimage.2005.01.016. [DOI] [PubMed] [Google Scholar]