Abstract
We report herein the application of the phosphoramidate ProTide technology to improve the metabolism of the DNA methytransferase inhibitor, zebularine (Z). Zebularine is a riboside that must undergo a complex metabolic transformation before reaching the critical 2’-deoxyzebularine-5’-triphosphate (dZTP). Because 2’-deoxyzebularine (dZ) is not phosphorylated and therefore inactive, the ProTide strategy was employed to bypass the lack of phosphorylation of dZ and the inefficient reduction of zebularine-5’-diphosphate by ribonucleotide-diphosphate reductase required for zebularine. Several compounds were identified as more potent inhibitors of DNA methylation and stronger inducers of p16 tumor suppressor gene than zebularine. However, their activity was dependent on the administration of thymidine to overcome the potent inhibition of thymidylate synthase (TS) and deoxycytidine monophosphate (dCMP) deaminase by dZMP, which deprives cells of essential levels of thymidine. Intriguingly, the activity of the ProTides was cell line-dependent and activation of p16 was manifest only in Cf-Pac-1 pancreatic ductal adenocarcinoma cells.
Introduction
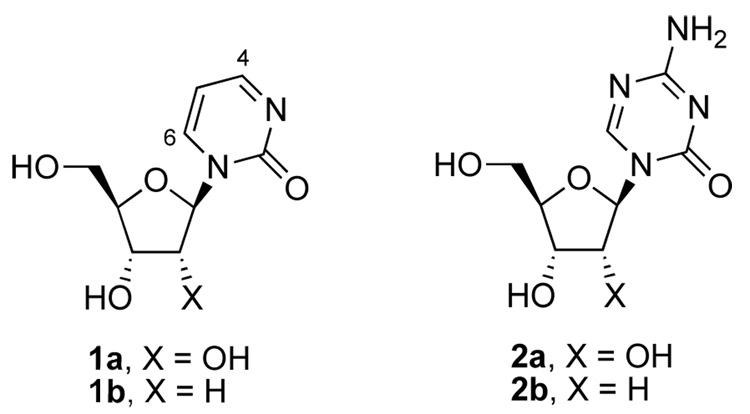
The initiation and progression of cancer is driven by both genetic and epigenetic changes. Epigenetic changes, which are independent of the primary DNA sequence, have received a great deal of attention for their key role in cancer initiation and tumor progression.1,2 In the epigenomics landscape, one of the most studied processes is DNA methylation, an event that controls the transcriptional silencing of a number of tumor suppressor genes. In cancer, such transcriptional repression is associated with the abnormal methylation of cytosines in CpG islands near the promoter regions of the genes and maintained through cellular division. Because these methylated CpG islands are incapable of initiating transcription unless the methylation signal is removed, therapeutic strategies to revert this process have been sought by the use of drugs that alter the transcriptional status of the affected tumor suppressor genes by inhibiting DNA methylation.3,4 Therefore, silenced tumor suppressor genes present themselves as obvious targets for reactivation by DNA methylation inhibitors such as 5-azacytosine nucleosides (2a,b) and more recently zebularine (1a).5,6
Zebularine (1-[b-D-ribofuranosyl]-1,2-dihydropyrimidin-2-one) is a cytidine analog that was initially developed as a cytidine deaminase (CDA) inhibitor7,8 and recently discovered to also inhibit DNA methylation.5 The simple removal of the 4-amino group from cytidine increases the electrophilicity of the resulting 2-(1H)-pyrimidinone aglycon, which explains the ease of nucleophilic attacks at C4 or C6 of the 2-oxopyrimidine ring that are responsible for zebularine’s activity as a potent inhibitor of both CDA and DNA methyltransferases, respectively.9 Once incorporated into DNA, the 2-(1H)-pyrimidinone aglycon forms a covalent complex with DNA methyltransferases via nucleophilic attack at C6 from a key conserved cysteine residue (Cys81)10 that results in the depletion of DNA methyltransferase 1 (DNMT1),11,12 the reactivation of hypermethylated genes in yeast and solid tumor cells,5 and antitumor activities in mouse xenografts5 and radiation-induced T-cell lymphomas in mice.13
In vitro inhibition of bacterial M.HhaI DNA methyltransferase activity by double-stranded oligodeoxynucleotides containing either 2’-deoxy-5-azacytidine (2b) or 2’-deoxyzebularine (1b) in the hemimethylated recognition sequence [5’-GXGC-3’/3’-CGMG-5’ (M = 5-methylcytosine, X = 5-azacytosine or 2-(1H)-pyrimidinone)], confirmed that the two heterocyclic moieties have comparable reactivity.14 In contrast, much higher doses of zebularine were required to achieve comparable levels of p16 reactivation in T24 cells relative to the 5-azacytidine nucleosides, which suggested that the differences in potency were due to other factors.5 Indeed, zebularine (1a) required doses10- to 100-fold higher than 5-azacytidine (2a) and 2’-deoxy-5-azacytidine (2b), respectively, to induce comparable levels of p16 expression.5 On the other hand, the stability and reduced toxicity of zebularine allowed it to be administered continuously to cells resulting in marked p16 expression.5
The incorporation of zebularine into DNA necessitates critical levels of 2′-deoxyzebularine-5′-triphosphate (dZTP) which is formed by a complex metabolic route that may explain its weaker potency.15 A quantitative assessment of the phosphorylation and DNA incorporation of zebularine in T24 cells using 2-[14C]-zebularine revealed that the drug is readily phosphorylated to the corresponding 5′-mono- (ZMP), 5’-di- (ZDP) and 5’-triphosphate (ZTP) in a dose- and time-dependent manner.15 Two additional zebularine-containing metabolites were also observed and identified as diphosphocholine (ZDP-Chol) and diphosphoethanolamine adducts. Intracellular concentrations of ZTP and ZDP-Chol were comparable and greatly exceeded those of the other metabolites.15
When DNA and RNA levels of incorporation were compared, RNA incorporation surpassed DNA incorporation by at least 7-fold.15 Thus, formation of zebularine riboside metabolites appears to be quite robust, but conversion of zebularine-5′-diphosphate (ZDP) to 2′-deoxyzebularine-5′-diphosphate (dZDP), which is catalyzed by ribonucleotide-diphosphate reductase (RNR), seems to be a rate-limiting step that could explain zebularine’s weaker potency.15 Unfortunately, the use of 2′-deoxyzebularine (1b) was unable to overcome this deficiency because it was completely ineffective, perhaps owing to lack of recognition by the activating enzyme, deoxycytidine kinase (dCK).5,15 In a biochemical sense zebularine behaves like a cytidine analogue,16 but its metabolic activation to form the dZTP required for DNA incorporation seems to be complex and inefficient.
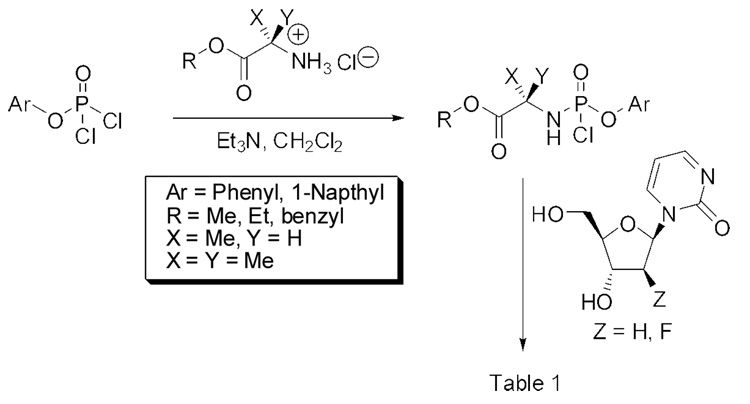
In considering the limitations of zebularine described above and the inactivity of 2’-deoxyzebularine, we decided to investigate a prodrug strategy for dZMP that could simultaneously bypass both the dCK step (needed for 2’-deoxyzebularine) and the RNR step (involved in the case of zebularine at its 5’-diphosphate anabolite stage) as a means of increasing DNA incorporation and enhancing potency. Thus, the intracellular delivery of dZMP in a membrane permeable “ProTide” form was explored by the well-known aryloxyphosphoramidate approach of McGuigan et al. 17,18 Through this technology the lipophilicity of the nucleotide is augmented making the ProTide able to penetrate the interior of the cell by passive diffusion. Once inside the cell, the nucleotide monophosphate (dZMP) is expected to be released at effective intracellular levels. The McGuigan ProTide approach already has produced significant enhancements in the antiviral activity of various nucleosides against HIV and HCV viruses compared to the parent nucleoside analogues in vitro.19,20 As far as we know, this work is the first attempt to utilize the ProTide technology for an anticancer agent.
Selection of ProTide targets and preliminary activity
A rapid survey with different aryloxyphosphoramidates was explored with the idea of correlating some key structural parameters with increases in lipophilicity calculated according to the atom-based program MOE SLog P21 as shown in Table 1. The objective was to quickly perform a limited structure-activity analysis to identify the most potent candidates for proof of concept. The biological response used to gauge our structure-activity analysis was the re-expression of the tumor suppressor gene p16 in Cf-Pac-1 pancreatic tumor cells using real time PCR analysis.11,22,23 For the purposes of our initial screen, the compounds in Table 1 were given a qualitative “yes” or “no” response to p16 reactivation.
Table 1.
Target aryloxyphosphoramidates synthesized for initial SAR analysis
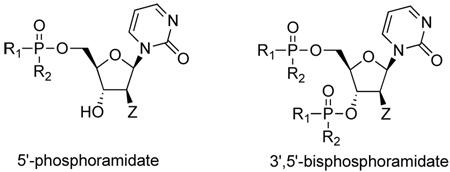 | |||||
|---|---|---|---|---|---|
| Compounda | Z | R1 | R2 | log Pb | p16 activationc |
| 3 | H | 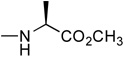 |
0.77 | no | |
| 4 | F | 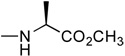 |
1.14 | no | |
| 5a | H | 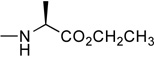 |
1.81 | yes | |
| 5b (bis) | 4.15 | yes | |||
| 6 | H | 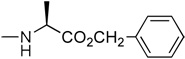 |
2.60 | no | |
| 7a | H | 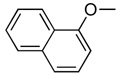 |
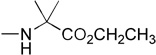 |
2.70 | yes |
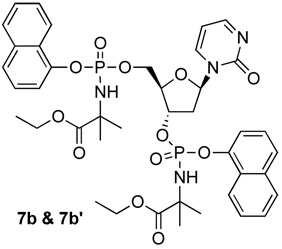
| 7b (bis) | 5.93 | yes | |||
| 7b’ (bis) | 5.93 | yes | |||
| 8 | F | 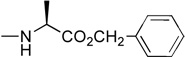 |
2.97 | no | |
| 9a | H | 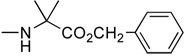 |
3.00 | yes | |
| 9b (bis) | 6.51 | yes | |||
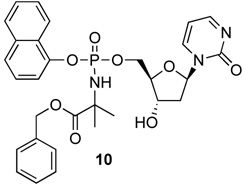
| 10 | H | 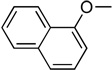 |
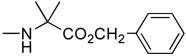 |
4.15 | yes |
Compounds 5b, 7b, 7b’ and 9b are 3’,5’-bisphosphoramidates
Calculated log P value
p16 activation in the presence of 100 mM thymidine
In the first and simplest ProTide (3) we combined a phenoxy group with the L-alanine methyl ester. The same two groups were also attached to a modified 2’-β-fluoro-2’-deoxyzebularine analogue (4) which was expected to function as a 2’-deoxy surrogate with increased lipophilicity. However, none of these compounds showed activity. A further increase in log P achieved with the p-chlorophenoxy group and the L-alanine ethyl ester in the next compound (5a) seems to suggest that a log P approaching 2 is a good threshold for activity as compound 5a was indeed able to induce p16 reactivation. Interestingly, the 3’,5’-bis-[4-chlorophenoxy(ethoxy-L-alanyl)] phosphoramidate (5b, log P = 4.15) obtained as a by-product also showed activity. This was unexpected on several grounds. Firstly, the presence of the bulky group at the 3’ position might be anticipated to possibly impede enzyme processing of the ProTide. Secondly, if only the 5’-ProTide moiety were processed, as intended, the dZMP-3’-ProTide would not be expected to be active per se. Thirdly, if both ProTide motifs were processed, the resulting 3’,5’-bis-phosphate would not be expected to be the ideal pharmacophore. Thus, the data seem to imply that 3’,5’-bis ProTides may have a surprising efficacy by a mechanism to be determined. The next two entries in Table 1 (compounds, 6 and 7) showed that contributions from the aryloxy and L-alanine ester sides are different and potency does not necessarily correlate with log P. In compound 6, a rise in log P to 2.60, achieved by increasing the lipophilicity of the L-alanine moiety from an ethyl to a benzyl ester, while leaving the phenoxy group intact, resulted in an inactive compound. On the other hand, augmenting the log P from the aryloxy side with the naphthyloxy group and adding an extra methyl group on the ester side with the dimethylglycine ethyl ester to reach a similar log P of 2.70 produced a very potent compound (7a) which together with the two diastereoisomers of the 3’,5’-bis-[naphthyloxy(ethyloxydimethylglycinyl)] phosphoramidate (7b and 7b’, log P = 5.93) induced strong p16 activation (vide infra). The next compound (8), which differs from 6 by the simple addition of fluorine, was also inactive despite having an adequate log P of 2.97.
Notwithstanding the fact that compound 6 failed to reactivate p16, the simple change of the L-alanine benzyl ester to the dimethylglycine benzyl ester was accompanied by a modest rise in log P to produce 9a, which was active. Similarly to compound 5b, the 3’,5’-bis-[phenoxy(benzyloxydimethylglycinyl)] phosphoramidate 9b (log P = 6.51) was active. The final compound of the initial series was compound 10, which evolved from 9a by replacing the phenyloxy moiety with the naphthyloxy group. This compound is the most lipophilic member of the 5’-ProTide series (log P = 4.15).
Chemistry
The synthesis of the phosphoramidates (3–10) was performed using either the THF/N-methylimidazole (NMI) or the tert-butyl magnesium chloride protocol (see Scheme 1 and experimental section). Because of the stereochemistry of the phosphorus center, each of the 5'-mono phosphoramidates 3, 4, 5a, 6, 7a, 8, 9a, and 10 were isolated as pairs of diastereoisomers and tested as such. Such isomerism was most evident from the presence of 2 closely spaced signals in the 31P NMR in a ratio of roughly 1:1. Other signals were also duplicated in the proton and 13C NMR spectra in each case. For the 3',5'-bis phosphoramidates, 4 stereoisomers (8 31P NMR peaks) are possible and multiple peaks were observed, consistent with mixtures being generated. In the case of 7 we achieved chromatographic separation of some of the 3',5'-bis phosphoramidate isomers and each fraction, 7b and 7b' gave only 2 31P NMR signals, this being consistent with each being a single diastereoisomer. As discussed later these compounds possessed different potencies in the activation of p16.
Scheme 1.
Synthesis of 2’-deoxyzebularine phosphoramidates
The four most potent compounds 7a, 7b, 7’b and 10 from Table 1 that were identified in the preliminary screen as strong promoters of p16 re-expression were fully characterized by chemical and spectroscopic means (see experimental) and used for additional biological studies.
Biological Studies
All ProTides were initially tested in T24 cells by treating continuously with the compounds for eight days. No induction of the p16 gene was observed at 1, 10, and 100 µM concentrations indicating that the compounds were unable to inhibit DNA methylation in T24 cells (data not shown). Similarly, all compounds failed to induce the p16 gene in Cf-Pac-1 pancreatic cancer cells (data not shown).
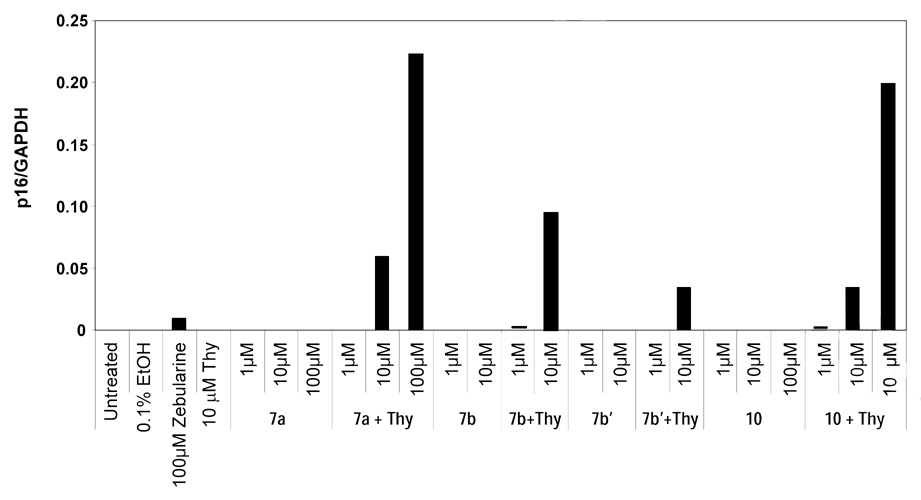
When the compounds were tested with Cf-Pac-1 cells supplemented with 100 µM thymidine activities began to emerge (Table 1). The rationale for including thymidine in the treatment was to maintain a pool of thymine monophosphate (TMP) to allow DNA synthesis to progress without cell-cycle arrest. 2’-Deoxyzebularine monophosphate (dZMP) is also known to inhibit two critical enzymes: (1) thymidylate synthase (TS),24 the enzyme responsible for converting dUMP into TMP via the salvage pathway, and (2) dCMP deaminase25 which prevents formation of dUMP, the substrate for TS to make TMP. By inhibiting TS and dCMP deaminase, dZMP could slow down DNA synthesis, which is essential for inhibition of DNA methylation. We therefore supplemented the cells with 100 µM thymidine to allow the cell cycle to progress without interference from the inhibition of TS and dCMP deaminase. Strong induction of p16 expression was observed in Cf-Pac-1 cells in the presence of 100 µM thymidine for compounds 7a and 10 at 100 µM (Figure 1). Importantly, these compounds induced measurable p16 expression still at 10 µM and displayed a significantly stronger response than zebularine at an equivalent 100 µM concentration. However, every active compound in Cf-Pac cells failed to induce p16 expression in T24 and HCT15 cancer cells even in the presence of thymidine (data not shown). Zebularine alone in contrast was effective in T24 cells and HCT15 cells.
Figure 1. Effect of 2’-deoxyzebularine ProTides 7a, 7b, 7b’ and 10 on p16 expression in Cf-Pac-1 pancreatic cancer cells.
Cf-Pac-1 cells were treated with ProTides continuously for 8 days. RNA was collected and the levels of p16 expression were measured by real time RT-PCR. GADPH was used as a reference gene. All compounds induce a strong p16 expression in the presence of 100 µM thymidine.
Of the two 3’,5’-bis-[naphthyloxy(ethyloxydimethylglycine)]phosphoramidate diastereoisomers (7b) was the most potent compound of the entire series showing stronger induction of p16 at 10 µM compared to zebularine at 100 µM (Figure 1). Also, this diastereoisomer was ca. 3-fold more potent than 7b’, indicating that the enzymatic release of dZMP was different and perhaps dependent on the stereochemistry of the phosphorus center.
Since thymidine is also known to block DNA synthesis at millimolar concentrations (1–2 mM), 26 we then tested to see if lower concentrations of thymidine were effective in facilitating the demethylating activity of the ProTides. However, the induction of p16 gene was observed only at 100 µM thymidine and not at lower concentrations, indicating that these cells must be supplemented with a 1:1 ratio of thymidine to ProTide. Replacement of 100 µM thymidine with 100 µM uridine did not aid the ProTides in promoting the induction of p16.
The activity of compounds, 7a and 10, as well as diastereoisomers 7b and 7b’ correlated well with increases in doubling time in cell culture (Table 2). However, 7b and 7b’ caused severe toxicity at 100 µM while 7a and 10 were less toxic at the same concentrations (data not shown).
Table 2.
Percent increase in doubling time of Cf-Pac-1 pancreatic carcinoma cells treated with dZMP prodrugs continuously for 8 days in the presence of 100 µM thymidine
| Compound | Concentration | Doubling Time (% increase) |
|---|---|---|
| Untreated control | — | 0 |
| Ethanol | 0.1% | 23 |
| Zebularine | 100 µM | 103 |
| Thymidine | 100 µM | 0 |
 |
1 µM | 58 |
| 10 µM | 81 | |
 |
1 µM | 13 |
| 10 µM | 116 | |
| 1 µM | 64 | |
| 10 µM | 33 | |
 |
10 µM | 36 |
| 100 µM | 23 |
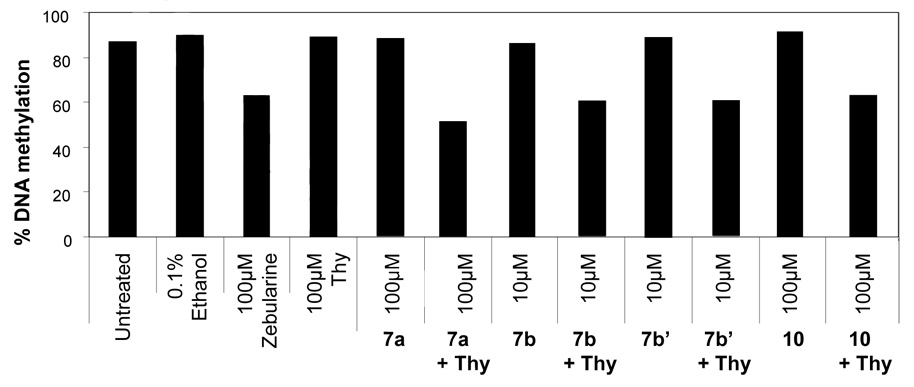
To confirm that these phosphoramidates were indeed hypomethylating the DNA in these cells, we analyzed the DNA methylation status of Cf-Pac-1 cells after treatment with 7a, 10, 7b and 7b’ at three different loci by Ms-SNuPE. 27 The regions analyzed were theCpG-rich promoter region of p16, the CpG-poor promoter region of MAGEA1, and a repeat sequence known as D4Z4. All three loci are hypermethylated and have previously been shown to lose DNA methylation after zebularine treatment. 11 The results of DNA methylation analyses at all three regions were similar: the dZMP ProTides caused a decrease in the methylation level in the presence of thymidine. The pattern is maintained whether the region is a CpG-rich, CpG-poor, or repetitive element containing regions. Figure 2 summarizes the DNA methylation status at D4Z4; all four compounds tested showed demethylation. As before, these drugs worked best in Cf-Pac-1 pancreatic cancer cells and marginally in HCT15 colon cancer cells (data not shown).
Figure 2. DNA methylation analysis of D4DZ in Cf-Pac-1 pancreatic cancer cells treated with 2’-deoxyzebularine ProTides 7a, 7b, 7b’ and 10.
Cf-Pac-1 cells were treated with ProTides continuously for 8 days and DNA was collected. DNA methylation status of the D4DZ repeats expressed in percent methylation was analyzed by quantitative Ms-SNuPE. Treatment with these compounds caused demethylation at the D4DZ locus.
Discussion
Zebularine is an effective inhibitor of DNA methylation capable of inducing the expression of methylation-silenced genes upon treatment. It has many properties that are advantageous as a chemotherapeutic agent, including stability in aqueous solution as well as low toxicity in animals and cultured cells.5,11, 28 However, zebularine must undergo several metabolic steps before DNA incorporation,15 which subsequently weakens the demethylating effect of the drug. A number of aryloxyphosphoramidate nucleotide prodrugs (ProTides) of 2’-deoxyzebularine (dZMP) were tested in search of a demethylating agent more potent and efficient than zebularine. The ProTides that were effective displayed good activity in Cf-Pac-1 pancreatic cancer cells only in the presence of 100 µM thymidine; they were marginally active in HCT15 colon cancer cells and inactive in T24 bladder cancer cells, even in the presence of thymidine. Such cell line specificity of the phosphoramidates may be due to the availability and differential activities of enzymes involved in the release of the dZMP moiety.
The unexpected finding of this investigation was that the phosphoramidates were not able to induce p16 expression in the absence of thymidine. Thymidine was used to prevent the inhibition of TS24 and dCMP deaminase25 by dZMP, which would deplete TMP levels. Since zebularine depends on the synthesis of new DNA to inhibit methylation and the inhibition of TS and dCMP would block DNA synthesis due to the shortage of TMP, it was imperative to supplement the cells with an outside source of thymidine.
Although these compounds demonstrated conceptually that the treatment with dZMP ProTides inhibits DNA methylation efficiently, the required administration of thymidine might particularly limit their use in a potential clinical setting. Nevertheless, it might be feasible to use this approach in the future.
In summary some dZMP ProTides were capable of inducing greater expression of the p16 gene than zebularine. Although they are very cell line-specific and require the presence of thymidine these drugs provide proof of concept that two enzymes, dCK and RNR, can be efficiently circumvented by these ProTides which represent a promising start for the development of future prodrug strategies for 2’-deoxyzebularine.
Materials and Methods
Cell lines and drug treatment
T24 bladder carcinoma cells and HCT116 colon carcinoma cells were obtained from the American Type Culture Collection (Rockville, MD) and cultured in McCoy’s 5A medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 units/mL penicillin, and 100µg/mL streptomycin (Invitrogen, Carlsbad, CA). Cf-Pac-1 pancreatic carcinoma cells also from American Type Culture Collection were cultured in IMDM medium (Gibco/Life Technologies Inc., Palo Alto, CA) supplemented with 10% heat-inactivated FCS, 100 units/mL penicillin, and 100µg/ml streptomycin (Invitrogen, Carlsbad, CA). All cells were cultured in a humidified incubator at 37°C in 5% CO2. Zebularine and 5-aza-CdR (Sigma-Aldrich, St. Louis, MO) were dissolved in PBS. Zebularine prodrugs were dissolved in PBS or ethanol, depending on solubility, and stored at −80°C. Cells were seeded (3×105 cells/10cm dish) and treated with various compounds after 24 hr. The medium was changed every 2–3 days after the initial treatment and supplemented with a fresh dose of drug.
Determination of doubling time
The cell number/dish was counted with a Z1 Coulter Particle Counter (Beckman Coulter Corporation, Hialeh, FL) every 2 to 3 days. Untreated cells were analyzed under similar conditions as a control. The average cell number from two plates was determined, and the mean cell numbers were plotted to define the cell population doubling times, where population doubling PD = log (number of cells harvested/ number of cells seeded)/log 2. Initial drug treatment was started 24 h after seeding.
Nucleic acid isolation
RNA was collected from T24 and HCT116 cells with the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. DNA was collected using the DNeasy Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol.
Quantitative RT-PCR
Total RNA (5µg) was reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA) and random primers (Invitrogen, Carlsbad, CA). The reverse transcription was carried out in a total volume of 50µL as previously described.23 The quantitation of mRNA levels was carried out by a real-time fluorescence detection method as described previously.11,22 All samples were normalized to the reference gene, GAPDH. The primer and probe sequences are as follows: for p16, sense 5’-CTG CCC AAC GCA CCG A-3’, probe 5’ 6-FAM –TGG ATC GGC CTC CGA CCG TAA CT BHQ-1 3’, and antisense 5’-CGC TGC CCA TCA TCA TGA C-3’; for SPANXA1, sense 5’-TGT GAT TCC AAC GAG GCC A-3’, probe 5’ 6-FAM-CGA GAT GAT GCC GGA GAC CCC A BHQ-1 3’, and antisense 5’-GCG GGT CTG AGT CCC CA-3’; for MAGEA1, sense 5’-GAA CCT GAC CCA GGC TCT GTG-3’, probe 5’ 6-FAM-CAA GGT TTT CAG GGG ACA GGC CAA C BHQ-1 3’, and antisense 5’-CCA CAG GCA GAT CTT CTC CTT C’-3’; for MAGEB2, sense 5’-CGG CAG TCA AGC CAT CAT G-3’, probe 5’ 6-FAM-TCG TGG TCA GAA GAG TAA GCT CCG TGC BHQ-1 3’, and antisense 5’-GCG GGT CTG AGT CCC CA-3’; for GAGE, sense 5’-GCT GAT AGC CAG GAA CAG GG-3’, probe 5’ 6-FAM-CAC CCA CAG ACT GGG TGT GAG TGT GA BHQ-1 3’, and antisense 5’-CCT GCC CAT CAG GAC CAT C-3’; for XAGEA1, sense 5’-TCC CCA GAC GGG ACC AG-3’, probe 5’ 5-FAM-AGA GGG ACG GCA TGA GCG ACA CAC BHQ-1 3’, and antisense 5’-CTG GCT GTG TGG TTC TGT GTT T-3’; for GAPDH, sense 5’-TGA AGG TCG GAG TCA ACG G-3’, probe 5’ 6-FAM –TTT GGT CGT ATT GGG CGC CTG G BHQ-1 3’, and antisense 5’-AGA GTT AAA AGC AGC CCT GGT G-3’. The conditions for real time RT-PCR are: 94°C for 9 min followed by 45 cycles at 94°C for 15 s and 60°C for 1 min.
Quantitation of DNA methylation
Genomic DNA (4µg) was treated with sodium bisulfite as previously described.11 Methylation analysis was performed using the methylation-sensitive single-nucleotide primer extension (Ms-SNuPE) assay for p16 5’ region as previously described.27 The PCR primers used are: for p16, sense 5’-TTT GAG GGA TAG GGT-3’ and antisense 5’-TCT AAT AAC CAA CCA ACC CCT CC-3’; for MAGEA1, sense 5’-GTT TAT TTT TAT TTT TAT TTA GGT AGG A-3 and antisense 5’-TTA CCT CCT CAC AAA ACC TAA A-3’; for MAGEB2, sense 5’-TTG AGG GAG GTG GGG GTA TTG T-3’ and antisense 5’-CTT CAA TTT ACA CTC AAA ATC CTC ACC T-3’; for D4Z4, sense 5’-GGG TTG AGG GTT GGG TTT AT-3’ and antisense 5’- AAC TTA CAC CCT TCC CTA CA-3’. An initial denaturation at 94°C for 3 min was followed by 94°C for 45 s, annealing for 45 s, 72°C for 45 s for 40 cycles. The annealing temperatures for each locus are: for p16, 65°C, for MAGEA1, 53°C, for MAGEB2, 62 °C, and for D4Z4, 58 °C. Primers used for Ms-SNuPE analysis are as follows: for p16, 5’-TTT TTT TGT TTG GAA AGA TAT-3’, 5’-TTT TAG GGG TGT TAT ATT-3’, and 5’-GTA GAG TTT AGT T-3’; for MAGEA1, 5’-AGG TTT TTA TTT TGA GGG A-3’, 5’-TGG GGT AGA GAG AAG-3’, and 5’-TTT TAT TTT TAT TTA GGT AGG ATT-3’; for MAGEB2, 5’-ATT GTT TGG AGG TTG G-3’, 5’-GAG GAT TTT TAG TGA AGA-3’, and 5’-GAT GTG GTT TAT TTT GAT TTT-3’; for D4Z4, 5’-TGA GGG TTG GGT TTA TAG T-3’, TAT ATT TTT AGG TTT AGT TTT GTA A-3’, 5’-GTG GTT TAG GGA GTG GG-3’, and 5’-GAA AGG TTG GTT ATG T-3’. Conditions for primer extension were: 94°C for 1 min, 50°C for 30 s, and 72°C for 20 s.
Synthesis
General Procedures
All experiments involving water-sensitive compounds were conducted under scrupulously dry conditions. Anhydrous tetrahydrofuran (THF) and dichloromethane were purchased from Aldrich and used directly. Column chromatography refers to flash column chromatography carried out using Merck silica gel 60 (40–60 µm) as stationary phase. Proton, carbon and phosphorus nuclear magnetic resonance (1H, 13C, 31P NMR) spectra were recorded on a Bruker Avance spectrometers operating either at 500, 125, and 202 MHz, or 300, 75 and 121 MHz. For compounds 3 and 4 the spectrometer was a Varian Unity Inova instrument operating at 400, 100 and 161.9 MHz. The solvents used are indicated for each compound. All 13C and 31P spectra were recorded proton decoupled. Chemical shifts for 1H and 13C spectra are in parts per million downfield from tetramethylsilane. Coupling constants are referred to as J values. Signal splitting patterns are described as singlet (s), doublet (d), triplet (t), quartet (q), broad signal (br), doublet of doublet (dd), doublet of triplet (dt), or multiplet (m). Chemical shifts for 31P spectra are in parts per million relative to an external phosphoric acid standard. Many proton and carbon NMR signals were split because of the presence of (phosphate) diastereoisomers in the samples. The mode of ionization for mass spectrometry for compounds 3 and 4 was fast atom bombardment (FAB) using MNOBA (m-nitrobenzylalcohol) as matrix. Electrospray mass spectra were obtained using a Waters LCT time of flight mass spectrometer coupled to a Waters M600 HPLC pump. Samples were dissolved in Methanol and injected into the solvent stream via a Rheodyne injector. The mobile phase used was Methanol at a flow rate of 200 µL/minute. The electrospray source was operated at a temperature of 130 °C with a desolvation temperature of 300 °C, a capillary voltage of 3kV and cone voltage of 30V. Data was collected in the continuum mode over the mass range 100–2000 amu and processed using Masslynx 4.1 software. Accurate mass measurements were facilitated by the introduction of a single lockmass compound of known elemental composition into the source concurrently with sample. The purity of crude compounds 7a, 7b, 7b’ and 10 was established by HPLC chromatography which ranged from 85 to 93%. Single peaks of pure compounds were collected dried and weighed accurately.
Standard Procedure A: synthesis of phosphorodichloridate
Phosphorus oxychloride (1.0 mmol) was added to a stirred solution of the appropriate phenol or naphthol (1.0 mmol) in dry ether. Then the solution was stirred at −78 °C and anhydrous triethylamine (1.0 mmol) was added dropwise. After 1 hr the reaction was left to rise to room temperature and stirred for about 3 hr, monitoring the formation of the desired compound by 31P-NMR. The triethylamine hydrochloride salt was filtered off and the filtrate reduced to dryness to give a crude oil as product that was used without further purification for the next step.
Standard Procedure B: synthesis of phosphorochloridate
Anhydrous triethylamine (2.0 mol eq.) was added dropwise at −78 °C to a stirred solution of the appropriate phosphorodichloridate (1.0 mol eq.) and the appropriate amino acid hydrochloride salt (1.0 mol eq.) in anhydrous dichloromethane (15–30 mL). After 1 hr the reaction was allowed to slowly warm to room temperature and the formation of the desired compound was monitored by 31P-NMR. The solvent was removed under reduced pressure and the crude residue was re-suspended in anhydrous ether and filtered under nitrogen. The corresponding filtrate was reduced to dryness and purified by flash chromatography (ethyl acetate/hexane 7/3) to give the product as an oil.
Standard Procedure C: synthesis of phosphoramidate (tBuMgCl method)
tert-Butylmagnesiumchloride (1M solution THF, 1.2 mmol) was added to a stirring suspension of the appropriate nucleoside (1.0 mmol) in dry THF (10 mL), under argon atmosphere. The appropriate phosphorochloridate (1.2 mmol) dissolved in dry THF was added dropwise and the reaction was left stirring overnight. Volatiles were evaporated and the residue was purified by flash chromatography (CH2Cl2/CH3OH) to give the desired product.
Standard Procedure D: synthesis of phosphoramidate (NMI method)
N-methylimidazole (NMI, 5.0 mmol) was added to a stirring suspension of the appropriate nucleoside (1.0 mmol) in dry THF (10 mL), under argon atmosphere. The appropriate phosphorochloridate (3.0 mmol) dissolved in THF was added dropwise and the reaction left stirring overnight. Volatiles were evaporated and the residue was dissolved in CH2Cl2 and washed with HCl 0.5 M. The organic layer was dried over MgSO4, filtered, reduced to dryness and purified by flash chromatography (CH2Cl2/CH3OH).
Standard Procedure E: synthesis of phosphoramidate (variant of the NMI method)
A stirred solution of the appropriate nucleoside (1.0 mmol) in anhydrous pyridine (5 mL) was treated with the appropriate phosphorochloridate (3.4 mmol) in dry THF (4 mL) under argon atmosphere. N-methylimidazole (NMI, 6.3 mmol) was then added and the reaction was stirred overnight. Additional amounts of phosphorochloridate (3.4 mmol) and NMI (6.3 mmol) were added and stirring continued for 4 additional hours. Volatiles were evaporated and the residue was dissolved in CH2Cl2 and chromatographed twice by flash column chromatography (CH2Cl2/CH3OH).
2’-Deoxyzebularine-5’-[phenyl(methoxy-L-alaninyl)] phosphate (3)
Prepared according to standard procedure E, using 2’-deoxyzebularine (0.09 g, 0.42 mmol), methylchlorophenylphosphoryl-L-alaninate (2 × 0.40 g, 2.88 mmol) and NMI (2 × 0.24 mL, 6 mmol). The crude was purified twice by column chromatography (CH2Cl2/CH3OH 93/7) and co-evaporated with ether (2 × 5 mL) to give the pure product as a white foamy solid (57 mg, 29.7% yield); 31P-NMR (CDCl3, 161.9 MHz): δ 3.23, 3.36; 1H-NMR (CDCl3, 400 MHz): δ 1.43 (3H, m, CH3), 2.00–2.10 (1H, m, H-2’), 2.60–2.70 (1H, m, H-2’), 3.50–3.70 (4H, m, H-4’, CO2CH3), 3.90–4.05 (1H, m, CH), 4.10 (1H, m, H-3’), 4.25–4.40 (2H, m, H-5’), 6.10 (1H, m, H-5), 6.22 (1H, m, H-1’), 7.10–7.30 (5H, m, PhO), 8.14 (1H, m, H-6), 8.50 (1H, br s, H-4); 13C-NMR (CDCl3, 100 MHz): δ 19.8 (CH3CH), 40.2 (C-2’), 49.3, 51.6 (CHCH3), 64.7 (CH3O), 68.7 (C-5’), 68.9 (C-3’), 86.6 (C-4’), 103.3, 109.1, 118.9, 119.1, 124.2, 128.8, 142.6, 149.4, 154.5, 164.6, 172.9, 173.1 (CO2Me); FAB-MS m/e: 454.1 (MH+, 15%); Anal. Calcd for C19H24N3O8P: C, 50.33; H, 5.34; N, 9.27. Found: C, 50.04; H, 5.59; N, 9.03.
2’-β-Fluro-2’-deoxyzebularine-5’-[phenyl(methoxy-L-alaninyl)] phosphate (4)
Prepared according to standard procedure E, using 2’-fluoro-2’-deoxyzebularine (0.230 g, 1 mmol), methylchlorophenylphosphoryl-L-alaninate (2 × 0.95 g, 6.8 mmol), NMI (2 × 0.5 mL, 12.54 mmol). The crude was purified twice by column chromatography (CH2Cl2/CH3OH 93/7) and co-evaporated with ether (2 × 5 mL) to give the pure product as a white foamy solid (160 mg, 34% yield); 31P-NMR (CDCl3, 161.9 MHz): δ 3.46, 3.82; 1H-NMR (CDCl3, 400 MHz): δ 1.30–1.36 (3H, 2 d, J = 5.4 Hz, CH3), 3.63 and 3.65 (3H, singlets, CO2CH3), 3.70–3.85 (1H, dt, J = 37.6, 10.0 Hz, H-2’), 3.91–4.04 (1H, m, CH), 4.22–4.40 (3H, m, H-5’, H-4’), 5.28 (1H, dm, J = 50.8 Hz, H-3’), 6.26 (1H, dt, J = 19.9, 2.9 Hz, H-1’), 6.33 (1H, br t, H-5), 7.05–7.30 (5H, m, PhO), 8.08–8.14 (1H, ddd, J = 12.7, 6.6, 1.9 Hz, H-6), 8.54 (1H, br s, H-4); 13C-NMR (CHCl3, 100 MHz): δ 19.6, 19.7, (CH3CH), 49.2 (CHCH3), 51.52, 64.6, 64.8 (C-5’), 73.4, 73.6 (C4’), 83.0, 83.1, 83.2, 83.3 (C3’), 86.12 (t), 93.0 (d, J = 195 Hz, C-2’), 103.3 (C-1’), 119.0, 119.1, 119.2, 119.3, 124.1, 128.7, 128.8, 144.3, 149.4, 149.5, 149.6, 154.3, 165.3, 172.9 (C=O); FAB-MS m/e: 472.4 (MH+, 70%); Anal. Calcd for C19H23FN3O8P•0.5H2O: C, 47.50; H, 5.04; N, 8.75. Found: C, 47.54; H, 5.06; N, 8.57.
2’-Deoxyzebularine-5’-[4-chlorophenyl(ethoxy-L-alaninyl)] phosphate (5a)
Prepared according to Standard Procedure D, from 2’-deoxyzebularine (0.19 g, 0.88 mmol), 4-chloro-phenyl(ethoxy-L-alaninyl) phosphorochloridate (0.86 g, 2.64 mmol), NMI (0.36 g, 4.40 mmol, 0.35 mL) and dry THF (10 mL). The crude was purified by column chromatography (CH2Cl2/CH3OH 95/5) to give the pure product as a white foamy solid (88 mg, 20.0% yield); 31P-NMR (CDCl3, 121 MHz): δ 3.23, 3.32; 1H-NMR (CDCl3, 300 MHz): δ 1.11–1.20 (3H, m, OCH2CH3), 1.39–1.42 (3H, m, CHCH3), 1.99–2.09 (1H, m, one of H-2’), 2.61–2.73 (1H, m, one of H-2’), 3.83–4.40 (9H, m, H-3’, H-4’, H-5’, OH-3’, CHNH, CHNH, OCH2CH3), 6.10–6.30 (2H, m, H-5, H-1’), 7.13–7.22 (4H, m, pCl-Ph), 8.09–8.19 (1H, m, H-4), 8.45–8.53 (1H, m, H-6); 13C-NMR (CDCl3, 75 MHz): δ 14.1 (CH3CH2O), 20.8 (CH3CH), 41.2 (C-2’), 50.3, 50.4 (CHCH3), 61.8 (CH3CH2O), 65.9 (C-5’), 69.8, 70.0 (C-3’), 85.4, 85.5 (C-4’), 87.7 (C-1’), 121.4, 121.5, 121.6, 129.8 (C-5, pCl-Ph), 130.5 (C-1 pCl-Ph), 143.6 (C-4), 149.0 (C-4 pCl-Ph), 155.5 (C-2), 165.7 (C-6), 173.6 (CO2Et). HPLC (H2O/CH3CN from 70/30 to 0/100 in 10 min): Rt 4.61 min.
2’-Deoxyzebularine-3’,5’-bis-[4-chlorophenyl(ethoxy-L-alaninyl)] phosphate (5b)
Isolated through column chromatography purification of 5a crude reaction mixture and purified by preparative reverse phase HPLC (40 mg, 9.1% yield) and obtained as a white, foamy solid; 31P-NMR (CDCl3, 121 MHz): δ 1.84, 1.89, 2.42, 2.47, 3.04, 3.09, 3.11; 1H-NMR (CDCl3, 300 MHz): δ 1.11–1.37 (12H, m, 2 CHCH3 2 OCH2CH3). 1.98–2.14 (1H, m, one of H-2’), 2.78–2.97 (1H, m, one of H-2’), 3.82–4.42 (9H, m, H-4’, H-5’, 2 CHNH, 2 CHNH, 2 OCH2CH3), 4.99–5.21 (1H, m, H-3’), 6.03–6.30 (2H, m, H-5, H-1’), 7.01–7.28 (8H, m, 2 pCl-Ph), 8.03–8.18 (1H, m, H-4), 8.42–8.51 (1H, m, H-6); 13C-NMR (CDCl3, 75 MHz): δ 14.1 (2 CH3CH2O), 20.6, 20.8, 20.9 (2 CH3CH), 39.8, 39.9, 40.0 (C-2’), 50.2, 50.3, 50.4, 50.5 (2 CHCH3), 61.8, 61.9 (2 CH3CH2O), 65.6, 65.7, 66.8, 66.9 (C-5’), 76.7, 77.0, 77.1 (C-3’), 84.2, 84.3 (C-4’), 87.3, 87.4 (C-1’), 121.3, 121.5, 121.6, 129.7, 129.8 (C-5, 2 pCl-Ph), 130.7 (C-1 pCl-Ph), 143.8, 144.0, 144.1 (C-4), 149.1, 149.2 (2 C-4 pCl-Ph), 155.3, 155.4 (C-2), 165.5, 165.6 (C-6), 173.4, 173.5, 173.7 (CO2Et). HPLC (H2O/CH3CN from 70/30 to 0/100 in 10 min): Rt 7.21 min.
2’-Deoxyzebularine-5’-[phenyl(benzoxy-L-alaninyl)] phosphate (6)
Prepared according to Standard Procedure D, from 2’-deoxyzebularine (0.20 g, 0.94 mmol), phenyl(benzoxy-L-alaninyl) phosphorochloridate (1.00 g, 2.83 mmol), NMI (0.39 g, 4.70 mmol, 0.37 mL) and dry THF (10 mL). The crude was purified by column chromatography (CH2Cl2/CH3OH 96/4) and preparative TLC (CH2Cl2/CH3OH 96/4) to give the pure product as a white foamy solid (70 mg, 14.1% yield); 31P-NMR (CDCl3, 121 MHz): δ 4.09, 4.28; 1H-NMR (CDCl3, 300 MHz): δ 1.42–1.45 (3H, m, CHCH3), 1.99–2.12 (1H, m, one of H-2’), 2.69–2.78 (1H, m, one of H-2’), 4.03–4.48 (7H, m, H-3’, H-4’, H-5’, OH-3’, CHNH, CHNH), 5.14–5.22 (2H, m, PhCH2), 6.18–6.32 (2H, m, H-5, H-1’), 7.20–7.38 (10H, m, PhO, PhCH2), 8.21–8.25 (1H, m, H-4), 8.46–8.54 (1H, m, H-6); 13C-NMR (CDCl3, 75 MHz): δ19.6,19.7,19.8 (CH3), 40.2 (C-2’), 40.3, 40.4, 40.5 (CHCH3), 64.6, 64.7, 64.8 (C-5’), 66.2, 66.3 (CH2Ph), 68.8, 68.9 (C-3’), 84.4, 84.5, 84.6, 84.7 (C-4’), 86.6 (C-1’), 103.3 (C-5), 119.0, 119.1, 119.3, 124.2, 127.2, 127.5, 127.6, 127.7, 128.7, 128.8 (Ph), 134.2 (‘ispo’ CH2Ph), 142.5, 142.6 (C-4), 149.4, 149.5 (‘ipso’ OPh), 164.6, 164.8 (C-6), 172.3, 172.4 (COOCH2Ph). HPLC (H2O/CH3CN from 100/0 to 0/100 in 20 min): Rt 12.37 min.
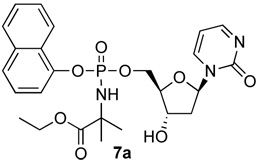
2’-Deoxyzebularine-5’-[naphthyl(ethoxy-dimethylglycinyl)] phosphate (7a)
Prepared adopting the standard procedure D using 2’-deoxyzebularine (0.13 g, 0.60 mmol) in dry THF (10 mL), NMI (0.24 mL, 3.0 mmol), naphthyl-(ethoxy-dimethylglycinyl)- phosphorochloridate (0.64 g, 1.8 mmol). The use of procedure D minimized the amount of 3’,5’-bisphosphoramidates, whereas procedure C failed to provide 7a. The crude was purified by column chromatography (CH2Cl2/CH3OH 96/4) to give the pure product as a white solid (35.0 mg, 11.0%); 31P-NMR (MeOD, 202 MHz): δ 2.93, 2.94; 1H-NMR (MeOD, 500 MHz): δ 1.12–1.15 (3H, m, CH2CH3) 1.42–1.45 (6H, m, CH3), 1.58–1.67 (1H, m, H-2’), 2.30–2.36 (1H, m, H-2’), 4.03–4.37 (6H, m, H-3’, H-4’, H-5’, CH2CH3) , 5.39–5.97 (1H, m, H-1’), 6.08–6.17 (1H, m, H-5), 7.26–8.07 (7H, m, Naph), 8.09–8.12 (1H, m, H-4), 8.31–8.37 (1H, m, H-6); 13C-NMR (MeOD, 125 MHz): δ 14.45 (CH3CH2), 27.56, 27.59, 27.99, 28.05, 28.11 (CH3), 42.21, 42.29 (C-2’), 62.66 (CH3CH2), 67.54, 67.58, 67.63 (C-5’), 71.73, 71.87 (C-3’), 87.57, 87.63, 87.69 (C-4’), 89.39, 89.46 (C-1’), 106.06 (C-5), 116.29, 116.31, 116.76, 116.78, 122.83, 122.95, 126.00, 126.10, 126.53, 126.58, 127.48, 127.53, 127.89, 128.95 (Naph), 136.31 (ipso Naph), 145.56 (C-4), 157.04 (C-2), 167.03, 167.12 (C-6), 176.81 (C=O); MS (ES) m/e: 554.4 (MNa+, 100%); Accurate mass: C25H30N3O8NaP required 554.1688, found 554.1664. HPLC (H2O/CH3CN from 100/0 to 0/100 in 20 min): Rt 11.92 min.
2’-Deoxyzebularine-3’,5’-bis-[naphthyl(ethoxy-dimethylglycinyl)] phosphate (7b and 7b’)
Prepared adopting the standard procedure C, using 2’-deoxyzebularine (0.15 g, 0.70 mmol) in dry THF (10 mL), tBuMgCl (1M solution THF, 0.84 mL, 0.84 mmol), naphthyl-(ethoxy-dimethylglycinyl)-phosphorochloridate (0.30 g, 0.84 mmol). The crude was purified by column chromatography (CH2Cl2/CH3OH 96/4) to give the pure product as a white solid (fast eluting fraction = 24.4 mg, 4.1%; slow eluting fraction = 19.4 mg, 3.2%).
Fast eluting fraction (7b)
31P-NMR (MeOD, 202 MHz): δ 2.60, 2.93; 1H-NMR (MeOD, 500 MHz): δ 1.17–1.26 (6H, m, CH2CH3), 1.42–1.57 (12H, m, CH3), 1.75–1.78 (1H, m, H-2’), 2.75–2.80 (1H, m, H-2’), 4.09–4.17 (4H, m, CH2CH3), 4.32–4.41 (3H, m, H-4’, H-5’), 5.22–5.24 (1H, m, H-3’), 5.95–5.98 (1H, m, H-1’), 6.06–6.08 (1H, m, H-5), 7.34–8.24 (15H, m, Naph, H-4), 8.39–8.40 (1H, m, H-6); 13C-NMR (MeOD, 125 MHz): δ 14.41, 14.47 (CH3CH2), 27.46, 27.55, 27.58, 27.96, 28.02, 28.13, 28.18 (CH3), 40.70, 40.74 (C-2’), 62.58, 62.66 (CH3CH2), 66.81, 66.85 (C-5’), 77.81, 77.85 (C-3’), 86.00, 86.07, 86.10 (C-4’), 89.14 (C-1’), 106.08 (C-5), 116.95, 123.00, 123.07, 126.13, 126.20, 126.53, 126.61, 127.51, 127.58, 127.84, 127.93, 128.89, 128.94 (Naph), 136.25, 136.37 (ipso Ph), 145.32 (C-4), 156.78 (C-2), 167.12 (C-6), 176.70, 176.88 (C=O); MS (ES) m/e: 873.4 (MNa+, 100%); Accurate mass: C41H48N4O12NaP2 required 873.2642, found 873.2661. HPLC (H2O/CH3CN from 100/0 to 0/100 in 20 min): Rt 18.04 min.
Slow eluting fraction (7b’)
31P-NMR (MeOD, 202 MHz): δ 2.14, 2.69; 1H-NMR (MeOD, 500 MHz): δ 1.04–1.14 (6H, m, CH2CH3), 1.36–1.45 (12H, m, CH3), 1.78 1.84 (1H, m, H-2’) , 2.69–2.73 (1H, m, H-2’), 3.95–4.05 (4H, m, CH2CH3) , 4.22–4.30 (3H, m, H-4’, H-5’), 5.09–5.11 (1H, m, H-3’ , 5.92–5.96 (1H, m, H-1’), 6.19–6.22 (1H, m, H-5), 7.21–8.13 (15H, m, Naph, H-4), 8.39–8.40 (1H, m, H-6); 13C-NMR (MeOD, 125 MHz): δ 14.41, 14.46 (CH3CH2), 27.55, 27.58, 27.61, 27.94, 28.00, 28.07, 28.13 (CH3), 40.83, 40.87 (C-2’), 62.61, 62.64 (CH3CH2), 67.31, 67.36 (C-5’), 78.81, 78.85 (C-3’), 86.37, 86.43, 86.47 (C-4’), 89.35, 89.45 (C-1’), 106.21 (C-5), 116.31, 116.33, 116.85, 116.88, 122.81, 123.03, 125.98, 126.14, 126.52, 127.51, 127.81, 127.84, 127.96, 128.01, 128.87, 128.91 (Naph), 136.27, 136.34 (ipso Ph), 145.55 (C-4), 156.99 (C-2), 167.40 (C-6), 176.72, 176.81 (C=O); MS (ES) m/e: 873.2 (MNa+, 100%); Accurate mass: C41H48N4O12NaP2 required 873.2642, found 873.2669. HPLC (H2O/CH3CN from 100/0 to 0/100 in 20 min): Rt 17.75 min.
2’-Deoxy-2’-β-fluoro zebularine-5’-[phenyl(benzoxy-L-alaninyl)] phosphate (8)
Prepared adopting the standard procedure D, using 2’-deoxy-2’-β-fluoro zebularine (200 mg, 0.87 mmol), in dry THF (10 mL), NMI (345 µL, 4.35 mmol), phenyl-(benzoxy-dimethylglycininyl)-phosphorochloridate 1 M in dry THF (2.61 mL, 2.61 mmol). The crude mixture was purified by flash chromatography (CH2Cl2/CH3OH gradient elution from 98/2 to 95/5) and semi-preparative TLC, to give the pure product as a white foamy solid (110 mg, 23%); 31P-NMR (MeOD, 121 MHz): δ 5.00, 5.21; 1H-NMR (MeOD, 300 MHz): δ 1.30–1.43 (3H, m, CH3CH), 3.96–4.40 (5H, m, H-3’, H-4’, H-5’, CHCH3), 5.06–5.26 (3H, m, H-2’, CH2Ph), 6.15–6.30 (1H, m, H-1’), 6.45–6.55 (1H, m, H-5), 7.13–7.43 (10H, m, Ph), 8.26–8.36 (1H, m, H-4), 8.56–8.65 (1H, m, H-6); 13C-NMR (MeOD, 75 MHz): δ 20.5, 20.6, 20.7 (CH3CH), 52.0 (CHCH3), 66.9, 67.0 (C-5’), 68.2 (CH2Ph), 75.3, 75.4, 75.6, 75.7 (C-4’), 85.4, 85.5, 85.6 (C-3’), 88.6, 88.8 (C-5), 95.7 (d, J = 195 Hz, C-2’), 106.2 (C-1’), 121.6, 121.7, 121.8, 126.5, 129.5, 129.6, 129.8, 131.0, 131.1, 131.2, 137.5 (Ph), 147.0 (C-4), 152.3, 152.4 (“ipso” OPh), 157.1 (C-2), 168.2, 168.4 (C-6), 174.8, 174.9, 175.0, 175.1 (C=O). HPLC (H2O/CH3CN from 100/0 to 0/100 in 10 min): Rt 7.05 min.
2’-Deoxyzebularine-5’-[phenyl(benzoxy-dimethylglycininyl)] phosphate (9a)
Prepared adopting the standard procedure D, using 2’-deoxyzebularine (135 mg, 0.63 mmol), in dry THF (10 mL), NMI (250 µL, 3.15 mmol), phenyl-(benzoxy-dimethylglycinyl)-phosphorochloridate 1 M in dry THF (1.89 mL, 1.89 mmol). As before, the use of procedure D minimized the amount of 3’,5’-bisphosphoramidates, whereas procedure C failed to provide 9a. The crude mixture was purified with flash chromatography (CH2Cl2/CH3OH gradient elution from 98/2 to 95/5), semi-preparative TLC and eventually HPLC (isocratic elution water/acetonitrile, 60/40) to give the pure product as a white foamy solid (25 mg, 7%); 31P-NMR (MeOD, 121 MHz): δ 2.52, 2.60; 1H-NMR (MeOD, 300 MHz): δ 1.49–1.55 (6H, m, (CH3)2C), 1.80–2.03 (1H, m, H-2’), 2.45–2.63 (1H, m, H-2’), 4.13–4.40 (4H, m, H-3’, H-4’, H-5’), 5.10–5.20 (2H, m, CH2Ph), 6.10–6.20 (1H, m, H-1’), 6.40–6.53 (1H, m, H-5), 7.13–7.43 (10H, m, Ph), 8.30–8.40 (1H, m, H-4), 8.50–8.60 (1H, m, H-6); 13C-NMR (MeOD, 75 MHz): δ 27.5, 27.8, 27.9, 28.0 ((CH3)2C), 42.3 (C-2’), 58.2 ((CH3)2C), 66.9 (C-5’), 67.3, 67.4 (OCH2Ph), 71.7 (C-3’), 87.5, 87.6, 87.7 (C-4’), 89.5, 89.6 (C-1’), 106.2 (C-5), 121.5, 121.6, 126.3, 128.0, 128.3, 129.3, 129.4, 129.5, 129.6, 129.9, 130.8, 132.4, 133.6, 137.4 (Ph), 145.9 (C-4), 152.1, 152.2, 152.3 (“ipso” OPh), 157.2 (C-2), 167.2, (C-6), 176.5 (C=O). HPLC (H2O/CH3CN 60/40): Rt 3.21 min.
2’-Deoxyzebularine-3’,5’-bis-[phenyl(benzoxy-dimethylglycinyl)]-phosphate (9b)
Prepared adopting the standard procedure C, using 2’-deoxyzebularine (0.15 g, 0.70 mmol) in dry THF (10ml), NMI (0.28 ml, 3.50 mmol), phenyl-(benzoxy-dimethylglycinyl)-phosphorochloridate (0.77 g, 2.10 mmol). The crude was purified by column chromatography (CH2Cl2/CH3OH 96/4) to give the pure product as a white solid (60 mg, 9.8%); 31P-NMR (MeOD, 202 MHz): δ 1.50, 1.59, 1.62, 1.88, 2.12, 2.26, 2.31, 2.37; 1H-NMR (MeOD, 500 MHz): δ 1.35–1.40 (12H, m, CH3), 1.76–1.86 (1H, m, H-2’), 2.57–2.73 (1H, m, H-2’), 4.15–4.27 (3H, m, H-4’, H-5’), 5.00–5.08 (5H, m, H-3’, CH2Ph), 5.92–6.02 (1H, m, H-1’), 6.26–6.37 (1H, m, H-5), 7.00–7.27 (20H, m, Ph), 8.16–8.21 (1H, m, H-4), 8.40–8.45 (1H, m, H-6); 13C-NMR (MeOD, 125 MHz): δ 27.43, 27.49, 27.52, 27.64, 27.81, 27.87, 27.90, 27.94, 27.96, 28.00, 28.08, 28.16 (CH3), 40.77, 40.81, 40.89, 40.92 (C-2’), 66.79, 66.83, 67.15, 67.19, 67.28 (C-5’), 68.22, 68.24, 68.27, 68.30 (CH2Ph), 78.36, 78.40, 78.56, 78.59, 78.67, 78.72 (C-3’), 85.95, 86.05, 86.12, 86.17, 86.33, 86.38 (C-4’), 89.21, 89.32, 89.43, 89.48 (C-1’), 106.27, 106.32 (C-5), 121.51, 121.55, 121.60, 121.62, 121.63, 121.66, 121.70, 121.75, 121.76, 121.80, 126.31, 126.35, 129.16, 129.19, 129.24, 129.29, 129.32, 129.34, 129.37, 129.64, 130.88 (Ph), 137.32, 137.38 (ipso Ph), 145.73, 145.84 (C-4), 152.07, 152.10, 152.13(ipso OPh), 157.01, 157.03, 157.06 (C-2), 167.48, 167.52 (C-6), 176.48 (C=O); MS (ES) m/e: 1771.4 (2M + Na+, 100%).
2’-Deoxyzebularine-5’-[naphthyl(benzoxy-dimethylglycinyl)]-phosphate (10)
Prepared adopting the standard procedure D, using 2’-deoxyzebularine (0.13 g, 0.60 mmol) in dry THF (10 mL), NMI (0.24 ml, 3.0 mmol), naphthyl-(ethoxy-dimethylglycinyl)-phosphorochloridate (0.76 g, 1.8 mmol). The crude was purified by column chromatography (CH2Cl2/CH3OH 96/4) to give the pure product as a white solid (7.0 mg, 1.7%); 31P-NMR (MeOD, 202 MHz): δ 2.92, 2.89; 1H-NMR (MeOD, 500 MHz): δ 1.56–1.59 (6H, m, CH3), 1.62–1.72 (1H, m, H-2’), 2.38–2.44 (1H, m, H-2’), 4.13–4.37 (4H, m, H-3’, H-4’, H-5’), 5.13–5.20 (2H, m, CH2Ph), 6.02–6.06 (1H, m, H-1’), 6.17–6.25 (1H, m, H-5), 7.29–8.19 (8H, m, H-4, Naph), 8.42–8.47 (1H, m, H-6); 13CNMR (MeOD, 125 MHz): δ 27.51, 27.54, 27.99, 28.06, 28.13(CH3), 42.20, 42.28 (C-2’), 67.51, 67.56, 67.60 (C-5’), 68.32 (CH2Ph), 71.69, 71.83 (C-3’), 87.52, 87.59, 87.65 (C-4’), 84.37, 89.43 (C-1’), 106.06 (C-5), 116.33, 116.80, 116.83, 122.82, 122.94, 125.99, 126.09, 126.50, 126.56, 127.46, 127.51, 127.87, 128.93, 129.33, 129.59 (Naph, Ph), 136.29 (ipso Ph), 137.31 (ipso Naph), 145.52 (C-4), 156.98 (C-2), 166.99, 167.08 (C-6), 176.56 (C=O); MS (ES) m/e: 616.3 (MNa+, 100%); Accurate mass: C30H32N3O8NaP required 616.1825, found 616.1818.
Acknowledgment
This research was supported in part by the Intramural Research Program of the NIH, Center for Cancer Research, NCI-Frederick.
Abbreviations
- CDA
cytidine deaminase
- dCK
deoxycytidine kinase
- dCMP
deoxycytidine monophosphate deaminase
- DNMT1
DNA methyltransferase 1
- dUMP
deoxyuridine monophosphate
- dZMP
2’-deoxyzebularine-5’-monophoshate
- dZTP
2’-deoxyzebularine-5’-triphosphate
- FCS
fetal calf serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IMDM
Iscove's Modified Dulbecco's Medium
- MAGEA1
melanoma antigen family A1
- MOE SLogP
Molecular Operating Environment SMART log P
- Ms-SNuPE
methylation-sensitive single-nucleotide primer extension
- NMI
N-methylimidazole
- NMR
nuclear magnetic resonance
- P
partition coefficient
- PBS
phosphate buffer saline
- PCR
polymerase chain reaction
- PD
population doubling
- RNR
ribonucleotide-diphosphate reductase
- RT-PCR
reverse transcriptase PCR, SAR, structure-activity relationship
- SAR
structure-activity relationship
- SMART
Smiles Arbitrary Target Specification
- SMILES
Simplified Molecular Input Line Entry Specification
- THF
tetrahydrofuran
- TMP
thymidine monophosphate
- TS
thymidylate synthase
- Z
zebularine
- ZMP
zebularine-5’-monophosphate
- ZDP
zebularine-5’-diphosphate
- ZTP
zebularine-5’-triphosphate
- ZDP-Chol
zebularine-5’-diphosphocholine
References
- 1.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat. Rev. Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 2.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baylin SB. DNA methylation and gene silencing. Nat. Clin. Pract. Oncol. 2005;2:S4–S11. doi: 10.1038/ncponc0354. [DOI] [PubMed] [Google Scholar]
- 4.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat. Rev.Drug Disc. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 5.Cheng JC, Matsen CB, Gonzalez FA, Ye W, Greer S, Marquez VE, Jones PA, Selker EU. Inhibition of DNA methylation and reactivation of silenced genes by zebularine. J. Natl. Cancer Inst. 2003;95:399–409. doi: 10.1093/jnci/95.5.399. [DOI] [PubMed] [Google Scholar]
- 6.Yoo CB, Cheng JC, Jones PA. Zebularine: a new drug for epigenetic therapy. Biochem. Soc. Trans. 2004;32:910–912. doi: 10.1042/BST0320910. [DOI] [PubMed] [Google Scholar]
- 7.McCormack JJ, Marquez VE, Liu PS, Vistica DT, Driscoll JS. Inhibition of cytidine deaminase by 2-oxopyrimidine riboside and related compounds. Biochem. Pharmacol. 1989;29:830–832. doi: 10.1016/0006-2952(80)90566-3. [DOI] [PubMed] [Google Scholar]
- 8.Frick L, Yang C, Marquez VE, Wolfenden R. Binding of pyrimidin-2-one ribonucleoside by cytidine deaminase as a transition-state analogue 3,4-dihydrouridine and contribution of the 4-hydroxyl group to its binding activity. Biochemistry. 1989;28:9423–9430. doi: 10.1021/bi00450a027. [DOI] [PubMed] [Google Scholar]
- 9.Marquez VE, Kelley JA, Agbaria R, Ben-Kasus T, Cheng JC, Yoo CB, Jones PA. Zebularine: A unique molecule for an epigenetically based strategy in cancer chemotherapy. Ann. N.Y. Acad. Sci. 2005;1058:246–254. doi: 10.1196/annals.1359.037. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Cheng X, Connolly BA, Dickman MJ, Hurd PJ, Hornby DP. Zebularine: a novel DNA methylation inhibitor that forms a covalent complex with DNA methyltransferases. J Mol Biol. 2002;321:591–599. doi: 10.1016/S0022-2836(02)00676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng JC, Weisenberger DJ, Gonzalez FA, Liang G, Xu G-L, Hu Y-G, Marquez VE, Jones PA. Continuous zebularine treatment effectively sustains demethylation in human bladder cells. Mol. Cell Biol. 2004;24:1270–1278. doi: 10.1128/MCB.24.3.1270-1278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng JC, Yoo CB, Weisenberger DJ, Chuang J, Wozniak C, Liang G, Marquez VE, Greer S, Orntoft TF, Thykjaer T, Jones PA. Preferential response of cancer cells to zebularine. Cancer Cell. 2004;6:151–158. doi: 10.1016/j.ccr.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Herranz M, Caballero J-M, Fraga MF, Ruiz-Cabello J, Flores JM, Marquez VE, Esteller M. Zebularine is a non-toxic DNA demethylating drug effective against the development of lymphoma. Blood. 2006;107:1174–1177. doi: 10.1182/blood-2005-05-2033. [DOI] [PubMed] [Google Scholar]
- 14.Marquez VE, Rao KVR, Barchi JJ, Jr, Kelley JA, Agbaria R, Ben-Kasus T, Cheng JC, Yoo CB, Jones PA. Zebularine: A unique molecule for an epigenetically-based strategy in cancer chemotherapy. The magic of its chemistry and biology. Nucleosides Nucleotides & Nucleic Acids. 2005;24:305–318. doi: 10.1081/ncn-200059765. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Kasus T, Ben-Zvi Z, Marquez VE, Kelley JA, Agbaria R. Metabolic activation of zebularine, a novel DNA methylation inhibitor in human bladder carcinoma cells. Biochem. Pharmacol. 2005;70:121–133. doi: 10.1016/j.bcp.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Dowd C, Sutch BT, Haworth IS, Eritja R, Marquez VE, Yang AS. Incorporation of zebularine from its 2’-deoxynucleoside triphosphate derivative and activity as a template-coding nucleobase. Nucleoside Nucleotides & Nucleic Acids. 2008;27:131–145. doi: 10.1080/15257770701795888. [DOI] [PubMed] [Google Scholar]
- 17.Balzarini J, Kruining J, Wedgwood O, Pannecouque C, Aquaro S, Perno CF, Naesens L, Witvrouw M, Heijtink R, De Clercq E, McGuigan C. Conversion of 2’,3’-dideoxyadenosine (ddA) and 2’,3’-didehydro-2’,3’-dideoxyadenosine (d4A) to their corresponding aryloxyphosphoramidate derivatives markedly potentiates their activity against human immunodeficiency virus and hepatitis B virus. FEBS Lett. 1997;410:324–328. doi: 10.1016/s0014-5793(97)00616-9. [DOI] [PubMed] [Google Scholar]
- 18.McGuigan C, Cahard D, Sheeka HM, De Clercq E, Balzarini J. Aryl phosphoramidate derivatives of d4T have improved anti-HIV efficacy in tissue culture and may act by the generation of a novel intracellular metabolite. J. Med.Chem. 1996;39:1748–1753. doi: 10.1021/jm950605j. [DOI] [PubMed] [Google Scholar]
- 19.McGuigan C, Wedgwood OM, De Clercq E, Balzarini J. Phosphoramidate derivatives of 2’,3’-didehydro-2,3’-dideoxyadenosine (d4A) have markedly improved anti-HIV potency and selectivity. Bioorg. Med. Chem. Lett. 1996;6:2359–2362. [Google Scholar]
- 20.Perrone P, Daverio F, Valente R, Rajyaguru S, Martin JA, Lévêque V, Le Pogam S, Najera I, Klumpp K, Smith DB, McGuigan C. First example of phosphoramidate approach applied to a 4’-substituted purine Nucleoside (4’-azidoadenosine): Conversion of an inactive nucleoside to a submicromolar compound versus hepatitis C virus. J. Med. Chem. 2007;50:5463–5470. doi: 10.1021/jm070362i. [DOI] [PubMed] [Google Scholar]
- 21.Wildman SA, Crippen GM. Prediction of physicochemical parameters by atomic contributions. J. Chem. Inf. Comput. Sci. 1999;39:868–873. http://iss.nci.nih.gov/cgi-bin/moe-front/moe.tcl). [Google Scholar]
- 22.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Danenberg PV, Laird PW. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res. 1999;59:2302–2306. [PubMed] [Google Scholar]
- 23.Gonzalez-Zulueta M, Bender CM, Yang AS, Nguyen T, Beart RW, Van Tornout J, Jones P. Methylation of the 5’-CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995;55:4531–4535. [PubMed] [Google Scholar]
- 24.Votruba I, Holy A, Wightman RH. The mechanism of inhibition of DNA synthesis in Escherichia coli by pyrimidine-2-one ß-D-ribofuranoside. Biochim Biophys. Acta. 1973;324:14–23. doi: 10.1016/0005-2787(73)90246-3. [DOI] [PubMed] [Google Scholar]
- 25.Barchi JJ, Jr, Cooney DA, Hao Z, Weinberg M, Taft C, Marquez VE, Ford H., Jr Improved synthesis of zebularine [1-(ß-D-ribofuranosyl)-dihydroxypyrimidin-2-one] nucleosides as inhibitors of human deoxycytidylate deaminase. J. Enzyme Inhib. 1995;9:147–162. doi: 10.3109/14756369509042814. [DOI] [PubMed] [Google Scholar]
- 26.Bjursell G, Reichard P. Effects of thymidine on deoxyribonucleoside triphosphate pools and deoxyribonucleic acid synthesis in Chinese hamster ovary cells. J. Biol.Chem. 1973;248:3904–3909. [PubMed] [Google Scholar]
- 27.Gonzalgo ML, Jones PA. Quantitative methylation analysis using methylation-sensitive single-nucleotide primer extension (Ms-SNuPE) Methods. 2002;27:128–133. doi: 10.1016/s1046-2023(02)00064-6. [DOI] [PubMed] [Google Scholar]
- 28.Yoo C, Chuang JC, Byun H-M, Egger G, Yang AS, Dubeau L, Long T, Laird PW, Marquez VE, Jones PA. Long-term epigenetic therapy with oral zebularine has minimal side effects and prevents intestinal tumors in mice. Cancer Prev. Res. 2008 doi: 10.1158/1940-6207.CAPR-07-0008. (published Online First on March 19, 2008 as 10.1158/1940-6207.CAPR-07-0008). [DOI] [PMC free article] [PubMed] [Google Scholar]