Abstract
The steroid hormone 20-hydroxyecdysone (20HE) is an essential signaling molecule that modulates molting response in insects and may function as a putative anabolic factor in vertebrate animals, although no mammalian 20HE receptor has been identified. Here we show that in H4IIE cell culture, 20HE treatment decreased expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), reduced glucose production, and induced Akt2 phosphorylation sensitive to the phosphoinositide-3 kinase pathway-specific inhibitor LY-294002. Daily oral administration of 20HE (10 mg/kg for 13 wk) ameliorated obesity and insulin resistance in C57BL/6J mice fed a high-fat diet and produced a significant decrease of body weight gain and body fat mass compared with nontreated animals as demonstrated by dual-energy X-ray absorptiometry analysis. In addition, plasma insulin levels and glucose tolerance were significantly lowered by 20HE treatment. These changes were accompanied by the reduced hepatic expression of PEPCK and G6Pase and increased adiponectin production by visceral fat tissue. These studies demonstrate the anti-obesity and anti-diabetic effects of 20HE and begin to elucidate its putative cellular targets both in vitro and in vivo.
Keywords: gluconeogenic enzymes, adiponectin, obesity and diabetes
ecdysteroids are polyhydroxylated steroids present in plants and invertebrates. So far, close to 120 ecdysteroids have been structurally characterized (18). The chemical structure of ecdysteroids is based on the C-27 cholesterol skeleton; however, they differ from the vertebrate steroids in their polarity and bulkiness. One of the most common and abundant ecdysteroids, 20-hydroxyecdysone (20HE), is found in many plants including widely cultivated species like Spinacia oleracea (spinach) (29). In insects, ecdysteroids are predominantly involved in the regulation of molting and metamorphosis, whereas in plants they may contribute to the deterrence of invertebrate predators (9).
Little information is available on the physiological roles of ecdysteroids in vertebrate animals. In addition to their anabolic effects (7, 8), ecdysteroids have been reported to have immunomodulatory (7), hepatoprotective (30), anti-arrythmic (21), and cholesterol-lowering properties (26). There is also some supporting evidence showing that 20HE affects glucose metabolism in vivo. Pretreatment with 20HE reduced hyperglycemia associated with administration of either glucagon or alloxan (25). Moreover, ecdysteroid-rich extract from Ajuga turkestanica administrated orally (5 mg/kg) was more effective in reducing hyperglycemia in an alloxan-induced diabetes model in rats than the reference drug manilil without causing a hypoglycemic effect in normal animals (22).
An important aspect of the ecdysteroids is their low toxicity in mammals. The median lethal dose (LD50) of 20HE in rodents is 6.4 g/kg body wt (for intraperitoneal injection), and it is >9 g/kg body wt when given orally (25).
Type 2 non-insulin-dependent diabetes mellitus is caused by a combination of genetic and environmental factors, notably diet and physical inactivity (34). Among environmental factors, the high-fat diet and sedentary lifestyle common to the Western world is considered a major cause of obesity-associated insulin resistance and impaired glucose tolerance (34). The frequency of type 2 diabetes mellitus is estimated to reach 300 million individuals by the year 2025 (40), with obesity being the leading cause. There is increasing evidence that adipose-derived cytokines and hormones are relevant for impaired insulin action in liver, fat, skeletal muscle, and pancreatic cells (28). The insulin, leptin, and adiponectin signaling pathways are known to share certain downstream molecules like phosphatidylinositol 3-kinase (PI3K), protein kinase B (PKB), mitogen-activated protein kinase (MAPK), and AMP-activated protein kinase (AMPK) (23). The present study was designed to investigate the effect of 20HE on glucose metabolism in H4IIE hepatoma cell line and to evaluate the relative efficacy and activity of chronic 20HE treatment in the murine model of diet-induced obesity. Effects on body weight, adipose mass, glucose homeostasis, insulin sensitivity, adiponectin production, and activity of the PI3K and AMPK signaling pathways were examined.
MATERIALS AND METHODS
Chemicals.
Ecdysterone was purchased from Bosche Scientific (New Brunswick, NJ). Dexamethasone, 8-(4-chlorophenylthio)-cAMP (cAMP), sodium lactate, and sodium pyruvate were purchased from Sigma Chemicals (St. Louis, MO). Human insulin (Humulin) was purchased from Eli Lilly (Indianapolis, IN) and compound C from EMD Biosciences (San Diego, CA); phospho-Akt2 and Akt2 rabbit mAbs were purchased from Cell Signaling Technology (Danvers, MA). All other chemicals, including cell culture media, were obtained from Invitrogen (Carlsbad, CA). Reagents and enzymes used for RT-PCR were obtained from Stratagene (La Jolla, CA) and Applied Biosystems (Foster City, CA). The H4IIE cell line (CRL-1548) was obtained from American Type Culture Collection (Manassas, VA).
Cell culture and treatment.
The H4IIE hepatoma cells were cultured in 24-well tissue culture plates (Greiner Bio One, Monroe, NC) and grown to near confluence in Dulbecco's modified Eagle's medium containing 2.5% (vol/vol) newborn calf serum and 2.5% (vol/vol) fetal calf serum. Cells were treated for 8 h with 500 nM dexamethasone and 0.1 mM 8-CTP-cAMP (Dex-cAMP) to induce phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) gene expression together with different concentrations of 20HE, or 10 nM insulin. Three wells were allocated for each treatment, including the negative control (untreated cells). For inhibitory assays, cells were pretreated for 30 min with either 20 μM LY-294002 or 40 μM compound C as specified, washed with phosphate-buffered saline, and incubated for an additional 7 h with Dex-cAMP together with different concentrations of 20HE, or 10 nM insulin.
Glucose production assay.
H4IIE rat hepatoma cells were serum starved overnight in the glucose production buffer (glucose-free Dulbecco's modified essential medium, pH 7.4, containing 20 mM sodium lactate and 2 mM sodium pyruvate without phenol red) and treated for 8 h with Dex-cAMP in the presence or absence of 10 nM insulin or different concentrations of 20HE for 8 h. At the end of the incubation, 0.5 ml of medium was taken to measure the glucose concentration in the culture medium using the Amplex Red glucose assay kit (Invitrogen). Corrections for cell number were made on the basis of the protein concentration measured using the BCA Protein assay kit (Pierce Biotechnology, Rockford, IL).
Total RNA extraction, purification, and cDNA synthesis.
Total RNA was extracted from H4IIE cells or liquid nitrogen-preserved murine tissues using Trizol reagent (Invitrogen) following the manufacturer's instructions. RNA was quantified spectrophotometrically by absorbance measurements at 260 and 280 nm using the NanoDrop system (NanoDrop Technologies, Wilmington, DE). Quality of RNA was assessed by separation in gel electrophoresis. To remove any traces of DNA contamination, RNA was then treated with DNase I (Invitrogen) following the manufacturer's guidelines. The cDNAs were synthesized using 2.5 μg of RNA for each sample, using Stratascript Reverse Transcriptase (Stratagene) following the manufacturer's protocol.
Quantitative PCR analysis of H4IIE rat hepatoma cells.
The synthesized cDNAs were diluted fourfold. Five microliters of each diluted sample were used for a PCR reaction of 25 μl final volume containing 0.5 μl of 6 μM gene-specific primers (IDT, Coralville, IA) and 12.5 μl of Brilliant SYBR green PCR master mix (Stratagene). ROX (Stratagene) was used as a reference dye. The primers were selected using the Primer Express version 2.0 software (Applied Biosystem) as follows: β-actin (NM_031144), forward primer 5′-GGG AAA TCG TGC GTG ACA TT-3′, reverse primer 5′-GCG GCA GTG GCC ATC TC-3′; PEPCK (NM_198780), forward primer 5′-GCA GAG CAT AAG GGC AAG GT-3′, reverse primer 5′-TTG CCG AAG TTG TAG CCA AA-3′; G6Pase (NM_013098.2), forward primer 5′-TCT ACC TTG CGG CTC ACT TTC-3′, reverse primer 5′-GAA AGT TTC AGC CAC AGC AAT G-3′; sterol regulatory element-binding protein-1 (SREBP-1) (NM_011480), forward primer 5′-GAT GTG CGA ACT GGA CAC AG-3′, reverse primer 5′-CAT AGG GGG CGT CAA ACA G-3′. qPCR amplifications were performed on the MX3000p system (Stratagene) using 1 cycle at 50°C for 2 min, 1 cycle of 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. The dissociation curve was completed with one cycle of 1 min at 95°C, 30 s of 55°C, and 30 s of 95°C. NRT (no RT) and NTC (no template control) were included in each experiment as quality control steps. Target mRNA expression was analyzed using the ΔΔCT method and normalized with respect to the expression of the β-actin housekeeping gene (32). The Dex-cAMP treatment (positive control) served as the calibrator sample in this study, and the target gene expression of the calibrator sample was assigned to a value of 1.0. All samples were run in triplicate. A P value of 0.05 was considered to be significant.
qPCR analysis of murine tissues.
The qRT-PCR amplifications were carried out in triplicate on an ABI 7300 Real-Time Detection System in a total volume of 20 μl containing 10 μl of SYBR Green 2× Supermix (Applied Biosystems), 5 μl of the 1:50 diluted cDNA, 1 μl of each specific primer, and 3 μl of PCR-grade water. The qRT-PCR program was 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 60 s. The corresponding primers were selected using the Primer Express version 2.0 software as follows: β-actin (NM_007393.3), forward primer: 5′-AAC CGT GAA AAG ATG ACC CAG AT-3′, reverse primer: 5′-CAC AGC CTG GAT GGC TAC GT-3′; PEPCK (NM_011044.2), forward primer 5′-CAG GAT CGA AAG CAA GAC AGT-3′, reverse primer 5′-AAG TCC TCT TCC GAC ATC CAG-3′; G6Pase (NM_008061.3), forward primer 5′-GAA AAA GCC AAC GTA TGG ATT CC-3′, reverse primer 5′-CAG CAA GGT AGA TCC GGG A-3′; Adiponectin (NM_009605), forward primer 5′-AGC CGC TTA TAT GTA TCG CTC A-3′, reverse primer 5′-TGC CGT CAT AAT GAT TCT GTT GG-3′. qRT-PCR data from three replicate samples were analyzed with a 7300 System SDS Software v1.3.0 (Applied Biosystems) to estimate transcript copy numbers for each sample. Target mRNA expression was analyzed using the ΔΔCT method and normalized with respect to the expression of the β-actin housekeeping gene (16). The high-fat diet (HFD) control group served as the calibrator sample in this study, and the target gene expression of the calibrator sample was assigned a value of 1.0. All samples were run in triplicate. The data obtained from qRT-PCR analysis were subjected to Student's t-test. A P value of 0.05 was considered to be significant.
AMPKα1 and -α2 activity assay.
AMPK activity was assayed as previously described (12). Briefly, AMPK was immunoprecipitated from 200 μg of H4IIE cell lysate using anti-AMPKα1 (Upstate Biotechnology, Lake Placid, NY) or -α2 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies in 500 μl of buffer A (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 50 mM NaF, 5 mM sodium pyrophosphate, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aproptin) at 4°C for 2 h. Immunocomplexes were washed with buffer A three times, buffer B containing 0.5 M NaCl and 62.5 mM NaF one time, and then the reaction buffer (50 mM HEPES, pH 7.4, 1 mM DTT) three times. AMPK activity of immunocomplexes was determined by phosphorylation of SAMS peptide in the reaction buffer containing 0.25 mM SAMS, 5 mM MgCl2, and 10 μCi of [r-32P]ATP for 10 min at 30°C with or without 200 μM AMP stimulation. The reaction was terminated by spotting reaction mixtures onto P81 filter paper and rinsing in 1% (vol/vol) phosphoric acid with gentle stirring to remove free ATP. The phosphorylated substrate was measured by scintillation counting.
Western blot analysis.
H4IIE cells were cultured as described above, and whole cell extracts were prepared in ice-cold lysis buffer [62.5 mM Tris·HCl (pH 6.8), 2% wt/vol SDS, 10% glycerol, 50 mM DTT, 0.01% wt/vol bromophenol blue] and centrifuged at 12,000 g for 20 min at 4°C. Equal amounts of protein (50 μg) from the supernatants were separated on SDS 10% polyacrylamide gels and blotted onto the nitrocellulose membrane. Western blot analysis was performed with monoclonal phospho-Akt (Ser473) antibodies according to the manufacturer's instructions (Cell Signaling Technology, Danvers, MA). After being washed, the blots were incubated with an anti-rabbit peroxidase-labeled secondary antibody and visualized using ECL Western Blotting Detection Reagent (GE Healthcare, Piscataway, NJ). After being stripped, the blots were probed with Akt2 (5B5) antibodies to visualize the total Akt (loading control).
Animal experiments.
All animal experiments were performed according to procedures approved by the Rutgers Institutional Animal Care and Use Committee. Six-week-old male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and maintained on either a low-fat diet (LFD; n = 10) containing 10% fat-derived calories (D12450B; Research Diets, New Brunswick, NJ) or an HFD (n = 10) containing 60% fat-derived calories (D12492, Research Diets) with 12-h light and dark cycles.
The HFD animals were further randomized into two groups. The control group (n = 10) was gavaged daily with a vehicle solution alone (10% DMSO in corn oil), and a treatment group (n = 10) was gavaged with 10 mg/kg body wt 20HE for 13 wk. To monitor gain and loss of body weight, the animals were weighed weekly for the duration of the experiment. The mice intrarectal temperature was measured weekly using a thermometer containing a probe (Oakton Instruments, Vernon Hills, IL). Plasma glucose concentrations were measured at weeks 4, 9, 10, 11, and 12 in submandibular vein blood samples using a glucometer (Lifescan, Johnson and Johnson, NJ). Plasma concentrations of insulin and adiponectin were determined at week 13 by rat/mouse insulin ELISA kit (Millipore, Billerica, MA) and adiponectin ELISA kit (Otsuka Phamaceuticals, Toyko, Japan), respectively.
To perform the glucose tolerance test at week 13 of the experiment, both LFD and HFD mice were fasted overnight (16 h) and injected intraperitoneally with 1.5 g/kg glucose solution. Plasma glucose levels were measured immediately before and 30, 60, and 120 min after the glucose challenge. At the end of the study, mice were killed and equal amounts of liver and visceral fat were removed. The fat mass and lean tissue were determined using dual-energy X-ray absorptiometry (DEXA) analysis on PIXImus equipment (Lunar, Madison, WI) as described elsewhere (27). The percentage of fat tissue was calculated as follows: %body fat = (fat mass/total body wt) × 100, where total body weight was the sum of lean mass and fat mass for each animal. The ratio between adipose mass and lean mass for each animal was calculated by dividing fat mass by lean mass.
RESULTS
20HE modulates glucose metabolism in rat hepatoma cells.
The production of glucose in response to 20HE was tested in H4IIE rat hepatoma cells incubated in medium containing pyruvate and lactate as substrates for gluconeogenesis.
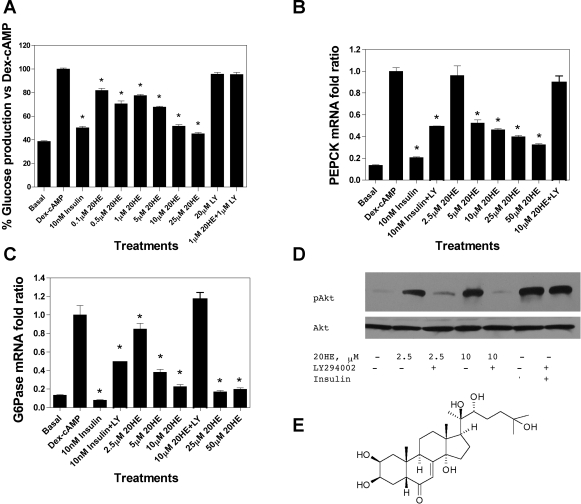
The cells were serum starved for 16 h and incubated with different concentrations of 20HE for 8 h with or without insulin. Absolute glucose production numbers were normalized to the total cell protein and transformed to represent relative glucose production units as percentage of Dex-cAMP-stimulated glucose production value (set as 100% for each experiment). 20HE decreased the glucose production in a dose-dependent manner with 20HE at 10 μM showing similar inhibition as insulin at physiological concentration (10 nM). The inhibitory effect of 20HE over glucose production was mediated by the activation of PI3K and could be reversed by specific PI3K inhibitor LY-294002 (Fig. 1A). A quantitative analysis of the mRNA expression patterns of PEPCK and G6Pase in Dex-cAMP-stimulated and 20HE-treated H4IIE cells was performed to determine whether the effect of 20HE on glucose production is related to its effect on PEPCK or G6Pase, two of the rate-limiting enzymes regulated by insulin in the hepatic gluconeogenic pathway. 20HE significantly decreased PEPCK and G6Pase gene expression in Dex-cAMP-induced cells in a dose-dependent manner (Fig. 1, B and C). Furthermore, the inhibitory effect of 20HE on the expression of both gluconeogenic enzymes was mediated by the activation of PI3K and could be reversed by specific PI3K inhibitor LY-294002 (Fig. 1, B and C). Untreated cells were used to measure the basal level of PEPCK and G6Pase expression, while the β-actin gene was chosen as an internal standard since the level of β-actin mRNA remained unaffected by the treatments. Moreover, 20HE at concentrations of 2.5 and 10 μM induced Akt2 phosphorylation within the carboxy terminus at Ser473. This effect was reversed by the PI3K inhibitor LY-294002 (Fig. 1D). Compound C, an ATP-competitive inhibitor of AMPK (26), did not reverse the effects of 20HE on Dex-cAMP-stimulated PEPCK gene expression in H4IIE cells (data not shown).
Fig. 1.
Effect of 20-hydroxyecdysone (20HE) treatment on glucose metabolism in H4IIE cells. A: 20HE lowers glucose production in rat hepatoma cells. The cells were treated with dexamethasone and 8-CTP-cAMP (Dex-cAMP) (except for negative control) in the presence of different concentrations of 20HE or 10 nM insulin for 8 h. The results are presented as percentages relative to the glucose produced by Dex-cAMP-treated H4IIE cells. B: dose-dependent inhibition of Dex-cAMP stimulated expression of phosphoenolpyruvate carboxykinase (PEPCK) (B) and glucose-6-phosphatase (G6Pase) (C) gluconeogenic enzymes. PEPCK and G6Pase mRNAs were normalized to β-actin mRNA, and the specific fold changes were calculated as a ratio of the target mRNA level relative to the response to Dex-cAMP activation. Data represent the mean of 3 experiments ± SE. D: 20HE stimulates phosphatidylinositol 3-kinase (PI3K)-dependent Akt2 activation in H4IIE rat hepatoma cells. Cells were pretreated with either 20 μM LY-294002 (LY) or vehicle for 30 min before treatment with either 2.5 and 10 μM 20HE or 10 nM insulin as a positive control. The cell lysates were prepared and used for Western blot analysis with phospho-Akt2 and total Akt antibodies. *P < 0.05. Student's t-test comparison for all treatments vs. Dex-cAMP controls. E: chemical structure of 20HE.
20HE activates AMPKα1 in rat hepatoma cells.
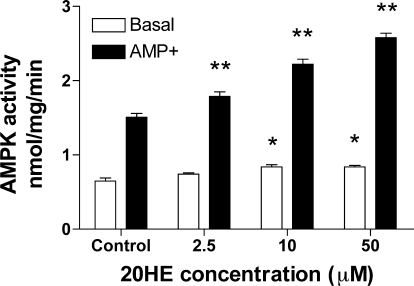
Liver AMPK is another PI3K-independent signaling pathway that decreases expression of glycolytic and lipogenic genes, as well as genes involved in hepatic glucose production (37). The effects of 20HE on basal and AMP-stimulated activity of AMPKα1 catalytic subunit in H4IIE rat hepatoma cells are shown in Fig. 2. AMP levels were significantly higher in cells treated with different concentrations of 20HE compared with control levels. Indeed, 20HE at 2.5, 10, and 50 μM increased AMPKα1 activation by 4, 14, and 32%, respectively, over the control values. On the other hand, AMPKα2 catalytic subunit activity was not affected by the 20HE treatment (data not shown).
Fig. 2.
Effect of different concentrations of 20HE on basal and AMP-stimulated activity of the AMP-activated protein kinase (AMPK)α1 catalytic subunit in H4IIE rat hepatoma cells. Cells were incubated with 0.1% DMSO (vehicle) and 2.5, 10, and 50 μM 20HE. The percentage of increase of AMPKα1 activation was 4, 14, and 32% for 2.5, 10, and 50 μM 20HE, respectively. Data represent the mean of 3 experiments ± SE. *P < 0.05, **P < 0.01. Student's t-test comparison for 20HE treatments vs. control.
Because AMPKα2 is the main AMPK catalytic subunit expressed in liver and muscle, it is likely that a moderate AMPK-mediated effect of 20HE on glucose metabolism is secondary to activation of the PI3K signaling pathway. Therefore, we focused on the PI3K-related mechanism on 20HE action in the subsequent animal studies.
20HE-treated mice resist diet-induced obesity.
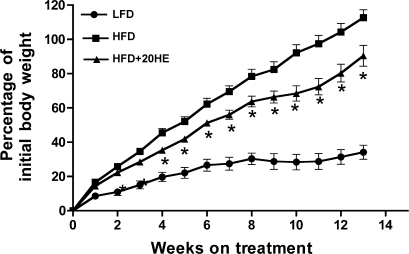
Obesity was induced by feeding mice for 13 wk with the HFD. The final body weights were 41.7 ± 1.66 vs. 26.7 ± 0.74 g for HFD and LFD animals, respectively. In the HFD animals receiving a daily oral gavage of 10 mg/kg 20HE, final body weights were markedly decreased (36.5 ± 1.56 g, P < 0.05 compared with HFD animals) (Fig. 3). Body weight gain relative to the initial body weight in mice treated with 20HE was 18% less compared with the HFD control (15.4 ± 0.5 vs. 18.8 ± 0.57 g, P < 0.05), while the body weight gain of the LFD mice remained low (5.18 ± 0.25 g). Reduction in body weight and body weight gain in the 20HE-treated group could not be attributed to changes in animal feeding habits, since food consumption rates remained unchanged throughout the treatment (2.2 ± 0.02 g/day for all groups). DEXA analysis (4) indicated that adipose mass in the 20HE-treated mice was decreased by 41% when compared with the HFD animals (13.1 ± 0.67 vs. 22.3 ± 1.0 g, P < 0.05), while lean mass was decreased by only 5% (21.3 ± 0.09 vs. 22.3 ± 0.72 g). The ratio between adipose mass and lean mass of the 20HE-treated animals was significantly reduced relative to that of the HFD animals (0.62 ± 0.05 vs. 1.0 ± 0.05, P < 0.05, respectively). For the LFD-fed animals, this ratio was 0.25 ± 0.07. Thus the augmentation in body weight gain in the 20HE-treated mice was predominantly due to decreased adipose mass and enhanced resistance to diet-induced obesity. There was no detectable change in body temperature in either the HFD or the HFD+20HE animals when measured at the end of the experiment.
Fig. 3.
Effect of 20HE on the relative body weight gain of C57/6J black mice. Six-week-old male mice were fed a low-fat diet (LFD), high-fat diet (HFD), or HFD combined with daily gavage with 10 mg/kg 20HE (HFD+20HE) for 13 wk. *P < 0.05, significantly different from HFD-fed vehicle-treated animal values. One-way ANOVA, Dunnett's post hoc test. This study is representative of 2 independent experiments.
Adiponectin expression and production are enhanced by 20HE treatment.
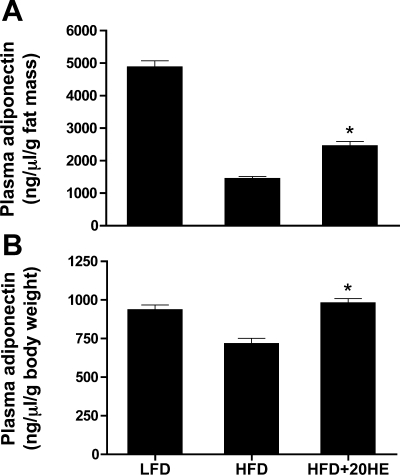
Adiponectin is involved in glucose and lipid metabolism, increases fatty acid oxidation in the muscle, and potentiates insulin inhibition of hepatic gluconeogenesis (36), and the circulating levels of adiponectin are reduced in obese subjects (33). Adiponectin mRNA was quantified in the adipose tissue of the LFD, HFD, and HFD-fed 20HE-treated animals. Thirteen weeks of daily oral treatment of HFD-fed C57BL/6J mice with 10 mg/kg 20HE resulted in a 7.9-fold increase in visceral fat adiponectin expression compared with the control animals fed an HFD with a fold ratio equal to 1. The LFD-fed animals displayed a 5.2-fold increase in adiponectin expression in visceral fat compared with the HFD-fed animals. Plasma adiponectin levels measured at the end of the study (week 13) did not change among the tested groups (data not shown); however, when plasma adiponectin levels were adjusted using either fat mass weight or body weight, a significant increase was found in the mice treated with 20HE (Fig. 4, A and B, and Table 1).
Fig. 4.
20HE increases relative circulating adiponectin levels in plasma. Circulating adiponectin levels were measured by ELISA and normalized to fat mass (A) or body weight (B) of the animals. Values are means ± SE. *P < 0.05. Student's t-test comparison for 20HE treatment vs. HFD control. This study is representative of 2 independent experiments.
Table 1.
Effect of daily oral treatment with 10 mg/kg 20HE on C57BL/6J mice after 13 wk on plasma insulin and adiponectin levels
| Group | Insulin, ng/ml | Plasma Adiponectin/Body Fat | Plasma Adiponectin/Body Weight |
|---|---|---|---|
| LFD | 0.63±0.03 | 934.3±33 | 4,872±200 |
| HFD | 1.74±0.14 | 715.6±36 | 1,441±73 |
| HFD+20HE | 0.38±0.03* | 978.3±30.2* | 2,500±16* |
Data represent the mean of 2 different experiments ± SE. Insulin concentration is expressed as ng/ml, and adiponectin concentrations are ng·μl−1·g body fat−1 and ng·μl−1·g body wt−1. LFD, low-fat diet; HFD, high-fat diet; 20HE, 20-hydroxyecdysone.
P < 0.05, Student's t-test comparison for 20HE treatments vs. HFD control.
Effect of 20HE on glucose metabolism and plasma insulin.
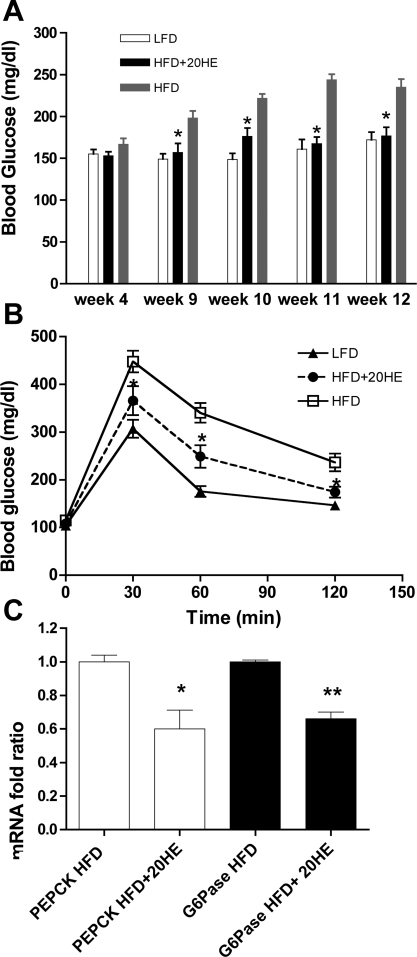
The baseline blood glucose levels did not differ in all groups 4 wk after the start of treatment (Fig. 5A). Starting on week 9, the blood glucose of the HFD group increased steadily while the 20HE-treated group maintained a constant level of blood glucose, similar to that of the LFD group. Table 1 shows that plasma insulin levels measured at the end of the study were significantly higher in the HFD animals compared with the LFD group. 20HE treatment resulted in a 4.5-fold decrease in plasma insulin compared with the HFD group and a 1.7-fold decrease compared with the basal insulin levels observed in the LFD animals. An intraperitoneal glucose tolerance test was performed in week 13 of the experiment. The HFD group plasma glucose levels were significantly higher at 30, 60, and 120 min after oral gavage compared with the LFD group, while the 20HE treatment significantly reversed this effect at 30 and 60 min (Fig. 5B). Expression of PEPCK and G6Pase gluconeogenic enzymes was determined in the liver tissue at the end of the study. In animals treated with 20HE, the RNA levels were significantly reduced for PEPCK and G6Pase, respectively (Fig. 5C).
Fig. 5.
Fasting plasma glucose, intraperitoneal glucose tolerance test (IGTT), and hepatic expression of gluconeogenic enzymes following the 20HE treatment. A: fasting blood glucose levels in animals fed with LFD (n = 10) or HFD (n = 10), or HFD animals treated with 10 mg/kg 20HE (n = 10) at weeks 4, 9, 10, 11, and 12. B: IGTT curves of groups fed either LFD or HFD, or HFD animals receiving the 20HE treatment. The study is representative of 2 independent experiments. C: PEPCK and G6Pase expression in liver tissue of HFD treated with 20HE or control HFD animals. Animals were fasted for 16 h. PEPCK and G6Pase mRNA were normalized to β-actin mRNA, and the data represent the average of 2 independent experiments. Values are means ± SE. *P < 0.05, **P < 0.01. One-way ANOVA followed by Dunnett's post hoc test.
To determine whether 20HE affects hepatic lipogenesis, we analyzed the pattern of expression of SREBP-1 in liver tissue; however, we did not notice any significant change in SREBP-1 mRNA expression in animals fed the HFD when compared with animals fed HFD and treated with 20HE (data not shown).
DISCUSSION
Earlier data suggest that 20HE has hypoglycemic effects both in vitro and in vivo (6, 38); however, no mechanism explaining this pharmacological activity had been proposed. Present studies have begun to reveal the anti-diabetic mode of action of 20HE in H4IIE rat hepatoma cell line and in a murine model of diet-induced obesity. Our study confirms that 20HE reduces glucose production in the hepatic cell culture (Fig. 1A) and suggests that this effect is regulated predominantly through PI3K-dependent signaling pathways. 20HE downregulated expression of PEPCK and G6Pase genes in a dose-dependent manner (Fig. 1, B and C). PEPCK is a key enzyme modulating hepatic gluconeogenesis, and its activity is closely correlated with hepatic glucose output (13), while G6Pase catalyzes the last step of hepatic glucose production by hydrolyzing glucose-6-phosphate into glucose (35). LY-294002 is a specific inhibitor of the PI3K pathway (31) known to decrease insulin-induced Akt2 phosphorylation on Ser473 and subsequently abolish the insulin inhibition of Dex-cAMP-induced PEPCK gene transcription in hepatoma cells (1). The inhibitory effect of 20HE on glucose production and expression of gluconeogenic enzymes was abolished in the presence of this specific PI3K inhibitor (Fig. 1, A–C), suggesting that 20HE suppresses glucose output by affecting the upstream components of the PI3K-dependent insulin signaling pathway. Indeed, LY-294002 significantly inhibits Akt2 phosphorylation induced by 20HE or insulin (Fig. 1D). The dose response of 20HE for glucose secretion appears biphasic with a significant effect at 0.1 μM (Fig. 1A), while no effect on PEPCK or G6Pase gene expression is observed at 2.5 μM (Fig. 1, B and C). It is therefore possible that at lower concentrations, there is an unknown non-PEPCK/G6Pase-mediated effect of 20HE on glucose output that remains LY-294002 sensitive.
The biguanidine drugs of plant origin, such as metformin, exert a PI3K-independent downregulation of basal PEPCK gene expression in hepatocytes (39) through an AMPK-dependent mechanism. This pathway leads to the insulin-independent suppression of hepatic gluconeogenesis by phosphorylation and cytoplasmic sequestration of the mammalian target of rapamycin complex-2 transcriptional coactivator (17). 20HE was capable of increasing basal activity of ubiquitously expressed AMPKα1 catalytic subunit, but had no effect on liver-specific AMPKα2 catalytic subunit (Fig. 2). Compound C, an ATP-competitive inhibitor of AMPK (26), did not reverse the effects of 20HE on Dex-cAMP-stimulated PEPCK gene expression in H4IIE cells (data not shown). Taken together, these data suggest that PI3K-independent activation of AMPKα1 by 20HE plays a minor role in regulation of the gluconeogenic enzymes and liver glucose production.
To test the effect of 20HE in the in vivo diabetes model, male C57BL/6J mice were fed an HFD (60% energy by fat) and treated daily by oral administration of 10 mg/kg 20HE for 13 wk. An HFD group treated with the vehicle solution and a LFD group (10% energy by fat) were used as controls. At the end of the treatment, the body weight of the HFD-20HE group was significantly lower than that of the HFD group (Fig. 3). Significant differences were also observed for body weight gain and adipose mass as estimated by DEXA; however, no differences in animal feeding behavior were noted in any of the groups. Weight differences were accompanied by lower blood glucose and plasma insulin as well as improved glucose tolerance (Fig. 5). It has been previously reported that ecdysone is rapidly eliminated from plasma within 30 min of administration (10). In the hepatic subcellular fractions, [3H]ecdysone was detected in large quantities in cytoplasm and microsomes and to a lesser degree in mitochondria and nuclei of hepatic cells (10). Yet another report showed that plant extract enriched with ecdysone had a moderate blood glucose lowering effect in healthy animals (11). Taken together with the lack of changes in food consumption or thermogenesis following the 20HE treatment, we propose that the sustained decrease in body weight of the animals results from modulation of glycemic control and regulation of adipose tissue development by 20HE. Compounds similar to 20HE counteract insulin-dependent growth in Drosophila (8); however, we did not observe any effect of 20HE administration on animal growth or body length. It is also know that ecdysone coordinates optic lobe neurogenesis in Drosophila via a nitric oxide signaling pathway; however, nitric oxide-mediated effects in animals have not been confirmed for 20HE (14, 19, 20).
To understand the anti-obesity effects of 20HE, we focused on adiponectin, an adipose tissue-specific secretory plasma protein with well-established effects in the regulation of insulin resistance, energy homeostasis, and obesity (3, 5, 24). Plasma concentrations of adiponectin are reduced in obese, insulin-resistant, and type 2 diabetic humans and rodents (15, 16, 33). In the present study, reduced adiponectin expression observed in HFD animals was completely reversed by 20HE treatment. Although the treatment did not lead to a marked increase in absolute plasma adiponectin levels, the relative circulating adiponectin increased significantly when adjusted to fat mass or body weight (Fig. 4, A and B). There is an inverse correlation between plasma adiponectin levels and body weight resulting from downregulation of the expression of adiponectin within adipose tissue as fat mass increases (2). Reduced adiponectin mRNA expression observed in the HFD animals compared with the LFD controls was completely reversed by 20HE treatment. 20HE significantly increased circulating adiponectin levels relative to fat mass or body weight of the treated animals (Fig. 4, A and B). Because adiponectin increases energy expenditure and fatty acid oxidation in liver and skeletal muscles (36), one could postulate that relative adiponectin levels elevated in 20HE-treated mice might confer resistance to diet-induced obesity and insulin resistance in these animals. Additionally, circulating adiponectin is known to suppress the expression of PEPCK and G6Pase genes by the activation of the AMPK signaling pathway (36).
In conclusion, our results shed new light on the hypoglycemic effect of 20HE in vitro and in vivo and indicate that this effect may be exerted through the PI3K-dependent regulation of gluconeogenic enzyme activity. Even more important, the data suggest that daily administration of 20HE can prevent obesity, insulin resistance, and associated hyperglycemia in animals by decreasing adipose depots, upregulating adiponectin expression in the adipose tissue, and increasing circulating adiponectin levels adjusted to body weight or fat mass.
GRANTS
Research was supported by the Fogarty International Center of the National Institutes of Health (NIH) under the U01-TW-006674 grant for the International Cooperative Biodiversity Groups; NIH Center for Dietary Supplements Research on Botanicals and Metabolic Syndrome, grant no. 1-P50-AT-002776-01; and Phytomedics (Jamesburg, NJ).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agati JM, Yeagley D, Quinn PG. Assessment of the roles of mitogen-activated protein kinase, phosphatidylinositol 3-kinase, protein kinase B, and protein kinase C in insulin inhibition of cAMP-induced phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 273: 18751–18759, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Barnea M, Shamay A, Stark AH, Madar Z. A high-fat diet has a tissue-specific effect on adiponectin and related enzyme expression. Obesity (Silver Spring) 14: 2145–2153, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Brommage R Validation and calibration of DEXA body composition in mice. Am J Physiol Endocrinol Metab 285: E454–E459, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bullen JW, Bluher S, Kelesidis T, Mantzoros CS. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 292: E1079–E1086, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Xia Y, Qiu Z. Effect of ecdysterone on glucose metabolism in vitro. Life Sci 78: 1108–1113, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Chiang HC, Wang JJ, Wu RT. Immunomodulating effects of the hydrolysis products of formosanin C and beta-ecdysone from Paris formosana Hayata. Anticancer Res 12: 1475–1478, 1992. [PubMed] [Google Scholar]
- 8.Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carre C, Noselli S, Leopold P. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310: 667–670, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Dinan L Phytoecdysteroids: biological aspects. Phytochemistry 57: 325–339, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Dzucharova MC, Sachidov AD, Kasimov B, Syrov VN, Takanaev AA, Saatov Z. Pharmacokinetics of ecdysterone. Chemico-Pharmacol J 10: 1163–1167, 1987. [Google Scholar]
- 11.El Hilaly J, Lyoussi B. Hypoglycaemic effect of the lyophilised aqueous extract of Ajuga iva in normal and streptozotocin diabetic rats. J Ethnopharmacol 80: 109–113, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Govorko D, Logendra S, Wang Y, Esposito D, Komarnytsky S, Ribnicky D, Poulev A, Wang Z, Cefalu WT, Raskin I. Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line. Am J Physiol Endocrinol Metab 293: E1503–E1510, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem 66: 581–611, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Harmatha J, Vokac K, Kmonickova E, Zidek Z. Lack of interference of common phytoecdysteroids with production of nitric oxide by immune-activated mammalian macrophages. Steroids 73: 466–471, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271: 10697–10703, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437: 1109–1111, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Koolman J Ecdysone: from Chemistry to Mode of Action. New York: Stuttgart, 1989.
- 19.Korkach Iu P, Kotsiuruba AV, Prysiazhna OD, Mohyl‘nyts’ka LD, Sahach VF. [NO-dependent mechanisms of ecdysterone protective action on the heart and vessels in streptozotocin-induced diabetes mellitus in rats]. Fiziol Zh 53: 3–8, 2007. [PubMed] [Google Scholar]
- 20.Korkach Iu P, Rudyk OV, Kotsiuruba AV, Prysiazhna OD, Sahach VF. [The role of nitric oxide and superoxide synthesis in protective mechanism of ecdysterone in the heart mitochondria of rats with streptozotocin-induced diabetes]. Fiziol Zh 53: 22–28, 2007. [PubMed] [Google Scholar]
- 21.Kurmukov AG, Ermishina OA. [The effect of ecdysterone on experimental arrhythmias and changes in the hemodynamics and myocardial contractility induced by coronary artery occlusion]. Farmakol Toksikol 54: 27–29, 1991. [PubMed] [Google Scholar]
- 22.Kutepova TA, Syrov VN, Khushbatkova ZA, Saatov Z. Hypoglycemic activity of the total ecdysteroid extract from Ajuga Turkestanica. Pharmaceut Chem J 35: 24–25, 2001. [Google Scholar]
- 23.Lazar MA How obesity causes diabetes: not a tall tale. Science 307: 373–375, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8: 731–737, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda H, Kawaba T, Yamamoto Y. [Pharmacological studies of insect metamorphotic steroids]. Nippon Yakurigaku Zasshi 66: 551–563, 1970. [PubMed] [Google Scholar]
- 26.Mironova VN, Kholodova IuD, Skachkova TF, Bondar' OP, Datsenko ZM. [Hypocholesterolemic effect of phytoecdysones during experimental hypercholesterolemia in rats]. Vopr Med Khim 28: 101–105, 1982. [PubMed] [Google Scholar]
- 27.Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res 8: 392–398, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Pirola L, Johnston AM, Van Obberghen E. Modulation of insulin action. Diabetologia 47: 170–184, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Schmelz EA, Grebenok RJ, Ohnmeiss TE, Bowers WS. Interactions between Spinacia oleracea and Bradysia impatiens: a role for phytoecdysteroids. Arch Insect Biochem Physiol 51: 204–221, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Syrov V, Sharapova R, Kurmukov A. Effect of Ecdysterone on the hematopoietic activity on the laboratory animals with experimental anemia. Issues Obstetr Gynecol 1: 62–63, 1976. [Google Scholar]
- 31.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269: 5241–5248, 1994. [PubMed] [Google Scholar]
- 32.Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem 277: 34933–34940, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86: 1930–1935, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Wolfram S, Raederstorff D, Preller M, Wang Y, Teixeira SR, Riegger C, Weber P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J Nutr 136: 2512–2518, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Wu C, Okar DA, Kang J, Lange AJ. Reduction of hepatic glucose production as a therapeutic target in the treatment of diabetes. Curr Drug Targets Immune Endocr Metabol Disord 5: 51–59, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Maika S, Craddock L, King JA, Liu ZM. Chronic activation of AMP-activated protein kinase-alpha1 in liver leads to decreased adiposity in mice. Biochem Biophys Res Commun 370: 248–253, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida T, Otaka T, Uchiyama M, Ogawa S. Effect of ecdysterone on hyperglycemia in experimental animals. Biochem Pharmacol 20: 3263–3268, 1971. [DOI] [PubMed] [Google Scholar]
- 39.Yuan L, Ziegler R, Hamann A. Inhibition of phosphoenolpyruvate carboxykinase gene expression by metformin in cultured hepatocytes. Chin Med J (Engl) 115: 1843–1848, 2002. [PubMed] [Google Scholar]
- 40.Zimmet P The burden of type 2 diabetes: are we doing enough? Diabetes Metab 29: 6S9–6S18, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]