Abstract
Psoriasis is an inflammatory skin disease that affects 1–3% of Caucasian populations and may be persistent, disfiguring and stigmatising. There is a range of severity, but even when the affected body surface area is relatively limited the impact on day‐to‐day activities and social interactions may be significant. An understanding of the psychological burden and an appreciation that many patients are currently dissatisfied with their management has driven the development of more effective treatment. In recent years psoriasis has been the focus of intense investigation resulting in an improved understanding of the immunopathogenesis, and the development of new, targeted biological treatments.
Keywords: biological, pathophysiology, psoriasis
Psoriasis encompasses a group of diseases of the skin and joints. It may start at any age and the incidence peaks in the second and third decades of life. Chronic plaque psoriasis is the most common manifestation, but guttate, flexural, pustular or erythrodermic forms may also present. The diagnosis is usually straightforward and is based on the recognition of well demarcated, erythematous, scaly plaques with a predilection for the scalp, extensor aspects of the limbs and the trunk. Histology reveals hyperkeratosis, hyperplasia of the epidermis (acanthosis), inflammatory cell infiltration of leucocytes into the dermis and epidermis, and dilatation of dermal capillaries. Nail changes, such as superficial pitting and onycholysis, are often present and may be useful diagnostically. The features of psoriasis, and the different types of the condition, are summarised in tables 1 and 2.
Table 1 Summary of features of psoriasis.
| Prevalence | 1–3% |
| Peak onset | Second and third decades |
| Clinical variants | Chronic plaque |
| Guttate | |
| Pustular | |
| Flexural | |
| Erythrodermic | |
| Other clinical features | Nail involvement (onycholysis; nail pitting) |
| Joint involvement (axial or peripheral skeleton) | |
| Associated risks | Cardiovascular disease |
| Malignancy | |
| Psychological – anxiety and depression | |
| Clinical features (chronic plaque psoriasis) | Well demarcated, erythematous scaly plaques (scalp, extensor aspect of elbows and knees, sacral area) |
| Tools for clinical assessment | PASI (Psoriasis Area and Severity Index) |
| DLQI (Dermatology Life Quality Index) | |
| Precipitants | Infection (especially streptococcal throat) |
| Drugs | |
| Trauma | |
| Stress | |
| Alcohol binge | |
| Genetic predisposition | Multifactorial |
| PSORS 1 accounts for 50% of heritability | |
| Factors involved in pathophysiology | Angiogenesis |
| T cells | |
| TNF (tumour necrosis factor) | |
| Innate immunity |
Table 2 Summary of different types of psoriasis.
| Type | Most common form |
|---|---|
| Chronic plaque psoriasis | May present at any age |
| Thick, scaly plaques affecting scalp, extensor aspect of limbs and trunk | |
| Responds well to coal tar, vitamin D analogues, ultraviolet B (UVB) and most second line and biological agents | |
| Guttate psoriasis | Affects young adults |
| Often streptococcal throat infection as precipitant | |
| Small scaly lesions affecting trunk, although may be widespread | |
| Responds well to UVB | |
| Pustular psoriasis | May arise de novo or on chronic plaque psoriasis background |
| Small sterile pustules on an inflammatory base | |
| Responds to topical steroids, vitamin D analogues and acitretin or ciclosporin | |
| Requires close monitoring | |
| Flexural psoriasis | Shiny erythematous plaques in axillae, groin, submammary areas—scaling not seen |
| Responds to topical steroids, calcitriol, topical tacrolimus | |
| Erythrodermic psoriasis | Widespread erythema |
| Requires inpatient monitoring | |
| May be associated fluid loss, thermoregulatory problems—therefore monitor vital signs | |
| Treat with emollients initially, followed by coal tar or second line agent |
Psoriatic arthritis, which is present in up to 30% of patients with psoriasis, is now recognised as a distinct entity that can affect either the axial or peripheral skeleton. The distribution is often asymmetrical, and enthesitis and dactylitis are characteristic features. Severe forms are erosive and mutilating. The onset of psoriasis usually precedes joint involvement and the severity of disease in the two compartments does not run in parallel.
Patients with psoriasis have a significantly increased risk of cardiovascular disease, with the risk greatest for young patients with severe disease.1 This relative risk is preserved, even when traditional risk factors known to be more prevalent in psoriasis, such as smoking,2,3 hyperlipidaemia,3 hypertension,3 diabetes3,4 and obesity2,3,4 are corrected for. The inflammatory changes involved in a Th1 type condition such as psoriasis may contribute to an increased risk of atherosclerosis.1 Effective treatment of psoriasis may reduce the risk as a cohort of patients treated with methotrexate were found to have reduced incidence of atherosclerosis.5 This effect may be due to the anti‐inflammatory effects of methotrexate, although the greatest reduction was seen in patients treated with concomitant folic acid.
An increased risk of malignancy has also been reported, and in patients with severe disease the risk is comparable to that seen in patients after organ transplantation.6,7,8 Significantly higher rates of non‐melanoma skin cancer and lymphoma have been reported; treatment with immunosuppressants and phototherapy appear to play some role. There is also a statistically significant association between psoriasis and Crohn's disease.9,10
Surprisingly, the effect of psoriasis on health related quality of life is comparable to that seen in patients with cardiac failure, cancer or diabetes. Persistent itch or pain in the skin, the mess and embarrassment of shedding scales, and the stigmatising effect of a disfiguring condition all contribute to the impact. Patients may suffer low self esteem and frequently have difficulty forming relationships and maintaining employment. The impact on quality of life may be disproportionate to the extent of psoriasis, particularly if affected sites are visible (for example, the face) or important functionally (for example, the hands). More than one third of patients experience depressive and anxiety disorders, and social phobia and alcohol dependence are common.11 Suicidal ideation among psoriasis patients is estimated as 2.5% for outpatients and 7.2% for inpatients,12 while the prevalence of alcohol abuse among psoriasis patients is 18% compared to 2% among patients with other dermatological conditions.13
Assessment of psoriasis severity may vary considerably between patient and physician. A multidimensional approach comprising objective dermatological assessment, an estimation of quality of life and treatment responsiveness is optimal. In clinical trials the biological severity of psoriasis at a given point in time is often quantified using the Psoriasis Area and Severity Index (PASI). This is a composite score incorporating a grading of erythema, induration and scaling of plaques, multiplied by the surface area of skin involved. This score has been validated in the clinical setting and although it has many shortcomings, remains the gold standard. Several questionnaires have been developed to quantify the impact of psoriasis on quality of life, including the Dermatology Life Quality Index (DLQI), which has been well validated in this setting. A PASI of 10 or more, or a DLQI of 10 or more, indicate severe disease.14 A reduction in the DLQI by 5 points and/or a 50% fall in the PASI with treatment represent a clinically meaningful response.15,16,17
Psoriasis genotype
Some of the variation in severity and phenotype of psoriasis is due to environmental factors such as ultraviolet exposure, streptococcal throat infections, alcohol, and physical and psychological stress. Nevertheless, twin studies indicate that the proportion of phenotypic variability due to genes (heritability) is about 80%.18 A lack of complete concordance in identical twins suggests multifactorial inheritance and an interaction between a genetic basis and environmental factors. A positive family history is found in as many as 71% of childhood cases,19 and occasionally large multigenerational pedigrees are seen, suggesting genetic heterogeneity, with a common low penetrance gene(s) and rare high penetrance genes(s).
The major genetic determinant, PSORS 1, has been robustly replicated in most populations studied and accounts for about 50% of the heritability of psoriasis.20,21 It has been localised to the region of the major histocompatibility complex (MHC) on chromosome 6p21.3, but due to the strength of linkage disequilibrium in this region it has proved difficult to map the gene at this site. One largely unbroken risk haplotype extends from HLA‐C to corneodesmosin, which are the strongest positional candidate genes.22,23,24 The PSORS 1 risk haplotype correlates with the clinical pattern of psoriasis: it is strongly associated with psoriasis of early onset, particularly guttate psoriasis, but is not associated with late onset psoriasis, palmoplantar pustulosis, or psoriatic arthritis.25
Linkage analysis in large pedigrees has revealed several other susceptibility loci (PSORS 2 to PSORS 9 and PSORAS 1), but most of these have not been independently replicated and may be geographically restricted. PSORS 2 on chromosome 17q25 contains at least three susceptibility loci, one of which is inherited as an autosomal dominant mendelian trait with high penetrance.26
Several loci overlap with risk loci for other immune mediated inflammatory diseases such as atopic disease,27 rheumatoid arthritis28 and Crohn's disease.29 Despite the known association between psoriasis and Crohn's disease and a shared locus on chromosome 16, polymorphisms in CARD15 at this site, which carry risk for Crohn's disease (IBD 1), are not psoriasis risk alleles.
Pathophysiology
As abnormal scaling and epidermal thickening are key clinical features, for many years psoriasis was considered primarily a disease of the keratinocyte. This view has received support from some recent animal models, notably the development of a psoriasiform phenotype, including arthritis, in transgenic mice with inducible targeted deletions in AP‐1 proteins in keratinocytes.30 The vascular nature of psoriasis is evident clinically by the erythematous plaques, which exhibit pinprick bleeding on removal of scale (Auspitz sign), and is seen histologically with dilated tortuous capillaries, a prominent early feature of plaques. Histological studies have shown a fourfold increase in surface area of the superficial vascular plexus in psoriasis, and endothelial cell proliferation is increased.31 Angiogenic factors over‐expressed by lesional keratinocytes include VEGF, interferon‐γ (IFN‐γ), and interleukin 8 (IL8). VEGF in particular appears to play a central role in angiogenesis in psoriasis. This cytokine is also a potent mediator of inflammation and increases vascular permeability. Its synthesis by keratinocytes, activated T cells and endothelial cells, is induced by cytokines such as tumour growth factor‐α (TGF‐α), IFN‐γ, and tumour necrosis factor‐α (TNF‐α). VEGF is over‐expressed in psoriatic epidermis, and its receptors (KDR and Flt‐1) are over‐expressed on papillary microvessels. Transgenic mice over‐expressing VEGF in the epidermis develop a psoriasis‐like condition.32 Serum concentrations of VEGF are increased in erythrodermic psoriasis,33 and some authors have found a correlation between plaque VEGF, PASI and serum VEGF values.34,35 The hyperpermeability induced by VEGF has been proposed as the cause for microalbuminuria and pulmonary oedema in patients with severe psoriasis.35
The observation, first made in the 1980s, that ciclosporin is highly effective in the treatment of psoriasis suggested a primary role for T cells. The importance of T cells is also supported by rare instances of the transfer of psoriasis to the recipients of bone marrow transplants from affected individuals,36 and also xenotransplant animal models. Psoriasis spontaneously develops in non‐lesional skin transplanted from individuals with psoriasis to AGR129 mice, which lack T and B cells and have a defective innate immune response.37 The development of these plaques corresponds to the proliferation of resident T cells within the graft.
The trigger to T cell activation in psoriasis remains unknown, and although a number of antigens have been proposed, antigen independent stimulation via innate immune mechanisms is plausible. Antigen presenting cells (APC) in the epidermis (Langerhan cells) and dermis (dermal dendritic cells) are activated and mature (characterised by enhanced expression of cell surface counter receptors responsible for stimulation of the naïve T cell—CD4 or CD8). These cell surface molecules include CD80, CD86, CD40 and ICAM1. Activated APCs then travel via lymphatics to lymph nodes where they encounter and activate naïve CD4 or CD8 cells. This requires presentation of the APC on MHC class I or II molecules, followed by co‐stimulation, which is a non‐antigen specific cell–cell interaction. CD 28 on the T cell is one of the important co‐stimulatory molecules which bind to counter receptors CD80 and CD86 on the APC. Other co‐stimulatory molecules on the T cell include LFA‐1 which binds to ICAM‐1 on the APC; CD2 on the T cell binds to LFA‐3; and CD 40 ligand on the T cell binds to CD 40 on the APC. Once these co‐stimulatory interactions have occurred, the naïve T cell proliferates and transforms into memory CD4 or CD8 T cells and induces expression of CD2, IL2 and IL2R, which are responsible for T cell proliferation and survival.
Activated T cells differentiate under the influence of cytokines IL12 and IFN‐γ; CD4 T cells differentiate into a Th1 phenotype and CD8 cells into a Tc1 phenotype that produce type 1 cytokines (IL2, TNF‐α, IFN‐γ).
Once T cells are activated they “home” to the skin to exert their effects. This requires interactions between activated T cells and the endothelium. T cells activated in lymph nodes express a new surface protein called cutaneous lymphocyte associated antigen, an adhesion molecule that allows tethering of T cells to the endothelium. T cells then roll slowly along endothelial cells, where they encounter chemokines, resulting in modifications of integrins on the T cell, including LFA‐1 and VFA‐4 which bind to intracellular adhesion molecule‐1 (ICAM‐1) and VCAM‐1, respectively, on blood vessels. Chemokines are stimulated by IFN‐γ and TNF‐α released from activated T cells. Chemokines IP‐10 and MIG produced by keratinocytes in response to IFN‐γ then induce Tc1 lymphocytes to migrate into the epidermis.
After exiting post‐capillary venules, Th1 (CD4+) lymphocytes encounter dendritic cells within the dermis and Tc1 (CD8+) lymphocytes encounter Langerhan cells within the epidermis, and subsequently release pro‐inflammatory cytokines such as TNF‐α and IFN‐γ. Other cytokines (IL1, IL2, IFN‐γ) further increase production of TNF‐α. The cytokines engage in a complex cascade, culminating in epidermal and vascular hyperproliferation and pro‐inflammatory effects. Release of TNF‐α and IFN‐γ from T cells triggers keratinocytes to produce IL8, which is the main chemotactic signal for recruitment of neutrophils into the epidermis.
Psoriasis is considered a Th1 condition, characterised by the production of IFN‐γ and TNF‐α under the influence of IL12. This immunological model is supported by the effectiveness in early clinical trials of a monoclonal antibody directed against the p40 subunit of IL12.38 However, there is increasing evidence of the importance of a novel T cell population, Th17 cells, in autoimmune disease. Th17 cells are stimulated by IL23 (which shares the p40 subunit with IL12) to produce IL17 and also IL22, which has recently been shown to be a major driver of acanthosis in psoriasis, and so is a novel target for treatment.39
Dendritic cells are numerous in psoriatic plaques and influence T cell differentiation and activation by the patterns of cytokines they produce. Dendritic cells are also a major source of TNF‐α, and the success of anti‐TNF‐α treatment for psoriasis has highlighted the important role played by this pro‐inflammatory cytokine. TNF‐α, which has key roles in both innate and adaptive immune responses, is elevated in psoriatic plaques, and serum values correlate with the severity of the disease.40 Other elements of the innate immune response implicated in psoriasis pathophysiology include natural killer T cells (NK T cells), neutrophilic granulocytes, keratinocytes, antimicrobial peptides, and toll‐like receptors (TLR). NK T cells are present in psoriatic plaques in significantly increased numbers compared with non‐lesional skin, whereas circulating concentrations tend to be reduced, in proportion to disease activity.41 TLR1 and TLR2 expression is increased in psoriatic keratinocytes,42 and indeed monomethylfumarate, an agent used to treat psoriasis, interferes with TLR signalling.43
Management
Optimal management of psoriasis depends on a number of factors including the severity of disease, co‐morbidities, and patient expectation. Patient education about the chronic nature of the condition and the need for realistic expectations is vital. Complete clearance of psoriasis may be unrealistic and recurrence is to be expected. A stepwise approach to treatment is often employed with initial use of topical treatment, proceeding to phototherapy and systemic therapy if required. With each step efficacy improves, but toxicity increases.
In a flare of psoriasis a precipitant should be sought, such as infection (particularly streptococcal throat infection in guttate psoriasis), a new drug, trauma, stress or recent alcohol binge. Most psoriasis patients are managed within a primary care setting and are treated with topical treatments alone. Patient compliance, especially for topical treatment, is often disappointing, and needs to be considered when advising on appropriate therapy. In those whom topical treatments prove ineffective, phototherapy or systemic therapy should be considered. Therapeutic responsiveness and safety vary unpredictably between patients and so a repertoire of treatments is required. As the field of pharmacogenetics expands, it may be possible to predict which patients will respond to, or be susceptible to toxicity from, psoriasis treatments. Early results suggest VEGF polymorphisms play a role in predicting response to acitretin in psoriasis,44 while several polymorphisms in the methylenetetrahydrofolate reductase gene predict methotrexate toxicity in patients with rheumatoid arthritis.45,46 Currently the choice of treatment is determined by patient preference, age, reproductive potential, co‐morbidities, and extent and pattern of disease, especially psoriatic arthritis. Each agent has contraindications and side effects, and so management of psoriasis is individualised. In an attempt to minimise long term toxicity, rotational and sequential approaches are sometimes employed. A strategy should be devised with the patient's involvement, where a realistic goal is set, comprising disease control, symptom relief, time involvement of treatment, and minimising toxicity. Table 3 summarises the available treatment modalities for psoriasis.
Table 3 Summary of treatment modalities for psoriasis.
| Topical | Phototherapy | Systemic | Biological |
|---|---|---|---|
| Vitamin D derivatives | UVB | Methotrexate | Efaluzimab |
| Corticosteroids | PUVA | Ciclosporin | Alefacept |
| Coal tar | Acitretin | Etanercept | |
| Dithranol | Infliximab | ||
| Adalimumab |
PUVA, psoralen plus ultraviolet A; UVB, ultraviolet B.
Topical treatments
Two thirds of psoriasis patients have mild disease that can be treated with topical therapy alone, which has a high efficacy to safety ratio. Unfortunately, concordance with topical therapeutic regimens is poor, with only 50% concordance in clinical trials where the patients are told that drug use is monitored. Patients sometimes complain of a lack of practical guidance by the doctor prescribing topical therapy and specialist nurses may help with advice about application of treatment, with supervised treatments where appropriate.
Vitamin D analogues and topical corticosteroids are the most frequently used topical agents. Vitamin D3 analogues (such as calcipotriol) are generally well tolerated and have a favourable long term safety record, although a maximum weekly dose of 100 g is suggested to avoid systemic hypercalcaemia. Placebo controlled comparisons show calcipotriol is more effective than dithranol, tar and other D3 analogues in treating chronic plaque psoriasis.47 Topical corticosteroids are effective and well tolerated in psoriasis, but the risk of skin atrophy or tachyphylaxis (a rapidly reduced response following repeated dosing) limits their use. Combination calcipotriol and betamethasone has been shown to be more effective than twice daily monotherapies of each agent.48 Crude coal tar and dithranol are established treatment but are mostly used under supervision as a day patient or inpatient due to the mess and inconvenience associated with their application.
Certain sites, such as the scalp, flexures and face, are particularly difficult to treat. In managing scalp psoriasis initial descaling with a preparation containing salicylic acid is followed by use of an anti‐inflammatory agent, such as calcipotriol or corticosteroids. Application to this area is difficult and patients may require nurse support. The face and flexures are particularly sensitive and calcipotriol use is not recommended. The use of steroids should be restricted due to potential risk of skin atrophy and perioral dermatitis. Calcineurin inhibitors (such topical tacrolimus 0.1%) have been shown to be effective in both these sites.49
Narrowband ultraviolet B
The introduction of Philips narrowband (311–312 nm) TL‐01 fluorescent tubes in 1984 represented a significant advance over previous broad spectrum (290–315 nm) ultraviolet B (UVB) tubes. Wavelengths between 300–311 nm have the greatest anti‐psoriatic therapy. The exact mechanism of action of UVB is not fully understood, but it is known to inhibit DNA synthesis and epidermal keratinocyte proliferation; induce T cell apoptosis, and induce immunosuppressive and anti‐inflammatory cytokines.
UVB is appropriate for patients who do not respond adequately to topical treatments or who have widespread disease—for example, guttate psoriasis. Treatment is given three times weekly and 60–80% of patients achieve clearance after 15–20 treatments.50,51 Long term risks include photodamage and a possible dose related increase in the incidence of skin cancer. The exact risk is unknown at present, although a meta‐analysis of studies using BB–UVB has estimated the excess annual risk of non‐melanoma skin carcinoma (NMSC) to be <2%.52 The risk is certainly considered to be less than that associated with psoralen plus ultraviolet A (PUVA). The role of iatrogenic immunosuppression is not fully known, but patients who are likely to require immunosuppression should not receive excess UVB. Current guidelines recommend limited treatment courses and shielding of face and genitalia. Inconvenience and cost to the patient of travelling to clinic for frequent treatments are considerations.
PUVA
Recent trials suggest the efficacy of narrowband UVB is similar to, but slightly lower than PUVA.53 PUVA involves oral or topical administration of a photosensitising psoralen followed by exposure to long wavelength (320–400 nm) UVA radiation. PUVA therapy is effective for most forms of psoriasis and induces complete or partial remission in 70–90% of patients with psoriasis.54,55 Combination retinoid plus PUVA therapy is more effective than either regimen alone, and the efficacy of PUVA is increased by concomitant topical treatment.56
Long term risks include skin cancer and premature ageing of skin. There is a clear relationship between cumulative PUVA exposure and increased risk of skin cancer. Minimising cumulative PUVA exposure may reduce the risk of adverse events. Careful selection of patients is important; PUVA is unsuitable for those with photosensitivity, skin cancer, aphakia, on immunosuppressive therapy, and in pregnancy and breastfeeding. British Association of Dermatologist guidelines recommend that PUVA should be limited to 150 lifetime treatments due to the increased risk of cutaneous malignancy. Caution should be applied when giving PUVA to younger patients as they may eventually require immunosuppressive systemic therapy. The risk of skin cancer associated with PUVA persists for at least 15 years after discontinuation of treatment57 and is increased when ciclosporin is given. Narrowband UVB is now considered first line phototherapy, with PUVA reserved for those who fail to respond adequately to this.
Systemic treatment
Methotrexate, acitretin and ciclosporin have been used to treat severe psoriasis for 40, 25 and 15 years, respectively, and are licensed for this indication in the UK. In mainland Europe fumaric acid esters are also licensed and have been used effectively since the 1960s. For those who fail to respond to these agents a variety of unlicensed second line systemic agents are used including hydroxycarbamide, azathioprine, leflunomide and mycophenolate mofetil. Each carries a risk of toxicity but there are few published data on the efficacy and safety of long term treatment. Biological therapies have been used over the past 5 years in increasing numbers of patients.
Methotrexate
Low dose methotrexate is the most frequently used systemic treatment for psoriasis worldwide and is also effective in treating psoriatic arthritis. The mechanism of action is not fully understood but it is believed to act primarily as an anti‐inflammatory and immunosuppressant drug. Patients with severe psoriasis achieve a 60% improvement in PASI during the first 6 months of methotrexate treatment.58 Major side effects include myelosuppression, hepatic fibrosis and pulmonary fibrosis. Mouth ulceration is a useful clinical indicator of toxicity/overdose. A particular concern has been the accidental prescription of daily methotrexate, which is responsible for 67% of all 137 patient safety incidents related to methotrexate dosing collected by the National Patient Safety Association between 1993 and 2002 (including 25 deaths and 26 episodes of serious harm to patients).59 Methotrexate is a teratogen and abortifacient and so is contraindicated in pregnancy and should be used with care in women of childbearing potential. It should also be avoided in men whose partners may become pregnant. Frequent monitoring of renal, hepatic and haematological parameters is essential.
Concern about hepatic fibrosis has limited the use of methotrexate in psoriasis, particularly in view of the association between severe psoriasis and alcohol consumption and diabetes, which are thought to increase this risk. Given the low sensitivity of serum liver enzymes in detecting early fibrosis, American guidelines have advised serial liver biopsy for monitoring every 1.5 g of methotrexate or every 2–3 years.60 Increasingly European dermatologists use serial measurements of serum aminoterminal peptide of type III procollagen (P3NP) to assess for ongoing liver fibrosis. Serial P3NP correlates with the presence and severity of liver fibrosis in psoriasis patients treated with methotrexate, although concentrations may be raised in childhood and perhaps also in the presence of psoriatic arthritis.61 Most British dermatologists give supplemental folate with methotrexate for psoriasis, as this has been shown to reduce liver enzyme abnormalities in patients with rheumatoid arthritis on methotrexate; however, a recent study of methotrexate in psoriasis patients suggests that supplemental folic acid may reduce the efficacy of methotrexate.62
Oral retinoids
The exact mechanism of action is unknown, but retinoids modulate epidermal proliferation and differentiation and have immunosuppressive and anti‐inflammatory activity. Acitretin is the only oral retinoid approved for psoriasis although other retinoid‐like drugs are undergoing clinical trials. Acitretin is effective in a smaller proportion of patients than ciclosporin or methotrexate, but has a favourable side effect profile for longer term use. The major safety concern is teratogenicity, which extends up to 3 years following discontinuation of treatment and precludes its use in many women. Treatment is discontinued in 10–20% of patients because of dose related side effects such as mucocutaneous drying and irritation, myalgia and hair loss. Mild elevation in liver enzymes and lipids are common and require regular monitoring.
Ciclosporin
Ciclosporin has been shown to be effective in treating both plaque and pustular psoriasis. In a recent comparative trial in moderate to severe psoriasis, 71% treated with ciclosporin and 60% treated with methotrexate achieved PASI 75 (a 75% reduction in PASI from baseline) at 16 weeks.63 In view of its rapid onset of action and cumulative toxicity, in particular nephrotoxicity and hypertension, it is better suited to short term treatment of psoriasis flares, rather than long term maintenance treatment. Current guidelines recommend that patients should receive continuous ciclosporin for no more than 2 years. Ciclosporin increases the risk of infection and, with long term use, non‐melanoma skin cancer, particularly in those previously exposed to significant doses of PUVA. Although there is an increased risk of systemic malignancy, including lymphoma, in transplant patients treated with ciclosporin, the degree of immunosuppression in these patients is far greater than for those treated for psoriasis.
Fumaric acid esters
Fumaric acid esters (FAE) (dimethylfumarate and monomethylfumarate) are thought to act by causing a shift towards a Th2 cytokine profile associated with a reduction in peripheral lymphocytes and inhibition of keratinocyte proliferation. FAE have been shown to be effective in treating psoriasis and since their use was first reported in 1959, have been of increasing popularity due to their relatively long term safety profile. Organ toxicity or myelosuppression is rare but flushing and gastrointestinal symptoms cause 30–40% of patients to stop treatment.64 FAE are not currently licensed in the UK.
Immunobiological treatment
An understanding of the immunopathogenesis of psoriasis has led to the emergence of new treatments targeting the immune system. Biological treatments block cellular or molecular steps in the pathogenesis of psoriasis and represent a valuable alternative for patients with severe psoriasis. Currently licensed treatments comprise T cell targeted agents (efaluzimab, alefacept) and TNF‐α antagonists (infliximab, etanercept, adalumimab).
T cell targeted treatment
The first biological treatment approved for psoriasis was alefacept, a fusion protein consisting of the extracellular domain of LFA‐3 fused to IgG. It binds CD2 on activated memory T cells, blocking co‐stimulatory signals from antigen presenting cells and inducing apoptosis in T cells to which it has bound. Alefacept is given as a 12 week course of weekly intramuscular injections which requires CD4 count monitoring during treatment. Efaluzimab is a monoclonal antibody against the CD11a subunit of the integrin LFA‐1 on T cells. This blocks the interaction between LFA1 and ICAM1, which is necessary in both T cell extravasation and also in establishing the immunological synapse between T cells and antigen presenting cells. Efaluzimab is licensed as continuous treatment by weekly subcutaneous injection. In contrast to alefacept, it is not T cell depleting and CD4 counts do not need to be monitored, but because of occasional thrombocytopenia, full blood count is monitored.
At 12 weeks, a response (PASI 75) is seen in 33% with alefacept65 and 27% with efalizumab.66,67 Both drugs appear to have a durable clinical response; improvement was maintained until 24 weeks with repeated dosing in most patients treated with efaluzimab and maintained in approximately 30% during 12 week follow up of a drug‐free period,66 while improvement was maintained in many for 7 months after a single course of alefacept and many patients treated with a second course showed improved clinical response.68 In general these co‐stimulation inhibitors are more effective in cutaneous psoriasis than in psoriatic arthritis.
TNF‐α antagonists
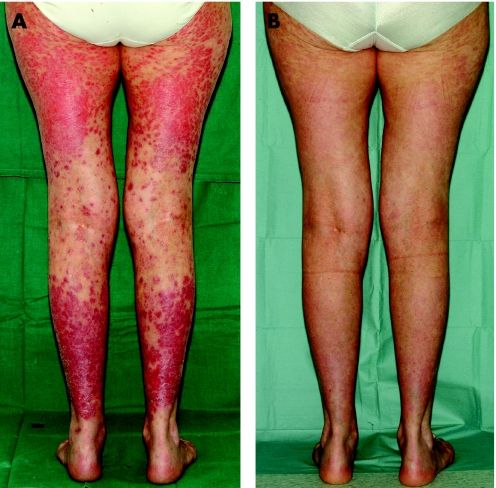
Adalimumab (a human anti‐TNF‐α monoclonal antibody), etanercept (a human recombinant TNF receptor p75 fusion protein) and infliximab (a chimeric anti‐TNF‐α monoclonal antibody) are all licensed for psoriasis and/or psoriatic arthritis. Their safety has been determined over many years in treatment registries when used to treat rheumatoid arthritis or Crohn's disease. However, the effective dose to clear psoriasis is generally higher than is needed in rheumatoid arthritis. As patients with severe psoriasis are more likely to have received high doses of ultraviolet phototherapy, treatment registries are being established in this population also to determine long term relative safety. Efficacy with these agents can be excellent (fig 1) with PASI 75 achieved at 12 weeks in 80%, 49% and 88% with adalimumab 40 mg weekly,69 etanercept 50 mg twice weekly70 or infliximab 5 mg/kg,71 respectively. These drugs are licensed as monotherapy although there may be synergy with conventional systemic agents such as methotrexate, which may also reduce the development of neutralising antibodies against infliximab. In contrast to the co‐stimulator inhibitors, TNF antagonists may be effective for psoriatic arthritis also.
Figure 1 A patient with severe psoriasis before (A) and after (B) treatment with a TNF‐α antagonist.
In Europe (in contrast to the USA) immunobiological treatments for psoriasis are only licensed in those with moderate to severe psoriasis who have failed other licensed systemic drugs (methotrexate, ciclosporin and PUVA) or in whom these drugs are contraindicated or not tolerated. The British Association of Dermatologists has produced useful guidelines on the management of psoriasis with biological treatments (http://www.bad.org.uk/healthcare/guidelines/Biological_Interventions.pdf). At the time of writing, the National Institute for Health and Clinical Evidence (NICE) has produced guidelines for the use of etanercept and efalizumab in psoriasis (http://guidance.nice.org.uk/TA103) and for etanercept or infliximab in psoriatic arthritis. In the case of cutaneous disease, this requires a PASI of 10 or greater and DLQI of 10 or greater, combined with a failure of other treatment modalities to qualify for treatment. Response is to be assessed at 12 weeks and the response criterion is a PASI 75 or PASI 50 and 5 point fall in DLQI. Using the NICE pharmacoeconomic model, etanercept is advised as first biological treatment, but only at 25 mg twice weekly (which will achieve PASI 75 in 34% as pulsed therapy)70 with efalizumab reserved for non‐responders. Advice on infliximab and adalimumab is awaited.
Summary
Managing psoriasis effectively remains a challenge for the dermatologist. Assessment of psoriatic patients must include an evaluation of impact on quality of life, in addition to the clinical appearance and extent of the condition. There is no standard therapeutic approach, as management requires a carefully balanced individualised approach, taking into consideration the efficacies and toxicities of each treatment. Biological therapies are in their early development in psoriasis treatment, but are being used increasingly in treatment resistant severe disease. Their role in the longer term is unknown. Further research into the pathophysiology of psoriasis is vital to suggest further ways to manipulate the biological process to a therapeutic end. In particular, identification of psoriasis susceptibility genes, and further pharmacogenetic profiling of the condition, may allow prediction of efficacy of agents according to genotype. For the present, an appreciation that severe psoriasis may have a major effect on morbidity and quality of life has developed in tandem with fascinating insights into disease pathomechanisms and hopeful new treatments.
Multiple choice questions (true (T)/false (F); answers after the references)
1. Psoriasis phenotype:
The peak incidence of psoriasis is in the fourth and fifth decades
Dilatation of dermal capillaries is a characteristic histological finding
Psoriatic arthritis affects 50% of patients
Psoriasis patients have an increased risk of ischaemic heart disease
Psoriasis patients have increased risk of lymphoma
2. Psoriasis genotype:
Phenotypic variability due to heritability of psoriasis is about 80%
A positive family history is found in 50% of childhood cases
PSORS 1 is found on chromosome 12
PSORS 1 is associated with early onset psoriasis
Psoriasis susceptibility loci overlap with loci for atopy
3. Pathophysiology of psoriasis:
Angiogenic factors are important in psoriasis pathophysiology
VEGF is over‐expressed in the psoriatic epidermis
Psoriasis may be transferred in bone marrow transplants from affected individuals
TNF concentrations are reduced in plaques of psoriasis
Circulating concentrations of natural killer (NK T) cells are increased in active psoriasis
4. Treatment of psoriasis:
Most psoriasis patients are managed with topical treatment alone
UVB wavelengths between 312–325 nm have the greatest anti‐psoriatic activity
PUVA increases the risk of skin cancer
Methotrexate should be given as a daily dose
Oral retinoids are treatment of choice for young females
5. Biological treatment for psoriasis:
Efaluzimab blocks the interaction between LFA 1 and ICAM 1
CD4 counts should be monitored during efaluzimab treatment
Infliximab has the greatest rates of clearance of psoriasis of current biological agents
Biological agents are often given with concurrent methotrexate
Response to biological treatment should be assessed at 6 weeks
Abbreviations
APC - antigen presenting cells
DLQI - Dermatology Life Quality Index
FAE - fumaric acid esters
ICAM - intracellular adhesion molecule
IFN‐γ - interferon‐γ
IL - interleukin
MHC - major histocompatibility complex
NK - natural killer
NMSC - non‐melanoma skin carcinoma
PASI - Psoriasis Area and Severity Index
PUVA - psoralen plus ultraviolet A
TGF‐α - tumour growth factor‐α
P3NP - aminoterminal peptide of type III procollagen
TLR - toll‐like receptors
TNF‐α - tumour necrosis factor‐α
UVA - ultraviolet A
UVB - ultraviolet B
VEGF - vascular derived endothelial growth factor
Answers
(A) F (B) T (C) F (D) T (E) T
(A) T (B) F (C) F (D) T (E) T
(A) T (B) T (C) T (D) F (E) F
(A) T (B) F (C) T (D) F (E) F
(A) T (B) F (C) T (D) T (E) F
Footnotes
Competing interests: None
References
- 1.Gelfand J M, Neimann A L, Shin D B.et al Risk of myocardial infarction in patients with psoriasis. JAMA 20062961735–1741. [DOI] [PubMed] [Google Scholar]
- 2.Lebwohl M, Callen J P. Obesity, smoking and psoriasis. JAMA 2006295208–210. [DOI] [PubMed] [Google Scholar]
- 3.Neimann A L, Shin D B, Wang X.et al Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 200655829–835. [DOI] [PubMed] [Google Scholar]
- 4.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol 199532982–986. [DOI] [PubMed] [Google Scholar]
- 5.Prodanowich S, Ma F, Taylor J R.et al Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol 200552262–267. [DOI] [PubMed] [Google Scholar]
- 6.Boffeta P. Cancer risk in a population‐based cohort of patients hospitalised for psoriasis in Sweden. J Invest Dermatol 20011171531–1537. [DOI] [PubMed] [Google Scholar]
- 7.Hannuksela‐Svahn A, Pukkala E, Laara E.et al Psoriasis, its treatment, and cancer in a cohort of Finnish patients. J Invest Dermatol 2000114587–590. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand J M, Berlin J, Van Voorhees A.et al Lymphoma rates are low but increased in patients with psoriasis: results from a population‐based cohort study in the United Kingdom. Arch Dermatol 20031391425–1429. [DOI] [PubMed] [Google Scholar]
- 9.Yates V M, Watkinson G, Kelman A. Further evidence for an association between psoriasis, Crohn's and ulcerative colitis. Br J Dermatol 1982106323–330. [DOI] [PubMed] [Google Scholar]
- 10.Ho P, Bruce I N, Silman A.et al Evidence for common genetic control pathways of inflammation for Crohn's disease and psoriatic arthritis. Arthritis Rheum 2005523596–3602. [DOI] [PubMed] [Google Scholar]
- 11.Woodruff P W R, Higgins E M, du Vivier A W P.et al Psychiatric illness in patients referred to a dermatology‐psychiatry clinic. Gen Hosp Psychiatry 19971929–35. [DOI] [PubMed] [Google Scholar]
- 12.Gupta M A, Gupta A K. Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis. Br J Dermatol 1998139846–850. [DOI] [PubMed] [Google Scholar]
- 13.Gupta M A, Gupta A K. Psychodermatology: an update. J Am Acad Dermatol 1996341030–1046. [DOI] [PubMed] [Google Scholar]
- 14.Finlay A Y. Current severe psoriasis and the Rule of Tens. Br J Dermatol 2005152861–867. [DOI] [PubMed] [Google Scholar]
- 15.Ashcroft D M, Li Wan Po A, Williams H C.et al Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol 1999141185–191. [DOI] [PubMed] [Google Scholar]
- 16.Lewis V, Finlay A Y. Ten years of experience of the Dermatology Life Quality Index (DLQI). J Invest Dermatol Symp Proc 20049169–180. [DOI] [PubMed] [Google Scholar]
- 17.Hongbo Y, Thomas C L, Harrison M A.et al Translating the science of quality of life into practice: what do Dermatology Life Quality Index scores mean? J Invest Dermatol 2005125659–664. [DOI] [PubMed] [Google Scholar]
- 18.Duffy D L, Spelman L S, Martin N G. Psoriasis in Australian twins. J Am Acad Dermatol 199329428–434. [DOI] [PubMed] [Google Scholar]
- 19.Morris A, Rogers M, Fischer G.et al Childhood psoriasis: a clinical review of 1262 cases. Pediatr Dermatol 200118188–198. [DOI] [PubMed] [Google Scholar]
- 20.Trembath R C, Clough R L, Rosbotham J L.et al Identification of a major susceptibility locus on chromosome 6p and evidence of further disease loci revealed by a two stage genome‐wide search in psoriasis. Hum Mol Genet 1997681–20. [DOI] [PubMed] [Google Scholar]
- 21.Burden A D, Javed S, Bailey M.et al Genetics of psoriasis: paternal inheritance and a locus on chromosome 6p. J Invest Dermatol 1998110958–960. [DOI] [PubMed] [Google Scholar]
- 22.Veal C D, Capon F, Allen M H.et al Family‐based analysis using a dense single‐nucleotide polymorphism based map defines genetic variation at PSORS 1, the major psoriasis susceptibility locus. Am J Hum Genet 200271554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair R P, Stuart P E, Nistor I.et al Sequence and haplotype analysis supports HLA‐C as the psoriasis susceptibility gene. Am J Hum Genet 200678827–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helms C, Saccone N L, Cao L.et al Localisation of PSORS 1 to a haplotype block harboring HLA‐C and distinct from corneodesmosin and HCR. Hum Genet 2005118466–476. [DOI] [PubMed] [Google Scholar]
- 25.Gudjonsson J E, Karason A, Runarsdottir E H.et al Distinct clinical differences between HLA‐Cw0602 positive and negative psoriasis patients – an analysis of 1019 HLA‐C and HLA‐B typed patients. J Invest Dermatol 2006126740–745. [DOI] [PubMed] [Google Scholar]
- 26.Hwu W L, Young C F, Fann C S J.et al Mapping of psoriasis to 17q terminus. J Med Genet 200542152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cookson W O, Ubbhi B, Lawrence R.et al Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nat Genet 200127372–373. [DOI] [PubMed] [Google Scholar]
- 28.Thompson S D, Moroldo M B, Guyeret al A genome‐wide scan for juvenile rheumatoid arthritis in affected sibpair families provides evidence of linkage. Arthritis Rheum 2004502920–2930. [DOI] [PubMed] [Google Scholar]
- 29.Low J H, Williams F A, Yang X.et al Inflammatory bowel disease is linked to 19p13 and associated with ICAM‐1. Inflamm Bowel Dis 200410173–181. [DOI] [PubMed] [Google Scholar]
- 30.Zenz R, Erfel R, Kenner L.et al Psoriasis‐like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 2005437369–375. [DOI] [PubMed] [Google Scholar]
- 31.Creamer D, Allen M H, Sousa A.et al Localisation of endothelial proliferation and microvascular expansion in active plaque psoriasis. Br J Dermatol 1997136859–865. [PubMed] [Google Scholar]
- 32.Xia Y P, Li B, Hylton D.et al Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood 2003102161–168. [DOI] [PubMed] [Google Scholar]
- 33.Creamer D, Allen M H, Groves R W.et al Circulating vascular permeability factor/vascular endothelial growth factor in erythroderma. Lancet 19963481101. [DOI] [PubMed] [Google Scholar]
- 34.Bhushan M, McLaughlin B, Weiss J B.et al Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br J Dermatol 1999141851–855. [DOI] [PubMed] [Google Scholar]
- 35.Creamer D, Allen M, Jaggar R.et al Mediation of systemic vascular hyperpermeability in severe psoriasis by circulating vascular endothelial growth factor. Arch Dermatol 2002138791–796. [DOI] [PubMed] [Google Scholar]
- 36.Snowden J A, Heaton D C. Development of psoriasis after syngeneic bone marrow transplant from psoriatic donor: further evidence for adoptive autoimmunity. Br J Dermatol 1997137130–132. [PubMed] [Google Scholar]
- 37.Boyman O, Hefti H P, Conrad C.et al Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumour necrosis factor‐alpha. J Exp Med 2004199731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kauffman C L, Aria N, Toichi E.et al A phase 1 study evaluating the safety, pharmacokinetics, and clinical response of a human IL‐12 p40 antibody in subjects with plaque psoriasis. J Invest Dermatol 2004123125–130. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Y, Danilenko D M, Valdez P.et al Interleukin 22 a T(H)17 cytokine, mediates IL‐23 dermal inflammation and acanthosis. Nature 2007445648–651. [DOI] [PubMed] [Google Scholar]
- 40.Mussi A, Bonifati C, Carducci M.et al Serum TNF‐alpha levels correlate with disease severity and are reduced by effective therapy in plaque type psoriasis. J Biol Regul Homeost Agents 199711115–118. [PubMed] [Google Scholar]
- 41.Cameron A L, Kirby B, Grifffiths C E. Circulating natural killer cells in psoriasis. Br J Dermatol 2003149160–164. [DOI] [PubMed] [Google Scholar]
- 42.Baker B S, Ovigne J M, Powles A V.et al Normal keratinocytes express toll‐like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol 2003148670–679. [DOI] [PubMed] [Google Scholar]
- 43.Litjens N H, Rademaker M, Ravensbergen B.et al Monomethylfumarate affects polarization of monocyte‐derived dendritic cells resulting in downregulated Th1 lymphocyte responses. Eur J Immunol 200434565–575. [DOI] [PubMed] [Google Scholar]
- 44.Young H S, Summers A M, Read I R.et al Interaction between genetic control of vascular endothelial growth factor production and retinoid responsiveness in psoriasis. J Invest Dermatol 2006126453–459. [DOI] [PubMed] [Google Scholar]
- 45.Berkun Y, Levartovsky D, Rubinow A.et al Methotrexate related adverse effects in patients with rheumatoid arthritis are associated with the A1298C polymorphism of the MTHFR gene. Ann Rheum Dis 2004631227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Ede A E, Laan R F, Blom H J.et al The C667T mutation in the methylenetetrahydrofolate reductase gene: a genetic risk factor for methotrexate‐related elevation of liver enzymes in rheumatoid arthritis patients. Arthritis Rheum 2001442525–2530. [DOI] [PubMed] [Google Scholar]
- 47.Mason J, Mason A R, Cork M J. Topical preparations for the treatment of psoriasis: a systemic review. Br J Dermatol 2002146351–364. [DOI] [PubMed] [Google Scholar]
- 48.Guenther L, van de Kerkhof P C, Snellman E.et al Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double blind, vehicle controlled clinical trial. Br J Dermatol 2002147316–322. [DOI] [PubMed] [Google Scholar]
- 49.Lebwohl M, Freeman A K, Chapman M S.et al Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol 200451723–730. [DOI] [PubMed] [Google Scholar]
- 50.Gordon P M, Diffey B L, Matthews J N S.et al A randomized comparison of narrowband TL‐01 phototherapy and PUVA photochemotherapy for psoriasis. J Am Acad Dermatol 199941728–732. [DOI] [PubMed] [Google Scholar]
- 51.Cameron H, Dawe R S, Yule S.et al A randomized, observer‐blinded trial of twice versus three times weekly narrowband UVB phototherapy for chronic plaque psoriasis. Br J Dermatol 2002147973–978. [DOI] [PubMed] [Google Scholar]
- 52.Pasker de Jong P C, Wieling G, van der Valk P G.et al Treatment with UVB for psoriasis and non‐melanoma skin cancer: a systemic review of the literature. Arch Dermatol 1999135834–840. [DOI] [PubMed] [Google Scholar]
- 53.Ibbotson S H, Bilsland D, Cox N H.et al An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatoloy Group Workshop Report. Br J Dermatol 2004151283–297. [DOI] [PubMed] [Google Scholar]
- 54.Lauharanta J. Photochemotherapy. Clin Dermatol 199715769–780. [DOI] [PubMed] [Google Scholar]
- 55.Morison W L, Baughman R D, Day R M.et al Consensus workshop on the toxic effects of long‐term PUVA therapy. Arch Dermatol 1998134595–598. [DOI] [PubMed] [Google Scholar]
- 56.Griffiths C E M, Clark C M, Chalmers R J G.et al A systematic review of treatments for severe psoriasis. Health Technol Assess 200041–125. [DOI] [PubMed] [Google Scholar]
- 57.Nijsten T E C, Stern R S. The increased risk of skin cancer is persistent after discontinuation of psoralen + ultraviolet A: a cohort study. J Invest Dermatol 2003121252–258. [DOI] [PubMed] [Google Scholar]
- 58.Leon A, Nguyen A, Letsinger J.et al An attempt to formulate an evidence‐based strategy in the management of moderate‐to‐severe psoriasis: a review of the efficacy and safety of biologics and prebiologic options. Expert Opin Pharmacother 20078617–632. [DOI] [PubMed] [Google Scholar]
- 59.National Patient Safety Agency http://www.npsa.nhs.uk (Accessed 4 April 2007)
- 60.Roenigk H H, Jr, Auerbach R, Maibach H.et al Methotrexate in psoriasis: consensus conference. J Am Acad Dermatol 199838478–485. [DOI] [PubMed] [Google Scholar]
- 61.Zachariae H, Aslam H M, Bjerring P.et al Serum aminoterminal propeptide of type III procollagen in psoriasis an psoriatic arthritis: relation to liver fibrosis and arthritis. J Am Acad Dermatol 19912550–53. [DOI] [PubMed] [Google Scholar]
- 62.Salim A, Tan E, Ilchyshyn A.et al Folic acid supplementation during treatment of psoriasis with methotrexate: a randomized, double‐blind, placebo‐controlled trial. Br J Dermatol 20061541169–1174. [DOI] [PubMed] [Google Scholar]
- 63.Heydendael V M, Spuls P I, Opmeeer B C.et al Methotrexate versus cyclosporine in moderate‐to‐severe chronic plaque psoriasis. N Engl J Med 2003349658–665. [DOI] [PubMed] [Google Scholar]
- 64.Naldi L, Rzany B. Chronic plaque psoriasis. Clin Evid 200281688–1708. [PubMed] [Google Scholar]
- 65.Ellis C N, Krueger G G. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med 2001345248–255. [DOI] [PubMed] [Google Scholar]
- 66.Lebwohl M, Tyring S K, Hamilton T K.et al A novel targeted T‐cell modulator, efaluzimab, for chronic plaque psoriasis. N Engl J Med 20033492004–2013. [DOI] [PubMed] [Google Scholar]
- 67.Gordon K B, Papp K A, Hamilton T K.et al Efaluzimab for patients with moderate‐to severe plaque psoriasis; a randomized controlled trial. JAMA 20032323073–3080. [DOI] [PubMed] [Google Scholar]
- 68.Krueger G G, Papp K A, Stough D B.et al A randomized, double‐blind, placebo‐controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol 200247821–833. [DOI] [PubMed] [Google Scholar]
- 69.Gordon K B, Papp K A, Hamilton T K.et al Efaluzimab for patients with moderate‐to severe plaque psoriasis; a randomized controlled trial. JAMA 20032323073–3080. [DOI] [PubMed] [Google Scholar]
- 70.Gordon K B, Langley R G, Leonardi C.et al Clinical response to adalimumab treatment in patients with moderate‐to‐severe psoriasis: double‐blind, randomized controlled trial and open‐label extension study. J Am Acad Dermatol 200655598–606. [DOI] [PubMed] [Google Scholar]
- 71.Leonardi C L, Powers J L, Matheson R T.et al Etanercept as monotherapy in patients with psoriasis. N Engl J Med 20033492014–2022. [DOI] [PubMed] [Google Scholar]
- 72.Gottlieb A B, Evans R, Li S.et al Infliximab induction therapy for patients with severe plaque‐type psoriasis: a randomized, double‐blind, placebo‐controlled trial. J Am Acad Dermatol 200451534–542. [DOI] [PubMed] [Google Scholar]