Abstract
Endothelium-derived hyperpolarizing factor (EDHF) plays an important role in the regulation of vascular microcirculatory tone. This study explores the role of estrogen in controlling EDHF-mediated vasodilation of uterine resistance arteries of the rat and also analyzes the contribution of endothelial cell (EC) Ca2+ signaling to this process. A parallel study was also performed with mesenteric arteries to provide comparison with a nonreproductive vasculature. Mature female rats underwent ovariectomy, with one half receiving 17β-estradiol replacement (OVX+E) and the other half serving as estrogen-deficient controls (OVX). Uterine or mesenteric resistance arteries were harvested, cannulated, and pressurized. Nitric oxide and prostacyclin production were inhibited with 200 μM NG-nitro-l-arginine and 10 μM indomethacin, respectively. ACh effectively dilated the arteries preconstricted with phenylephrine but failed to induce dilation of vessels preconstricted with high-K+ solution. ACh EC50 values were decreased by estrogen replacement by five- and twofold in uterine and mesenteric arteries, respectively. As evidenced by fura-2-based measurements of EC cytoplasmic Ca2+ concentration ([Ca2+]i), estrogen replacement was associated with increased basal and ACh-stimulated EC [Ca2+]i rise in uterine, but not mesenteric, vessels. These data demonstrate that EDHF contributes to endothelium-dependent vasodilation of uterine and mesenteric resistance arteries and that estrogen controls EDHF-related mechanism(s) more efficiently in reproductive vs. nonreproductive vessels. Enhanced endothelial Ca2+ signaling may be an important underlying mechanism in estrogenic modulation of EDHF-mediated vasodilation in small resistance uterine arteries.
Keywords: endothelium-derived hyperpolarizing factor, acetylcholine, fura-2, high potassium
the vascular endothelium plays an important role in the control of blood vessel tone through release of relaxing and contracting factors in response to mechanical or agonist-induced stimulation of endothelial cells (ECs). Nitric oxide (NO) and prostacyclin are two well-characterized key mediators of endothelium-dependent vasodilation of large-conduit blood vessels (14, 16, 36, 48). However, as evident from recent studies, resistance vessels retain the ability to dilate in response to endothelial stimulation after blockade of NO and prostacyclin production, implicating the contribution of another factor(s) to endothelium-dependent vasodilation (7). Because endothelium-dependent NO- and prostacyclin-resistant dilation is associated with hyperpolarization of vascular smooth muscle cells (SMCs), the third mediator (or mechanism) was defined as endothelium-derived hyperpolarizing factor (EDHF) (6, 7, 10, 11, 13, 14, 28). The importance of EDHF increases with decreasing vessel size, and it is especially prominent in small-diameter arteries and arterioles, which predominantly regulate regional vascular resistance and blood flow (11, 14, 36, 47).
Although the exact nature of EDHF remains unknown, the rise in cytosolic Ca2+ in response to mechanical or chemical stimulation of ECs is considered to be the first crucial step in EDHF-mediated vasodilation. In our previous study, loading ECs of pressurized uterine arteries with the Ca2+-chelating agent BAPTA resulted in abolition of ACh-induced cytoplasmic Ca2+ concentration ([Ca2+]i) rise and associated vasodilation (17). Therefore, in uterine arteries the production of NO, prostacyclin, and EDHF requires elevation of endothelial [Ca2+]i. In pressurized rat gracilis muscle arterioles, loading with BAPTA prevented endothelial [Ca2+]i elevation and EDHF-mediated vasodilation in response to ACh (51).
Several potential Ca2+-induced mechanisms underlying EDHF responses were recently proposed: 1) Ca2+-dependent activation of arachidonic acid causes subsequent formation of epoxyeicosatrienoic acids (EETs), which then diffuse from ECs and result in hyperpolarization of underlying SMCs through opening Ca2+-activated K+ channels. This mechanism was first recognized in coronary arteries of different animal species as well as in humans (9, 15). 2) Elevation of endothelial [Ca2+]i also leads to activation of endothelial small (SKCa)- and intermediate (IKCa)-conductance potassium channels with resultant hyperpolarization of ECs (6, 7, 11, 14, 28, 30). It has been proposed that K+ ions, by diffusing from ECs through opened SKCa and IKCa channels, cause SMC hyperpolarization and relaxation due to the activation of inward-rectifier K+ channels and stimulation of the Na+-K+ pump of the SMC plasma membrane (13). 3) Electrotonic spreading of hyperpolarization from ECs to SMCs through myoendothelial gap junctions may also be a mechanism of EDHF that does not require any diffusible chemical factor (6, 7, 10, 14, 28, 30). All of the major proposed mechanisms are not mutually exclusive and can differentially contribute to EDHF-mediated responses depending on the type of vascular activation and levels of expression of gap junction proteins, potassium channels, or the Na+-K+ pump.
It is acknowledged that sex is an important factor modulating EDHF-mediated vasodilation in different vascular beds (22, 37). In mesenteric resistance arteries from nitric oxide synthase (NOS) III/cyclooxygenase (COX)-1 double-knockout mice, the contribution of EDHF to endothelium-dependent vasodilation was markedly reduced in male compared with female animals in association with significant mean arterial blood pressure elevation in males but not in females (46). This study suggested the importance of EDHF in the control of systemic blood pressure and implicated sex steroid hormones in the regulation of mechanisms underlying EDHF-mediated vasodilation.
Estrogen effects are especially prominent in the reproductive vasculature. Marked estrogen-induced sensitization of the uterine artery to ACh was first demonstrated by C. Bell (4). It was later found that the high estrogen state of pregnancy increases endothelial NOS (eNOS) activity in the main uterine artery of the guinea pig (53). Endogenous estrogen during pregnancy also increases NO-dependent modulation of vessel tone and arterial distensibility in the smaller, resistance arteries of the rat mesentery (55).
In this study we hypothesized that EDHF contributes to endothelium-dependent vasodilation of smaller uterine resistance arteries of the rat, and that estrogen can upregulate EDHF-mediated responses. Considering EC [Ca2+]i rise to be a key step in EDHF-mediated dilation, we postulated that estrogen replacement may enhance endothelial [Ca2+]i responses to ACh and, through this mechanism, modulate EDHF-dependent vasodilation. For comparative purposes, and in view of earlier findings, we also tested the effects of estrogen in a nonreproductive vasculature—mesenteric resistance arteries. In evaluating the data, it should be noted that we did not attempt to directly compare uterine and mesenteric arteries in view of differences in size and location. Rather, by using pharmacological sensitivity [concentration required to produce half-maximal vasodilation (EC50) values] as a tool, we evaluated the relative shifts in sensitivity within each vessel type as an indicator of the magnitude of the estrogenic effect. The results clearly indicate that the absence versus presence of estrogen has a much larger effect in uterine than mesenteric arteries in terms of its modulation of vasodilator sensitivity to both NO and EDHF.
METHODS
Animals.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, revised 1996), and the experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Vermont.
Female Sprague-Dawley rats (n = 63) underwent ovariectomy at 11 wk of age (Taconic, Germantown, NY). At the time of ovariectomy, one-half of the rats received 21-day timed-release pellets containing 0.5 mg of 17β-estradiol (Innovative Research of America, Sarasota, FL) to approximate physiological levels of estrogen in pregnancy (50).
The rats were then shipped to the University of Vermont, where they acclimatized during the following week. The animals were housed in the University of Vermont small animal care facility, which is accredited by the American Association for Accreditation of Laboratory Animal Care. Both groups of rats received free access to food and water and were kept in a climate-controlled room. The animals were studied between postoperative days 17 and 21. At the time of study, each rat was anesthetized by an intraperitoneal injection of Nembutal (50 mg/kg) and killed by decapitation. Trunk blood was immediately collected and centrifuged. The resultant serum was stored at −80°C until further analysis. Circulating estrogen levels were determined by liquid chromatography-tandem mass spectrometry with a previously established method (3).
Arterial preparation.
After the abdominal wall was transected, the entire uterus or a portion of the small intestine with its vasculature was rapidly removed and pinned in a dissecting dish filled with aerated physiological salt solution (PSS; see Solutions and drugs for composition). Third-order branches of the superior mesenteric artery running in parallel to the intestinal surface or third-order uterine (radial) arteries were dissected free of surrounding connective tissue and cannulated on both ends in an arteriograph. Arterial segments were continuously superfused at 3 ml/min with aerated (10% O2, 5% CO2, and 85% N2) PSS at 37°C and pH 7.4. The arteriograph was placed on the stage of an inverted microscope with an attached video camera. Lumen diameter was monitored with the SoftEdge Acquisition Subsystem (IonOptix, Milton, MA).
Vessels were tested for leaks before experimentation. Vessels that failed to maintain pressure (50 mmHg) were not used for the study. Intraluminal pressure was controlled with a servo pressure system (Living System Instrumentation, Burlington, VT). All experiments were performed under no-flow conditions.
Protocol for studying EDHF-mediated vasodilation.
To minimize mechanical stimulation of ECs within the arterial wall during the equilibration period, cannulated arteries were initially pressurized to 10 mmHg for 1 h at 37°C. Intraluminal pressure was then increased to 50 mmHg, and vessel diameter was allowed to stabilize for 10 min. The arteries were then incubated with NG-nitro-l-arginine (l-NNA; NOS inhibitor) and indomethacin (COX inhibitor) for an additional 20 min to inhibit the production of NO and prostacyclin, respectively. A number of published observations have demonstrated a nearly complete abolition of agonist-induced endothelial NO production in the presence of 100–200 μM l-NNA measured with the NO-sensitive dye 4,5-diaminofluorescein (DAF-2) (12, 42, 49). Pretreatment of mesenteric arteries with a combination of 100 μM l-NNA, 10 μM indomethacin, 500 nM apamin, and 100 nM charybdotoxin resulted in a complete suppression of ACh-induced responses (40). On the basis of these observations, we used the combination of 200 μM l-NNA and 10 μM indomethacin for effective inhibition of NO and prostacyclin production, respectively.
Phenylephrine (Phe) was added in increasing concentrations to produce a constriction of 50–70% relative to the initial diameter. Once a steady state of constriction was achieved, ACh was added over 5-min intervals in increasing concentrations (0.01–10 μM). At the completion of the experiment, papaverine (100 μM, a phosphodiesterase inhibitor) and diltiazem (10 μM, a calcium channel blocker) were applied to determine maximal passive arterial diameter at 50 mmHg (Dmax). ACh-induced vasodilation was expressed as the percentage of maximal dilation induced by a combined treatment with papaverine and diltiazem.
Endothelial cell loading with fura-2 and measurement of endothelial cell [Ca2+]i in pressurized arteries.
In a separate set of experiments, after a 20-min equilibration period at 10 mmHg and 37°C, ECs were loaded with the Ca2+-sensitive dye fura-2 (5 μM) by intraluminal perfusion of fura-2 AM-containing solution at room temperature for 5 min, followed by 10 min of washout with regular PSS. Background fluorescence and arterial autofluorescence were measured before the loading procedure was started. A similar experimental approach has been successfully used in studies aimed at selectively loading ECs of pressurized vessels with fura-2 (17, 18, 26, 39). Ratiometric measurements of fura-2 fluorescence from ECs were performed with a photomultiplier system (IonOptix). Background-corrected ratios of 510-nm emission were obtained at a sampling rate of 5 Hz from arteries alternately excited at 340 and 380 nm. The arterial lumen diameter was simultaneously monitored with the SoftEdge Acquisition Subsystem (IonOptix). All experimental protocols were started after an additional 15-min equilibration period at 10 mmHg to allow intracellular deesterification of fura-2 AM. l-NNA and indomethacin were added during this equilibration period.
In the first set of experiments, intraluminal pressure was elevated to 50 mmHg, and Phe was applied to produce 50–70% constriction of mesenteric arteries from rats treated with ovariectomy + 17β-estradiol replacement (OVX+E). After stabilization of sustained constrictor response, ACh was added in two concentrations to study the correlation between endothelial [Ca2+]i rise and vasodilation. All solutions contained 200 μM l-NNA and 10 μM indomethacin.
In the second set of experiments utilizing uterine and mesenteric arteries from ovariectomized (OVX) and OVX+E rats, intraluminal pressure was elevated to 50 mmHg. After stabilization of arterial diameter (typically 5–7 min), ACh was applied in four increasing concentrations (0.03, 0.1, 0.3, and 10 μM) for a period of 5 min each; 200 μM l-NNA and 10 μM indomethacin were added to all solutions.
Solutions and drugs.
PSS contained (in mM) 119 NaCl, 4.7 KCl, 24.0 NaHCO3, 1.2 KH2PO4, 1.6 CaCl2, 1.2 MgSO4, 0.023 EDTA, and 11.0 glucose. High-potassium solution was made by replacing Na+ with an equivalent amount of K+. For the fura-2 calibration procedure, we used a solution of the following composition: 140 mM KCl, 20 mM NaCl, 5 mM HEPES, 5 mM EGTA, 1 mM MgCl2, 5 μM nigericin, and 10 μM ionomycin, pH = 7.1.
All chemicals were purchased from Sigma (St. Louis, MO), with the exception of ionomycin and nigericin, which were obtained from Calbiochem (La Jolla, CA). Fura-2 AM and pluronic acid were purchased from Invitrogen (Carlsbad, CA). Fura-2 AM was dissolved in dehydrated DMSO as a 1 mM stock solution, frozen in small aliquots, and used within 1 wk of preparation. Stock solutions of Phe, l-NNA, ACh, and papaverine were prepared on the day of the experiment in deionized water and were used on the same day only. Diltiazem and indomethacin were dissolved in deionized water and ethanol, respectively, and were kept refrigerated until use. Ionomycin and nigericin were prepared as 10 mM stock solutions in methanol and kept at −20°C until use.
Calculations and statistical analysis.
EC [Ca2+]i was calculated with the equation (19) [Ca2+]i = Kdβ(R − Rmin)/(Rmax − R), where R is the experimentally measured ratio (340/380 nm) of fluorescence intensities, Rmin is a ratio in the absence of [Ca2+]i, Rmax is a ratio at Ca2+-saturated fura-2 conditions, and β is a ratio of the fluorescence intensities at 380-nm excitation wavelength at Rmin and Rmax. Rmin, Rmax, and β were determined by an in situ calibration procedure from arteries treated with nigericin (5 μM) and ionomycin (10 μM). Calibration was performed with vessels loaded intraluminally with fura-2 (n = 9). These values were then pooled and used to convert the ratio values into [Ca2+]i. Kd (the dissociation constant for fura-2) was 282 nM, as determined by in situ titration of Ca2+ in fura-2-loaded small arteries (27). Arterial diameter, pressure, and ratio values were simultaneously recorded with an IonOptix data acquisition program and imported into Sigma Plot and Sigma Stat programs for graphical representation, calculations, and statistical analysis.
Statistical analysis of treatment differences in body weight, uterine weight, baseline diameter, and estradiol level was performed with the unpaired Student's t-test. The concentration of ACh required to produce half-maximal vasodilation (EC50) was determined for each tested artery by standard curve analysis from data imported into the Sigma Plot program. Comparative analysis of concentration-response curves was performed by repeated-measures ANOVA. P < 0.05 was considered statistically significant. Data are expressed as means ± SE, where n is the number of arterial segments studied. One mesenteric or uterine artery from each rat was used for a particular experimental protocol.
RESULTS
Characterization of animal model.
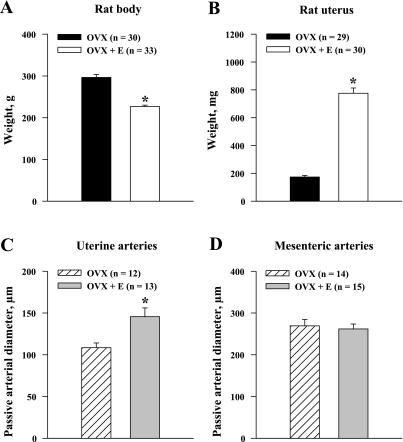
Ovariectomy was associated with very low levels of circulating estradiol in the OVX rats (10.1 ± 7.1 pg/ml). Chronic administration of estrogen induced a marked elevation of its circulating levels in the OVX+E rats (180.8 ± 70.5 pg/ml). OVX animals were significantly heavier than their OVX+E counterparts (297 ± 7 and 227 ± 3 g, respectively; P < 0.05) (Fig. 1A). Estrogen supplementation also caused a significant increase in uterine weight (OVX group 174 ± 12 mg and OVX+E group 776 ± 38 mg; P < 0.05), indicating the effectiveness of the estrogen replacement (Fig. 1B). The average Dmax of uterine arteries were significantly increased by estrogen (Fig. 1C). There was no significant difference in Dmax of the mesenteric vessels between the OVX and OVX+E groups (Fig. 1D).
Fig. 1.
Effects of long-term exposure of ovariectomized (OVX) rats to estrogen (OVX+E) on body weight (A), uterine weight (B), and passive diameters of uterine (C) and mesenteric (D) arteries. Passive diameters were determined in arteries pressurized at 50 mmHg in the presence of 10 μM diltiazem and 100 μM papaverine. n = no. of tested arteries. *Significantly different at P < 0.5 (unpaired Student's t-test).
Estrogen replacement enhances EDHF-mediated vasodilation.
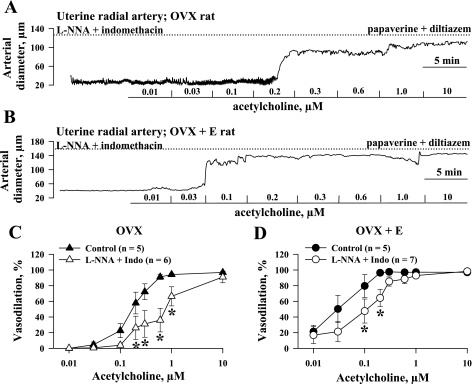
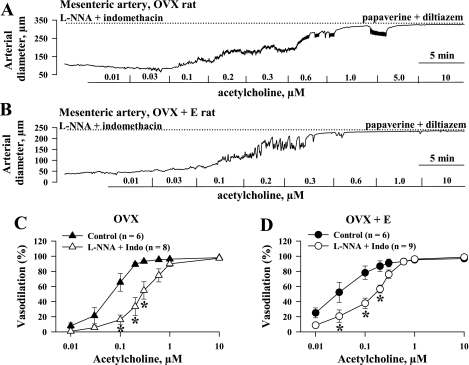
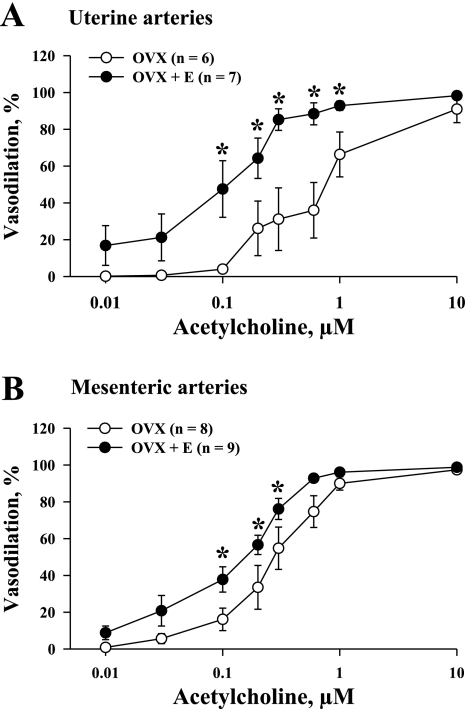
The NO- and prostacyclin-resistant responses of all four groups of vessels to cumulative application of ACh were studied next. Additional experiments were performed to characterize ACh-induced responses in vessels untreated with l-NNA and indomethacin (control). Figure 2 shows representative ACh-induced changes in the diameters of pressurized uterine arteries obtained from OVX (Fig. 2A) and OVX+E (Fig. 2B) rats. In the presence of l-NNA and indomethacin, ACh effectively dilated vessels from both groups of rats in a concentration-dependent manner to nearly 100% of their maximal diameter. Summary graphs demonstrating the degree of vasodilation as a function of ACh concentration for untreated arteries and arteries treated with l-NNA and indomethacin are shown in Fig. 2, C and D. This indicates that inhibition of NO and prostacyclin production in uterine arteries of OVX and OVX+E rats significantly reduces endothelium-dependent vasodilation. Maximal vasodilation occurs in both the l-NNA/indomethacin and control groups with very high concentrations of ACh. Similar experiments were performed with mesenteric arteries from OVX and OVX+E rats. Original tracings are shown in Fig. 3, A and B. Summary graphs demonstrate significant attenuation of endothelium-mediated vasodilation after inhibition of NO and prostacyclin production in mesenteric vessels as well (Fig. 3, C and D). As shown in Fig. 4A, the concentration-response curve for ACh in the presence of l-NNA and indomethacin was shifted to the left after chronic estrogen administration, indicating a significant enhancement of EDHF-mediated vasodilation. A similar but less striking effect of estrogen was also observed in mesenteric arteries (Fig. 4B).
Fig. 2.
Estrogen replacement potentiates nitric oxide (NO)- and prostacyclin-resistant vasodilation of uterine arteries in response to ACh. A and B: representative changes in arterial diameter of uterine arteries from ovariectomized, estrogen-deficient (OVX, A) and ovariectomized, estrogen-replaced (OVX+E, B) rats induced by a cumulative application of ACh in concentrations from 0.01 to 10 μM. All arteries were pretreated for 20 min with 200 μM NG-nitro-l-arginine (l-NNA) and 10 μM indomethacin and preconstricted with phenylephrine before testing with ACh. Solid lines indicate the time of administration of ACh at different concentrations. Dotted lines show maximally dilated diameters of the arteries measured in a relaxing solution containing 10 μM diltiazem and 100 μM papaverine. C and D: summary graphs demonstrating effect of NO and prostacyclin inhibition on ACh-induced vasodilation of uterine arteries from OVX (C) and OVX+E (D) rats. n = no. of tested arteries. *Significant difference between groups at P < 0.05 (2-way repeated-measures ANOVA).
Fig. 3.
Effects of estrogen supplementation on ACh-induced dilation of mesenteric arteries. A and B: representative changes in diameter of mesenteric arteries from OVX (A) and OVX+E (B) rats induced by a cumulative application of ACh. Arteries were pretreated for 20 min with 200 μM l-NNA and 10 μM indomethacin and preconstricted with phenylephrine before testing with ACh. Solid lines indicate the time of administration of ACh at different concentrations. Dotted lines show maximally dilated diameters of arteries measured in a relaxing solution containing 10 μM diltiazem and 100 μM papaverine. C and D: summary graphs showing effect of NO and prostacyclin inhibition on ACh-induced vasodilation of mesenteric arteries from OVX (C) and OVX+E (D) rats. n = no. of tested arteries. *Significant difference between groups at P < 0.5 (2-way repeated-measures ANOVA).
Fig. 4.
Chronic administration of estrogen to ovariectomized rats enhances endothelium-derived hyperpolarizing factor (EDHF)-mediated vasodilation of mesenteric and uterine arteries. Vasodilator responses of uterine (A) and mesenteric (B) arteries from OVX and OVX+E rats are shown as a function of ACh concentration. Vasodilation is expressed as % of maximal dilator response to papaverine and diltiazem. n = no. of tested arteries. All experiments were performed in the presence of 200 μM l-NNA and 10 μM indomethacin. *Significant difference between groups at P < 0.05 (2-way repeated-measures ANOVA).
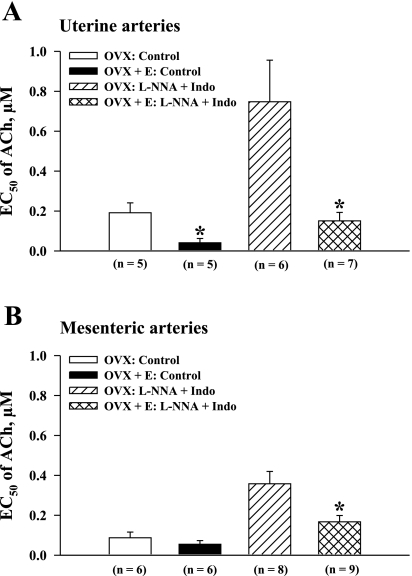
To further characterize the effect of estrogen on arterial sensitivity to ACh, the EC50 values calculated for uterine and mesenteric vessels were compared between the OVX and OVX+E groups. In control vessels, the EC50 values for uterine arteries of OVX rats were significantly higher than those of OVX+E rats (0.20 ± 0.05 μM vs. 0.04 ± 0.02 μM; P < 0.05; Fig. 5A). This difference remained very striking in uterine arteries after inhibition of NO and prostacyclin production, where the EC50 of vessels from OVX rats (0.75 ± 0.2 μM) was five times higher than those of vessels from OVX+E rats (0.15 ± 0.04 μM; P < 0.05). Sensitivity of mesenteric arteries to ACh was altered by estrogen replacement to a much lesser degree. EC50 calculated for control arteries of OVX and OVX+E rats were not significantly different (Fig. 5B). In arteries treated with l-NNA and indomethacin, estrogen administration resulted in a twofold reduction in EC50 from 0.36 ± 0.06 μM to 0.17 ± 0.03 μM (P < 0.05). These data demonstrate that estrogen produces a greater enhancement of endothelium-dependent dilation in arteries from reproductive (uterine) versus nonreproductive (mesenteric) tissues.
Fig. 5.
Estrogen replacement increases the vasodilator sensitivity of uterine and mesenteric arteries to ACh. Summary graphs demonstrate an increase in the concentration of ACh producing half-maximal dilation (EC50) of uterine (A) and mesenteric (B) arteries from estrogen-deficient (OVX) vs. estrogen-replaced (OVX+E) rats before and after blockade of NO and prostacyclin production. n = no. of tested arteries. *Significantly different at P < 0.05 (unpaired Student's t-test).
NO- and prostacyclin-resistant vasodilation is abolished by treatment of arteries with high-K+ solution.
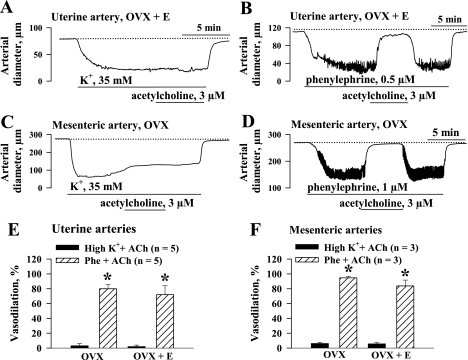
One of the important characteristics of EDHF-induced hyperpolarization is its dependence on the activation of K+ channels (7). Elevation of extracellular K+ results in a diminished driving force for K+ ions and abolishes hyperpolarization induced by activation of K+ channels (2). This common methodological approach was used in our study to confirm that NO- and prostacyclin-resistant uterine and mesenteric vasodilation is induced through EDHF-related mechanisms. In these experiments we used ACh at a concentration of 3 μM that resulted in similar and near-maximal dilation of all groups of vessels preconstricted with Phe. As shown in Fig. 6A, pretreatment of a uterine artery with 35 mM K+ in the presence of l-NNA and indomethacin abolished ACh-induced vasodilation. When the uterine artery was preconstricted with Phe, ACh application resulted in near-complete dilation (Fig. 6B). Similar data were obtained in our experiments using mesenteric vessels (Fig. 6, C and D). The graphs in Fig. 6, E and F, summarize the data for uterine and mesenteric arteries from the OVX and OVX+E groups. These experiments provide additional evidence that l-NNA- and indomethacin-resistant vasodilation is mediated through an EDHF-related mechanism.
Fig. 6.
Abolition of EDHF-mediated arterial responses by treatment with high-K+ solution. A: application of 3 μM ACh results in no vasodilation of a uterine artery preconstricted with 35 mM K+. B: ACh-induced dilation of a uterine artery preconstricted with phenylephrine. l-NNA (200 μM) and indomethacin (10 μM) were present throughout whole experiment. Dotted line shows maximal level of dilation in response to application of 10 μM diltiazem and 100 μM papaverine. C and D: responses of mesenteric arteries to the same concentration of ACh (3 μM) preconstricted with high-K+ solution (C) or phenylephrine (D) in the presence of 200 μM l-NNA and 10 μM indomethacin. E and F: effects of ACh in uterine (E) and mesenteric (F) arteries preconstricted with high-K+ solution or phenylephrine. Vasodilation is expressed as % of maximal response obtained in a relaxing solution containing diltiazem and papaverine. n = no. of tested arteries. *Significant difference at P < 0.05 (unpaired Student's t-test).
Estrogen enhances ACh-induced elevation of EC [Ca2+]i in uterine but not in mesenteric arteries.
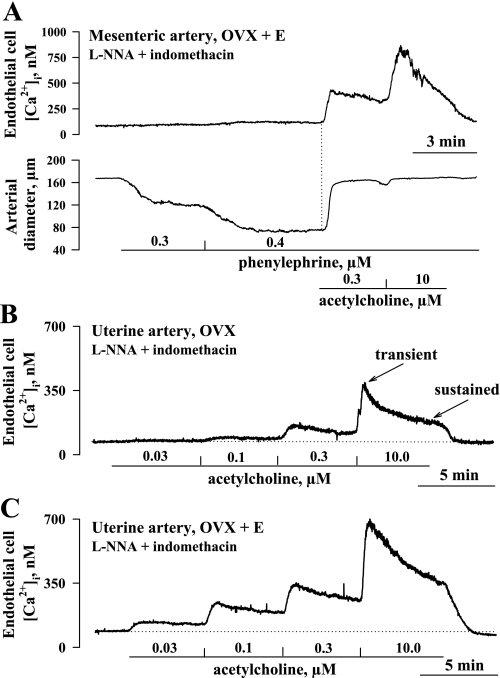
Agonist-induced elevation of intracellular [Ca2+]i is a key event in EC hyperpolarization and EDHF-induced vasodilation in a variety of arteries, including those from the uterine circulation (7, 17). Figure 7A shows a simultaneous recording of changes in EC [Ca2+]i and diameter of a mesenteric artery from an OVX+E rat in response to 0.3 and 10 μM ACh. It is evident that the EC [Ca2+]i response closely precedes vasodilation. In a series of similar experiments on mesenteric vessels from OVX+E rats, the onset of dilation was delayed from the [Ca2+]i response to 0.3 and 10 μM ACh by only 14 ± 0.2 s and 7 ± 2 s, respectively (n = 5). These data provide additional evidence for the importance of Ca2+ rise in EDHF-mediated vasodilation.
Fig. 7.
A: temporal correlations between ACh-induced elevations in cytoplasmic concentration of Ca2+ in endothelial cells (EC [Ca2+]i) and associated vasodilation. Representative changes in EC [Ca2+]i (top) and diameter of a mesenteric artery (bottom) from an OVX+E rat to phenylephrine and ACh are shown. Artery was preconstricted with phenylephrine to 50% of initial diameter in the presence of 200 μM l-NNA and 10 μM indomethacin. ACh induced a rise in EC [Ca2+]i followed by immediate vasodilation. Dotted line indicates the starting point of the EC [Ca2+]i rise, while solid lines show the time the artery was exposed to phenylephrine and ACh. B and C: representative changes in EC [Ca2+]i in response to increasing concentrations of ACh in pressurized uterine arteries from an estrogen-deficient (OVX, B) and an estrogen-replaced (OVX+E, C) rat. Arteries were pretreated with 200 μM l-NNA and 10 μM indomethacin. Solid lines indicate time of exposure of arteries to ACh, while dotted lines show basal levels of EC [Ca2+]i. Transient and sustained (5 min after ACh application) components of the responses are shown as well. n = no. of tested arteries.
We next tested the hypothesis that estrogen replacement increases ACh-induced EC [Ca2+]i responses of uterine and mesenteric arteries treated with l-NNA and indomethacin. It has been shown that Phe-induced activation of SMCs can stimulate endothelial Ca2+ signaling. A diffusion of inositol 1,4,5-trisphosphate (IP3) from SMCs to ECs through myoendothelial gap junctions most likely mediates this effect (29). To minimize the involvement of this mechanism in our experiments, we performed all [Ca2+]i measurements in response to ACh without preconstriction of the vessels with Phe.
Representative changes in EC [Ca2+]i of uterine arteries from OVX and OVX+E rats in response to increasing concentrations of ACh are shown in Fig. 7, B and C, respectively. Administration of ACh in increasing concentrations resulted in a marked elevation of EC [Ca2+]i above basal levels. The ACh-induced response consisted of a transient [Ca2+]i rise followed by a relatively steady-state [Ca2+]i elevation (sustained response). Both transient and sustained components of [Ca2+]i responses were markedly enhanced by estrogen replacement.
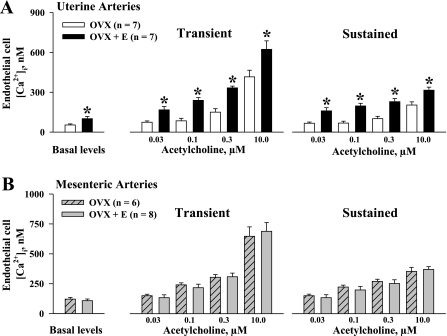
Figure 8 summarizes our data on the basal levels of EC [Ca2+]i and transient and sustained [Ca2+]i responses to ACh obtained from uterine and mesenteric arteries of both the OVX and OVX+E groups. Basal levels of EC [Ca2+]i were significantly higher in uterine vessels from the OVX+E group (102 ± 17 nM) compared with those from the OVX group (54 ± 8 nM; P < 0.05). Significant differences were also found for transient and sustained [Ca2+]i elevations in response to ACh between these two groups (Fig. 8A). In contrast, neither basal levels of EC [Ca2+]i nor transient and sustained [Ca2+]i responses to ACh were significantly different in mesenteric arteries of OVX versus OVX+E rats (Fig. 8B).
Fig. 8.
Estrogen replacement results in significant enhancement of ACh-induced elevation in EC [Ca2+]i in uterine but not in mesenteric arteries. A: summary graphs show a significant increase in basal levels of EC [Ca2+]i, and transient and sustained [Ca2+]i responses to application of ACh in uterine arteries of OVX vs. OVX+E rats. B: summary graphs demonstrate lack of differences in EC [Ca2+]i responses to ACh in mesenteric arteries of OVX vs. OVX+E rats. All experiments were conducted in the presence of l-NNA and indomethacin. n = no. of tested arteries. *Significantly different at P < 0.05 (2-way repeated-measures ANOVA).
DISCUSSION
The findings of the present study provide the first evidence that upregulation of EC Ca2+ signaling is one of the underlying mechanisms for estrogenic enhancement of EDHF-mediated uterine artery vasodilation. This conclusion is based on the following observations: 1) Resistance vessels of the mesentery and uterus from OVX and OVX+E rats can be effectively dilated by ACh via an NO- and prostacyclin-independent mechanism; this vasodilation is abolished by high K+ depolarization, confirming the role of EDHF in mediating this response. 2) Chronic administration of 17β-estradiol to OVX rats enhances EDHF-mediated vasodilation more markedly in reproductive (uterine) versus nonreproductive (mesenteric) vasculature. 3) Estrogen replacement significantly increases basal levels and ACh-stimulated EC [Ca2+]i responses in uterine but not in mesenteric arteries.
This study utilized small, resistance-size (<300 μm) uterine and mesenteric arteries. It should be noted, however, that comparison of sensitivities in mesenteric versus uterine vessels per se was not of interest, since both region- and size-dependent differences in arterial reactivity are well established. Rather, our intent was to specifically evaluate the influence of estrogen on the shift in dilator sensitivity within each vessel type, because this ratio can be viewed as an index of the functional estrogenic effect.
Our data demonstrate that supplementation of OVX rats with estrogen resulted in a fivefold increase in sensitivity to ACh of uterine arteries under control conditions as well as after inhibition of NO and prostacyclin production (Fig. 5A). In contrast, mesenteric artery sensitivity to ACh was increased only twofold (Fig. 5B). Administration of estrogen to OVX sheep caused a significant enhancement of bradykinin-induced vasodilation of uterine but not renal arteries (52). After chronic infusion of estrogen in OVX sheep, the relative uterine vascular responses significantly exceeded systemic hemodynamic changes (35). Together, our findings and previously published observations demonstrate that the influence of estrogen replacement is more prominent in the vasculature of reproductive versus nonreproductive organs.
In control untreated vessels, estrogen replacement also increased the sensitivity of uterine arteries to ACh, as evident from the reduction in EC50 values for ACh-induced vasodilation (Fig. 5A); conversely, this effect of estrogen supplementation was not significant in mesenteric arteries (Fig. 5B). Pretreatment of arteries with l-NNA and indomethacin resulted in a rightward shift in concentration-response curves for both uterine and mesenteric vessels, demonstrating a significant contribution of NO and prostacyclin to endothelium-mediated vasodilation in both types of vessels. At the same time, vessels from both OVX and OVX+E rats were markedly dilated by ACh after blockade of NO and prostacyclin production, implicating an important role for EDHF in mediating endothelium-dependent dilation.
It has been reported that maximal EDHF-induced vasodilatory responses of large mesenteric (superior mesenteric) and uterine (main uterine and arcuate arteries) arteries are significantly reduced after ovariectomy (44, 54). In contrast, the EDHF-mediated vasodilation of smaller resistance mesenteric and uterine arteries observed in the present study was nearly complete (Figs. 2 and 3). These differences most likely result from an increasing contribution of EDHF in the control of vascular tone in the uterine and mesenteric circulation with decreasing vessel size. Our data are in agreement with ample evidence that EDHF is an important mediator of endothelial influences in microcirculation of animals and humans (6, 7, 9, 11, 13, 25, 28, 30, 33, 36, 45–47).
In the present study, chronic administration of estrogen to OVX rats significantly enhanced NO- and prostacyclin-independent vasodilation of small pressurized mesenteric arteries in response to ACh. These data correlate well with previously published observations utilizing isometric force measurements from arterial rings of larger mesenteric vessels (31, 40). Enhancement of EDHF-mediated vasodilation of uterine radial arteries by estrogen replacement is a novel observation. A previous study indicated that EDHF-mediated mesenteric vasodilation in response to nonreceptor stimulation with the Ca2+ ionophore A-23187 is potentiated by estrogen (32). Also, estrogen replacement produced no change in the expression of muscarinic receptors in the rat uterus (1). Together, these data suggest that it is unlikely that estrogenic enhancement of ACh-evoked responses is induced by increased expression of endothelial muscarinic receptors.
Long-term exposure to estrogen increases the expression of estrogen receptors (ER)-α and -β in different vascular beds (23). Expression of ERs in ECs of uterine and mammary arteries was markedly elevated in OVX sheep exposed to systemic estrogen (8). In contrast, no significant change in ER expression was found in omental or renal vessels (8). Therefore, the differences in estrogen-induced modulation of ACh responses in the uterine versus mesenteric vessels may result, in part, from differential upregulation of ERs in reproductive versus nonreproductive vasculature. According to recently published data, ER-β mediates a beneficial effect of estrogen on EDHF-induced vasodilation of small femoral arteries of female compared with male mice (34). This observation suggests that estrogen-induced specific upregulation of endothelial ER-β might be responsible for the enhancement of EDHF-mediated vasodilation found in the present study.
The role of the endothelium in mediating the beneficial effects of estrogen is well documented in animal and human studies (22, 37). Estrogen-induced upregulation of eNOS expression is an important mechanism for increased NO-dependent vasodilation in large uterine and systemic arteries (48). Several recent studies suggest that estrogen can also regulate vascular tone through its modulation of EDHF-mediated vascular responses, although the underlying mechanisms are not clearly defined (18, 22, 31, 38, 44, 46). It is generally accepted that elevation of intracellular Ca2+ in endothelial cells is an early step in the signaling process that leads to vasodilation in response to mechanical or chemical stimulation (6, 7, 14, 17, 30). In this regard, basal endothelial calcium levels were higher in ECs of females versus males, suggesting an important role of estrogen in mediating these sex differences (26, 43). Our study provides novel and direct evidence for estrogenic modulation of EC [Ca2+]i, because estrogen administration resulted in a significant elevation of basal calcium in uterine vessels from OVX+E compared with OVX rats (Fig. 8). Circulating levels of estrogen are increased manyfold during rat gestation (50). Therefore, estrogen is most likely also responsible for the pregnancy-induced upregulation of endothelial Ca2+ signaling in resistance uterine arteries found in our previous study (17).
Although the mechanism by which estrogen augments endothelial Ca2+ signaling is not known, the present results indicate that both basal [Ca2+]i and ACh-induced [Ca2+]i responses of uterine arteries were enhanced by estrogen, suggesting that some common pathway may be responsible. For example, estrogen-induced endothelial membrane hyperpolarization could enhance both basal and stimulated Ca2+ influx by increasing the electrochemical gradient for Ca2+ ions (30, 41). In this regard, several lines of evidence in different cell types have linked estrogen to potassium channel expression and function via direct genomic effects. Chronic exposure to estrogen produced transcriptional upregulation of SKCa (SK3) channel in L6 cells, a rat skeletal muscle cell line that expresses SK3 and ERs (24). Injection of estrogen into OVX guinea pigs was followed by an increase in the levels of SK3 mRNA in the hypothalamus (5). Therefore, modulation of EC membrane potential through estrogen-induced upregulation of potassium channels might be a plausible mechanism for increased endothelial Ca2+ signaling in uterine arteries and deserves further investigation.
In our study, while dilation to ACh was increased in mesenteric vessels, EC [Ca2+]i signaling was not altered, indicating that mechanisms regulating EDHF may be different in blood vessels of reproductive versus nonreproductive organs. In support of this, estrogen-induced changes in EDHF-mediated responses of cerebral arteries—another nonreproductive vasculature—did not correlate with changes in Ca2+ signaling of ECs (18). These data suggest that there is some alternative mechanism(s) responsible for enhancement of EDHF-mediated vasodilation by estrogen. For example, it is agreed that in small mesenteric vessels ACh-induced activation of SKCa and IKCa channels results in EC hyperpolarization that can be electrotonically transmitted to SMCs through myoendothelial gap junctions. Gap junctions are composed of specific membrane proteins, connexins, whose density in the vascular wall has been correlated with the extent of EDHF-mediated vasodilation (20, 45). Recent studies using mesenteric arteries demonstrate that the expression of connexin 43 or connexin 40 is greatly reduced by ovariectomy and is restored by treatment with estrogen (32, 40). These data strongly suggest that estrogen-induced upregulation of connexins and an increase in gap junction density can be responsible for enhancing EDHF-mediated vasodilation of mesenteric vessels. In contrast, there was no change in the expression of connexins in the aorta of ovariectomized rats (21). Although sex differences in EDHF-mediated vasodilation of small femoral arteries were absent in ER-β-knockout compared with wild-type mice, immunostaining for connexins 43, 37, and 40 revealed no differences in their vascular expression (34). Hence, the role of altered expression of vascular wall connexins in estrogen-related changes in EDHF-mediated uterine vasodilation remains to be determined.
In conclusion, our data demonstrate that estrogen can significantly enhance EDHF-mediated vasodilation of small resistance vessels, and this effect is especially prominent in the uterine circulation. The results of this study provide strong evidence in favor of estrogen as a key molecule in regulating endothelial Ca2+ signaling and associated production of endothelium-derived uterine vasodilators, including EDHF.
Further characterization of the relationship between estrogen and EDHF-mediated vasodilation has potential clinical implications. For example, recent work has shown that a compromise in normal regulation of EDHF function in pregnancy may be related to the development of preeclampsia (25). Outside of pregnancy complications, endothelial dysfunction is typical of a number of cardiovascular diseases, and a better understanding of estrogenic vascular effects is needed to allow the development of new methods for improving endothelial function in both sexes.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-067250, HL-088245, and HL-073895.
Acknowledgments
We thank Sara Tourville for technical assistance with preparation of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abdalla FM, Marostica E, Picarelli ZP, Abreu LC, Avellar MC, Porto CS. Effect of estrogen on muscarinic acetylcholine receptor expression in rat myometrium. Mol Cell Endocrinol 213: 139–148, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Adeagbo AS, Triggle CR. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J Cardiovasc Pharmacol 21: 423–429, 1993. [PubMed] [Google Scholar]
- 3.Anari MR, Bakhtiar R, Zhu B, Huskey S, Franklin RB, Evans DC. Derivatization of ethinylestradiol with dansyl chloride to enhance electrospray ionization: application in trace analysis of ethinylestradiol in rhesus monkey plasma. Anal Chem 74: 4136–4144, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bell C Oestrogen-induced sensitization of the uterine artery of the guinea-pig to acetylcholine. Br J Pharmacol 49: 595–601, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch MA, Kelly MJ, Ronnekleiv OK. Distribution, neuronal colocalization, and 17beta-E2 modulation of small conductance calcium-activated K+ channel (SK3) mRNA in the guinea pig brain. Endocrinology 143: 1097–1107, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Bryan RM, You J, Golding EM, Marrelli SP. Endothelium-derived hyperpolarizing factor: a cousin to nitric oxide and prostacyclin. Anesthesiology 102: 1261–1277, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–380, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Byers MJ, Zangl A, Phernetton TM, Lopez G, Chen DB, Magness RR. Endothelial vasodilator production by ovine uterine and systemic arteries: ovarian steroid and pregnancy control of ERalpha and ERbeta levels. J Physiol 565: 85–99, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49: 590–596, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Cohen RA The endothelium-derived hyperpolarizing factor puzzle: a mechanism without a mediator? Circulation 111: 724–727, 2005. [DOI] [PubMed] [Google Scholar]
- 11.de Wit C, Wolfle SE. EDHF and gap junctions: important regulators of vascular tone within the microcirculation. Curr Pharm Biotechnol 8: 11–25, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Dedkova EN, Blatter LA. Nitric oxide inhibits capacitative Ca2+ entry and enhances endoplasmic reticulum Ca2+ uptake in bovine vascular endothelial cells. J Physiol 539: 77–91, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: past beliefs and present facts. Ann Med 39: 495–516, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension 47: 629–633, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989. [PubMed] [Google Scholar]
- 17.Gokina NI, Goecks T. Upregulation of endothelial cell Ca2+ signaling contributes to pregnancy-enhanced vasodilation of rat uteroplacental arteries. Am J Physiol Heart Circ Physiol 290: H2124–H2135, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Golding EM, Ferens DM, Marrelli SP. Altered calcium dynamics do not account for attenuation of endothelium-derived hyperpolarizing factor-mediated dilations in the female middle cerebral artery. Stroke 33: 2972–2977, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 20.Hill CE, Rummery N, Hickey H, Sandow SL. Heterogeneity in the distribution of vascular gap junctions and connexins: implications for function. Clin Exp Pharmacol Physiol 29: 620–625, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Hortovanyi E, Varbiro S, Tokes AM, Illyes G, Szekacs B, Paku S, Kerenyi T, Kadar A. Connexin 43 expression in rat aortic smooth muscle after ovariectomy and hormonal replacement. Pathol Res Pract 197: 109–112, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation 11: 9–38, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res 91: 814–820, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP. Determinants contributing to estrogen-regulated expression of SK3. Biochem Biophys Res Commun 303: 660–668, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. Differential mechanisms of endothelium-dependent vasodilator responses in human myometrial small arteries in normal pregnancy and pre-eclampsia. Clin Sci (Lond) 103: 67–73, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Knot HJ, Lounsbury KM, Brayden JE, Nelson MT. Gender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activity. Am J Physiol Heart Circ Physiol 276: H961–H969, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohler R, Hoyer J. The endothelium-derived hyperpolarizing factor: insights from genetic animal models. Kidney Int 72: 145–150, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Lamboley M, Pittet P, Koenigsberger M, Sauser R, Beny JL, Meister JJ. Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Calcium 37: 311–320, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Liu MY, Hattori Y, Fukao M, Sato A, Sakuma I, Kanno M. Alterations in EDHF-mediated hyperpolarization and relaxation in mesenteric arteries of female rats in long-term deficiency of oestrogen and during oestrus cycle. Br J Pharmacol 132: 1035–1046, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu MY, Hattori Y, Sato A, Ichikawa R, Zhang XH, Sakuma I. Ovariectomy attenuates hyperpolarization and relaxation mediated by endothelium-derived hyperpolarizing factor in female rat mesenteric artery: a concomitant decrease in connexin-43 expression. J Cardiovasc Pharmacol 40: 938–948, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Luksha L, Nisell H, Kublickiene K. The mechanism of EDHF-mediated responses in subcutaneous small arteries from healthy pregnant women. Am J Physiol Regul Integr Comp Physiol 286: R1102–R1109, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Luksha L, Poston L, Gustafsson JA, Hultenby K, Kublickiene K. The oestrogen receptor beta contributes to sex related differences in endothelial function of murine small arteries via EDHF. J Physiol 577: 945–955, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magness RR, Rosenfeld CR. Local and systemic estradiol-17β: effects on uterine and systemic vasodilation. Am J Physiol Endocrinol Metab 256: E536–E542, 1989. [DOI] [PubMed] [Google Scholar]
- 36.McGuire JJ, Ding H, Triggle CR. Endothelium-derived relaxing factors: a focus on endothelium-derived hyperpolarizing factor(s). Can J Physiol Pharmacol 79: 443–470, 2001. [PubMed] [Google Scholar]
- 37.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 308: 1583–1587, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Morton JS, Jackson VM, Daly CJ, McGrath JC. Endothelium dependent relaxation in rabbit genital resistance arteries is predominantly mediated by endothelial-derived hyperpolarizing factor in females and nitric oxide in males. J Urol 177: 786–791, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Muller JM, Davis MJ, Kuo L, Chilian WM. Changes in coronary endothelial cell Ca2+ concentration during shear stress- and agonist-induced vasodilation. Am J Physiol Heart Circ Physiol 276: H1706–H1714, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Nawate S, Fukao M, Sakuma I, Soma T, Nagai K, Takikawa O, Miwa S, Kitabatake A. Reciprocal changes in endothelium-derived hyperpolarizing factor- and nitric oxide-system in the mesenteric artery of adult female rats following ovariectomy. Br J Pharmacol 144: 178–189, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Pittner J, Liu R, Brown R, Wolgast M, Persson AE. Visualization of nitric oxide production and intracellular calcium in juxtamedullary afferent arteriolar endothelial cells. Acta Physiol Scand 179: 309–317, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Rahimian R, Wang X, van Breemen C. Gender difference in the basal intracellular Ca2+ concentration in rat valvular endothelial cells. Biochem Biophys Res Commun 248: 916–919, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Sakuma I, Liu MY, Sato A, Hayashi T, Iguchi A, Kitabatake A, Hattori Y. Endothelium-dependent hyperpolarization and relaxation in mesenteric arteries of middle-aged rats: influence of oestrogen. Br J Pharmacol 135: 48–54, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res 90: 1108–1113, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111: 796–803, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol Regul Integr Comp Physiol 272: R441–R463, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Sylvester FA, Stepp DW, Frisbee JC, Lombard JH. High-salt diet depresses acetylcholine reactivity proximal to NOS activation in cerebral arteries. Am J Physiol Heart Circ Physiol 283: H353–H363, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Taya K, Greenwald GS. In vivo and in vitro ovarian steroidogenesis in the pregnant rat. Biol Reprod 25: 683–691, 1981. [DOI] [PubMed] [Google Scholar]
- 51.Ungvari Z, Csiszar A, Koller A. Increases in endothelial Ca2+ activate KCa channels and elicit EDHF-type arteriolar dilation via gap junctions. Am J Physiol Heart Circ Physiol 282: H1760–H1767, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Veille JC, Li P, Eisenach JC, Massmann AG, Figueroa JP. Effects of estrogen on nitric oxide biosynthesis and vasorelaxant activity in sheep uterine and renal arteries in vitro. Am J Obstet Gynecol 174: 1043–1049, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci USA 91: 5212–5216, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wight E, Kung CF, Moreau P, Takase H, Bersinger NA, Luscher TF. Aging, serum estradiol levels, and pregnancy differentially affect vascular reactivity of the rat uterine artery. J Soc Gynecol Investig 7: 106–113, 2000. [PubMed] [Google Scholar]
- 55.Zhang Y, Stewart KG, Davidge ST. Endogenous estrogen mediates vascular reactivity and distensibility in pregnant rat mesenteric arteries. Am J Physiol Heart Circ Physiol 280: H956–H961, 2001. [DOI] [PubMed] [Google Scholar]