Abstract
An increasing body of evidence suggests that the nucleus accumbens (NAcc) is engaged in both incentive reward processes and in adaptive responses to conditioned and unconditioned aversive stimuli. Yet, it has been argued that NAcc activation to aversive stimuli may be a consequence of the rewarding effects of their termination, i.e., relief. To address this question we used fMRI to delineate brain response to the onset and offset of unpleasant and pleasant auditory stimuli in the absence of learning or motor response. Increased NAcc activity was seen for the onset of both pleasant and unpleasant stimuli. Our results support the expanded bivalent view of NAcc function and call for expansion of current models of NAcc function that are solely focused on reward.
The role of the nucleus accumbens (NAcc) in behavior has tended to focus largely on responses toward rewarding and appetitive stimuli and events. However, based on evidence from both human neuroimaging studies and animal-based research a broader role for the NAcc function has been proposed in behavior modulated by aversive events. This imaging study has been designed to test the proposed bivalent function of the NAcc in behavior by addressing two arguments that have been raised against this idea: 1) that activation of the NAcc to aversive stimuli is secondary to some kind of relief as a result of the termination of that event; and 2) that it is a consequence of preparation and regulation of instrumental motor action.
The NAcc has been viewed as the key site for transference of motivational and other emotional signals received from the prefrontal cortex, amygdala and hippocampus to adaptive behavioral responses, and dopamine has been strongly implicated in facilitating this process (Laviolette, 2007; Meredith, 1999). However, this role has largely focused on rewarding appetitive processes (Day and Carelli, 2007), which has tended to overshadow work that has also demonstrated the involvement of the NAcc and the dopaminergic system in aversive emotional processes (Blackburn et al., 1992). For example, dopaminergic midbrain neurons increase firing in response to conditioned and unconditioned aversive stimuli (Guarraci and Kapp, 1999; Horvitz, 2000; but see, Ungless et al., 2004 and Schultz, 1997), as well as novel or unpredicted stimuli (Miller et al., 1981; Rasmussen et al., 1986). Moreover, while the abuse potential of drugs such as amphetamine and cocaine is largely attributed to their rewarding actions, the effects of these drugs are not always hedonic, and there is evidence that their anxiogenic and psychomimetic effects (Mathias et al., 2008; Raven et al., 2000) might also be mediated by the NAcc and its dopaminergic innervation (Broderick et al., 2003; Hunt et al., 2005; Koob et al., 1989; Miczek et al., 1999).
Indeed, the function of the NAcc in gating and modulating goal-directed action (Cardinal et al., 2002) requires the detection of both safety and danger cues in the environment. Thus, an expanded, bivalent view of NAcc function has been advocated, whereby the NAcc is engaged in processing of both rewarding and aversive stimuli (Becerra et al., 2001; Jensen et al., 2003; Reynolds and Berridge, 2002). Consistent with the idea that the NAcc role in behavior is bivalent is that it is richly innervated, not only by the amygdala, which signals the salience of both positive and negative stimuli (Breiter et al., 1996; Demos et al., 2008; Hamann and Mao, 2002; Hamann et al., 2002; Paton et al., 2006), but also by other regions that process both aversive and reward information, such as the orbitofrontal cortex, insula, cingulate cortex, and the midline- and intra-laminar thalamic nuclei (Bourgeais et al., 2001; Haber et al., 2006; Hsu et al., 2000; Vogt, 2005). In turn the NAcc can affect expression of emotion via two routes: 1) It can influence motor action in response to emotive stimuli via its projections to the substantia nigra and ventromedial globus pallidus, which belong to the basal ganglia system involved in motor programming (Zahm and Heimer, 1993); and 2) It can induce significant changes in autonomic and physiological processes to these same stimuli, since it also projects to the lateral hypothalamus, which participates in the autonomic and endocrine expression of emotion (Kirouac and Ganguly, 1995).
The functional significance of the anatomical connectivity of the NAcc is reflected at the physiological level where different neurons in the NAcc can respond to either aversive or appetitive stimuli (Roitman et al., 2005; Setlow et al., 2003; Wheeler et al., 2008; Wilson and Bowman, 2005; Yanagimoto and Maeda, 2003). In addition, behavioral studies in both rodents and non-human primates have shown that the NAcc plays a critical role in aversive conditioning and active avoidance behavior (Ammassari-Teule et al., 2000; Hoebel et al., 2007; Iordanova et al., 2006; Levita et al., 2002; Schwienbacher et al., 2004), and an increasing number of human imaging studies have shown enhanced activity in this region in response to both conditioned and unconditioned aversive stimuli (Becerra et al., 2001; Gottfried et al., 2002; Jensen et al., 2003). Finally, in a manner similar to that previously demonstrated for the amygdala (Etkin et al., 2004; Stein et al., 2007; Straube et al., 2007), studies in non-human primates and humans have demonstrated that the positive association between anxiety levels and responses to aversive and anxiety-evoking stimuli is also modulated by the degree of NAcc activation (Kalin et al., 2005; Sturm et al., 2003).
A role for the NAcc in negative contexts is further supported by studies implicating the NAcc in contextual Pavlovian aversive conditioning (Haralambous and Westbrook, 1999; Levita et al., 2002; Westbrook et al., 1997), as well as conditioned inhibition of ongoing instrumental action (Parkinson et al., 1999). The latter is consistent with evidence implicating the NAcc in mediating the interaction between Pavlovian and instrumental contingencies (Hall et al., 2001). However, in contrast to the detrimental effect of lesions of the amygdala on fear learning (for review see, Maren, 2001; Phelps and LeDoux, 2005), some studies have failed to find an effect of lesions or pharmacological manipulations of the NAcc on discrete cue Pavlovian aversive conditioning (e.g., Levita et al., 2002; Westbrook et al., 1997). This finding is also mirrored in a number of human imaging studies failing to show NAcc activation in response to conditioned aversive stimuli (Chandrasekhar et al., 2008; Hamann and Mao, 2002; Phelps et al., 2004). Moreover, it could be argued that NAcc activity observed in anticipation of, or response to, negative events, is due to the rewarding effects of termination of an aversive event rather than a result of a response to the noxious stimuli (Ikemoto and Panksepp, 1999). Additionally, since the NAcc influences instrumental behavior by allowing Pavlovian conditioned stimuli (CSs) to affect the level of instrumental responding (Cardinal et al., 2002), the engagement of the NAcc in some studies may reflect its role in modulating instrumental motor actions dissociable from emotion.
To address these two possibilities, we designed an fMRI study in which we could dissociate brain activation to the initiation and termination of unpleasant and pleasant auditory stimuli in the absence of learning or a motor response. Consequently, in this study subjects were required to passively listen to pleasant and unpleasant auditory stimuli that were randomly presented in a long-event related design while skin conductance response (SCR) was recorded. To dissociate activation related to onset versus offset, the duration of positively and negatively valenced auditory stimuli were jittered and regressors were created for onset and offset of the negative and positive stimuli. We predicted that NAcc activation would be observed for the initiation, but not termination of the unpleasant sounds, results that would be consistent with the idea of bivalent NAcc function.
Materials and Methods
Subjects
Twenty right-handed adults (10 male, 10 female; age: range 20-31, mean 25.7 ± 0.6; IQ = 116 ± 2.7) took part in the study. Subjects were free of any medical or neurological problems, and had no current or previous diagnosis of psychiatric or neurological disorder. All subjects gave informed consent in accordance with Weill Medical College of Cornell University IRB committee, and were paid for their participation.
Stimuli and Apparatus
Auditory stimuli consisting of two unpleasant (negative tones: n1, n2) and two pleasant (positive tones: p1, p2) tones were used in this study. These tones were presented for 2, 4, and 6 seconds. The auditory stimuli used were modified and generated using the digital audio editors: Audacity 1.2.6 (http://audacity.sourceforge.net) and PRAAT Version 4.5.08 (www.praat.org). The auditory stimuli generated were of 2 seconds duration and were looped to generate 4 and 6 second segments. Stimuli: n1, a combined 1000 Hz tone and white noise, which was intensity tiered for smooth onset and offset; n2, four bursts of a 1000 Hz square wave tone, duration 0.4s, and silence 0.1s; p1, a wind chime recording that was modified for a smooth rise and fall; p2, a second chime recording amplified and modified, like p1, for a smooth rise and fall. All stimuli were modified so they would have the same intensity (95 dB in scanner; Headphones; fMRI Devices Corporation, Waukesha, WI). These stimuli were chosen after a pilot study was run to select the most pleasant and unpleasant sounds from a selection of eight. In the pilot study a randomly mixed sequence of the eight sounds was presented three times to 11 subjects (age 26-36) who rated them individually on a 20-point unpleasantness–pleasantness scale. There was a significant difference in rating the pleasant and unpleasant sounds (p < 0.001). Average rating for the aversive sounds was 3.7 ± 0.33 and 17.1 ± 0.31 for pleasant sounds. From these eight sound stimuli the two sounds that were rated as most unpleasant and the two sounds that were rated as most pleasant were selected for the imaging study.
Skin Conductance Response
A skin conductance response (SCR) MRI compatible system (SCR100C Biopac, Goleta, CA) together with the AcqKnowledge (Biopac) software was used to monitor the SCR as it varied with the eccrine sweat gland activity. The computer running AcqKnowledge and the computer running E-prime (Psychology Software Tools, Inc, Pittsburgh, PA) were interfaced allowing generation of digital TTL timestamps for each stimulus on the Biopac channel recording, so that stimuli presentations during scan were co-registered with SCR record. The SCR was sampled at 200 Hz using disposable electrodermal gel electrodes (Biopac model EL507) attached to the distal phalanx of the pointer and middle fingers of the left hand. The electrodes were connected to an MRI compatible cable set (MECMRI-TRANS) that interfaced with the SCR100C amplifier and the control panel. The SCR100C used a constant voltage (0.5 V) to measure skin conductance. The SCR was digitized at the electrodes and 1 Hz filter applied (Gain 2 μmho/V). Subjects were asked to wash their hands with water and dry them gently before the electrodes were attached. SCRs were analyzed by subtracting the peak skin conductance response occurring in a time window of 1-5 sec after stimulus onset from a baseline measure just prior to the stimulus onset. The small number of subjects which we successfully recorded SCR from (n = 7) precluded the inclusion of the SCR measures in our fMRI analysis.
Experimental Task
Subjects completed a passive listening task in which they heard pleasant and unpleasant sounds. Stimuli duration varied between 2, 4, and 6 seconds in order to deconvolve stimulus onset and offset BOLD responses. The interstimulus interval was 12 seconds (Figure 1A). The entire experiment consisted of 5 runs, each lasting 212 seconds. A total of 60 stimuli were presented, 30 negative and 30 positive sounds. The stimuli were presented in a pseudorandom order, with never more than two sounds of the same valence type following each other. Before the start of the experiment participants were told that they would hear sounds that were pleasant and unpleasant in nature and that no action was required on their part except to continue to pay attention to tones that would be presented. In addition, participants were instructed to close their eyes throughout the experiment, and were reminded of this at the beginning of each run. At the end of the experiment, while still in the scanner, subjects heard the auditory stimuli presented throughout the experiment, and rated their subjective experience of each tone on a 5-point scale (1 as most unpleasant and 5 as the most pleasant). The scanner was on during subjective rating so that the sounds would be experienced under the same conditions as during the experimental task. Subjects made their responses on a five button response glove. Stimuli and response collection (valence ratings of stimuli) were presented with the integrated functional imaging system (IFIS; PST, Pittsburgh) using an LCD video display in the bore of the MR scanner and a fiber optic response collection device. Self report ratings of state and trait anxiety were measured using the Spielberger’s State-Trait Anxiety Inventory (Spielberger, 1983) administered following the scanning session.
Figure 1.
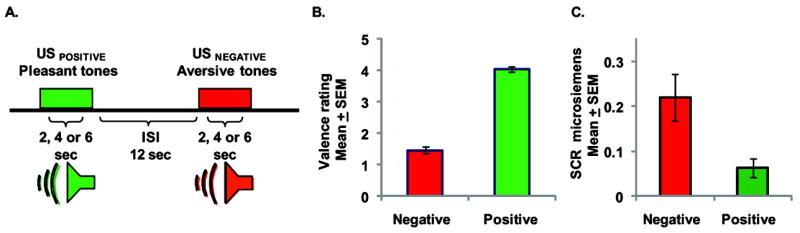
Behavioral task and validation of stimulus valence. A. The paradigm consisted of presentations of negative and positive sound stimuli of variable duration (2, 4 or 6 s) presented with a fixed inter-stimulus interval (ISI) of 12s. In total 30 negative and 30 positive auditory stimuli were presented in a pseudorandom order. B. While in the scanner subjects rated the sounds as either pleasant or unpleasant on a 5 point rating scale, 1 being most unpleasant, and 5 being most pleasant. C. Skin conductance response (SCR) to the negative and positive auditory stimuli.
Image Acquisition
Subjects were scanned with a General Electric Signa Excite 3.0 Tesla fMRI scanner (General Electric Medical Systems, Milwaukee, WI) with a quadrature head coil. Foam padding placed around the head was used to reduce motion. A whole brain, high resolution, T1 weighted anatomical scan (a 3D SPGR; 256 × 256 in-plane resolution, 240 mm field of view [FOV]; 124 1.5-mm axial slices) was acquired for each subject for transformation and localization of functional data into Talairach space (Talairach and Tournoux, 1988). A spiral in and out sequence (Glover and Thomason, 2004) was used to collect functional data (TR = 2000, TE = 30, FOV = 200 mm, Flip angle = 90 and 64 × 64 matrix). We obtained 29, 5 mm thick coronal slices with an in-plane resolution of 3.125 × 3.125 mm that covered the entire brain except for the posterior portion of occipital lobe.
Imaging data analysis
Functional imaging data were preprocessed and analyzed using the AFNI software package (Cox, 1996). The first 4 volumes (8 s) from each run were discarded to allow the scanner to reach magnetization equilibrium. Following slice time correction, images were registered to the first image volume following the high-resolution anatomical dataset using rigid body transformations and smoothed using an isotropic 6 mm Gaussian kernel. Head motion was examined to confirm that all subjects had less than 2 mm of translation or 2° of rotational movement. Time series were normalized to percent signal change to allow comparisons across runs and individuals by dividing signal intensity at each time point by the mean intensity for that voxel and multiplying the result by 100. Four regressors were created for onset and offset of negative and positive sounds by convolving the stimulus timing files with a gamma-variant hemodynamic response function. Linear regression modeling was performed to fit the percent signal change time courses to each regressor. Linear and quadratic trends were modeled in each voxel time course to control for correlated drift. Motion parameters were included in the GLM as covariates of no interest. The resulting regression coefficients represent an estimate of percent signal change from the mean.
Group level analyses were conducted on the regression coefficients from the individual analysis after transformation into the standard coordinate space of Talairach and Tournoux (1988), using parameters obtained from the transformation of each subjects’ high-resolution anatomical scan. Talairached transformed images had a re-sampled resolution of 3 cubic mm. Normalization to Talairach space was done using automatic talairach transformation in AFNI, where the anatomical volume was warped using 12-parameter affine transform to a template volume (TT_N27) in talairch space. An omnibus 2 (Valence; negative/positive) × 2 (Time; onset/offset) ANOVA that included subject as a random factor was conducted to determine the main effects of Valence, Time, and Valence × Time interaction. Correction for multiple comparisons was applied at the cluster level following Monte Carlo simulations conducted in the AlphaSim program within AFNI. Clusterwise false-positive rates of p < 0.05 corrected for multiple comparisons were determined for whole brain analyses. Additional simulations were restricted to the NAcc and amygdala based on the size of these regions unilaterally derived from the Talairach atlas included in the AFNI distribution (Volume: amygdala ~890 mm3; Nucleus accumbens ~1000 mm3). Whole brain simulations were conducted at individual voxel α probabilities set at 0.01, 0.001 and 0.0001 to allow identification of both broad and focal activations. Individual voxel α probabilities were set at 0.025 for simulations within the amygdala and NAcc. Only clusters with more than three voxels were considered for analysis.
For functional region of interest (ROI) analyses, anatomical masks of the NAcc and amygdala ROIs were defined from the Talairach atlas included in the AFNI software distribution. Voxels within these masks that showed activation above the threshold of p < 0.025 for the valence × time interaction at the group level were included in the functional ROI analysis. To address the concern that our group level functional ROI (defined by the average group level of activation) represented the same anatomical region in different participants we talairach transformed individual anatomical ROIs for the NAcc and amygdala generated with FreeSurfer to compare the individual FreeSurfer ROIs with the talairached group functional ROIs. Parcellation of the subcortical anatomy into regions of interest was performed using the FreeSurfer software suite (Fischl et al., 2004). These tools delineate anatomical divisions via automatic parcellation methods in which the statistical knowledge base derives from a training set incorporating the anatomical landmarks and conventions described by Duvernoy (1991). The resulting segmentation maps were viewed and the FreeSurfer derived-segmentation of regions of interest were evaluated and manually edited when found to be incorrect. We found that all subjects showed overlap within each of the two regions of interest, demonstrating that our group fROIs fit the individual subject anatomical data.
To examine the time course of activation, mean BOLD responses were plotted for selected clusters. To that end we applied functional masks of these clusters (based on the group level analysis) to extract individual time series averaged across voxels for each subject’s fMRI time series. From these, percent signal change for each event was calculated relative to the mean of the two TRs prior to stimulus onset in a time window of 0 to 12 seconds. We also analyzed our data using the mean as the baseline which did not appear to change any of the results. In addition, BOLD signal attenuation or enhancement with repeated presentations of the negative and positive stimuli was examined in individual subjects in two regions of interest, the NAcc and amygdala. In this analysis the peak hemodynamic response for each stimulus in each run was measured in the significant clusters observed after group analysis in the right NAcc and right amygdala. Mean of peak BOLD signal in early runs (1 & 2) and late runs (3 & 4) in these regions was calculated and a slope of best fit for early versus late trial peak response was generated. The gradient of the slope was taken as a measure of either habituation or sensitization to repeated presentation of stimuli across the experiment. Positive slopes are indicative of an increase in activation (sensitization) with time, whereas negative slopes are indicative of a decrease in activation (habituation) to the repeatedly presented stimuli.
Moreover, the interaction of the activity in the NAcc and amygdala in response to the onset of the negative and positive stimuli with the activity of other brain regions was characterized by performing a functional connectivity analysis. This analysis was computed in AFNI and performed by using the right amygdala functional ROI (derived from group analysis results valence × time). Individual time series were extracted from the amygdala cluster for each subject’s fMRI time series and averaged across voxels. Linear trend removal was first conducted on the entire time-series. Deconvolution was then run on the seed time series, and an interaction regressor created [deconvolved seed time series X events of interest – the onset of the negative (ni) and positive (pi) stimuli]. Single subject GLM was then run exactly as for a regular analysis, but here, adding the two additional regressors of interest. In addition, all of the original regressors of interest (events) and no interest (motion parameters) were included to account for all sources of variability in the dataset. The resulting correlation coefficients for the interaction regressors were transformed to a normal distribution using Fisher’s z transformation before group analyses on these values.
All Statistical analysis of the data was conducted in SPSS 15.0 (SPSS Inc. Chicago, IL).
Results
Validation of Stimulus Valence
At the end of the experimental session, while still in the scanner, subjects rated the auditory stimuli presented with respect to their positive and negative stimulus valence. There was a significant difference in valence ratings between the positive and negative stimuli presented (Figure 1B), where positive sounds were rated as pleasant and negative sounds were rated as unpleasant (Z = -3.9, p ≤ 0.001). No gender differences were found in valence rating, nor did valence ratings differ with respect to the duration of the stimuli presentation (2, 4, or 6 sec).
Skin Conductance Response
Dissociation between the positive and negative sound stimuli was also found at the physiological level. Significantly greater skin conductance response (SCR) was observed to the negative versus the positive auditory stimuli (Figure 1C; Z = -2.67, p = 0.008). Measurement noise caused by the scanner environment prohibited reliable SCR in the majority of subjects tested, consequently the small number of subjects with robust SCR (n = 7) precluded the inclusion of the SCR measures in our fMRI analysis. No evidence was found of a SCR to the tone offset.
Imaging Results
Whole brain analysis
The fMRI data were analyzed using a generalized linear model (GLM) that evaluated BOLD responses to the initiation and termination of the auditory stimuli. While this study was focused on NAcc activation, we first performed a whole-brain analysis to determine regions activated by negative versus positive stimuli at onset and offset using a 2 (Valence; negative versus positive) × 2 (Time; onset versus offset) ANOVA. The complete list of brain regions showing main effects and interactions is given in Table 1 and in Supplementary Tables 1 and 2. Whole-brain contrast analysis probing the main effect of valence revealed greater activation to negative rather than positive auditory stimuli in the greater part of the striatal complex, as well as in the right amygdala (Table 2, and supplemental Figure 1). Moreover, whole brain contrast analysis for the main effect of time (stimulus offset minus onset) for the negative as well as the positive stimuli did not reveal any brain regions showing activation on termination of either of these stimuli (data not shown).
Table 1.
Interaction of Valence (positive vs. negative) × Time (onset vs. offset)
| Talairach coordinates (CM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Brain region | Side | RL | AP | IS | Size (mm3) | F Stats | Onset | Offset |
| Medial Frontal Gyrus | L | 6.2 | -1.7 | 19.1 | 1026 | 29.9 | n > p | n > p |
| Inferior Frontal Gyrus | L | 43.8 | -17.1 | 5.5 | 108 | 32.1 | n > p | n = p |
| Temporal Gyrus & Insula | L | 39.3 | 24.5 | 13.2 | 1350 | 27.6 | n > p | n > p |
| Insula | R | -43.5 | 24.9 | 15.5 | 810 | 27.0 | n > p | n = p |
| Insula | L | 31.6 | -22.6 | 9.2 | 513 | 27.6 | n > p | n > p |
| Cingulate Gyrus | L | 8.8 | -13.5 | 36.4 | 270 | 28.4 | n > p | n > p |
| Cingulate Gyrus | R | -8.7 | -10.3 | -10.3 | 270 | 27.8 | n > p | n > p |
| Insula | L | 33.5 | -7.1 | 5.8 | 243 | 30.5 | n > p | n > p |
| Insula | R | -38.3 | 12.3 | 6.1 | 243 | 28.1 | n > p | n > p |
| Globus Pallidus | L | 9.8 | 3.5 | 1.9 | 243 | 26.6 | n > p | n > p |
| Thalamus | L | 11.7 | 17.3 | 11.3 | 1350 | 29.4 | n = p | n > p |
| Thalamus | R | -5.9 | 11.2 | 10.8 | 108 | 25.1 | n = p | n > p |
| Cerebellum | R | -22.9 | 48.5 | -23.3 | 189 | 29.0 | n > p | n > p |
| Brain stem | 3.1 | 27.0 | 15.7 | 1431 | 29.7 | n > p | n > p | |
| Nucleus Accumbens | R | -12.9 | -4.5 | -1.1 | 189* | 8.65 | n > p | n = p |
| Amygdala | R | -25.7 | 3.3 | -14.3 | 702* | 11.75 | n = p | n > p |
| Amygdala | L | 19.1 | 4.5 | -14.2 | 270* | 8.04 | n > p | n > p |
Group analysis ANOVA. CM, center of mass; L, Left; R, Right. Whole brain p = 0.0001, corrected to p < 0.05.
Region of interest correction p = 0.025, corrected to p < 0.05.
Table 2.
Whole-brain contrast analysis probing the main effect of valence
| Talairach coordinates (CM) | ||||||
|---|---|---|---|---|---|---|
| Brain region Negative > Positive* |
Side | RL | AP | IS | Size (mm3) | T stats |
| Striatum, insula, globus pallidus, thalamus & brainstem | R&L | -3.8 | 14.7 | 4.3 | 41094 | 4.67 |
| Amygdala | R | -28.9 | -0.2 | -17.2 | 189 | 4.35 |
| Cingulate Gyrus | R&L | -0.9 | -9.3 | 39.6 | 5265 | 4.47 |
| Posterior cingulate gyrus | R&L | -0.5 | -23.0 | 40.8 | 324 | 44.5 |
| Insula & Inferior frontal gyrus | L | 37.5 | -11.8 | 9.8 | 375 | 4.33 |
| Cerebellum | R&L | 35.2 | 49.5 | -25.9 | 594 | 4.31 |
| Cerebellum | R | -23.6 | 47.2 | -25.6 | 459 | 4.21 |
Contrast Analysis: positive minus negative. CM, center of mass; L, Left; R, Right. Whole brain p > 0.001, corrected to p > 0.05.
No brain regions with greater activation to the positive tones were found.
Valence × time interaction (Figure 2) revealed greater activation for the negative auditory stimuli in the inferior frontal gyrus, cingulate cortex (Figure 2B), anterior and posterior insula (Figure 2C), globus pallidus, and cerebellum, all associated with greater relative deactivation on the offset of the negative stimulus. In contrast, the peak magnitude of onset BOLD activation was equivalent for both valence types in clusters observed in areas of the thalamus (Figure 2D) and the superior temporal gyrus (STG). However, these thalamic and STG activations were associated with greater relative deactivation on the offset of the negative stimulus.
Figure 2.
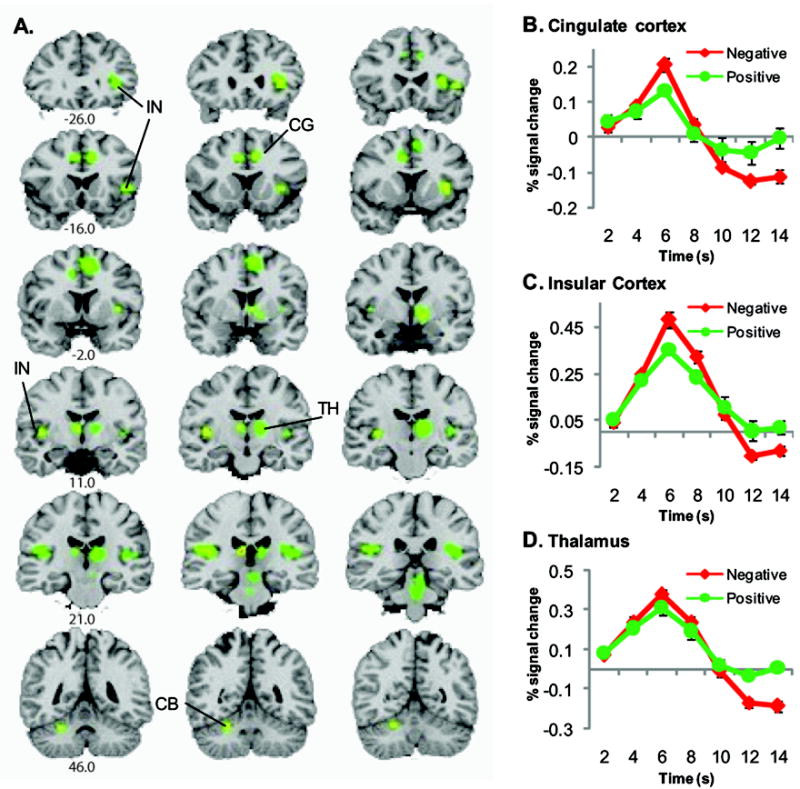
Activation of nociceptive/emotive brain regions. A. Coronal sections illustrating regions that showed significant activation in group analysis of valence × time (p < 0.0001, corrected to p < 0.05). Activations are displayed over a Talairach-normalized coronal templates in radiological convention (right = left). Greater activation was observed for the negative aversive stimuli in the (B) cingulate cortex (CIG), (C) insular cortex (IN), and cerebellum (CB). D. No difference in activation to negative and positive sounds was observed in thalamus (TH). Line plots represent mean ± standard error of the mean (SEM) across participants. Anterior/posterior y-coordinates are specified below the coronal sections.
Functional region of interest analysis
We conducted a functional region of interest (ROI) analysis on the NAcc to test our prior hypothesis regarding activation in response to positively and negatively valenced stimuli. Voxels within the NAcc that showed activation at the group level for the valence × time interaction were included in this functional ROI. For this analysis, an anatomical mask of the NAcc was defined from the Talairach atlas included in the AFNI software distribution. Voxels within the NAcc were corrected for multiple comparisons at the p < 0.05 level using cluster thresholds determined by AlphaSim. The AlphaSim Monte Carlo simulations were run using an individual voxel threshold of p < 0.025 within an anatomical mask of the NAcc taken from the AFNI Talairach atlas.
Within the NAcc there was a main effect of time, whereby bilateral activation of the NAcc was observed in response to the onset of the positive and negative stimuli. No main effect of valence was observed. However, there was a significant interaction between valence and stimulus onset and offset in the right NAcc (Figure 3 A & B, Table 1), with significantly greater response to unpleasant than pleasant sound stimuli (t = -3.61 p = 0.002) at onset. Moreover, no evidence for activation of NAcc activity on offset of the positive or negative stimuli was found (Figure 3B). Further analysis of activation of the NAcc revealed a positive correlation between the mean BOLD activation to the onset of negative and positive stimuli, such that subjects who showed a high response magnitude to the positive stimuli, also showed a heightened response to the negative stimuli (Figure 3D; Pearson’s r = 0.881; p < 0.001; n = 20). We also found that NAcc activation to the negative and positive stimuli remained largely constant throughout the experiment, as measured by comparing mean response magnitude during early, middle and late trials (Valence, F1,38 = 2.71; Time F1,38 = 0.43, Valence × Time, F1,38 = 0.001; Supplemental Figure 2).
Figure 3.
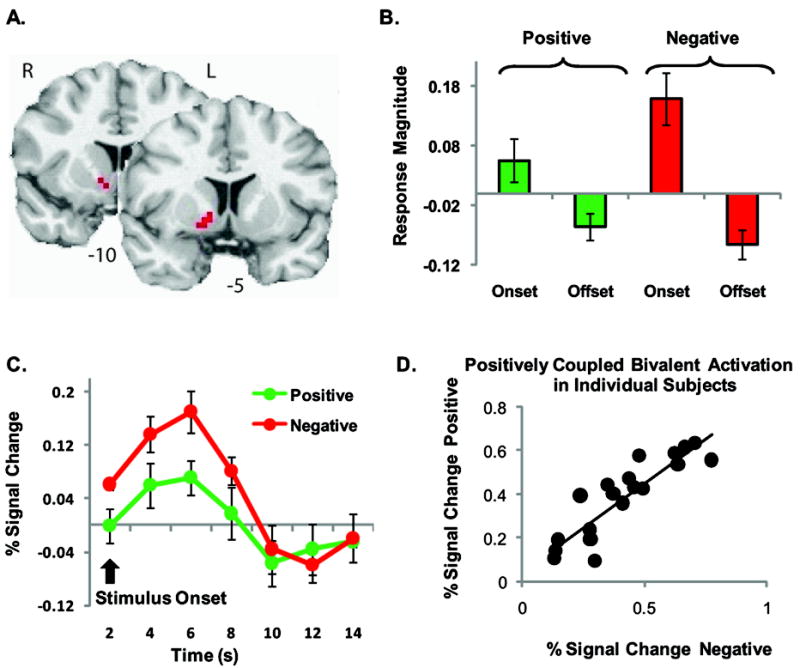
Bivalent activation of the nucleus accumbens. A. NAcc activation map in the coronal plane (p < 0.05, corrected). B. Activation in right NAcc on onset but not offset of both negative and positive events. C. Time course for the hemodynamic response in the right NAcc cluster. Image is in radiological format (right = left). D. Scatter plot of the positive correlation of individual subject’s mean peak activation of the NAcc to the positive and negative stimuli throughout the experiment. Peak activation was defined for each individual by plotting the bold response for each event, and taking the peak value observed in resulting hemodynamic response function. Bar & line plots represent mean ± standard error of the mean (SEM).
To examine regional specificity in response to aversive and pleasant stimuli, we investigated the response of the amygdala, a region implicated in affective processes and which sends significant projections to the NAcc. This analysis was performed in the same manner in which we conducted the functional ROI analysis for the NAcc. The amygdala functional ROI was defined by voxels showing a significant valence × time interaction using small volume correction within an anatomical mask of the amygdala at p < 0.05. Within the amygdala there was a main effect of time as well as valence × time interaction, but no main effect of valence. Post hoc t-tests on the main effect of time (onset vs. offset) revealed bilateral activation of the amygdala to both the negative and positive stimuli, as did valence × time interaction (Figure 4, Table 1, and Supplementary Table 2). We also found sensitization of the amygdala, as indicated by increased amygdala activity to repeated presentations of the negative stimuli, but not positive stimuli. This increase was associated with self-ratings of anxiety, such that an increased amygdala activity with repeated presentations of the negative stimuli predicated greater state and trait anxiety in individual subjects (State; Pearson’s r = 0.674; p = 0.001; Trait; Pearson’s r = 0.642, p = 0.003, Figure 4C). No such association was found for the NAcc (State; Pearson’s r = 0.051; Trait; Pearson’s r = -0.094).
Figure 4.
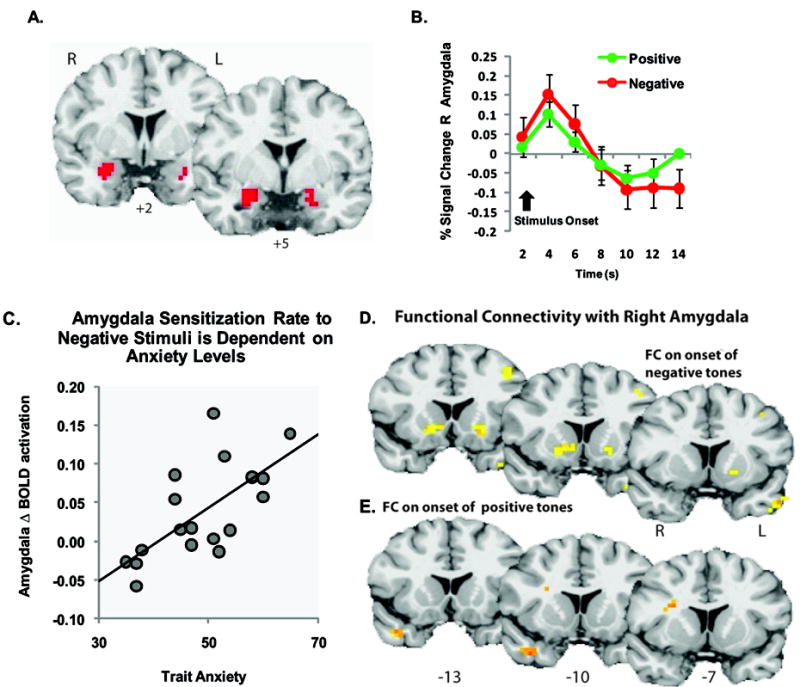
Bivalent amygdala activation. A. Coronal slice showing right and left amygdala clusters. B. Time course for the hemodynamic response in the right amygdala cluster. Images are in radiological format (right = left). Line plot represent mean ± standard error of the mean (SEM) across participants. C. Scatter plot of the correlation between trait anxiety and change in right amygdala activation through time. Trait anxiety scores were positively correlated with increased amygdala activity on repeated presentations of the negative auditory stimuli. The y-axis represents rate of change in amygdala activation, positive values indicate sensitization, and negative scores indicate habituation. The x-axis represents trait anxiety score. D & E. Functional connectivity analysis with the right amygdala fROI cluster set as the seed region; Significant functional connectivity was observed between the right NAcc and right amygdala during presentation of the negative (D) but not positive tones (E). FC, functional connectivity; R, right; L, left.
Based on anatomical data demonstrating NAcc-amygdala connectivity, we also conducted functional connectivity analysis with the right amygdala cluster set as the seed region (Figure 4A). We found a positive correlation between activity in the right amygdala and the right NAcc during the presentation of the negative, but not positive stimuli (Figure 4 D & E; and Supplemental Table 3).
Notably in this study auditory stimuli of different durations were presented to allow us to deconvolve stimulus onset versus offset. Plots of the hemodynamic response to the positive and negative stimuli of different durations revealed that only regions such as the superior temporal gyrus, which is involved in auditory perception, were sensitive to stimulus duration. In contrast, this was not the case in our regions of interest, the NAcc or amygdala, where the BOLD hemodynamic response was independent of stimulus duration (Figure 5, and Supplemental Figure 3).
Figure 5.
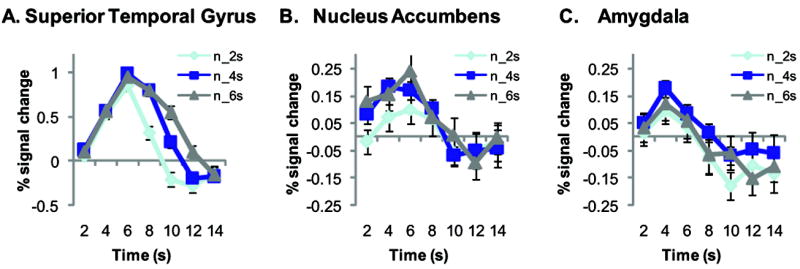
Nucleus accumbens and amygdala activation are not sensitive to stimulus duration. Time course of the hemodynamic response on presentation of the different duration negative (n) stimuli (2, 4, and 6 s) in the right superior temporal gyrus (A), right nucleus accumbens (B) and right amygdala (C) clusters from group analysis valence × time interaction. Line plots represent mean ± standard error of the mean (SEM).
Discussion
We found that the NAcc responds to the onset of both positive and negative stimuli. Onset and offset analysis of activation of the NAcc to pleasant and unpleasant sounds in a passive listening paradigm confirmed a direct activation of this region by aversive stimuli, rather than an effect secondary to some kind of relief, or a result of preparation and regulation of instrumental motor action. These results support the expanded view of NAcc function, whereby the NAcc plays a key role in modulating behavior to aversive and painful stimuli, and not just to stimuli that are rewarding in nature. Our findings are consistent with several studies that have reported striatal activity, including the NAcc, for primary and conditioned aversive stimuli (Blazquez et al., 2002; Ravel et al., 1999; Williams et al., 1993), as well as enhanced dopamine release in this region in response to similar events (Horvitz, 2002; Salamone et al., 2005). Moreover, consistent with the idea that the BOLD activations observed in this study did not reflect a simple sensory percept but rather valence, we found that neither the amygdala nor NAcc were sensitive to stimulus duration, unlike the superior temporal gyrus, a region involved in auditory perceptual processes (Pandya, 1995). Bivalent activation of the NAcc in this study is further supported by the clear dissociation in subjects’ subjective valence rating of the positive and negative stimuli as being pleasant and unpleasant, respectively, which was also reflected in dissociable physiological response (SCR) to these stimuli. Nevertheless, it is possible that the NAcc was responding to the arousing or attention-grabbing quality of the stimuli presented rather than their valence, which would be consistent with studies that have suggested that the NAcc maybe responding to stimulus salience (Zink et al., 2006), or with other studies which find that both valence and salience are critical for NAcc activation (Cooper and Knutson, 2008). However, in this study, the responses we observe in the NAcc are not solely a reflection of stimuli’s salience, since in auditory sensory areas as well as the thalamus we see equivalent activation to the positive and negative tones presented to the participants, suggesting matching of stimuli in terms of salience.
In this study we did not find activation of the NAcc on the offset of an aversive event. However, our negative result on offset of an aversive event needs to be interpreted with caution. It is possible that subjects would never have felt relief on the offset of the aversive sound, since the scanner environment may in itself have been an unpleasant setting.
Notably, in this study we did not observe a dissociable temporal activation profile for NAcc activation in response to positive and negative primary auditory stimuli, as previously reported for conditioned aversive stimuli (Gottfried et al., 2002). Gottfried et al (2002) found that the NAcc showed significant activation to the aversively conditioned stimulus (CS+) early but not late in learning, the reverse being the case for the appetitive CS+. These temporal differences may relate to cue learning rather than unconditioned stimuli (US) responses as investigated in this study. They may also explain the failure to observe NAcc activation in some human imaging aversive conditioning studies (Chandrasekhar et al., 2008; Delgado et al., 2006; Hamann and Mao, 2002), since activation of the region in response to aversive CS+ may be masked if time is not a factor in the analysis.
Previous studies have suggested that the NAcc may play a role in the expression of anxiety (Kalin et al., 2005; Sturm et al., 2003). However, here we did not find an association between the rate of habituation of the NAcc to the negative auditory stimuli and subjects’ self-rating of anxiety. Yet, consistent with previous studies (Etkin et al., 2004; Hare et al., 2008) the change in amygdala activity to the negative stimuli over time was associated with subjects’ anxiety levels. The amygdala, specifically the basolateral nucleus, sends significant projections to the NAcc (Nauta, 1982) and hence if high anxiety levels enhance output from the amygdala, it might be that target sites like the NAcc would also show the same phenotype. Indeed, functional connectivity analysis revealed a positive coupling between the amygdala and the NAcc when subjects were exposed to the aversive, but not positive stimuli. However, while amygdala input can affect NAcc function (Cardinal et al., 2004; Setlow et al., 2002), other pathways to the NAcc can act to offset or change the degree by which the amygdala can drive this region (Everitt et al., 1999; Grace, 2000; Jackson and Moghaddam, 2001; Setlow et al., 2002). Moreover, a lack of correlation between levels of anxiety and NAcc activation may be a result of the passive nature of our task. Thus, while our task design enabled us to examine the response of the NAcc to emotive stimuli in the absence of possible confounds stemming from motor responses, it did not allow us to examine fully the functional significance of these activations in modulation of behavior. Tasks that involve instrumental approach-avoidance behavior may be more likely to demonstrate correlations between individual anxiety levels and both task performance and the degree of brain activation in the NAcc.
In this study, the amygdala, like the NAcc, showed a bivalent pattern of activation, consistent with a large body of evidence demonstrating that while the amygdala responds most reliably to negative stimuli (Hariri et al., 2000; Phelps et al., 2001; Whalen et al., 1998), it also performs operations such as signaling the salience of positive stimuli (Breiter et al., 1996; Demos et al., 2008; Hamann and Mao, 2002; Hamann et al., 2002). However, while the NAcc and amygdala respond to similar types of bivalent information, and are intimately connected, they belong to functionally dissociable neural circuits: 1) An amygdala-centered circuit that acts as a rapid response module that can engage affective response units even prior to conscious stimulus identification (Morris et al., 2001); and 2) A NAcc-centered circuit that can only fully engage down-stream sites for action-selection once stimulus identity has been established and its significance evaluated. Thus, while NAcc neurons respond to emotion-eliciting stimuli, they do so in a manner that is largely dependent on individual stimulus identity, i.e., object-specific, rather than responding to a single common physical or psychological property of these stimuli (Roitman et al., 2005; Setlow et al., 2003; Wilson and Bowman, 2005; Yanagimoto and Maeda, 2003). This is in sharp contrast to neurons in the amygdala which tend to respond to a single common psychological property (Belova et al., 2007; Maeda et al., 1993; Paton et al., 2006; Salzman et al., 2007).
The stimulus-identity dependency of NAcc neurons is consistent with the NAcc being a part of an approach-avoidance behavior network. Such a system must first be able to process information about the identity and value of unconditioned stimuli that can act either as rewards or punishers, and that once these events occur, motor systems must redirect behavior to gain maximal utility from rewarding events (Day and Carelli, 2007), or be engaged in a way that will allow the organism to avoid threat and aversive outcomes (Faure et al., 2008; Reynolds and Berridge, 2001). This idea is consistent with the role of the NAcc in both negative and positive reinforcement processes, for example in humans anticipating monetary gain and loss (Cooper and Knutson, 2008), and the damaging effect of NAcc lesions and pharmacological manipulations in tasks that require behavioral inhibition and modulation of instrumental action to optimize reward gain and avoid risk (Cardinal et al., 2004; Christakou et al., 2004; Martinez et al., 2002; Salamone et al., 1997; Wadenberg et al., 1990).
Concluding Remarks
In this study we were able to show that just as the amygdala is not solely responsive to negative events, the NAcc is not only responsive to anticipated positive rewards, but also aversive events. These results support models of NAcc function that are not solely focused on reward. This broader bivalent role for the NAcc is consistent with the anatomical connectivity of the NAcc that allows it to integrate a substantial amount of information from regions that process both positive and negative valence. Future work needs to investigate the precise role of this integration in emotional regulation via outputs of the NAcc to motor, cognitive and autonomic centers.
Supplementary Material
Greater activation to the negative and positive auditory stimuli was observed in the greater part of the striatal complex. A. Activation is overlaid on coronal slices from a talairached structural template. Anterior/posterior y-coordinates are specified below images. Contrast analysis: onset negative minus onset positive p < 0.001, corrected to p < 0.05. The colors indicate different clusters generated during whole brain volume correction. Images are in radiological format (right = left). Note significant activation in right amygdala, as well as insula, and cingulate cortex. B. Bar graph showing response magnitude in the striatum to the onset and offset of the negative and positive sound stimuli. Response magnitudes presented are an average across all the voxels showing significant activation across the whole striatum (Mean ± SEM).
Time course of the hemodynamic response on presentation of the different duration positive (p) stimuli (2, 4, and 6 s) in the right superior temporal gyrus (A), right nucleus accumbens (B) and right amygdala (C) clusters from group analysis valence × time interaction. Line plots represent mean ± SEM.
Sustained activation of right NAcc during the experiment. Bar graph of response magnitude to the initiation of the negative and positive stimuli during the early, middle and late trials (mean ± SEM).
Acknowledgments
We would like to thank Bruce McCandliss and Jason Zevin for their thoughtful discussions about this work. This research was supported in part by the National Institute of Drug Abuse Grant R01 DA018879 (BJC), NIH P50 MH52196 & MH079513, the Mortimer D. Sackler family and Dewitt-Wallace Reader’s Digest Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ammassari-Teule M, Passino E, Restivo L, de Marsanich B. Fear conditioning in C57/BL/6 and DBA/2 mice: variability in nucleus accumbens function according to the strain predisposition to show contextual- or cue-based responding. Eur J Neurosci. 2000;12:4467–4474. [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol. 1992;39:247–279. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- Blazquez PM, Fujii N, Kojima J, Graybiel AM. A network representation of response probability in the striatum. Neuron. 2002;33:973–982. doi: 10.1016/s0896-6273(02)00627-x. [DOI] [PubMed] [Google Scholar]
- Bourgeais L, Monconduit L, Villanueva L, Bernard JF. Parabrachial internal lateral neurons convey nociceptive messages from the deep laminas of the dorsal horn to the intralaminar thalamus. J Neurosci. 2001;21:2159–2165. doi: 10.1523/JNEUROSCI.21-06-02159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Broderick PA, Rahni DN, Zhou Y. Acute and subacute effects of risperidone and cocaine on accumbens dopamine and serotonin release using in vivo microvoltammetry on line with open-field behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1037–1054. doi: 10.1016/S0278-5846(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar PV, Capra CM, Moore S, Noussair C, Berns GS. Neurobiological regret and rejoice functions for aversive outcomes. Neuroimage. 2008;39:1472–1484. doi: 10.1016/j.neuroimage.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci. 2004;24:773–780. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. Neuroimage. 2008;39:538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. Neuroscientist. 2007;13:148–159. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Ryan SL, Davis FC, Whalen PJ. Human Amygdala Sensitivity to the Pupil Size of Others. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Thomason ME. Improved combination of spiral-in/out images for BOLD fMRI. Magn Reson Med. 2004;51:863–868. doi: 10.1002/mrm.20016. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Kapp BS. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behav Brain Res. 1999;99:169–179. doi: 10.1016/s0166-4328(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13:15–19. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol Sci. 2002;13:135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Haralambous T, Westbrook RF. An infusion of bupivacaine into the nucleus accumbens disrupts the acquisition but not the expression of contextual fear conditioning. Behav Neurosci. 1999;113:925–940. doi: 10.1037//0735-7044.113.5.925. [DOI] [PubMed] [Google Scholar]
- Hare T, Tottenham N, Galvan A, Voss H, Glover G, Casey B. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7:617–627. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav Brain Res. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Hsu MM, Kung JC, Shyu BC. Evoked responses of the anterior cingulate cortex to stimulation of the medial thalamus. Chin J Physiol. 2000;43:81–89. [PubMed] [Google Scholar]
- Hunt MJ, Kessal K, Garcia R. Ketamine induces dopamine-dependent depression of evoked hippocampal activity in the nucleus accumbens in freely moving rats. J Neurosci. 2005;25:524–531. doi: 10.1523/JNEUROSCI.3800-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Westbrook RF, Killcross AS. Dopamine activity in the nucleus accumbens modulates blocking in fear conditioning. Eur J Neurosci. 2006;24:3265–3270. doi: 10.1111/j.1460-9568.2006.05195.x. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci. 2001;21:676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ, Ganguly PK. Topographical organization in the nucleus accumbens of afferents from the basolateral amygdala and efferents to the lateral hypothalamus. Neuroscience. 1995;67:625–630. doi: 10.1016/0306-4522(95)00013-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Wall TL, Bloom FE. Nucleus accumbens as a substrate for the aversive stimulus effects of opiate withdrawal. Psychopharmacology (Berl) 1989;98:530–534. doi: 10.1007/BF00441954. [DOI] [PubMed] [Google Scholar]
- Laviolette SR. Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr Bull. 2007;33:971–981. doi: 10.1093/schbul/sbm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Dalley JW, Robbins TW. Disruption of Pavlovian contextual conditioning by excitotoxic lesions of the nucleus accumbens core. Behav Neurosci. 2002;116:539–552. doi: 10.1037//0735-7044.116.4.539. [DOI] [PubMed] [Google Scholar]
- Maeda H, Morimoto H, Yanagimoto K. Response characteristics of amygdaloid neurons provoked by emotionally significant environmental stimuli in cats, with special reference to response durations. Can J Physiol Pharmacol. 1993;71:374–378. doi: 10.1139/y93-057. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Martinez G, Ropero C, Funes A, Flores E, Landa AI, Gargiulo PA. AP-7 into the nucleus accumbens disrupts acquisition but does not affect consolidation in a passive avoidance task. Physiol Behav. 2002;76:205–212. doi: 10.1016/s0031-9384(02)00696-0. [DOI] [PubMed] [Google Scholar]
- Mathias S, Lubman DI, Hides L. Substance-induced psychosis: a diagnostic conundrum. J Clin Psychiatry. 2008;69:358–367. doi: 10.4088/jcp.v69n0304. [DOI] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH, van Erp AM, Blank AD, McInerney SC. d-amphetamine “cue” generalizes to social defeat stress: behavioral sensitization and attenuated accumbens dopamine. Psychopharmacology (Berl) 1999;147:190–199. doi: 10.1007/s002130051160. [DOI] [PubMed] [Google Scholar]
- Miller JD, Sanghera MK, German DC. Mesencephalic dopaminergic unit activity in the behaviorally conditioned rat. Life Sci. 1981;29:1255–1263. doi: 10.1016/0024-3205(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Morris JS, Buchel C, Dolan RJ. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage. 2001;13:1044–1052. doi: 10.1006/nimg.2000.0721. [DOI] [PubMed] [Google Scholar]
- Nauta WJ. Limbic innervation of the striatum. Adv Neurol. 1982;35:41–47. [PubMed] [Google Scholar]
- Pandya DN. Anatomy of the auditory cortex. Rev Neurol (Paris) 1995;151:486–494. [PubMed] [Google Scholar]
- Parkinson J, Robbins T, Everitt B. Selective excitotoxic lesions of the nucleus accumbens core and shell differentially affect aversive Pavlovian conditioning to discrete and contextual cues. Psychobiology. 1999;27:256–266. [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Strecker RE, Jacobs BL. Single unit response of noradrenergic, serotonergic and dopaminergic neurons in freely moving cats to simple sensory stimuli. Brain Res. 1986;369:336–340. doi: 10.1016/0006-8993(86)90546-9. [DOI] [PubMed] [Google Scholar]
- Ravel S, Legallet E, Apicella P. Tonically active neurons in the monkey striatum do not preferentially respond to appetitive stimuli. Exp Brain Res. 1999;128:531–534. doi: 10.1007/s002210050876. [DOI] [PubMed] [Google Scholar]
- Raven MA, Necessary BD, Danluck DA, Ettenberg A. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp Clin Psychopharmacol. 2000;8:117–124. doi: 10.1037/1064-1297.8.1.117. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Paton JJ, Belova MA, Morrison SE. Flexible neural representations of value in the primate brain. Ann N Y Acad Sci. 2007;1121:336–354. doi: 10.1196/annals.1401.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schwienbacher I, Fendt M, Richardson R, Schnitzler HU. Temporary inactivation of the nucleus accumbens disrupts acquisition and expression of fear-potentiated startle in rats. Brain Res. 2004;1027:87–93. doi: 10.1016/j.brainres.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Setlow B, Holland PC, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned responses. Behav Neurosci. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- Setlow B, Schoenbaum G, Gallagher M. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron. 2003;38:625–636. doi: 10.1016/s0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkotter J. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg ML, Ericson E, Magnusson O, Ahlenius S. Suppression of conditioned avoidance behavior by the local application of (-)sulpiride into the ventral, but not the dorsal, striatum of the rat. Biol Psychiatry. 1990;28:297–307. doi: 10.1016/0006-3223(90)90657-n. [DOI] [PubMed] [Google Scholar]
- Westbrook RF, Good AJ, Kiernan MJ. Microinjection of morphine into the nucleus accumbens impairs contextual learning in rats. Behav Neurosci. 1997;111:996–1013. doi: 10.1037//0735-7044.111.5.996. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rolls ET, Leonard CM, Stern C. Neuronal responses in the ventral striatum of the behaving macaque. Behav Brain Res. 1993;55:243–252. doi: 10.1016/0166-4328(93)90120-f. [DOI] [PubMed] [Google Scholar]
- Wilson DI, Bowman EM. Rat nucleus accumbens neurons predominantly respond to the outcome-related properties of conditioned stimuli rather than their behavioral-switching properties. J Neurophysiol. 2005;94:49–61. doi: 10.1152/jn.01332.2004. [DOI] [PubMed] [Google Scholar]
- Yanagimoto K, Maeda H. The nucleus accumbens unit activities related to the emotional significance of complex environmental stimuli in freely moving cats. Neurosci Res. 2003;46:183–189. doi: 10.1016/s0168-0102(03)00058-0. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29:977–983. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Greater activation to the negative and positive auditory stimuli was observed in the greater part of the striatal complex. A. Activation is overlaid on coronal slices from a talairached structural template. Anterior/posterior y-coordinates are specified below images. Contrast analysis: onset negative minus onset positive p < 0.001, corrected to p < 0.05. The colors indicate different clusters generated during whole brain volume correction. Images are in radiological format (right = left). Note significant activation in right amygdala, as well as insula, and cingulate cortex. B. Bar graph showing response magnitude in the striatum to the onset and offset of the negative and positive sound stimuli. Response magnitudes presented are an average across all the voxels showing significant activation across the whole striatum (Mean ± SEM).
Time course of the hemodynamic response on presentation of the different duration positive (p) stimuli (2, 4, and 6 s) in the right superior temporal gyrus (A), right nucleus accumbens (B) and right amygdala (C) clusters from group analysis valence × time interaction. Line plots represent mean ± SEM.
Sustained activation of right NAcc during the experiment. Bar graph of response magnitude to the initiation of the negative and positive stimuli during the early, middle and late trials (mean ± SEM).


