Abstract
Heartwater, a major constraint to improved livestock production in Zimbabwe, threatens to invade areas which have been previously unaffected. To monitor its spread in Zimbabwe, an immunoblotting diagnostic assay based on the responses of animals to the immunodominant, conserved 32-kDa protein of Cowdria ruminantium was evaluated. In this assay, no false reactions were detected with sera known to be positive and negative, but sera from some cattle, sheep, and goats from heartwater-free areas of Zimbabwe reacted strongly with the 32-kDa protein, suggesting that either these animals had previous exposure to heartwater or they were false positives. To investigate the possibility of previous exposure to heartwater, 11 immunoblot-positive and 6 immunoblot-negative sheep from heartwater-free areas of Zimbabwe were compared regarding their susceptibilities to challenge with C. ruminantium. Prior to challenge, C. ruminantium could not be detected in any sheep by transmission to Amblyomma hebraeum ticks or by the polymerase chain reaction (PCR) conducted with plasma samples. All sheep were equally susceptible to the challenge, and infection was confirmed by brain biopsy, necropsy, PCR, and transmission of C. ruminantium to ticks. Our data suggest that the immunoblot-positive reactions of sera from heartwater-free areas were due not to previous C. ruminantium infection but rather to antigenic cross-reactivity between C. ruminantium and another agent(s) such as Ehrlichia species. In conclusion, the immunodominant 32-kDa protein is not antigenically specific to C. ruminantium and its use in serological diagnosis of heartwater requires reevaluation.
Full text
PDF

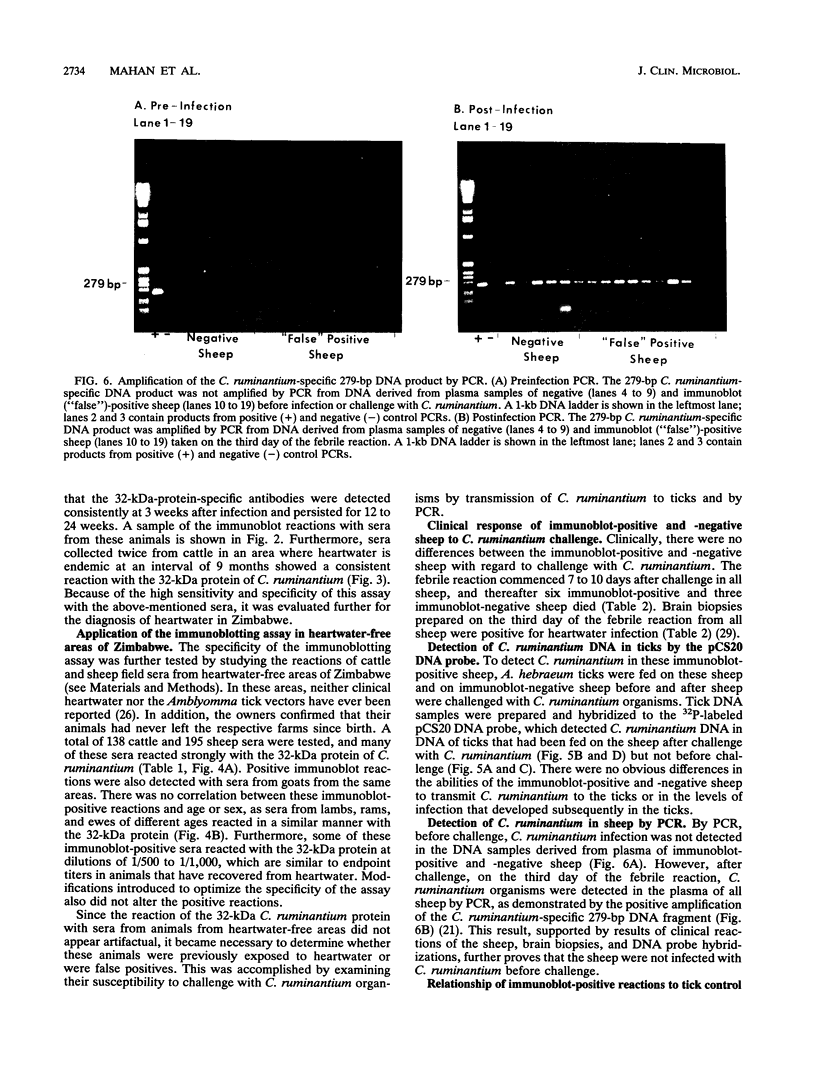



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew H. R., Norval R. A. The carrier status of sheep, cattle and African buffalo recovered from heartwater. Vet Parasitol. 1989 Dec;34(3):261–266. doi: 10.1016/0304-4017(89)90056-3. [DOI] [PubMed] [Google Scholar]
- Barré N., Uilenberg G., Morel P. C., Camus E. Danger of introducing heartwater onto the American mainland: potential role of indigenous and exotic Amblyomma ticks. Onderstepoort J Vet Res. 1987 Sep;54(3):405–417. [PubMed] [Google Scholar]
- Byrom B., Yunker C. E. Improved culture conditions for Cowdria ruminantium (Rickettsiales), the agent of heartwater disease of domestic ruminants. Cytotechnology. 1990 Nov;4(3):285–290. doi: 10.1007/BF00563789. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Mahan S. M., Yowell C. A. Phylogenetic relationship of Cowdria ruminantium, agent of heartwater, to Anaplasma marginale and other members of the order Rickettsiales determined on the basis of 16S rRNA sequence. Int J Syst Bacteriol. 1992 Apr;42(2):270–274. doi: 10.1099/00207713-42-2-270. [DOI] [PubMed] [Google Scholar]
- Dawson J. E., Rikihisa Y., Ewing S. A., Fishbein D. B. Serologic diagnosis of human ehrlichiosis using two Ehrlichia canis isolates. J Infect Dis. 1991 Mar;163(3):564–567. doi: 10.1093/infdis/163.3.564. [DOI] [PubMed] [Google Scholar]
- Du Plessis J. L. A method for determining the Cowdria ruminantium infection rate of Amblyomma hebraeum: effects in mice injected with tick homogenates. Onderstepoort J Vet Res. 1985 Jun;52(2):55–61. [PubMed] [Google Scholar]
- Du Plessis J. L., Camus E., Oberem P. T., Malan L. Heartwater serology: some problems with the interpretation of results. Onderstepoort J Vet Res. 1987 Sep;54(3):327–329. [PubMed] [Google Scholar]
- Du Plessis J. L., Malan L. The application of the indirect fluorescent antibody test in research on heartwater. Onderstepoort J Vet Res. 1987 Sep;54(3):319–325. [PubMed] [Google Scholar]
- Jongejan F., Thielemans M. J., De Groot M., van Kooten P. J., van der Zeijst B. A. Competitive enzyme-linked immunosorbent assay for heartwater using monoclonal antibodies to a Cowdria ruminantium-specific 32-kilodalton protein. Vet Microbiol. 1991 Jul;28(2):199–211. doi: 10.1016/0378-1135(91)90093-u. [DOI] [PubMed] [Google Scholar]
- Jongejan F., Thielemans M. J. Identification of an immunodominant antigenically conserved 32-kilodalton protein from Cowdria ruminantium. Infect Immun. 1989 Oct;57(10):3243–3246. doi: 10.1128/iai.57.10.3243-3246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongejan F., Wassink L. A., Thielemans M. J., Perie N. M., Uilenberg G. Serotypes in Cowdria ruminantium and their relationship with Ehrlichia phagocytophila determined by immunofluorescence. Vet Microbiol. 1989 Nov;21(1):31–40. doi: 10.1016/0378-1135(89)90016-3. [DOI] [PubMed] [Google Scholar]
- Logan L. L., Holland C. J., Mebus C. A., Ristic M. Serological relationship between Cowdria ruminantium and certain ehrlichia. Vet Rec. 1986 Nov 1;119(18):458–459. doi: 10.1136/vr.119.18.458. [DOI] [PubMed] [Google Scholar]
- Logan L. L., Whyard T. C., Quintero J. C., Mebus C. A. The development of Cowdria ruminantium in neutrophils. Onderstepoort J Vet Res. 1987 Sep;54(3):197–204. [PubMed] [Google Scholar]
- Mahan S. M., Waghela S. D., McGuire T. C., Rurangirwa F. R., Wassink L. A., Barbet A. F. A cloned DNA probe for Cowdria ruminantium hybridizes with eight heartwater strains and detects infected sheep. J Clin Microbiol. 1992 Apr;30(4):981–986. doi: 10.1128/jcm.30.4.981-986.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller Kubold A., Martinez D., Camus E., Jongejan F. Distribution of heartwater in the Caribbean determined on the basis of detection of antibodies to the conserved 32-kilodalton protein of Cowdria ruminantium. J Clin Microbiol. 1992 Jul;30(7):1870–1873. doi: 10.1128/jcm.30.7.1870-1873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norval R. A. Tick infestations and tick-borne diseases in Zimbabwe Rhodesia. J S Afr Vet Assoc. 1979 Dec;50(4):289–292. [PubMed] [Google Scholar]
- Perreau P., Morel P. C., Barre N., Durand P. Existence de la cowdriose (heartwater) à Cowdria ruminantium chez les ruminants des Antilles françaises (la Guadeloupe) et des Mascareignes (la Réunion et Ile Maurice). Rev Elev Med Vet Pays Trop. 1980;33(1):21–22. [PubMed] [Google Scholar]
- Rossouw M., Neitz A. W., de Waal D. T., du Plessis J. L., van Gas L., Brett S. Identification of the antigenic proteins of Cowdria ruminantium. Onderstepoort J Vet Res. 1990 Dec;57(4):215–221. [PubMed] [Google Scholar]
- Semu S. M., Mahan S. M., Yunker C. E., Burridge M. J. Development and persistence of Cowdria ruminantium specific antibodies following experimental infection of cattle, as detected by the indirect fluorescent antibody test. Vet Immunol Immunopathol. 1992 Sep;33(4):339–352. doi: 10.1016/0165-2427(92)90005-b. [DOI] [PubMed] [Google Scholar]
- Uilenberg G. Heartwater (Cowdria ruminantium infection): current status. Adv Vet Sci Comp Med. 1983;27:427–480. [PubMed] [Google Scholar]
- Waghela S. D., Rurangirwa F. R., Mahan S. M., Yunker C. E., Crawford T. B., Barbet A. F., Burridge M. J., McGuire T. C. A cloned DNA probe identifies Cowdria ruminantium in Amblyomma variegatum ticks. J Clin Microbiol. 1991 Nov;29(11):2571–2577. doi: 10.1128/jcm.29.11.2571-2577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunker C. E., Mahan S. M., Waghela S. D., McGuire T. C., Rurangirwa F. R., Barbet A. F., Wassink L. A. Detection of Cowdria ruminantium by means of a DNA probe, pCS20 in infected bont ticks, Amblyomma hebraeum, the major vector of heartwater in southern Africa. Epidemiol Infect. 1993 Feb;110(1):95–104. doi: 10.1017/s095026880005072x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet A. H., Jongejan F., van der Zeijst B. A. Phylogenetic position of Cowdria ruminantium (Rickettsiales) determined by analysis of amplified 16S ribosomal DNA sequences. Int J Syst Bacteriol. 1992 Jul;42(3):494–498. doi: 10.1099/00207713-42-3-494. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff A. J., Uilenberg G. Heartwater: cross-immunity studies with strains of Cowdria ruminantium isolated in West and South Africa. Trop Anim Health Prod. 1981 Aug;13(3):160–164. doi: 10.1007/BF02237914. [DOI] [PubMed] [Google Scholar]