Abstract
Transcranial Doppler sonongraphy is a non‐invasive, non‐ionising, inexpensive, portable and safe technique that uses a pulsed Doppler transducer for assessment of intracerebral blood flow. This article deals with the principles and technique of transcranial Doppler sonography. It gives a brief overview of its use in evaluation of intracranial steno‐occlusive disease, subarachnoid haemorrhage, and extracranial diseases (including carotid artery disease and subclavian steal syndrome). The role of transcranial Doppler in detection of microembolic signals and evaluation of right to left shunts is also dealt with. Finally, its use in acute stroke is briefly outlined.
Keywords: stroke, transcranial Doppler ultrasoound
Ultrasound has been used for the evaluation of cerebrovascular disease for over a decade and has made considerable progress. Transcranial Doppler sonography is a non‐invasive, non‐ionising, inexpensive, portable and safe technique that uses a pulsed Doppler transducer for assessment of intracerebral blood flow.
With the advent of thrombolytic treatment for acute ischaemic stroke, the internist would probably benefit from having a knowledge of transcranial Doppler ultrasound (TCD), which is a useful tool for the detection of occlusion of intracranial vasculature. In addition, success of thrombolytic treatment can also be assessed by TCD.
This review article aims to provide a basic understanding about the use of TCD in clinical practice. A brief outline is provided of the principles and techniques of TCD and its role in acute ischaemic stroke, including abnormalities affecting both intracranial and extracranial parts of vessels supplying the brain. We then explore the role of TCD in the detection of microembolic signals, which help in stratification of risk of recurrence of stroke or transient ischaemic attack (TIA), and its role of in the detection and quantification of right‐to‐left shunts. We also outline the possible role of TCD in subarachnoid haemorrhage and subclavian steal syndrome. Finally, the role of TCD during carotid endarterectomy is discussed (box 1).
Box 1: Use of transcranial Doppler ultrasound
Detection of site/degree of stenosis/occlusion of cerebral vasculature
Assessment of recanalisation following occlusion (with/without thrombolytic treatment)
Assessment of collateral flow in intracranial vasculature in cases of critical carotid artery stenosis (extracranial)
Detection of microemboli: stratification of risk of recurrence of stroke/TIA
Detection and quantification of right to left shunts
Detection of degree of vasospasm following subarachnoid haemorrhage
Complementary to duplex carotid scan in diagnosis of subclavian steal syndrome
Intraoperative monitoring of carotid endarterectomy
Principles
The “Doppler effect”, which was enunciated by Christian Andreas Doppler in 1842, forms the basis of TCD. In 1982, Aaslid and colleagues introduced a 2 Mhz Doppler which allowed adequate penetration through the intact skull.1 A pulsed wave transducer emits (insonant) waves, and then receives their reflections off the surfaces of the red blood cells within the intracranial vasculature. This information is analysed by a computer to give us both numerical and visual output, which is useful for inferring the flow characteristics within a blood vessel.
A particular vessel may be traced superficially or deep (that is, proximally and distally)—for example, the middle cerebral artery may be traced at a depth of between 30–60 mm via the transtemporal window. Transcranial Doppler spectra obtained may be single‐gated TCD or multigated TCD (M mode Doppler).
The peak systolic and diastolic velocities, mean flow velocity and Gosling pulsatility index (PI) are routinely calculated and displayed. Transcranial Doppler diagnoses are based on the detection of altered blood flow velocity, absence of blood flow, changes in the spectral waveform, or changes in pulsatility in a specific vessel.
Technique
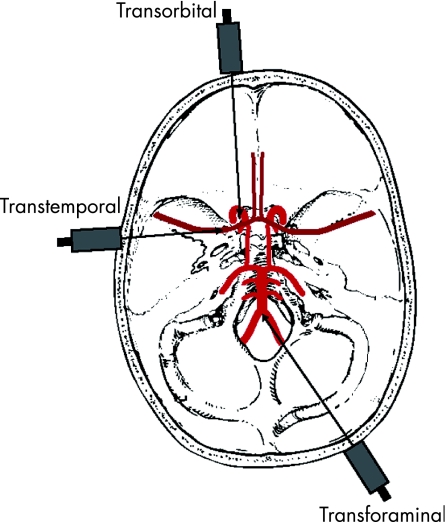
TCD is performed at the bedside. The first step is to localise a cranial “window” where the ultrasound beam can penetrate without being excessively dampened. The three main windows for accessing the intracranial arteries are listed below and shown in fig 1:
Figure 1 The three main windows for accessing the intracranial arteries.
Transtemporal window—found between the angle of the eye and the pinna above the zygomatic ridge and is the major route for insonating the anterior, middle and posterior cerebral arteries
Transorbital window—through the eye for insonation of the ophthalmic artery and the siphon of the internal carotid artery
Transforaminal window—through the foramen magnum insonated from the top of the neck below the occiput for the basilar artery and the intracranial segments of the vertebral arteries.
TCD is a blind technique. The various blood vessels are identified from the window used, the depth of insonation, the direction of blood flow with respect to the probe, and characteristics of the TCD waveform.
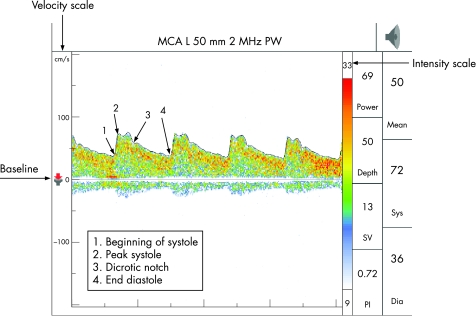
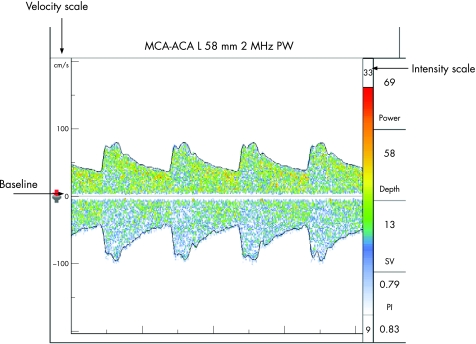
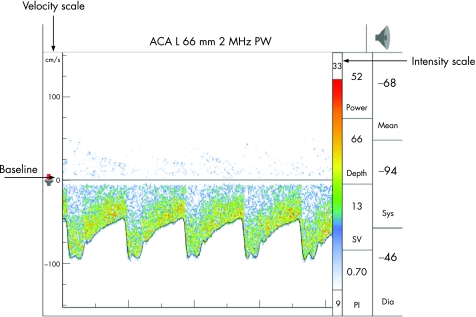
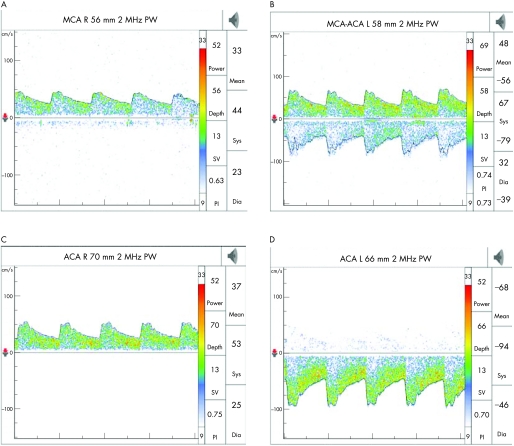
The middle cerebral artery (MCA) is identified through the transtemporal window, with the flow direction normally towards the probe, about 30–60 mm from the skull surface. At about 60 mm, the internal carotid artery (ICA) divides into the MCA and anterior cerebral artery (ACA), which flows away from the probe, and this bifurcation is one of the most important reference points for TCD. ACA is insonated between 65–80 mm with the probe directed anterosuperiorly through the transtemporal window. The posterior cerebral artery (PCA) is insonated at 55–70 mm with the probe directed posteroinferiorly through the transtemporal window. At times, provocative manoeuvres are used to confirm the identity of the vessels. Anomalies of the circle of Willis are common and a thorough knowledge of the anatomy of the blood vessels is an essential requisite for accurate interpretation. The technique is highly operator dependent and requires considerable skill and experience (figs 2–4)
Figure 2 A typical transcranial Doppler spectra with velocity and intensity scale on the left and right axis, respectively. The wave above baseline reflects flow towards the probe (normal middle cerebral artery (MCA) tracing at depth 50 mm—transtemporal window).
Figure 3 Middle cerebral artery (MCA)–anterior cerebral artery (ACA) tracing at the division of the internal carotid artery into the MCA (tracing above baseline) and the ACA (below baseline).
Figure 4 Normal anterior cerebral artery (ACA) tracing below the baseline reflecting flow away from the probe at a depth of 66 mm (transtemporal window).
TCD can be performed at the bedside and repeated as needed (even for continuous monitoring). It is non‐invasive and does not need a contrast agent. Its chief limitations are that it is a operator dependent technique and attenuation of ultrasound occurs through the skull and soft tissues. The mean loss of power through the skull can be up to 80%.2 The transtemporal window can be absent in up to 5–20% of the patients, and the vessels cannot be properly insonated due to inadequacy of the acoustic windows.3
However, with skill and experience in detecting accurate signals, TCD can be used at the bedside to help with stroke management. The pros and cons of TCD and its use in clinical practice are summarised in box 2.
Box 2: Pros and cons of transcranial Doppler ultrasound
Usefulness
Safe, non‐invasive
Bedside technique providing useful information on intracerebral vasculature
Detection of micro‐emboli—useful for prediction of early recurrence of stroke/TIA
Useful tool in investigation of cryptogenic stroke—with use of echo contrast agent
Adjunct to extracranial duplex carotid sonography in determining the effects on cerebral haemodynamics
Drawbacks
Inaccuracy due to poor acoustic window (in up to 5–20%)
Highly operator dependent and requires considerable skill and experience for accurate interpretation
Acute ischaemic stroke
Cerebral angiography shows acute occlusion in 76% of acute MCA territory infarcts within 6 h of stroke onset.4 Follow up studies show spontaneous recanalisation in the majority by the end of 48 h and in up to 86% by 2 weeks.5 TCD can detect these angiographic occlusions with high sensitivity and specificity, and has a high positive predictive value.6
TCD has a specificity of 90% in demonstrating MCA occlusions in patients with acute MCA stroke within 5 h. Alexandrov et al have shown major arterial occlusions in 69% of patients with acute hemispheric stroke, who may be eligible for thrombolytic treatment.7 Recanalisation can be inferred by TCD by the appearance of flow in the vessel or an improvement in the flow, with or without reduction in the PI in the proximal segments of the vessel. Thus, in the setting of acute ischaemic stroke, TCD can reveal the presence of arterial occlusion and it can also show whether recanalisation has occurred following intravenous thrombolysis.
TCD is also useful in prognostication of stroke.
A normal TCD at 6 h post‐ischaemic stroke is an independent predictor of early improvement.8
TCD may predict occurrence of spontaneous haemorrhagic transformation in ischaemic infarcts. MCA occlusions within 6 h of stroke onset may be an independent predictor of spontaneous haemorrhagic transformation with a positive predictive value of 72%.9
In acute MCA stroke, blood flow velocity on TCD of <30 cm/s within 12 h after stroke correlated with poor recovery.10
Microemboli are an important independent predictor of early ischaemic recurrence in patients with stroke or TIA of arterial origin (see later).11
Use of TCD in evaluating intracranial arterial disease
Intracranial steno‐occlusive disease
Intracranial atherosclerosis is responsible for up to 10% of TIA and strokes.12,13 Sensitivity, specificity, and positive and negative predictive value of TCD are generally higher in detecting abnormalities of the anterior circulation than in the vertebrobasilar circulation, as the latter has more anatomical variations and can be difficult to localise for TCD insonation.14
TCD can ascertain higher grades of stenosis (based upon elevated blood flow velocities) fairly accurately. However, its role in diagnosing milder grades of stenosis is still uncertain. The primary sign of stenosis is a focal increase in mean flow velocity at the site of luminal narrowing. Secondary TCD signs of stenosis include decreased velocity and increased pulsatility upstream from the lesion and abnormal flow immediately downstream from the lesion (box 3).15,16,17
Box 3: Signs of stenosis and occlusion
Primary signs of stenosis
Focal increase in mean flow velocity at the site of luminal narrowing
Secondary signs of stenosis
Decreased velocity and increased pulsatility upstream from the lesion
Abnormal flow immediately downstream from the lesion
Signs of occlusion
Absence of signal from artery
Sonographic evidence of collateral flow
With a tight narrowing (>50% reduction in lumen diameter), the velocity increases dramatically at the site of narrowing (mean flow velocity ⩾80 cm/s and a velocity difference of ⩾30% compared to the control side).18 In addition, there is lowering of signal intensity because of compromised blood flow due to narrowing. MCA stenosis has been most widely studied and can be diagnosed with a sensitivity of 86% and a specificity of 99% using TCD.7
Distal MCA stenosis, however, can be missed with TCD, as the distal branches are not well visualised.19 Stenosis‐like waveforms also occur in other conditions like arterial spasm, as in subarachnoid haemorrhage (SAH) or intracerebral haemorrhage, or due to increased collateral flow through hypoplastic communicators. In the case of spasm the changes tend to occur over a longer segment and/or involve multiple arteries.20
Intracranial arterial occlusions have been diagnosed using TCD with even greater accuracy and Demchuk et al have noted detailed diagnostic criteria for occlusion of large arteries.21 They have used the TIBI (Thrombolysis In Brain Ischaemia) classification to describe changes in the waveform found in association with recanalisation during recombinant tissue plasminogen activator treatment at or just beyond the site of occlusion.21
In general, the criteria for diagnosing an occluded artery based on TCD include: (1) absence of signal from the artery, taking care that a good acoustic window has been obtained by confirming flow in other vessels from the same window; and (2) sonographic evidence of collateral flow.
In general TCD has a high specificity, though sensitivity of TCD is lower in detecting all intracranial stenosis and occlusions.22
Use of TCD in evaluation of extracranial abnormalities
Extracranial carotid lesions
Extracranial ICA disease is a significant cause of neurologic deficits ranging from TIAs to progressive ischaemic eye disease and cerebral infarction. The risk rises as the degree of stenosis increases. Carotid duplex sonography is a sensitive and specific technique for demonstrating the presence and degree of narrowing of the proximal ICA.23 TCD is a useful adjunct to this investigation in evaluating its haemodynamic consequences on the intracranial circulation. In patients with haemodynamically significant extracranial ICA disease TCD may demonstrate: (1) a decrease in mean blood flow velocity, with diminished pulsatility in the ipsilateral MCA together with normal flow in the contralateral MCA24,25; (2) diminished flow acceleration in the ipsilateral MCA; (3) increased blood flow in potential collateral routes of circulation, typically the contralateral ACA, PCA, anterior communicating (Acom) and posterior communicating (Pcom) arteries; and (4) reversed direction of flow in the ipsilateral ACA and PCA and ophthalmic arteries (fig 5).
Figure 5 Effect of extracranial internal carotid artery (ICA) stenosis on cerebral haemodynamics (patient had high grade stenosis of proximal right ICA). (A) Decrease in mean flow velocity of right (ipsilateral) middle cerebral artery (MCA). (B) Normal flow in left (contralateral) MCA with increased flow in left (contralateral) anterior cerebral artery (ACA) (due to collateral flow). (C) Reversal of flow in right (ipsilateral) ACA. (D) Increased flow in left (contralateral) ACA.
TCD and transient microembolic signals
Microemboli in the cerebral circulation can originate from atherothrombotic lesions of the carotid arteries and cardiac sources. Interventional procedures such as cerebral angiography, carotid angioplasty, carotid endarterectomy and cardiopulmonary bypass may also give rise to microemboli. In addition, in right to left shunts microemboli may occur.
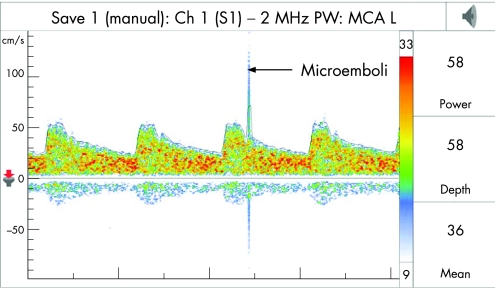
Gaseous or solid microemboli within the MCA can be detected by TCD as high intensity transient signals (HITS), also called microembolic signals (MES). They are characterised by: (1) having a duration <300 ms; (2) an amplitude that is 3 dB higher than the background blood flow signal; (3) are typically unidirectional and occur randomly within the cardiac cycle; and (4) produce a characteristic sound like a “moan” or “chirp” on audio signal (fig 6). The vessels have to be monitored for at least 30–60 min for detection of MES. An individual MES does not cause neurological symptoms but may represent an early warning sign for greater risk of future TIA/stroke. Regarding characterisation of the nature of emboli, it has been shown that gaseous emboli have signals of higher amplitude and intensity compared to formed solid particles.26
Figure 6 Transcranial Doppler wave form demonstrating an emboli.
MES correlate well with prior ischaemic events and may represent a higher risk for future recurrence ischaemic events. Higher prevalence rates of MES have been reported in strokes caused by large vessel disease and in cardioembolic strokes as compared to lacunar strokes.27 In asymptomatic patients with a critical ICA stenosis, when the microembolic signal rate is >2/hour in the ipsilateral MCA, there is an associated increased risk of development of ischaemia.28 MES arising from cardiac sources have higher total signal power, longer signal duration, and higher percentage of signals occurring in diastole as compared to those seen in carotid artery disease.29
TCD in ischaemic strokes due to right‐to‐left shunts
In young patients with cryptogenic stroke, right‐to‐left shunts (including patent foramen ovale (PFO)) are thought to be a risk factor for ischaemic stroke. However, whether it is only a chance association or a risk factor of stroke remains to be determined. Until recently, transoesophageal echocardiography (TOE) with a contrast agent was the only means of diagnosing right‐to‐left intracardiac shunts. It had many limitations—being a semi‐invasive technique it relied on the compliance of the patient to a great extent and also could not always detect latent shunts. TCD with a gaseous contrast medium is now used for the diagnosis of right‐to‐left shunts.
When a gaseous contrast agent is injected into the peripheral vein of a patient with shunts, microbubbles pass from the right to the left circulation during cardiac cycle, and enter the systemic circulation; TCD picks up the microbubbles as microembolic signals in the MCA. Two main contrast agents are in use: agitated saline containing tiny air bubbles, and a galactose based agent. Various authors have used different procedures regarding injection route, the type of contrast agent, and timing with respect to the provocative manoeuvre (Valsalva manoeuvre).30,31,32
Box 4 Quantification of right‐to‐left shunt
Test negative: no microbubble
Low grade shunt: 1–10 microbubbles
Medium grade shunt: >10 microbubbles but without “curtain effect”
High grade shunt: curtain effect, seen when the microbubbles are so numerous as to be no longer distinguishable separately47
The test is deemed positive if the appearance of at least one microbubble is recorded on the TCD trace within 40 s of terminating the injection. For quantification of right‐to‐left shunt, the results are classified as shown in box 4.
TOE is still considered the gold standard for PFO diagnosis. TOE can actually locate the anatomical location of the right‐to‐left shunt at the interatrial level and detect the coexistence of an associated atrial septal aneurysm. Compared to TOE, TCD has a sensitivity of 95% and a specificity of 75% in detecting PFO.33 TCD is a more sensitive tool than TOE for the detection of right‐to‐left shunt, and a shunt at non‐cardiac level (for example, patent ductus arteriosus) can be detected by this method. It can be repeated and can provide functional information on the quantification of the shunt which can be helpful in assessing the severity of the risk for stroke in patients with PFO.34,35
TCD in subarachnoid haemorrhage
Focal or diffuse cerebral vasospasm may follow in about 30% of patients having SAH due to rupture of an intracranial aneurysm or other pathologic conditions. The temporal course of vasospasm varies. In most cases it develops on the third or fourth day and gradually increases for a week, and then peaks between the 11th and 17th day, before gradually abating.36,37 TCD monitoring enables identification of patients at particular risk of developing ischaemic events secondary to vasospasm. This could be avoided by adequate early medical treatment.38 TCD is a sensitive and specific technique for the detection of vasospasm. There is an increase in blood flow velocity due to reduction in the luminal cross‐sectional area of the affected vessels. This is best appreciated in vasospasm of the proximal MCA.39,40
Altered blood flow velocities can be found in a wide variety of physiologic and pathologic conditions affecting intracranial arteries. Therefore, it is important to determine the underlying cause. Most studies indicate that patients with very high velocities (>200 cm/s) consistently have significant angiographic vasospasm and that some degree of vasospasm is present when the blood flow velocity in the MCA reaches 120 cm/s.
Subclavian steal syndrome
TCD (transforaminal window) can be used in conjunction with duplex vertebral sonography in the diagnosis of this condition. Incomplete steal causes a decrease in systolic blood flow velocity, and in severe cases alternating directions of blood flow occurs in the vertebral artery on the side of the subclavian steal.41 Complete flow reversal is noted in the vertebral artery in cases of complete steal.42,43 TCD findings may be potentiated by exercise of the arm on the affected side.
TCD during carotid endarterectomy
Intraoperative TCD involves monitoring the velocity of flow of blood in the MCA during carotid endarterectomy. It assesses the adequacy of cerebral blood flow while the carotid artery is cross‐clamped during carotid endarterectomy.44,45
Ischaemia during cross clamping is a classic complication and is considered severe if reduction in flow velocity is >85%, mild to moderate if reduction is between 60–85%, and absent if there is <60% reduction.
Hyperaemic phenomena may occur and result in a sudden increase in blood flow velocity in the MCA.
Microemboli (MES/HITS) caused by operative manoeuvres such as shunt insertion or removal can be documented in real time with TCD.
Postoperatively TCD may help in diagnosing hyperperfusion syndromes caused by deficient autoregulation.46
Conclusion
TCD provides insight into a wide range of intracranial and extracranial vascular pathologic conditions and their deleterious effects on cerebral haemodynamics in a way not possible with other imaging or diagnostic techniques. TCD is available for non‐invasive examination of intracranial arteries for the detection and quantification of stenosis. Newer developments in TCD have established the role of ultrasound in perioperative and postoperative carotid surgery, in detection of HITS for the diagnosis of microembolism, in the diagnosis of right‐to‐left shunts in suspected paradoxical embolism, and in the assessment of intracranial vasospasm. With rapid advancements being made in technology, we are likely to witness better, wider and newer applications of TCD for quantitative assessment of cerebral blood flow in the near future.
Acknowledgements
We are indebted to Sarah Syme and Steve Palmer (Medical Photography) for their help.
Abbreviations
ACA - anterior cerebral artery
HITS - high intensity transient signals
ICA - internal carotid artery
MCA - middle cerebral artery
MES - microembolic signals
PCA - posterior cerebral artery
PFO - patent foramen ovale
PI - pulsatility index
SAH - subarachnoid haemorrhage
TCD - transcranial Doppler ultrasound
TIA - transient ischaemic attack
TIBI - Thrombolysis In Brain Ischaemia
TOE - transoesophageal echocardiography
Footnotes
Competing interests: None
References
- 1.Aaslid R, Markwalder T, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocities in basal cerebral arteries. J Neurosurg 198257769–774. [DOI] [PubMed] [Google Scholar]
- 2.Grolimund P. Transmission of ultrasound through the temporal bone. In:Aaslid R, ed. Transcranial Doppler sonography. Vienna; Springer 1986
- 3.Santalucia P, Feldmann E. The basic transcranial Doppler examination: technique and anatomy. In: Babikian V, Wechler L, eds. Transcranial Doppler ultrasonography, 2nd ed. Boston: Butterworth Heinemann 199913–31.
- 4.Fieschi C, Argentino C, Lenzi G L.et al Clinical and instrumental evaluation of patients with ischemic stroke within the first six hours. Ital J Neurol Sci 198991311–321. [DOI] [PubMed] [Google Scholar]
- 5.Alexandrov A V, Bladin C F, Norris J W. Intracranial blood flow velocities in acute cerebral ischemia. Stroke 1994251378–1383. [DOI] [PubMed] [Google Scholar]
- 6.Wijman C A C, Babikian V L, Matjucha I C A.et al Cerebral microembolism in patients with retinal ischaemia. Stroke 1998291139–1143. [DOI] [PubMed] [Google Scholar]
- 7.Alexandrov A V, Bladin C F, Norris J W. Intracranial blood flow velocities in acute cerebral ischemia. Stroke 1994251378–1383. [DOI] [PubMed] [Google Scholar]
- 8.Toni D, Fiorelli M, Zanette E M.et al Early spontaneous improvement and deterioration of ischemic stroke patients; a serial study with transcranial Doppler ultrasonography. Stroke 1998291144–1188. [DOI] [PubMed] [Google Scholar]
- 9.Alexandrov A V, Black S E, Ehrlich L E.et al Predictors of hemorrhagic transformation occurring spontaneously and on anticoagulants in patients with acute ischemic stroke. Stroke 1997281198–1202. [DOI] [PubMed] [Google Scholar]
- 10.Halsey J H., Jr Prognosis of acute hemiplegia estimated by transcranial Doppler ultrasonography. Stroke 198819648–649. [DOI] [PubMed] [Google Scholar]
- 11.Valton L, Larrue V, Pavy le Traon A.et al Microembolic signals and risk of early recurrence in patients with stroke or transient ischaemic attack. Stroke 1998292125–2128. [DOI] [PubMed] [Google Scholar]
- 12.Goodin D S, Edlund W.Process for developing technology assessments. American Academy of Neurology Therapeutics and Technology Subcommittee 19991–35.
- 13.Wityk R J, Lehman D, Klag M.et al Race and sex differences in the distribution of cerebral atherosclerosis. Stroke 1996271974–1980. [DOI] [PubMed] [Google Scholar]
- 14.Wijman C A C, Babikian V L, Matjucha I C A.et al Cerebral microembolism in patients with retinal ischaemia. Stroke 1998291139–1143. [DOI] [PubMed] [Google Scholar]
- 15.Rajamani K, Gorman M. Transcranial Doppler in stroke. Biomed Pharmacother 200155247–258. [DOI] [PubMed] [Google Scholar]
- 16.Spencer M P. Hemodynamics of arterial stenosis. In: Spencer MP, ed. Ultrasonic diagnosis of cerebrovascular disease. Dordrecht, The Netherlands: Nijhoff, 1987117–146.
- 17.Ley‐Pozo J, Ringelstein E B. Noninvasive detection of occlusive disease of the carotid siphon and middle cerebral artery. Ann Neurol 199028640–647. [DOI] [PubMed] [Google Scholar]
- 18.Alexandrov A V, Neumyer M M. Diagnostic criteria for cerebrovascular ultrasound. In: Alexandrov AV, ed. Cerebrovascular ultrasound in stroke prevention and treatment. Oxford: Blackwell Publishing, 200481–129.
- 19.Suwanwela N C, Suwanwela N, Phanthumchinda K. Comparison of transcranial Doppler ultrasound and computed tomography angiography in symptomatic middle cerebral artery stenosis. Australas Radiol 200044174–177. [DOI] [PubMed] [Google Scholar]
- 20.Syme P D. The use of Transcranial Doppler ultrasonography as a ‘cerebral stethoscope' for the assessment and treatment of acute stroke. J R Coll Physicians Edinburgh 20063617–28. [Google Scholar]
- 21.Demchuk A M, Burgin W S, Christou I.et al Thrombolysis in brain ischemia (TIBI) Transcranial Doppler flow grades predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator. Stroke 20013289–93. [DOI] [PubMed] [Google Scholar]
- 22.Rajamani K, Gorman M. Transcranial Doppler in stroke. Biomed Pharmacother 200155247–258. [DOI] [PubMed] [Google Scholar]
- 23.Clarke W M, Hatten H P., Jr Noninvasive screening of extracranial carotid disease: duplex sonography with angiographic correlation. AJNR Am J Neuroradiol 19812443–444. [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider P A, Rossman M E, Torem S.et al Transcranial Doppler in the management of extracranial cerebrovascular disease: implication in diagnosis and monitoring. J Vasc Surg 19887223–231. [DOI] [PubMed] [Google Scholar]
- 25.Lindegaard K F, Bakke S J, Grolimund P.et al Assessment of intracranial hemodynamics in carotid artery disease by transcranial Doppler ultrasound. J Neurosurg 198563890–898. [DOI] [PubMed] [Google Scholar]
- 26.Georgiadis D, Mackay T G, Kelman A W.et al Differentiation between gaseous and formed embolic materials in vivo: application in prosthetic heart valve patients. Stroke 1994251559–1563. [DOI] [PubMed] [Google Scholar]
- 27.Grosset D G, Georgiadis D, Abdallah I.et al Doppler emboli signals vary according to stroke subtype. Stroke 199425382–384. [DOI] [PubMed] [Google Scholar]
- 28.Forteza A M, Babikian V L, Hyde C.et al Effect of time and cerebrovascular symptoms on the prevalence of microembolic signals in patients with cervical carotid stenosis. Stroke 199627687–690. [DOI] [PubMed] [Google Scholar]
- 29.Grosset D G, Georgiadis D, Kelman A W.et al Quantification of ultrasound emboli signals in patients with cardiac and carotid disease. Stroke 1993241922–1924. [DOI] [PubMed] [Google Scholar]
- 30.Schminke U, Ries S, Daffertshofer M.et al Patent foramen ovale: a potential source of cerebral embolism. Cerebrovasc Dis 19955133–138. [Google Scholar]
- 31.Steiner M M, Di Tullio M R, Rundek T.et al Patent foramen ovale size and embolic brain imaging findings among patients with ischaemic stroke. Stroke 199829944–948. [DOI] [PubMed] [Google Scholar]
- 32.Schwarze J J, etal Methodological parameters influence the detection of right‐to‐left shunts by contrast transcranial doppler. Stroke 1999301234–1239. [DOI] [PubMed] [Google Scholar]
- 33.Droste D W, Kriete J U, Stypmann J.et al Contrast transcranial Doppler ultrasound in the detection of right‐to‐left shunts: comparison of different procedures and different contrast agents. Stroke 1999301827–1832. [DOI] [PubMed] [Google Scholar]
- 34.De Castro S, Cartoni D, Fiorelli M.et al Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke 2000312407–2413. [DOI] [PubMed] [Google Scholar]
- 35.Kerut E K, Norfeet W T, Plotnick G D.et al Patent foramen ovale: a review of associated conditions and the impact on physiological size. J Am Coll Cardiol 200138613–623. [DOI] [PubMed] [Google Scholar]
- 36.Seiler R W, Newell D W. Subarachnoid hemorrhage and vasospasm. In: Newell DW, Aaslid R, eds. Transcranial Doppler. New York: Raven, 1992101–107.
- 37.Harders A G, Gilsbach J M. Time course of blood velocity changes related to vasospasm in the circle of Willis measured by transcranial Doppler ultrasound. J Neurosurg 198766718–728. [DOI] [PubMed] [Google Scholar]
- 38.LeRoux P D, Winn H R. Intracranial Aneurysms and subarachnoid hemorrhage: management of the poor grade patient. Acta Neurochir 199972(suppl 7)265. [DOI] [PubMed] [Google Scholar]
- 39.Newell D W, Winn H R. Transcranial Doppler in cerebral vasospasm. Neurosurg Clin N Am 19901319–328. [PubMed] [Google Scholar]
- 40.Sloan M A, Haley E C, Kassel N F.et al Sensitivity and specificity of transcranial Doppler ultrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology 1989391514–1518. [DOI] [PubMed] [Google Scholar]
- 41.Lupetin Anthony R.et al Transcranial Doppler sonography part 2. evaluation of intracranial and extracranial abnormalities and procedural monitoring. Radiographics 199515193–209. [DOI] [PubMed] [Google Scholar]
- 42.Von Reutern G M, Pourcelot L. Cardiac cycle dependent alternating flow in vertebral arteries with subclavian artery stenosis. Stroke 19789229–236. [DOI] [PubMed] [Google Scholar]
- 43.Klingelhofer J, Conrad B, Benecke R.et al Transcranial Doppler ultrasonography of carotidobasilar collateral circulation in subclavian steal. Stroke 198514721–728. [DOI] [PubMed] [Google Scholar]
- 44.Powers A D, Smith R B, Graeber M C. Transcranial Doppler monitoring of cerebral flow velocities during surgical occlusion of the carotid artery. Neurosurgery 198925383–389. [DOI] [PubMed] [Google Scholar]
- 45.Sundt T M, Sharbough F W, Anderson R E.et al Cerebral blood flow measurements and electroencephalographic changes during carotid endarterectomy. J Neurosurg 197441310–320. [DOI] [PubMed] [Google Scholar]
- 46.Steiger H J, Newell D W, Aaslid R. Monitoring for carotid surgery. In: eds. Transcranial Doppler. New York: Raven, 1992197–204.
- 47.Serena J, Segura T, Perez‐Ayuso M J.et al The need to quantify right‐to‐left shunt in acute ischaemic stroke: a case‐control study. Stroke 1998291322–1328. [DOI] [PubMed] [Google Scholar]