Abstract
The introduction of multidetector computed tomography (MDCT) is considered a dramatic development in CT imaging that has direct implication in the imaging of various systems, in particular the cardiovascular system. The advantages of MDCT are an enormous increase in imaging acquisition speed, more coverage of the patient, and high spatial resolution. This article reviews the recent developments in CT angiography and discusses the clinical application relevant to diagnosis and endovascular treatment of cardiovascular diseases.
Keywords: spiral computed tomography, multidetector CT, angiography, cardiovascular system, radiology
The introduction of multidetector computed tomography (MDCT) is considered a dramatic development in CT imaging that has direct implications in the imaging of various systems, in particular the cardiovascular system. The advantages of MDCT are an enormous increase in imaging acquisition speed, more coverage of the patient, and high spatial resolution.
There are two main differences between conventional spiral CT and MDCT. Firstly, MDCT has a high acquisition speed (0.37 s rotation speed vs 1 s rotation speed for conventional CT); secondly, and probably more importantly, MDCT acquires volume data instead of individual slice data. These two factors together with thin section slices enable the new technique to provide almost isotropic data that can be arranged in different planes without compromising the spatial resolution of the original axial images.
This article reviews the recent developments in CT angiography (CTA) and discusses the clinical application relevant to diagnosis and endovascular treatment of cardiovascular diseases.
Principles of CT angiography
There are four main principles of CTA:
Achieving good arterial contrast enhancement during image acquisition
Providing adequate cephalocaudal coverage of the arterial system during optimum contrast opacification
Imaging of the arterial tree during the first circulation of contrast to avoid venous artefact
Efficient handling of the huge amount of acquired data using various post‐processing algorithms including surface shaded display (SSD), volume rendering technique (VRT), multiplanar reconstruction (MPR) and maximum intensity projection (MIP).
Imaging of aorto‐iliac disease
The indications for imaging of aorta and iliac system are summarised in box 1.
Box 1: Clinical applications of CT angiography
Thoraco‐abdominal aorta
Diagnosis of congenital and degenerative aortic diseases
Assessment of acute aortic injuries and dissections
Evaluation of visceral arteries (coeliac, superior mesenteric and renal arteries)
Preoperative planning and follow up
Tumour staging and surgical planning
Renal arteries
Assessment of anatomy for donor transplants
Diagnosis of renal artery stenosis in hypertensives or deteriorating renal function
Assessment of renal arteries post‐intervention (renal artery stenting)
Peripheral arterial system
Assessment of peripheral vascular disease
Assessment of bypass grafts
Carotid/intracranial circulation
Characterisation of the atherosclerotic disease
Assessment of aortic arch vessels
Verification of internal carotid artery stenosis
Preoperative planning of endovascular and surgical treatment of intracranial aneurysms and vascular malformations
Cardiac imaging
Atypical chest pain
Patients with intermediate risk
Young patients with high risk for coronary disease
Coronary artery anomalies
Non‐invasive follow‐up following percutaneous transluminal angioplasty and stenting
Assessment of myocardial scars, aneurysms, tumours and thrombi
Assessment of coronary artery bypass grafts
Assessment of the pulmonary veins before and following radiofrequency ablation
Using the new generation of CT scanners the thoraco‐abdominal aorta can be covered within a single breath hold in between 11–22 s.1 The amount of injected contrast ranges from 50–110 ml, depending on a patient's respiratory status, left ventricular function and the degree of anatomical details of the aorta branches needed clinically. Retrospective or prospective ECG synchronisation can be applied to obtain artefact‐free images.2 This is of particular clinical interest when aortic dissection is suspected.
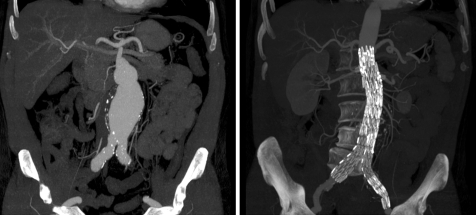
Until recently open surgery was the only option to treat patients with aortic aneurysms. The endovascular stent graft has evolved in the last decade to provide an effective alternative to surgery in selected patients with aortic diseases (fig 1).3 The key to successful aortic stent grafting is patient selection and appropriate planning. The factors that need to be taken into consideration in endovascular planning are: aneurysm neck of at least 1.5 cm below the renal arteries; absence of thrombus in the neck; neck angulations <65°; and favourable iliac anatomy. However, the arrival of fenestrated and branched stents allows treatment of short‐necked and juxta‐renal aneurysms. The stent graft should also be of appropriate length. Conventional angiography has long been the gold standard technique for evaluation of the aorto‐iliac region. However, it is invasive and fails to assess accurately the mural thrombus, the exact diameter of the aortic aneurysm and the status of the aortic wall and surrounding structures.
Figure 1 Abdominal aortic aneurysm. Multidetector computed tomography (MDCT) coronal reconstructions show a large abdominal aortic aneurysm pre‐ (left) and post‐endovascular stenting (right).
CTA is a less invasive technique, more reliable in determining the aneurysm sac and more sensitive in detecting mural thrombus. CT scanning can also visualise the aortic wall to assess for inflammatory changes or detect rupture of the aortic aneurysm. The available software reconstructs hundreds of images and displays them in various three dimensional and two dimensional planes providing a very powerful tool for preoperative planning and postoperative follow up. The most important point in the follow up of stent grafts is to exclude an endoleak into the aneurysm sac. It was found that CT angiography is more sensitive and specific than conventional angiography in detecting the presence of both endoleaks and faulty stents.4 The superior image quality with the current MDCT angiography, especially those with 16 and 64 channel detectors, means that diagnostic angiography is rarely used for the planning or follow‐up of endovascular aortic aneurysm repair at our centre.
Imaging of the renal arteries
The two main indications for renal angiography are to image the renal anatomy of live donors and to identify possible renal artery stenoses (RAS) in cases of suspected renovascular hypertension or in some cases of deteriorating renal function (box 1).
When imaging the renal anatomy of live donors the importance is in detecting any anatomical variations such as accessory arteries, early renal artery branching or venous anomalies, as these affect the difficulty and risk of complications in an operation which is being conducted for altruistic reasons.5 It is also important to identify incidental renal pathology on the donor side such as renal cysts.
Studies looking at the sensitivity and specificity for the detection of accessory renal arteries using MDCT angiography have either used operative findings or digital subtraction angiography (DSA) as the gold standard. Studies report sensitivity between 80–100% with specificity between 96–100% in the detection of accessory renal arteries.5,6,7,8 The sensitivity and specificity of the detection of early arterial branching was reported as 100% in one study,6 although the sensitivity in another study was only 89%, with a specificity of 100%.5 The different studies varied as to what they defined as venous anomalies with a sensitivity of between 97–100%5,6,7; however, one study had a relatively low sensitivity of 78% for minor venous variants.7
Renal artery disease is a curable cause of hypertension effecting 1–5% of patients with secondary hypertension.9 Doppler ultrasound (DUS) is used to look for RAS although it has the disadvantages of requiring an expert user, relying on a reasonable patient body habitus, and being unreliable in detecting accessory renal arteries. DUS compared to DSA has a sensitivity of 75% and a specificity of 89.6% for RAS.10 Both magnetic resonance angiography (MRA) and MDCT angiography are used to evaluate RAS. In one study there was no significant difference between the two, with sensitivities of 93% and 92%, respectively, and specificities of 100% and 99%, respectively, although in this study patient acceptance was higher for MDCT angiography.11 MDCT angiography can also be used post‐stenting to assess for recurrent RAS.
For evaluating the renal anatomy of liver donors MDCT angiography has the advantage over MRA in that it is more acceptable to patients and misses fewer accessory renal arteries.10 Although MDCT angiography involves radiation and a nephrotoxic contrast agent, kidney donors by their nature have good renal function where a single CTA has minimal negative effect when compared to the risk of complication due to not identifying an accessory renal artery. MDCT angiography ideally should not be used when a patient has poor renal function or an iodine allergy, although there is now concern about the use of some MRA contrast agents and the risk of nephrogenic systemic fibrosis.
Imaging of peripheral vascular disease
The indications for imaging of the peripheral arterial tree are summarised in box 1. DUS and pressure measurement studies are widely used to assess peripheral vascular disease (PVD). However, the presence of diffuse disease and heavy vascular calcification makes using these studies alone suboptimal in many cases. DUS is also operator dependent and can therefore not provide reliable evidence of extraluminal abnormalities such as cystic adventitial disease, popliteal entrapment and the bony status in trauma cases.
Although digital subtraction angiography remains the gold standard technique for evaluating PVD, CTA provides a relatively quick and non‐invasive technique, which covers a wide region of the body with high spatial resolution. The additional advantages of CTA are the visualisation of the extraluminal pathology, including aneurysms, as well as comprehensive review of visceral injuries and fractures in trauma patients. MDCT angiography provides better assessment of eccentric lesions and visualisation of more arterial segments, particularly in occlusive arterial disease. The sensitivity and specificity of MDCT angiography in comparison to DSA are reported to be between 93–100%.12,13,14,15 In a recent systematic review of 10 studies, including 400 patients, comparing angiography and MDCT angiography, the pooled sensitivity and specificity were, respectively, 92% (95% confidence interval (CI) 87% to 95%) and 91% (95% CI 87% to 95%) for the aorto‐iliac level, 96% (95% CI 94% to 99%) and 85% (95% CI 73% to 89%) for the femoro‐popliteal level, and 91% (95% CI 85% to 97%) and 85% (95% CI 72% to 97%) for infra‐popliteal level. However, there has been significant heterogeneity among the studies in terms of scan generation and scan protocol, and probably patient selection bias. This meta‐analysis concluded that MDCT angiography has high diagnostic value when compared with the reference standard test. The challenges for CTA in this part of the body include: injection of a large amount of contrast (120–160 ml); speed coverage, which should not exceed 50 mm/s to avoid the image acquisition running ahead of contrast column; and the need for imaging post‐processing since the number of the acquired slices is so large that they cannot be handled by viewing the axial images alone. Several protocols have been suggested to optimise vessel enhancement and reduce the total amount of injected contrast.16,17,18
The available post‐processing algorithms still have their own limitations. The MIP (maximum intensity projection) and VDR (vessel density ratio) exaggerate the degree of stenosis when there is calcified plaque. The MPR (multi‐planar reconstruction) cannot follow the tortuous or off plane course of some arteries, as is usually the case with the lower limb arteries. Several solutions are being investigated to overcome these technical problems including curved MPR and digital subtraction. Also, the current state of the art generation of 64 MDCT has not been thoroughly investigated.
Imaging of pulmonary arteries
Although selective pulmonary angiography is considered the standard reference test,19 there is strong doubt about the clinical usefulness and reproducibility of this examination. Pulmonary angiography is an invasive modality20,21 with significant inter‐observer disagreement rates ranging between 34–55%.22,23
Some centres still consider a ventilation–perfusion (VQ) scan the first line modality in diagnosis of pulmonary embolism (PE). However, this test suffers from a high percentage of intermediate results (73%)24 and poor inter‐observer agreement.25 Nevertheless, its role might improve if revised criteria of interpretation of VQ scan26,27 and newer technology such as single photon emission CT (SPECT) are introduced in practice.28 MRA has shown suboptimal resolution in detection of small PEs.29,30 Moreover, MRA is not widely available and a relatively long examination time is involved.
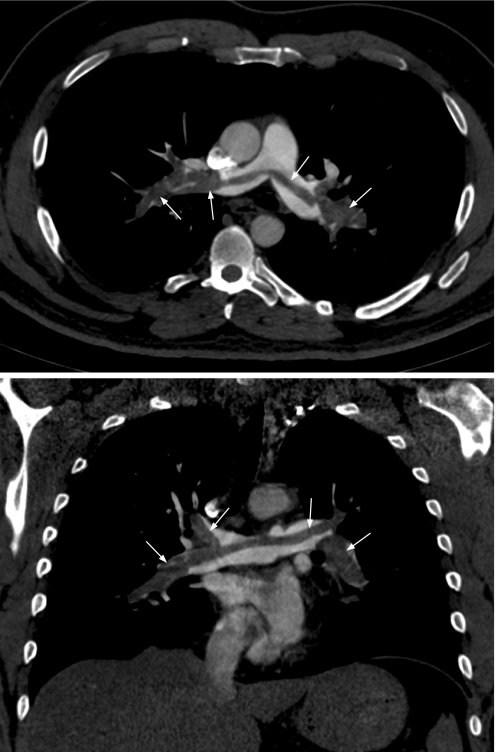
MDCT has the ability to cover the pulmonary artery tree with sub‐millimetre slices in a 10 s breath hold. This feature makes CT pulmonary angiography a first‐line diagnostic imaging modality in the management of PE in many centres (fig 2).
Figure 2 Pulmonary embolism. Contrast enhanced axial (top) and coronal (bottom) CT images showing a large saddle embolus within the main pulmonary trunk and the pulmonary arteries bilaterally (arrows).
The main advantage of this technique is the ability to evaluate the pulmonary vessels together with lung parenchyma and mediastinum. Studies have shown that more than two thirds of patients with a provisional diagnosis of PE may receive either an alternative or additional diagnosis such as aortic dissection or pneumothorax.31,32
In addition CT has demonstrated better inter‐observer agreement than nuclear medicine.33 Moreover, CT is more cost effective than other investigations that do not include CT, such as pulmonary angiography and VQ studies.34
MDCT with 16‐row detectors can cover the whole thorax with millimetre or sub‐millimetre resolution within a short breath‐hold of less than 10 s, which is crucial for sick patients. This high spatial resolution of 1 mm makes evaluation of the pulmonary tree down to the sixth order pulmonary artery branches possible with a lower percentage of non‐diagnostic studies.35,36 Also the near isotropic data with in‐plane and through‐plane resolution make two and three dimensional visualisation a reality and enables the images to be displayed in all planes without compromising spatial resolution.
Although the development of MDCT has led to improved detection of small peripheral pulmonary emboli, the clinical significance of such findings is uncertain. It is unclear whether treating patients with very small pulmonary emboli will improve final outcome.37,38,39 Nevertheless, investigators have suggested that withholding anticoagulation in patients with negative CT pulmonary angiography is a safe practice.40
Carotid arteries
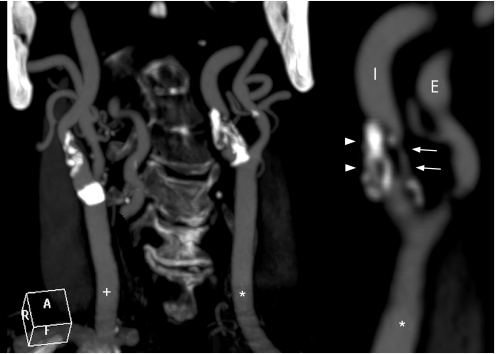
Atherosclerotic disease of the carotid arteries is responsible for a significant number of ischaemic strokes. Carotid intervention in both symptomatic and in some asymptomatic patients has proven beneficial in preventing further strokes (fig 3).41,42
Figure 3 Coronal MDCT angiography of the carotid arteries showing a 90% stenosis (arrows) of the left internal carotid artery (I) with calcification of the origin (arrowheads). The right (+) and left (*) common carotid arteries, left internal (I) and external (E) carotid arteries can also be seen.
Accurate estimation of the degree of stenosis is crucial for optimising the benefits from carotid surgery or stenting. DSA is the current gold standard; however, it carries a small but significant risk of complications (1.3%).43
Beside other non‐invasive imaging modalities such as DUS and MRA, MDCT angiography can provide an important diagnostic tool. The intracranial as well as extracranial neurovascular axis can be evaluated in the same sitting. In a recent study of 37 patients and 73 vessels, the reported sensitivity and specificity for high grade stenosis were 75% and 96%, respectively, and for moderate stenosis 88% and 82%, respectively.44 Furthermore, MDCT can assess the composition of the atherosclerotic plaque and the haemodynamics of the brain circulation by using the CT brain perfusion.45,46 Nevertheless, the exact role of MDCT in the management of carotid disease needs to be further defined.
Cardiac imaging
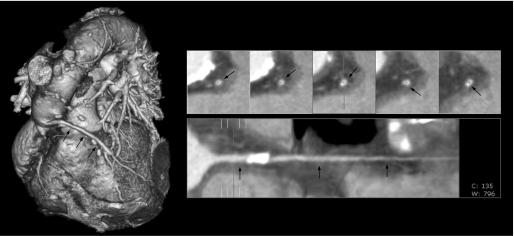
Ischaemic heart disease is the most common cause of premature death in western society. The need for an early screening tool is necessary to reduce the high mortality associated with acute cardiac events. MDCT can be a helpful screening modality to detect and quantify coronary calcium scoring, detect haemodynamically significant stenoses, and study the atherosclerotic plaque non‐invasively (fig 4).
Figure 4 CT coronary angiography. Reconstructed CT images using proprietary software which automatically provides a three dimensional cardiac image and allows selection of specific cardiac vessels and in this case a vein graft (arrows). This generates an opened‐out reconstruction to identify stenoses. Some vessel calcification is seen but no stenosis.
MDCT with a very short rotation time of 0.37 ms allows acquisition of up to 64 contiguous slices combined with prospective or retrospective ECG triggering to obtain artefact‐free images. The examination is completed in a single breath hold. Due to the ultrafast scanning time, MDCT is able to obtain several sets of data in one cardiac cycle. With retrospective ECG gating the heart volume is covered continuously with 1 mm collimation providing temporal resolution of 83 ms and isotropic spatial resolution of 0.4 mm.
The sensitivity and specificity of calcium scoring in predicting a cardiac event underwent thorough investigation in several large trials.47,48,49 Calcium scoring can be used as an additional tool for risk stratification and it is particularly suited for patients with intermediate risk. The current indications for cardiac imaging are summarised in box 1.
Main points
MDCT angiography is used to assess the cardiovascular system either before or after treatment and is useful in planning and confirming the need for endovascular procedures.
DSA is still considered the gold standard; however, non‐invasive angiographic techniques are approaching, and in some cases reaching, 100% sensitivity and specificity.
The contraindications for MDCT angiography are identical to conventional CT, and although the radiation dose is higher for MDCT angiography than for EBCT, it is lower than it is for DSA and requires a smaller amount of iodinated contrast.
CT coronary angiography is competing with the established but invasive technique of percutaneous coronary angiography. Ropers et al studied 84 patients with suspected coronary disease using 64 MDCT. The reported sensitivity and specificity were 95% and 93%, respectively, with negative predictive value of 98%.43 In another study involving 103 patients using 16 MDCT, the sensitivity and specificity were 95% and 97%, respectively, with negative predicative value of 94%.44 However, several challenges are still facing the visualisation of coronary arteries in CT scanning, including a heart rate more than 60 beats/min, cardiac arrhythmias, severe vessel calcification, vessel diameter <1.6 mm, and visualisation of all coronary artery segments. Maruyama et al showed that MDCT had sensitivity and specificity for visualised segment stenosis of 90% and 99%, respectively. However, MDCT could only visualise 74% of diseased segments, with conventional coronary angiography being the reference standard.50
As compared to conventional coronary angiography the current generation of 16 and 64 slice MDCT scanners and the available reformatting techniques still have lower sensitivity and specificity for detecting atherosclerosis. Nevertheless, the introduction of the newest technology in this field, such as 256 slice MDCT and dual source CT, makes this imaging modality a very promising screening as well as diagnostic tool for cardiac patients. Clinically the role of MDCT is likely to be limited in patients with a high pre‐test probability of having significant disease, since those patients will inevitably need percutaneous intervention. However, non‐invasive CT coronary angiography would be preferable for patients with intermediate or low risk of significant disease.
Points for the non‐specialist
Multidetector CT scanners have an increase in acquisition speed and high spatial resolution resulting in high quality angiographic imaging.
Evaluation of the cardiovascular system is therefore possible without using invasive conventional angiographic techniques.
In some cases, such as pulmonary angiography, MDCT has completely replaced conventional angiographic techniques.
MDCT angiography is particularly useful in clinical situations where there is a low incidence in the at‐risk population, such as renal artery stenosis, and therefore only a small proportion with the diagnosis need to go on to have an invasive endovascular procedure.
Radiation dose
As the use of MDCT becomes widespread, the importance of radiation dose has been highlighted. Initial studies in cardiac CT by Hunold et al demonstrated that both calcium scoring and coronary artery CT angiography involve a lower average effective dose with electron beam CT (EBCT) than with MDCT.51 Catheter coronary angiography has a higher effective dose, on average, than EBCT angiography, although lower than that of MDCT. Although effective dose increases, both scan time and the amount of nephrotoxic iodinated contrast needed for an MDCT angiogram is reduced when compared to EBCT.52 A number of methods have been used to reduce radiation dose when doing cardiac scans with MDCT. The technique of ECG pulsing involves modulating the mAs of the scanner depending on the cardiac cycle, and this has been shown in a review article to approximately halve the effective dose of an MDCT coronary angiogram.53
Unlike coronary angiography, peripheral angiography shows a general decreased dose when carried out using MDCT as opposed to conventional DSA techniques. An early study by Rubin et al18 demonstrated that the radiation exposure for DSA of the lower extremity arteries was approximately 3.9 times greater than for MDCT angiography, a fact, if not a figure, confirmed elsewhere.54,55 Further studies comparing EBCT with MDCT, and four and eight channel MDCT angiography, confirms that eight channel scanners are quicker and involve less contrast than either EBCT or four channel scanners, with a similar radiation dose.52,56
General dose reducing techniques have been shown to provide successful dose reduction without compromising diagnostic success when conducting MDCT angiography. Decreasing mAs has been shown not to affect the diagnostic sensitivity of MDCT angiography, with a substantial reduction in radiation dose.57,58 Wintersperger et al59 demonstrated that a reduction in kVp, from 120 to 100, significantly reduced patient dose. A reduced kVp reduces the signal‐to‐noise ratio. This reduction is compensated for by the attenuation level of iodine, leading to diagnostic images. While these techniques help to keep dose “As Low As Reasonably Achievable” (ALARA), some advances have to be made before MDCT coronary angiography is on a par with DSA.
Conclusion
The revolution in CT technology, including high scanning speed, wider coverage area and high temporal and spatial resolution, is providing a credible, non‐invasive diagnostic tool for a wide range of cardiovascular diseases and will likely replace catheter diagnostic angiography in most vascular beds in the near future. However, cost effectiveness studies and strong evidence data on its clinical utility are still lacking.
Abbreviations
ALARA - as low as reasonably achievable
CTA - computed tomographic angiography
DSA - digital subtraction angiography
DUS - Doppler ultrasound
EBCT - electron beam computed tomography
MDCT - multidetector computed tomography
MIP - maximum intensity projection
MPR - multiplanar reconstruction
MRA - magnetic resonance angiography
PE - pulmonary embolism
PVD - peripheral vascular disease
RAS - renal artery stenoses
SPECT - single photon emission computed tomography
SSD - surface shaded display
VDR - vessel density ratio
VQ - ventilation–perfusion
VRT - volume rendering technique
Footnotes
Funding: None
Competing interests: None
References
- 1.Flohr T, Stierstorfer K, Bruder H.et al New technical developments in multislice CT‐Part 1: Approaching isotropic resolution with sub‐millimeter 16‐slice scanning. Rofo 2002174839–845. [DOI] [PubMed] [Google Scholar]
- 2.Schoepf U J, Becker C R, Ohnesorge B M.et al CT of coronary artery disease. Radiology 200423218–37. [DOI] [PubMed] [Google Scholar]
- 3.Harris P L, Buth J. An update on the important findings from the EUROSTAR EVAR registry. Vascular 20041233–38. [DOI] [PubMed] [Google Scholar]
- 4.Armerding M D, Rubin G D, Beaulieu C F.et al Aortic aneurysmal disease: assessment of stent‐graft treatment‐CT versus conventional angiography. Radiology 2000215138–146. [DOI] [PubMed] [Google Scholar]
- 5.Laugharne M, Haslam E, Archer L.et al Multidetector CT angiography in live donor renal transplantation: experience from 156 consecutive cases at a single centre. Transpl Int 200720156–166. [DOI] [PubMed] [Google Scholar]
- 6.Schlunt L B, Harper J D, Broome D R.et al Improved detection of renal vascular anatomy using multidetector CT angiography: Is 100% detection possible? Journal of Endourology/Endourological Society 20072112–17. [DOI] [PubMed] [Google Scholar]
- 7.Raman S S, Pojchamarnwiputh S, Muangsomboon K.et al Utility of 16‐MDCT angiography for comprehensive preoperative vascular evaluation of laparoscopic renal donors. AJR Am J Roentgenol 20061861630–1638. [DOI] [PubMed] [Google Scholar]
- 8.Kim T, Murakami T, Takahashi S.et al Evaluation of renal arteries in living renal donors: comparison between MDCT angiography and gadolinium‐enhanced 3D MR angiography. Radiat Med 200624617–624. [DOI] [PubMed] [Google Scholar]
- 9.Dieter R S, Schmidt W S, Pacanowski J P., Jret al Renovascular hypertension. Expert Review of Cardiovascular Therapy 20053413–422. [DOI] [PubMed] [Google Scholar]
- 10.Rountas C, Vlychou M, Vassiou K.et al Imaging modalities for renal artery stenosis in suspected renovascular hypertension: prospective intraindividual comparison of color Doppler US, CT angiography, GD‐enhanced MR angiography, and digital substraction angiography. Ren fail 200729295–302. [DOI] [PubMed] [Google Scholar]
- 11.Willmann J K, Wildermuth S, Pfammatter T.et al Aortoiliac and renal arteries: prospective intraindividual comparison of contrast‐enhanced three‐dimensional MR angiography and multi‐detector row CT angiography. Radiology 2003226798–811. [DOI] [PubMed] [Google Scholar]
- 12.Mishra A, Bhaktarahalli J N, Ehtuish E F. Imaging of peripheral arteries by 16‐row multidetector computed tomography angiography: a feasible tool? Eur J Radiol 200761528–533. [DOI] [PubMed] [Google Scholar]
- 13.Mesurolle B, Qanadli S D, El Hajjam M.et al Occlusive arterial disease of abdominal aorta and lower extremities: comparison of helical CT angiography with transcatheter angiography. Clinical Imaging 200428252–260. [DOI] [PubMed] [Google Scholar]
- 14.Romano M, Mainenti P P, Imbriaco M.et al Multidetector row CT angiography of the abdominal aorta and lower extremities in patients with peripheral arterial occlusive disease: diagnostic accuracy and interobserver agreement. Eur J Radiol 200450303–308. [DOI] [PubMed] [Google Scholar]
- 15.Romano M, Amato B, Markabaoui K.et al Multidetector row computed tomographic angiography of the abdominal aorta and lower limbs arteries. A new diagnostic tool in patients with peripheral arterial occlusive disease. Minerva Cardioangiol 2004529–17. [PubMed] [Google Scholar]
- 16.Fleischmann D, Rubin G D, Bankier A A.et al Improved uniformity of aortic enhancement with customized contrast medium injection protocols at CT angiography. Radiology 2000214363–371. [DOI] [PubMed] [Google Scholar]
- 17.Fleischmann D. Multiple detector‐row CT angiography of the renal and mesenteric vessels. Eur J Radiol 200345(Suppl 1)S79–S87. [DOI] [PubMed] [Google Scholar]
- 18.Rubin G D, Schmidt A J, Logan L J.et al Multi‐detector row CT angiography of lower extremity arterial inflow and runoff: initial experience. Radiology 2001221146–158. [DOI] [PubMed] [Google Scholar]
- 19.Hull R D, Hirsh J, Carter C J.et al Pulmonary angiography, ventilation lung scanning, and venography for clinically suspected pulmonary embolism with abnormal perfusion lung scan. Ann Intern Med 198398891–899. [DOI] [PubMed] [Google Scholar]
- 20.Stein P D, Athanasoulis C, Alavi A.et al Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation 199285462–468. [DOI] [PubMed] [Google Scholar]
- 21.Zuckerman D A, Sterling K M, Oser R F. Safety of pulmonary angiography in the 1990s. J Vasc Interv Radiol 19967199–205. [DOI] [PubMed] [Google Scholar]
- 22.Diffin D C, Leyendecker J R, Johnson S P.et al Effect of anatomic distribution of pulmonary emboli on interobserver agreement in the interpretation of pulmonary angiography. AJR Am J Roentgenol 19981711085–1089. [DOI] [PubMed] [Google Scholar]
- 23.Stein P D, Henry J W, Gottschalk A. Reassessment of pulmonary angiography for the diagnosis of pulmonary embolism: relation of interpreter agreement to the order of the involved pulmonary arterial branch. Radiology 1999210689–691. [DOI] [PubMed] [Google Scholar]
- 24.PIOPED Investigators Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA 19902632753–2759. [DOI] [PubMed] [Google Scholar]
- 25.Blachere H, Latrabe V, Montaudon M.et al Pulmonary embolism revealed on helical CT angiography: comparison with ventilation‐perfusion radionuclide lung scanning. AJR Am J Roentgenol 20001741041–1047. [DOI] [PubMed] [Google Scholar]
- 26.Stein P D, Relyea B, Gottschalk A. Evaluation of individual criteria for low probability interpretation of ventilation‐perfusion lung scans. J Nucl Med 199637577–581. [PubMed] [Google Scholar]
- 27.Stein P D, Gottschalk A. Review of criteria appropriate for a very low probability of pulmonary embolism on ventilation‐perfusion lung scans: a position paper. Radiographics 20002099–105. [DOI] [PubMed] [Google Scholar]
- 28.Bajc M, Bitzen U, Olsson B.et al Lung ventilation/perfusion SPECT in the artificially embolized pig. J Nucl Med 200243640–647. [PubMed] [Google Scholar]
- 29.Gupta A, Frazer C K, Ferguson J M.et al Acute pulmonary embolism: diagnosis with MR angiography. Radiology 1999210353–359. [DOI] [PubMed] [Google Scholar]
- 30.Oudkerk M, van Beek E J, Wielopolski P.et al Comparison of contrast‐enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet 20023591643–1647. [DOI] [PubMed] [Google Scholar]
- 31.Hull R D, Raskob G E, Ginsberg J S.et al A noninvasive strategy for the treatment of patients with suspected pulmonary embolism. Arch Intern Med 1994154289–297. [PubMed] [Google Scholar]
- 32.van Rossum A B, Pattynama P M, Mallens W M.et al Can helical CT replace scintigraphy in the diagnostic process in suspected pulmonary embolism? A retrolective‐prolective cohort study focusing on total diagnostic yield. Eur Radiol 1998890–96. [DOI] [PubMed] [Google Scholar]
- 33.van Rossum A B, van Erkel A R, van Persijn van Meerten E L.et al Accuracy of helical CT for acute pulmonary embolism: ROC analysis of observer performance related to clinical experience. Eur Radiol 199881160–1164. [DOI] [PubMed] [Google Scholar]
- 34.van Erkel A R, van Rossum A B, Bloem J L.et al Spiral CT angiography for suspected pulmonary embolism: a cost‐effectiveness analysis. Radiology 199620129–36. [DOI] [PubMed] [Google Scholar]
- 35.Ghaye B, Szapiro D, Mastora I.et al Peripheral pulmonary arteries: how far in the lung does multi‐detector row spiral CT allow analysis? Radiology 2001219629–636. [DOI] [PubMed] [Google Scholar]
- 36.Remy‐Jardin M, Tillie‐Leblond I, Szapiro D.et al CT angiography of pulmonary embolism in patients with underlying respiratory disease: impact of multislice CT on image quality and negative predictive value. Eur Radiol 2002121971–1978. [DOI] [PubMed] [Google Scholar]
- 37.Remy‐Jardin M, Remy J, Deschildre F.et al Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 1996200699–706. [DOI] [PubMed] [Google Scholar]
- 38.Novelline R A, Baltarowich O H, Athanasoulis C A.et al The clinical course of patients with suspected pulmonary embolism and a negative pulmonary arteriogram. Radiology 1978126561–567. [DOI] [PubMed] [Google Scholar]
- 39.Goodman L R, Lipchik R J, Kuzo R S.et al Subsequent pulmonary embolism: risk after a negative helical CT pulmonary angiogram‐prospective comparison with scintigraphy. Radiology 2000215535–542. [DOI] [PubMed] [Google Scholar]
- 40.Swensen S J, Sheedy P F, 2nd, Ryu J H.et al Outcomes after withholding anticoagulation from patients with suspected acute pulmonary embolism and negative computed tomographic findings: a cohort study. Mayo Clin Proc 200277130–138. [DOI] [PubMed] [Google Scholar]
- 41.NASCET Investigators Clinical alert: benefit of carotid endarterectomy for patients with high‐grade stenosis of the internal carotid artery. National Institute of Neurological Disorders and Stroke Stroke and Trauma Division. North American Symptomatic Carotid Endarterectomy Trial (NASCET) investigators. Stroke 199122816–817. [DOI] [PubMed] [Google Scholar]
- 42.Halliday A, Mansfield A, Marro J.et al Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 20043631491–1502. [DOI] [PubMed] [Google Scholar]
- 43.Willinsky R A, Taylor S M, TerBrugge K.et al Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 2003227522–528. [DOI] [PubMed] [Google Scholar]
- 44.Silvennoinen H M, Ikonen S, Soinne L.et al CT angiographic analysis of carotid artery stenosis: comparison of manual assessment, semiautomatic vessel analysis, and digital subtraction angiography. AJNR Am J Neuroradiol 20072897–103. [PMC free article] [PubMed] [Google Scholar]
- 45.de Weert T T, Ouhlous M, Meijering E.et al In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vascul Biol 2006262366–2372. [DOI] [PubMed] [Google Scholar]
- 46.Wintermark M, Fischbein N J, Smith W S.et al Accuracy of dynamic perfusion CT with deconvolution in detecting acute hemispheric stroke. AJNR Am J Neuroradiol 200526104–112. [PMC free article] [PubMed] [Google Scholar]
- 47.Kondos G T, Hoff J A, Sevrukov A.et al Electron‐beam tomography coronary artery calcium and cardiac events: a 37‐month follow‐up of 5635 initially asymptomatic low‐ to intermediate‐risk adults. Circulation 20031072571–2576. [DOI] [PubMed] [Google Scholar]
- 48.Shaw L J, Raggi P, Schisterman E.et al Prognostic value of cardiac risk factors and coronary artery calcium screening for all‐cause mortality. Radiology 2003228826–833. [DOI] [PubMed] [Google Scholar]
- 49.Hecht H S, Budoff M J, Berman D S.et al Coronary artery calcium scanning: Clinical paradigms for cardiac risk assessment and treatment. Am Heart J 20061511139–1146. [DOI] [PubMed] [Google Scholar]
- 50.Maruyama T, Yoshizumi T, Tamura R.et al Comparison of visibility and diagnostic capability of noninvasive coronary angiography by eight‐slice multidetector‐row computed tomography versus conventional coronary angiography. Am J Cardiol 200493537–542. [DOI] [PubMed] [Google Scholar]
- 51.Hunold P, Vogt F M, Schmermund A.et al Radiation exposure during cardiac CT: effective doses at multi‐detector row CT and electron‐beam CT. Radiology 2003226145–152. [DOI] [PubMed] [Google Scholar]
- 52.Rubin G D, Shiau M C, Leung A N.et al Aorta and iliac arteries: single versus multiple detector‐row helical CT angiography. Radiology 2000215670–676. [DOI] [PubMed] [Google Scholar]
- 53.Gerber T C, Kuzo R S, Morin R L. Techniques and parameters for estimating radiation exposure and dose in cardiac computed tomography. Int J Card Imaging 200521165–176. [DOI] [PubMed] [Google Scholar]
- 54.Jakobs T F, Wintersperger B J, Becker C R. MDCT‐imaging of peripheral arterial disease. Semin Ultrasound CT MR 200425145–155. [DOI] [PubMed] [Google Scholar]
- 55.Willmann J K, Mayer D, Banyai M.et al Evaluation of peripheral arterial bypass grafts with multi‐detector row CT angiography: comparison with duplex US and digital subtraction angiography. Radiology 2003229465–474. [DOI] [PubMed] [Google Scholar]
- 56.Karcaaltincaba M, Foley D. Four‐ and eight‐channel aortoiliac CT angiography: a comparative study. Cardiovasc Intervent Radiol 200528169–172. [DOI] [PubMed] [Google Scholar]
- 57.Fraioli F, Catalano C, Napoli A.et al Low‐dose multidetector‐row CT angiography of the infra‐renal aorta and lower extremity vessels: image quality and diagnostic accuracy in comparison with standard DSA. Eur Radiol 200616137–146. [DOI] [PubMed] [Google Scholar]
- 58.Bae K T, Hong C, Whiting B R. Radiation dose in multidetector row computed tomography cardiac imaging. J Magn Reson Imaging 200419859–863. [DOI] [PubMed] [Google Scholar]
- 59.Wintersperger B, Jakobs T, Herzog P.et al Aorto‐iliac multidetector‐row CT angiography with low kV settings: improved vessel enhancement and simultaneous reduction of radiation dose. Eur Radiol 200515334–341. [DOI] [PubMed] [Google Scholar]