Abstract
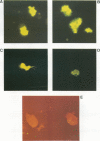
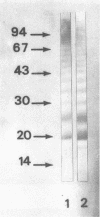
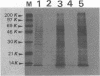
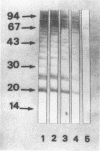
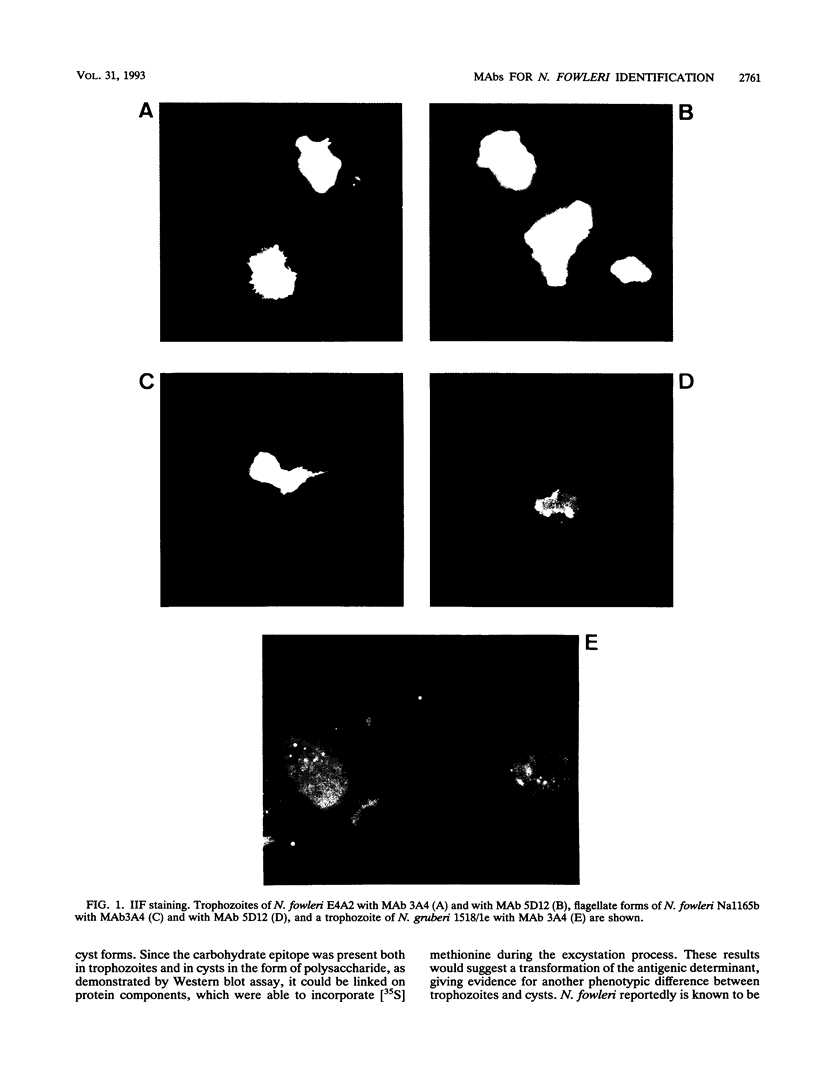
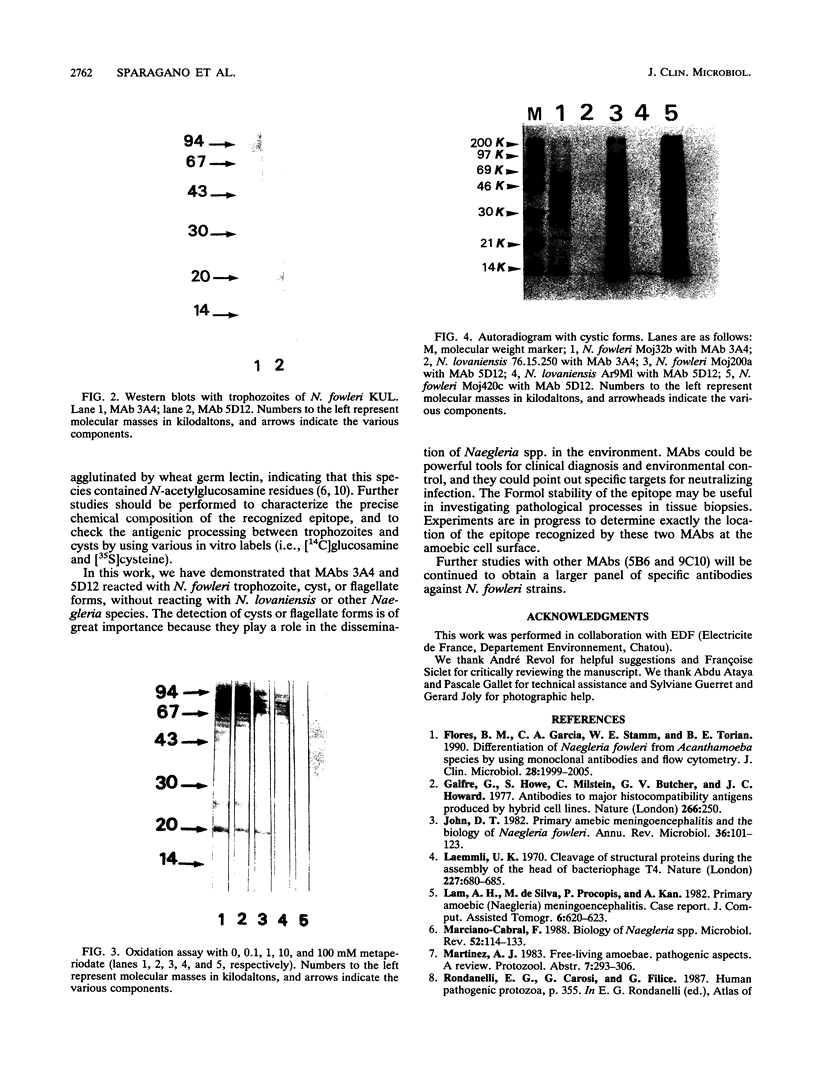
Monoclonal antibodies (MAbs) reactive to the pathogenic amoeba Naegleria fowleri were analyzed by enzyme-linked immunosorbent assay (ELISA), indirect immunofluorescence assay, Western blotting (immunoblotting), and radioimmunoprecipitation assay (RIPA). Two MAbs (3A4 and 5D12) showed reactivity by ELISA with all N. fowleri strains tested and no reactivity with the five other Naegleria species, N. lovaniensis, N. gruberi, N. australiensis, N. jadini, and N. andersoni. These MAbs reacted with the three morphological forms of N. fowleri (trophozoites, cysts, and flagellates). The reactivity on Western blots was suppressed by treatment with metaperiodate, suggesting a carbohydrate epitope. Differences in reactivity patterns between trophozoites and cysts observed with radioimmunoprecipitation assay might reflect differences in biological properties. The formalin stability of the epitope may be useful in detecting N. fowleri in fixed biopsies and in investigating the pathological process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Flores B. M., Garcia C. A., Stamm W. E., Torian B. E. Differentiation of Naegleria fowleri from Acanthamoeba species by using monoclonal antibodies and flow cytometry. J Clin Microbiol. 1990 Sep;28(9):1999–2005. doi: 10.1128/jcm.28.9.1999-2005.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D. T. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu Rev Microbiol. 1982;36:101–123. doi: 10.1146/annurev.mi.36.100182.000533. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam A. H., de Silva M., Procopis P., Kan A. Primary amoebic (Naegleria) meningoencephalitis. J Comput Assist Tomogr. 1982 Jun;6(3):620–623. doi: 10.1097/00004728-198206000-00032. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F. Biology of Naegleria spp. Microbiol Rev. 1988 Mar;52(1):114–133. doi: 10.1128/mr.52.1.114-133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. R., Shulman S. T., Lansen T. A., Cichon M. J., Willaert E. Primary amoebic meningoencephalitis: a report of two cases and antibiotic and immunologic studies. J Infect Dis. 1981 Feb;143(2):193–199. doi: 10.1093/infdis/143.2.193. [DOI] [PubMed] [Google Scholar]
- Stevens A. R., Stein S. Analyses of Pathogenic and nonpathogenic Acanthamoeba and Naegleria for lectin-induced agglutination. J Parasitol. 1977 Feb;63(1):151–152. [PubMed] [Google Scholar]
- Valenzuela G., López-Corella E., De Jonckheere J. F. Primary amoebic meningoencephalitis in a young male from northwestern Mexico. Trans R Soc Trop Med Hyg. 1984;78(4):558–559. doi: 10.1016/0035-9203(84)90084-1. [DOI] [PubMed] [Google Scholar]
- Visvesvara G. S., Peralta M. J., Brandt F. H., Wilson M., Aloisio C., Franko E. Production of monoclonal antibodies to Naegleria fowleri, agent of primary amebic meningoencephalitis. J Clin Microbiol. 1987 Sep;25(9):1629–1634. doi: 10.1128/jcm.25.9.1629-1634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]