Abstract
Normal β-cells adjust their function to compensate for any decrease in insulin sensitivity. Our aim was to explore whether a prolonged fast would allow a study of the effects of changes in circulating free fatty acid (FFA) levels on insulin secretion and insulin sensitivity and whether any potential effects could be reversed by the antilipolytic agent acipimox. Fourteen (8 female, 6 male) healthy young adults (aged 22.8–26.9 yr) without a family history of diabetes and a body mass index of 22.6 ± 3.2 kg/m2 were studied on three occasions in random order. Growth hormone and FFA levels were regularly measured overnight (2200-0759), and subjects underwent an intravenous glucose tolerance test in the morning (0800-1100) on each visit. Treatment A was an overnight fast, treatment B was a 24-h fast with regular administrations of a placebo, and treatment C was a 24-h fast with regular ingestions of 250 mg of acipimox. The 24-h fast increased overnight FFA levels (as measured by the area under the curve) 2.8-fold [51.3 (45.6–56.9) vs. 18.4 (14.4–22.5) *104 μmol/l*min, P < 0.0001], and it led to decreases in insulin sensitivity [5.7 (3.6–8.9) vs. 2.6 (1.3–4.7) *10−4 min−1 per mU/l, P < 0.0001] and the acute insulin response [16.3 (10.9–21.6) vs. 12.7 (8.7–16.6) *102 pmol/l*min, P = 0.02], and therefore a reduction in the disposition index [93.1 (64.8–121.4) vs. 35.5 (21.6–49.4) *102 pmol/mU, P < 0.0001]. Administration of acipimox during the 24-h fast lowered FFA levels by an average of 20% (range: −62 to +49%; P = 0.03), resulting in a mean increase in the disposition index of 31% (P = 0.03). In conclusion, the 24-h fast was accompanied by substantial increases in fasting FFA levels and induced reductions in the acute glucose-simulated insulin response and insulin sensitivity. The use of acipimox during the prolonged fast increased the disposition index, suggesting a partial reversal of the effects of fasting on the acute insulin response and insulin sensitivity.
Keywords: free fatty acid, growth hormone, first phase insulin secretion, disposition index, lipotoxicity
numerous studies have shown that insulin resistance precedes the manifestation of hyperglycemia in subjects who go on to develop type 2 diabetes (T2D; Ref. 31). However, given that normal β-cells adjust their function to compensate for decreases in insulin sensitivity (SI; Refs. 3, 16), it is increasingly being recognized that T2D only develops once β-cells are no longer able to sustain an adequate compensatory response (24). It is not known when β-cell dysfunction first appears in the development of T2D, but recent evidence (15) suggests it occurs when glucose tolerance is still classified as normal.
Lipotoxic mechanisms are likely to play an important role in the development of β-cell dysfunction, which is supported by extensive in vitro and animal model data (11, 36, 38, 51). Experimental studies (6, 8, 10, 17, 21, 43, 50) in humans to date have made use of lipid/heparin infusions to study the effect of manipulating circulating free fatty acid (FFA) levels on glucose metabolism. However, these infusions led to highly variable increases in FFA levels and yielded somewhat conflicting results with regard to their effect on insulin secretion (8, 10, 17, 21, 43, 50).
Our aim was to explore whether a prolonged fast would increase plasma FFA levels sufficiently to study the effect of elevated levels on the glucose-stimulated acute insulin response (AIR) and SI and whether any potential effects could be reversed by lowering FFA levels with the antilipolytic drug acipimox (7, 13, 45). We studied 14 healthy volunteers without a family history of diabetes to test the hypothesis that increased plasma FFA levels are directly linked to changes in AIR and SI.
METHODS
Subjects and Investigative Protocol
Fourteen healthy participants (8 female, 6 male) aged 22.8–26.9 yr were recruited to the study at the University Department of Paediatrics, Addenbrooke's Hospital (Cambridge, UK). None of the subjects' parents and siblings had diabetes. The study was approved by the Suffolk Local Research Ethics Committee, and written informed consent was obtained from each volunteer.
In random order, subjects were admitted to the Wellcome Trust Clinical Research Facility (WTCRF), Addenbrooke's Hospital, at 2000 on three occasions (treatment A, B, or C), which were 8–14 days apart. The participants were instructed not to consume alcohol and refrain from exercise for 48 h before each study visit. During treatment A, subjects were given a standard meal at 2100 and then remained fasted till completion of the study protocol at 1100 the following morning. During treatments B and C, subjects had breakfast between 0800 and 0815 on the day of admission to the WTCRF and then fasted till 1100 the following morning. In addition, participants ingested a placebo tablet or 250 mg of the antilipolytic drug acipimox at 1100, 1500, 1900, 2300, 0300, and 0700 in a double-blind design.
After admission on each visit, two intravenous cannulas were sited in an antecubital vein of each arm, one of which was warmed with a heating blanket to allow sampling of arterialized blood. Baseline samples for FFA, growth hormone (GH), and insulin levels were taken at 2200. Sampling for FFA continued overnight at 60-min intervals, while samples for GH were taken every 15 min. Final samples of the overnight protocol were taken at 0759. Additional samples for glucose and insulin were taken at 0750 and 0755. At 0800, an intravenous bolus (0.3 g/kg) of glucose was administered through the second cannula to commence an intravenous glucose tolerance test (IVGTT) on each study visit. At 0820, an intravenous bolus (0.02 U/kg) of insulin was given. During the IVGTT, blood samples for GH were taken every 15 min until 1100 and for FFA at 0810, 0819, 0830, 0845, 0900, 0930, 1000, 1030, and 1100. Glucose and insulin levels were measured 2, 3, 4, 5, 6, 8, 10, 15, 19, 22, 23, 25, 27, 30, 35, 40, 60, 90, 120, 150, and 180 min after the intravenous glucose bolus. Subjects were then given breakfast, and both cannulas were removed before subjects were discharged from the research unit.
Dual-Energy X-Ray Absorptiometry
A dual-energy X-ray absorptiometry scan was performed at the WTCRF after the subjects' final study visit. Data on body composition were gathered with a Lunar Prodigy machine using a constant pixel size of 1.2 × 1.2 cm and Lunar software programs (version 8.1, Lunar). The effective radiation dose was 0.2 μSv. The scan yielded measures of whole-body fat and fat-free mass and therefore percent whole-body fat mass.
Assays
Plasma FFA levels were analyzed using an enzymatic colorimetric kit (Alpha Laboratories) adapted to an ILab 600 clinical chemistry analyzer (Instrumentation Laboratories). The intra-assay coefficient of variation (CV) was 2.2% at levels of 559 and 1,143 μmol/l. The equivalent interassay CV was 2.5%. GH levels were measured using an ELISA (Oxford Bio-Innovations, UK) according to the manufacturer's instructions. The assay was calibrated to the WHO First International Standard (80/505). The intra-assay CV was 9.7% at 0.7 ng/ml and 6.5% at 6.4 ng/ml. The equivalent interassay CVs were 10.4 and 5.5%, respectively. Insulin levels were measured using an ELISA (Dako) according to the manufacturer's instructions. The intra-assay CV was 4.3% at 82 pmol/l, 3.0% at 402 pmol/l, and 5.7% at 907 pmol/l. The equivalent interassay CVs were 4.3, 5.1, and 5.4% respectively. Glucose levels were measured using 25-μl whole blood samples on a YSI 2300 stat plus analyzer (Lynchford House, UK). The intra-assay CV was 1.5% at 4.1 mmol/l. The equivalent interassay CV at this glucose concentration was 2.8% and 1.7% at 14.1 mmol/l.
Minimal Model of Glucose Kinetics During the IVGTT
The minimal model defines SI (the ability of insulin to enhance net glucose disappearance from plasma) and glucose effectiveness (SG, ability of glucose to promote its own disposal; Ref. 4) and is described by the following two differential equations
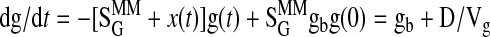 |
(1) |
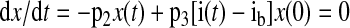 |
(2) |
where g(t) is glucose concentration, i(t) is insulin concentration, x(t) is insulin action above basal. The gb and ib are the end-test glucose and insulin concentrations, D is the amount of exogenous glucose injected at time 0, and SGMM, p2, p3, and Vg are model parameters. The parameter Vg represents the distribution volume of glucose. SIMM is calculated as p3/p2.
Bayesian population analysis was performed using WinBUGS version 1.4 (28, 49) on all subjects' data simultaneously. This provided both individual- and population-level parameter estimates for 1) minimal model derived postintravenous glucose insulin sensitivity, SIMM, 2) glucose effectiveness, SGMM, 3) the rate of provision of insulin action, p3, 4) the fractional transfer rate associated with remote insulin, p2, and 5) the volume of glucose distribution, Vg. The transfer rate p2 quantifies how fast insulin action dissipates, while the ratio ln(2)/p2 represents the time needed for insulin action to attain its half-maximum value. Individual-level estimates were based on fitting a minimal model of glucose and insulin kinetics (4) to each IVGTT. The minimal model was implemented in WinBUGS's WBDiff interface version 1.9.4 (www.winbugs-development.org.uk) following our earlier work (1). Differences between the subjects' parameters were simultaneously accounted for by a standard statistical model (27), which provided estimates of the variability between subjects as well as the population-level parameters. These in turn provided further information about the individual-level estimates, and the Bayesian approach allowed estimates of parameters to be “strengthened” via the incorporation of external knowledge regarding their approximate values.
Calculations
Body mass index (BMI) was calculated as [weight (kg)]/[height (m)]2. The area under the curve (AUC) for FFA levels between 2200 and 0759 was calculated according to the trapezium rule and gave a measure of exposure to FFAs before the IVGTT. The area under the insulin curve between 0800 and 0810 above basal insulin levels is the AIR and provided a proxy for first phase insulin secretion (26). Insulin AUC was multiplied with SIMM to calculate the disposition index, which is an assessment of the adequacy of AIR for the degree of SI (5, 25). Glucose AUC above basal glucose levels was calculated for the period of the IVGTT (0800-1100). A biexponential regression line was fitted to insulin levels during the IVGTT to model slow and fast insulin clearance phases (23). The metabolic clearance rate (MCR) for the total insulin volume of distribution was then calculated as the sum of the slow and fast exponential decay coefficients and normalized to body weight. The model implementation and parameter estimations were carried out in SAAM II version 1.2.1 (SAAM Institute, University of Washington).
Analysis of GH Secretion
GH levels were analyzed using the Easy Time Series Analysis program (Oxford University). After smoothing of data using a three-point moving average, probit analysis was used to quantify the distribution of GH levels present in the overnight profiles (2200-0759), giving unbiased estimates of basal, median, and maximal GH levels (12). Fourier transformation (FT) was used to assess occurrence of different oscillatory frequencies of secretion within the GH profiles. FT delineates the frequency component of complex signals by resolving data into a series of sine and cosine components. Each frequency has an amplitude attribute showing the power of that frequency within the data array. A spectral peak at any frequency indicates a greater than random tendency for peaks and troughs in the time series to recur at that frequency. AUC of the absolute FT provided a total measure of power, i.e., amplitude, of the GH signal.
Statistics
Data were analyzed for normality using the Kolmorgorov-Smirnov test and log-transformed to normal distributions wherever necessary to allow use of parametric analysis. Results are reported as means (95% confidence interval) unless stated otherwise. Dependent t-tests were used to assess differences between treatments A, B, and C. Associations between overnight levels of FFA, insulin, and glucose; GH secretion; AIR; and SIMM were tested using the Pearson's correlation coefficient (r). Two-tailed significance was set to P < 0.05. Analyses were performed using SPSS for Windows version 14.0.
RESULTS
Overnight (Treatment A) vs. 24-h Fast (Treatment B)
Cohort characteristics are summarized by sex in Table 1.
Table 1.
Cohort characteristics by sex
| Male | Female | |
|---|---|---|
| Weight, kg | 71.7 (64.2–79.1) | 58.0 (51.4–64.7) |
| Height, kg | 175.1 (166.1–184.0) | 163.7 (161.2–166.3) |
| Body mass index, kg/m2 | 23.5 (20.4–26.6) | 21.7 (19.1–24.3) |
| Whole-body fat mass, kg | 12.6 (2.9–22.4) | 17.3 (12.6–22.1) |
| Whole-body fat-free mass, kg | 55.9 (48.2–63.7) | 38.3 (36.2–40.5) |
| %Whole-body fat mass | 17.9 (4.9–30.9) | 30.7 (25.2–36.1) |
Data are means [95% confidence interval (CI)].
Overnight.
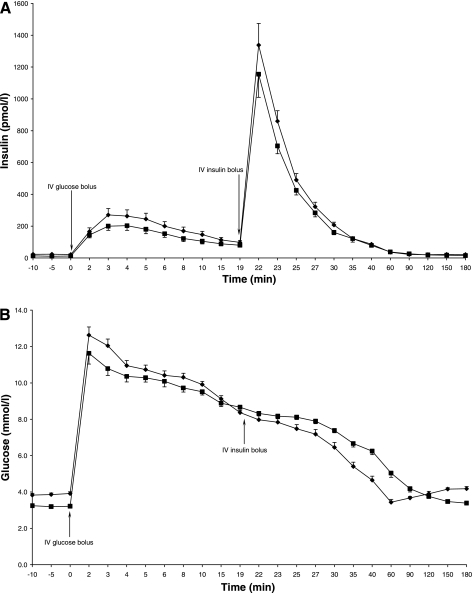
Overnight (2200-0759) FFA levels, as measured by the AUC, were 2.8-fold higher during the 24-h fast (P < 0.0001; Table 2). Overnight basal GH levels were similar for treatments A and B (P = 0.4; Table 2). Maximal GH levels and GH pulse amplitudes were increased during the prolonged fast (P = 0.04 and P = 0.02, respectively; Table 2), and there was a trend toward increased mean GH levels with the long fast (P = 0.07; Table 2). Fourier transformation showed similar GH pulse periodicity on treatments A and B [209.5 (139.1–280.0) vs. 181.0 (155.3–206.7) min; P = 0.4]. Fasting (pre-IVGTT) glucose and insulin levels were lower during the 24-h fast (P < 0.0001 and P = 0.001, respectively; Table 2; Fig. 1, A and B).
Table 2.
Overnight (2200-0759) FFA and GH levels, fasting (0759) glucose and insulin levels, AIR, SIMM, disposition index and glucose AUC during the IVGTT, and insulin MCR, p3, p2, SGMM, and Vg for treatments A (overnight fast) and B (24-h fast)
| Overnight Fast | 24-h Fast | P | |
|---|---|---|---|
| FFA AUC, *104 μmol/l*min | 18.4 (14.4–22.5) | 51.3 (45.6–56.9) | <0.0001 |
| Basal GH, ng/ml | 0.20 (−0.05–0.43) | 0.45 (0.07–0.84) | 0.4 |
| Mean GH, ng/ml | 2.2 (1.2–3.2) | 4.0 (1.9–6.2) | 0.07 |
| Maximal GH, ng/ml | 12.8 (5.8–19.9) | 19.5 (10.9–28.1) | 0.04 |
| GH amplitude, ng/ml*min | 48.2 (25.4–71.0) | 84.1 (53.2–114.9) | 0.02 |
| Fasting glucose, mmol/l | 3.9 (3.7–4.1) | 3.2 (3.0–3.4) | <0.0001 |
| Fasting insulin, pmol/l | 21.6 (16.2–27.0) | 12.2 (9.9–14.5) | 0.001 |
| AIR, *102 pmol/l*min | 16.3 (10.9–21.6) | 12.7 (8.7–16.6) | 0.02 |
| SIMM, *10−4 min−1 per mU/l | 6.1 (4.9–7.4) | 2.9 (2.2–3.5) | <0.0001 |
| Disposition index, *102 pmol/mU | 93.1 (64.8–121.4) | 35.5 (21.6–49.4) | <0.0001 |
| Glucose AUC, mmol/l*min | 151.6 (125.1–178.1) | 330.3 (246.6–413.9) | 0.001 |
| Insulin MCR, l min−1 per kg | 0.07 (0.05–0.09) | 0.06 (0.04–0.08) | 0.4 |
| p3, *10−6 min−2 per mU/l | 35.5 (26.8–44.2) | 8.8 (5.8–11.8) | <0.0001 |
| p2, *10−2 min−1 | 5.8 (5.0–6.7) | 2.8 9 (2.3–3.3) | <0.0001 |
| SGMM, *10−2 min−1 | 2.0 (1.7–2.2) | 1.7 (1.6–1.8) | 0.07 |
| Vg, l/kg | 0.21 (0.20–0.23) | 0.22 (0.20–0.24) | 0.1 |
Data are means (95% CI). FFA, free fatty acid; AUC, area under the curve; GH, growth hormone; AIR, acute insulin response; SIMM, minimal model derived postintravenous glucose insulin sensitivity; MCR, metabolic clearance rate; p3, rate of provision of insulin action; p2, fractional transfer rate associated with remote insulin; SGMM, glucose effectiveness; Vg, volume of glucose distribution.
Fig. 1.
A: insulin levels 10 min before and during the 3-h intravenous glucose tolerance test (IVGTT). ⧫Treatment A; ▪treatment B. Data are means ± 1SE. B: glucose levels 10 min before and during IVGTT. ⧫Treatment A; ▪treatment B. Data are means ± 1SE.
The increase in overnight FFA levels from treatment A to B tended to be associated with increases in GH pulse amplitude (r = 0.466; P = 0.09) and decreases in insulin levels (r = −0.457; P = 0.1).
IVGTT.
AIR decreased by an average of 18% from treatment A to B (P = 0.02; Table 2; Fig. 1A). SIMM, p3, and p2 were also lower during the 24-h fast (P < 0.0001, P < 0.0001, and P < 0.0001, respectively; Table 2). The disposition index decreased by an average of 59% from treatment A to B (P < 0.0001; Table 2). Glucose AUC above basal glucose levels was greater during the prolonged fast (P = 0.001; Table 2). Insulin MCR (P = 0.4), SGMM (P = 0.07), and Vg (P = 0.1) were similar for treatments A and B (Table 2).
The decrease in AIR from treatment A to B was associated with the increase in overnight FFA levels, independent of reductions in fasting glucose levels (r = 0.603; P = 0.03). The decrease in SIMM from treatment A to B was not related to overnight changes in FFA levels (r = −0.293; P = 0.3) but correlated with increases in mean GH levels (r = −0.63; P = 0.02) independent of changes in overnight FFA levels (r = −0.595; P = 0.04). The reduction in p3 from treatment A to B correlated with the increase in mean levels of GH (r = −0.629; P = 0.02) but not FFAs (r = 0.031; P = 0.9). The change in p2 from treatment A to B showed no association to changes in GH or FFA levels.
Effects of acipimox during the 24-h fast (treatment B vs. C).
This part of the analysis only applies to 13 subjects, as one (male) subject failed to undertake treatment C.
Overnight.
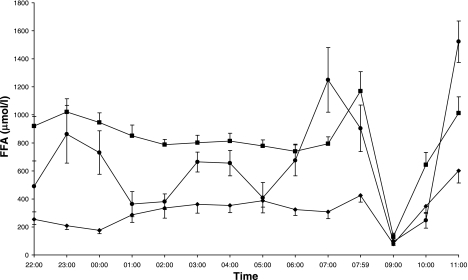
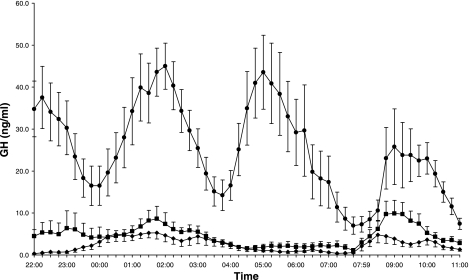
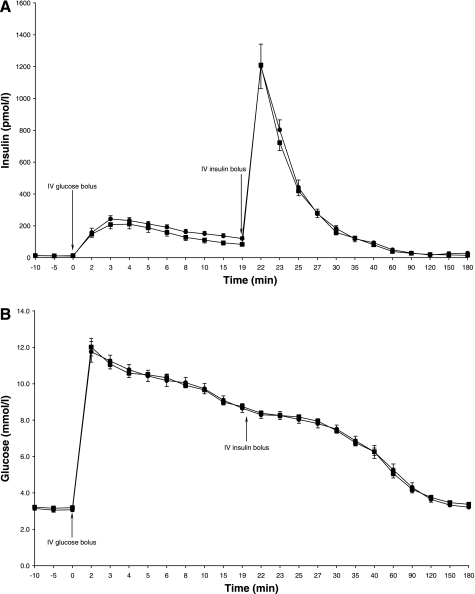
The average change in overnight FFA levels from treatment B to C was −20% and ranged from −62 to +49% (P = 0.03; Table 3; Fig. 2). FFA levels during treatment C [18.5 (14.0–22.9) *104 μmol/l*min] remained above those observed during the overnight fast (P < 0.0001; Fig. 2). GH levels were markedly higher on treatment C than on treatments B and A (Table 3; Fig. 3). Fasting glucose and insulin levels were similar for treatments B and C (P = 0.4 and P = 0.6, respectively; Table 3; Fig. 4, A and B).
Table 3.
Overnight (2200-0759) FFA and GH levels, fasting (0759) glucose and insulin levels, AIR, SIMM, disposition index and glucose AUC during the IVGTT, and insulin MCR, p3, p2, SGMM, and Vg for treatments B (24-h fast with placebo) and C (24-h fast with acipimox)
| 24-h Fast + Placebo | 24-h Fast + Acipimox | P | |
|---|---|---|---|
| FFA AUC, *104 μmol/l*min) | 51.4 (45.2–57.5) | 40.0 (29.7–50.3) | 0.03 |
| Basal GH, ng/ml | 0.48 (0.07–0.90) | 5.45 (0.94–9.97) | 0.001 |
| Mean GH, ng/ml | 4.0 (1.7–6.3) | 27.2 (18.2–36.3) | <0.0001 |
| Maximal GH, ng/ml | 18.7 (9.5–27.9) | 127.1 (80.8–173.3) | <0.0001 |
| GH amplitude, ng/ml*min | 81.7 (48.5–114.9) | 432.3 (298.5–566.1) | <0.0001 |
| Fasting glucose, mmol/l | 3.2 (3.0–3.4) | 3.1 (2.9–3.3) | 0.4 |
| Fasting insulin, pmol/l | 12.1 (9.6–14.5) | 11.1 (7.3–14.9) | 0.6 |
| AIR, *102 pmol/l*min | 13.2 (9.1–17.3) | 15.9 (12.9–18.9) | 0.2 |
| SIMM, *10−4 min−1 per mU/l | 2.9 (2.1–3.7) | 4.5 (2.3–6.7) | 0.07 |
| Disposition index, *102 pmol/mU | 36.9 (22.0–51.8) | 60.5 (40.0–81.1) | 0.03 |
| Glucose AUC, mmol/l*min | 337.9 (257.8–418.0) | 357.3 (290.7–424.0) | 0.6 |
| Insulin MCR, l min−1 per kg | 0.06 (0.04–0.08) | 0.06 (0.05–0.08) | 0.9 |
| p3, *10−6 min−2 per mU/l | 8.9 (5.4–12.5) | 8.0 (0.5–15.6) | 0.2 |
| p2, *10−2 min−1 | 2.9 (2.2–3.5) | 1.3 (0.7–1.8) | <0.0001 |
| SGMM, *10−2 min−1 | 1.7 (1.6–1.9) | 1.7 (1.5–1.9) | 0.9 |
| Vg, l/kg | 0.21 (0.21–0.22) | 0.21 (0.20–0.22) | 0.7 |
Data are means (95% CI).
Fig. 2.
Free fatty acid (FFA) levels overnight (2200-0759) and during the IVGTT (0800-1100) for treatments A (⧫), B (▪), and C (•). Data are means ± 1SE.
Fig. 3.
Growth hormone (GH) levels overnight (2200-0759) and during the IVGTT (0800-1100) for treatments A (⧫), B (▪), and C (•). Data are means ± 1SE.
Fig. 4.
A: insulin levels 10 min before and during the IVGTT. ▪Treatment B; •treatment C. Data are means ± 1SE. B: glucose levels 10 min before and during IVGTT. ▪Treatment B; •treatment C. Data are means ± 1SE.
IVGTT.
AIR and SIMM were similar during treatments B and C (P = 0.02 and P = 0.07, respectively; Table 3; Fig. 4A). However, the disposition index increased by an average of 31% with acipimox during the 24-h fast (P = 0.03; Table 3). Nevertheless, glucose AUC was similar for treatments B and C (P = 0.6; Table 3; Fig. 4B). The p2 was lower with acipimox (P < 0.0001; Table 3), but p3 (P = 0.2), insulin MCR (P = 0.9), SGMM (P = 0.9), and Vg (P = 0.7) were similar for treatments B and C (Table 3).
The reduction in p2 from treatment B to C showed no association to the changes in FFA and GH levels during treatment with acipimox.
DISCUSSION
In this group of healthy adults with normal BMI and no family history of diabetes, we explored the effect of a prolonged fast with and without acipimox on glucose metabolism. The central finding is that 24 h of fasting led to a 2.8-fold increase in circulating FFA levels compared with an overnight fast and was accompanied by decreases in the glucose-stimulated AIR and SIMM and therefore a reduced disposition index.
Given the hyperbolic equilibrium between insulin secretion and SI (5, 25), reduced first phase insulin secretion relative to the degree of SI, reflected in a decreased disposition index, is considered to be responsible for the development of impaired glucose tolerance (18). Continuing deteriorations in β-cell function, which ultimately lead to T2D (30), have been shown to involve lipotoxic mechanisms in vitro and manifest as reductions in glucose-stimulated insulin secretion (54), impaired insulin gene expression (20), and β-cell apoptosis (29). These findings have been replicated in animal models (19), but studies in humans have led to conflicting results. After 24- or 48-h lipid/heparin infusions, healthy subjects, unlike those with T2D (6), increased insulin secretion (determined through an IVGTT or by deconvolution of plasma C-peptide levels) to compensate for the concomitant fall in SI (8, 21). In contrast, Carpentier et al. (10) reported that hyperglycemic clamp-derived measures of insulin secretion were increased after an acute (90-min) lipid/heparin infusion while insulin secretion was impaired after a 48-h infusion. Similarly, first phase insulin secretion, as assessed with an IVGTT, was increased after 6 h and decreased after 24 h of infusing lipid/heparin (43). However, in another study of nine healthy women, first phase insulin and C-peptide levels were unchanged after 5.5 h of intravenous lipid/heparin (17). Furthermore, Stoorgard et al. (50) observed no change in insulin secretion during an IVGTT in eight healthy men after 2 and 24 h of lipid/heparin. The increase in circulating FFA levels after 24-h lipid infusions differed up to sevenfold in these studies, which makes comparisons difficult. In addition, the infusions were continued throughout the assessment of insulin secretion, which is rather unphysiological as it counteracts the decrease FFA levels that is normally observed when circulating insulin levels increase in healthy individuals. We achieved substantial elevations in plasma FFA levels in a physiological manner by letting our subjects fast for 24 h, thereby exploiting the effects of decreasing insulin levels and GH-induced increases in the rate of lipolysis during periods of energy restriction (39).
Elevated circulating GH levels have been shown to reduce hepatic and peripheral SI (40, 47), therefore sparing glucose and reducing the need for gluconeogenic precursors from muscle protein during periods of nutritional deprivation (41). Our experimental design did not allow an accurate assessment of hepatic SI, and given the relative hypoglycemia during the 24-h fast, we were unable to use a surrogate index such as the homeostasis model assessment either. However, SIMM, predominantly a measure of peripheral SI, was reduced after the 24-h fast. This was associated with increases in mean GH levels, independent of changes in FFA levels, which may signify a direct effect of elevated GH levels on SI (32, 33, 46). Despite the decline in SIMM, AIR was impaired, which correlated with the rise in FFA levels independent of reductions in pre-IVGTT fasting glucose levels. However, we had no measure of hepatic insulin extraction, which may have changed during the prolonged fast, so that AIR constitutes only a crude proxy for first phase insulin secretion. Furthermore, we did not measure levels of ketones, amino acids, glucagon, cortisol, and catecholamines (39), which all may have contributed to the decreases in AIR and SIMM that led to a reduction in the disposition index and therefore elevated glucose levels during the IVGTT, without changes in insulin MCR and SGMM. An apparent FFA-induced downregulation of AIR despite the development of insulin resistance may reflect an essential mechanism that allows sparing of glucose for glucose-dependant tissues during the first meal after a period of nutritional deprivation (38). The dynamics of the insulin response also appeared to be different, because the 24-h fast induced marked reductions in p3 and p2, suggesting a decreased rate of provision of insulin action, and a delayed onset but prolonged duration of insulin action. This could indicate a slowdown in the insulin signaling cascade due to enhanced activation of the GH receptor, which shares intracellular substrates with the insulin receptor (14, 35, 48). However, only the decrease in p3 correlated with the increase in GH levels during the prolonged fast, and Nielsen et al. (37) recently showed that there appears to be no cross-talk between the GH receptor and insulin signaling pathway in humans.
Previous studies in healthy human subjects without a family history of diabetes, which explored the effects of manipulating FFA levels with the antilipolytic agent nicotinic acid (9) or its longer-acting analog acipimox (2), yielded inconsistent findings with respect to changes in insulin secretion. Hyperglycemic clamp-derived measures of insulin secretion were unchanged after acipimox was administered (45). However, with the use of nicotinic acid and lipid/heparin infusions, two other studies showed that decreases in plasma FFA levels were associated with reductions in basal and glucose-stimulated insulin secretion (7, 13). The four, hourly administrations of 250 mg of acipimox during the 24-h fast of our subjects resulted in a relative reduction in overnight FFA levels. This was associated with an increased disposition index, suggesting partial improvements of AIR and SIMM, but these were not large enough to reach statistical significance. Return of these parameters to values obtained during the overnight fast was not possible because the interval between successive administrations of acipimox was too large and hence FFA levels were still elevated compared with the overnight fast. Furthermore, five subjects exhibited overnight FFA levels that were identical to or higher than those during the 24-h fast with placebo. Sporadic rebound lipolysis during treatment with acipimox over a comparable time period has previously been described (42, 53) and may involve a desensitization to drug action (53) or be due to insufficient plasma levels (52) if metabolized quickly. Acipimox at the given dose exerts maximum efficacy 2 h after ingestion, and plasma concentrations tail off sharply thereafter (34). Corresponding nadirs in circulating FFA levels were seen 2 h after each tablet ingestion in our subjects. These were mirrored by peaks in the GH secretion pattern on top of already highly elevated GH levels, owing to the feedback drive from lowered FFA levels (44), which was potentiated by the long fast (22). The vastly increased GH levels during the 24-h fast with acipimox may have counteracted some of the beneficial effects of reducing FFA levels on AIR and SIMM. Interestingly, p2 was reduced during treatment with acipimox, but this was not related to the rise in GH levels and may therefore, as all of our observations, be due to a direct effect of acipimox.
In conclusion, our results showed that prolonged fasting is accompanied by substantial increases in fasting FFA levels and that it induces reductions in AIR and SIMM and therefore a decrease in the disposition index. The use of acipimox during the prolonged fast led to an incomplete suppression of lipolysis, but the disposition index nevertheless increased with treatment, suggesting a partial reversal of the effects of fasting on AIR and SIMM.
GRANTS
This study was supported by the National Institute for Health Research Cambridge Biomedical Research Centre. At the time of the study, B. Salgin was a MB/PhD student at the School of Clinical Medicine, University of Cambridge, UK, and M. L. Marcovecchio was a Novo Nordisk Research Fellow.
Acknowledgments
We thank the Addenbrooke's Charitable Trust for funding this study. We also thank the study participants and research nurses at the WTCRF.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agbaje OF, Luzio SD, Albarrak AI, Lunn DJ, Owens DR, Hovorka R. Bayesian hierarchical approach to estimate insulin sensitivity by minimal model. Clin Sci (Lond) 105: 551–560, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Aktories K, Schultz G, Jakobs KH. Inhibition of adenylate cyclase and stimulation of a high affinity GTPase by the antilipolytic agents, nicotinic acid, acipimox and various related compounds. Arzneimittelforschung 33: 1525–1527, 1983. [PubMed] [Google Scholar]
- 3.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 51, Suppl 1: S212–S220, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol Endocrinol Metab Gastrointest Physiol 236: E667–E677, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68: 1456–1467, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boden G, Chen X. Effects of fatty acids and ketone bodies on basal insulin secretion in type 2 diabetes. Diabetes 48: 577–583, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Boden G, Chen X, Iqbal N. Acute lowering of plasma fatty acids lowers basal insulin secretion in diabetic and nondiabetic subjects. Diabetes 47: 1609–1612, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44: 1239–1242, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Carlson LA, Oro L. The effect of nicotinic acid on the plasma free fatty acid; demonstration of a metabolic type of sympathicolysis. Acta Med Scand 172: 641–645, 1962. [DOI] [PubMed] [Google Scholar]
- 10.Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol Endocrinol Metab 276: E1055–E1066, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier AC Postprandial fatty acid metabolism in the development of lipotoxicity and type 2 diabetes. Diabetes Metab 34: 97–107, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Diem K, Sedrup J. Geigy Scientific Tables Volume 2. Toms River, NJ: Ciba-Geigy, 1982.
- 13.Dobbins RL, Chester MW, Daniels MB, McGarry JD, Stein DT. Circulating fatty acids are essential for efficient glucose-stimulated insulin secretion after prolonged fasting in humans. Diabetes 47: 1613–1618, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Dominici FP, Argentino DP, Munoz MC, Miquet JG, Sotelo AI, Turyn D. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm IGF Res 15: 324–336, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 90: 493–500, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Ferrannini E, Mari A. Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia 47: 943–956, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Frias JP, Basabe L, Macaraeg G, Kruszynska YT. Lack of effect of a physiological elevation of plasma non-esterified fatty acid levels on insulin secretion. Diabetes Metab 26: 133–139, 2000. [PubMed] [Google Scholar]
- 18.Gerich JE Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 51, Suppl 1: S117–S121, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Goh TT, Mason TM, Gupta N, So A, Lam TK, Lam L, Lewis GF, Mari A, Giacca A. Lipid-induced β-cell dysfunction in vivo in models of progressive β-cell failure. Am J Physiol Endocrinol Metab 292: E549–E560, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Hagman DK, Hays LB, Parazzoli SD, Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem 280: 32413–32418, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen CB, Storgaard H, Holst JJ, Dela F, Madsbad S, Vaag AA. Insulin secretion and cellular glucose metabolism after prolonged low-grade intralipid infusion in young men. J Clin Endocrinol Metab 88: 2775–2783, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest 79: 207–213, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolly MC, Hovorka R, Godsland I, Amin R, Lawrence N, Anyaoku V, Johnston D, Robinson S. Relation between insulin kinetics and insulin sensitivity in pregnancy. Eur J Clin Invest 33: 698–703, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Kahn SE The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46: 3–19, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, and et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42: 1663–1672, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Korytkowski MT, Berga SL, Horwitz MJ. Comparison of the minimal model and the hyperglycemic clamp for measuring insulin sensitivity and acute insulin response to glucose. Metabolism 44: 1121–1125, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Lunn DJ, Best N, Thomas A, Wakefield J, Spiegelhalter D. Bayesian analysis of population PK/PD models: general concepts and software. J Pharmacokinet Pharmacodyn 29: 271–307, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS–a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10: 325–337, 2000. [Google Scholar]
- 29.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 51: 1437–1442, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Marchetti P, Del Prato S, Lupi R, Del Guerra S. The pancreatic beta-cell in human type 2 diabetes. Nutr Metab Cardiovasc Dis 16, Suppl 1: S3–S6, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340: 925–929, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Moller N, Butler PC, Antsiferov MA, Alberti KG. Effects of growth hormone on insulin sensitivity and forearm metabolism in normal man. Diabetologia 32: 105–110, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Moller N, Jorgensen JO, Schmitz O, Moller J, Christiansen J, Alberti KG, Orskov H. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am J Physiol Endocrinol Metab 258: E86–E91, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Musatti L, Maggi E, Moro E, Valzelli G, Tamassia V. Bioavailability and pharmacokinetics in man of acipimox, a new antilipolytic and hypolipemic agent. J Int Med Res 9: 381–386, 1981. [DOI] [PubMed] [Google Scholar]
- 35.Napoli R, Cittadini A, Chow JC, Hirshman MF, Smith RJ, Douglas PS, Horton ES. Chronic growth hormone treatment in normal rats reduces post-prandial skeletal muscle plasma membrane GLUT1 content, but not glucose transport or GLUT4 expression and localization. Biochem J 315: 959–963, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newsholme P, Keane D, Welters HJ, Morgan NG. Life and death decisions of the pancreatic beta-cell: the role of fatty acids. Clin Sci (Lond) 112: 27–42, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen C, Gormsen LC, Jessen N, Pedersen SB, Moller N, Lund S, Jorgensen JO. Growth hormone signaling in vivo in human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J Clin Endocrinol Metab 93: 2842–2850, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Nolan CJ, Prentki M. The islet beta-cell: fuel responsive and vulnerable. Trends Endocrinol Metab 19: 285–291, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Norrelund H The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm IGF Res 15: 95–122, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Norrelund H, Djurhuus C, Jorgensen JO, Nielsen S, Nair KS, Schmitz O, Christiansen JS, Moller N. Effects of GH on urea, glucose and lipid metabolism, and insulin sensitivity during fasting in GH-deficient patients. Am J Physiol Endocrinol Metab 285: E737–E743, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Norrelund H, Moller N, Nair KS, Christiansen JS, Jorgensen JO. Continuation of growth hormone (GH) substitution during fasting in GH-deficient patients decreases urea excretion and conserves protein synthesis. J Clin Endocrinol Metab 86: 3120–3129, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Norrelund H, Nielsen S, Christiansen JS, Jorgensen JO, Moller N. Modulation of basal glucose metabolism and insulin sensitivity by growth hormone and free fatty acids during short-term fasting. Eur J Endocrinol 150: 779–787, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Paolisso G, Gambardella A, Amato L, Tortoriello R, D'Amore A, Varricchio M, D'Onofrio F. Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia 38: 1295–1299, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Quabbe HJ, Bratzke HJ, Siegers U, Elban K. Studies on the relationship between plasma free fatty acids and growth hormone secretion in man. J Clin Invest 51: 2388–2398, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qvigstad E, Mostad IL, Bjerve KS, Grill VE. Acute lowering of circulating fatty acids improves insulin secretion in a subset of type 2 diabetes subjects. Am J Physiol Endocrinol Metab 284: E129–E137, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Rabinowitz D, Klassen GA, Zierler KL. Effect of human growth hormone on muscle and adipose tissue metabolism in the forearm of man. J Clin Invest 44: 51–61, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizza RA, Mandarino LJ, Gerich JE. Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes 31: 663–669, 1982. [DOI] [PubMed] [Google Scholar]
- 48.Smith TR, Elmendorf JS, David TS, Turinsky J. Growth hormone-induced insulin resistance: role of the insulin receptor, IRS-1, GLUT-1, and GLUT-4. Am J Physiol Endocrinol Metab 272: E1071–E1079, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Spiegelhalter D, Thomas A, Best N, Lunn D. WinBUGS User Manual. Cambridge, UK: Medical Research Council Biostatistics Unit, 2003.
- 50.Storgaard H, Jensen CB, Vaag AA, Volund A, Madsbad S. Insulin secretion after short- and long-term low-grade free fatty acid infusion in men with increased risk of developing type 2 diabetes. Metabolism 52: 885–894, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Unger RH Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 44: 863–870, 1995. [DOI] [PubMed] [Google Scholar]
- 52.Vaag AA, Beck-Nielsen H. Effects of prolonged acipimox treatment on glucose and lipid metabolism and on in vivo insulin sensitivity in patients with non-insulin dependent diabetes mellitus. Acta Endocrinol (Copenh) 127: 344–350, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Worm D, Henriksen JE, Vaag A, Thye-Ronn P, Melander A, Beck-Nielsen H. Pronounced blood glucose-lowering effect of the antilipolytic drug acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. J Clin Endocrinol Metab 78: 717–721, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab 80: 1584–1590, 1995. [DOI] [PubMed] [Google Scholar]