Abstract
Myofibroblasts (MF) play an important role in intestinal wound healing. A compromised epithelial barrier exposes intestinal subepithelial MF to luminal bacterial products. However, responses of murine intestinal MF to bacterial adjuvants and potential roles of intestinal MF in innate immune responses are not well defined. Our aims in this study were to determine innate immune responses and intracellular signaling pathways of intestinal MF exposed to LPS, a prototypic Toll-like receptor (TLR) ligand. Expression of TLR4 in primary murine intestinal MF cultures was confirmed by RT-PCR and Western blotting. LPS-induced secretion of prostaglandin E2 (PGE2), interleukin (IL)-6, and keratinocyte-derived chemokines (KC) was measured by ELISA. Intracellular responses to LPS were assessed by Western blotting for NF-κB p65, Iκ-Bα, Akt, p38 MAP kinase, and cyclooxygenase-2 (COX-2). LPS induced rapid phosphorylation of NF-κB p65, Akt, and p38 MAPK and degradation of Iκ-Bα. LPS induced expression of COX-2 and secretion of PGE2 (2.0 ± 0.8-fold induction vs. unstimulated cells), IL-6 (6.6 ± 0.4-fold induction), and KC (12.5 ± 0.4-fold induction). Inhibition of phosphoinositide-3 (PI3)-kinase, p38 MAPK, or NF-κB pathways reduced LPS-induced PGE2, IL-6, and KC secretion. These studies show that primary murine intestinal MF respond to LPS, evidenced by activation of NF-κB, PI3-kinase, and MAPK signaling pathways and secretion of proinflammatory molecules. Inhibition of these pathways attenuated LPS-dependent PGE2, IL-6, and KC production, indicating that LPS activates MF by multiple signaling pathways. These data support the hypothesis that MF are a component of the innate immune system and may exert paracrine effects on adjacent epithelial and immune cells by responding to luminal bacterial adjuvants.
Keywords: intestinal mucosa, Toll-like receptor-4, innate immunity
the intestinal mucosa is constantly exposed to a variety of environmental antigens and molecules, primarily derived from ingested food or from the commensal bacteria that colonize the intestinal tract (46). The commensal bacteria population is comprised of at least 400 species, with a load of as many as 1012 bacteria per gram of intestinal contents (13). Surprisingly, in a healthy individual, these bacteria typically fail to cause inflammatory responses in mucosal epithelial and immune cells. In fact, humans have developed a mutualistic relationship with our commensal bacteria, which promote normal development of the gut and its associated lymphoid tissues, aid in digestion, and help prevent colonization by pathogenic bacteria (35). However, in genetically susceptible individuals or in situations where the intestinal epithelial barrier is compromised, commensal bacteria cause inflammatory responses. This inflammatory response is important to contain and eliminate infections and to clear invading bacteria (46). However, in situations such as inflammatory bowel diseases (IBD), the inflammatory response is inappropriately sustained, resulting in chronic disease. Defining the specific cell types and intra- and intercellular mechanisms that regulate the immune response to bacteria are important steps toward better understanding of IBD and developing better treatments for these conditions.
The intestinal mucosa is populated by many distinct cell types. Intestinal subepithelial myofibroblasts (MF) form a network directly under the intestinal epithelium (39–41). Intestinal MF, like functionally related cells in other tissues such as the skin and liver, have structural and phenotypic similarities to both fibroblasts and smooth muscle cells (40, 41). For example, MF express α-smooth muscle actin (SMA) and vimentin but not desmin. The location of intestinal MF directly beneath the epithelial layer suggests that these cells may regulate the function and proliferation of adjacent epithelial cells. In support of this hypothesis, studies from several laboratories have shown that intestinal MF act through paracrine mechanisms to promote epithelial restitution (31), enhance transepithelial resistance in vitro (5), and promote epithelial differentiation and normal intestinal development (14). Mediators of these paracrine actions include cyclooxygenase (COX) enzyme-derived arachidonic acid metabolites such as prostaglandin E2 (PGE2) (5). MF are thus likely to play an important role in regulating epithelial wound healing and barrier function in IBD. Indeed, COX-2 is upregulated in the mucosa of patients with IBD (24) as well as in subepithelial MF near tumors in a colitis-associated cancer model (48). In addition, several studies show that intestinal MF produce immunomodulatory mediators including interleukin (IL)-6 and IL-8 (2, 45), suggesting that these cells may have multiple functions in intestinal inflammation. Intestinal MF from humans or mice can be isolated as primary cells and maintained in culture for several passages (36, 51), providing an excellent ex vivo/in vitro model system to study the potential inflammatory responses of these cells.
Signaling through Toll-like receptor (TLR) family members is an important component of inflammation in IBD (reviewed in Ref. 46). TLR4 is the receptor for the lipid A moiety from LPS present in the cell walls of Gram-negative bacteria. TLR4 expression and LPS-induced secretion of cytokines including IL-6 and IL-8 have been described in primary human intestinal MF (36, 45, 57) and a human intestinal MF cell line (36) but have not previously been reported in primary MF derived from murine intestine. When LPS binds to TLR4, multiple intracellular signaling pathways are activated, including the classical nuclear factor κ-B (NF-κB) pathway (49), as well as the mitogen-activated protein kinase (MAPK) (8) and phosphoinositide 3 (PI3)-kinase (18) pathways. The relative degree of activation of each of these pathways and the functional consequences differ among cell types and experimental systems. Our studies were designed to test the hypothesis that MF derived from murine small intestine and colon may contribute to innate immune responses in the intestine when exposed to LPS. We examined expression of TLR4 target genes including the proinflammatory cytokine IL-6 and keratinocyte-derived chemokine (KC), a murine functional homolog of IL-8. Both IL-6 and KC are known to be increased in the colon in experimental models of IBD (12, 50). Expression of COX-2 and secretion of PGE2 was also assessed because induction of COX-2 has previously been shown to result from stimulation of intestinal MF with proinflammatory cytokines such as IL-1 (33). In addition, we used selective inhibitors of the NF-κB, p38 MAPK, and PI3-kinase signaling pathways to determine which of these pathways contributed to LPS-induced secretion of proinflammatory mediators.
MATERIALS AND METHODS
Materials.
Phenol-extracted Escherichia coli (E. coli) serotype 0111:B4 LPS was purchased from Sigma-Aldrich (St. Louis, MO). Because this preparation of LPS contains a small amount of contaminating proteins and nucleic acids that can activate other TLRs, ultrapure (phenol-TCA-DOC-purified) LPS (E. coli serotype 0111:B4) purchased from InvivoGen (San Diego, CA) was used for signaling experiments. LPS purified by this method is free of lipoprotein contaminants and activates only the TLR4 pathway (19). Antibodies against COX-2, TLR4, and Iκ-Bα were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies recognizing phosphorylated and total p38 MAPK, phosphorylated and total Akt, and phosphorylated NF-κB p65 were purchased from Cell Signaling Technology (Danvers, MA). The SMA antibody was purchased from R&D Systems (Minneapolis, MN). The PI3-kinase pathway inhibitor wortmannin, the Akt/PKB-specific inhibitor Akt inhibitor IV, the specific p38 MAPK inhibitor SB239063, and NF-κB-inhibitory NF-κB-essential modulator (NEMO)-binding domain and negative control peptides were all purchased from Calbiochem (San Diego, CA). Concentrations of inhibitors were as follows: wortmannin, 100 nM; Akt inhibitor IV, 1 μM; SB239063, 10 μM; NEMO-binding domain and negative control peptides, 20 μM. Concentrations were chosen on the basis of the manufacturer's reported IC50 and, for wortmannin and SB239063, on published reports in intestinal epithelial cells (25). All inhibitors were dissolved in DMSO to make 1,000× stock solutions so that the concentration of DMSO for experimental conditions was 0.1%. This concentration of DMSO did not alter any of the experimental parameters tested (data not shown).
Animal care.
Cells were isolated from mice housed in a germ-free environment to minimize the possibility of contamination of primary cultures with bacteria. Germ-free 129/SvEv mice were maintained in flexible plastic isolators at the National Gnotobiotic Rodent Resource Center and the Gnotobiotics Animal Facility of the Center for Gastrointestinal Biology and Disease at the University of North Carolina. The GF status of the mice was monitored by biweekly aerobic and anaerobic culture and Gram stain of stools from each isolator. In addition, stools were cultured from mice at the end of each experiment to confirm that the experimental mice remained sterile. Male and female mice were used at 6–12 wk of age. Specific pathogen-free (SPF) C3H/HeJ and C3H/HeOUJ mice were purchased from Jackson Laboratories (Bar Harbor, ME). All experimental procedures were approved by the University of North Carolina Animal Care and Use Committee.
Isolation and culture of intestinal MF.
Isolation of primary MF from the small intestine and colon was performed according to a previously published protocol by Theiss et al. (51). The distal ileum or the entire colon was dissected, and contents were flushed thoroughly with DMEM (Sigma-Aldrich) supplemented with 100 U/ml penicillin-streptomycin. The intestinal segment was then opened longitudinally, cut into 0.5-cm pieces, and shaken at room temperature in DMEM to remove remaining contents. The tissue fragments were minced and digested in DMEM supplemented with 300 U/ml collagenase I and 0.1 mg/ml dispase (both from Worthington Biochemicals, Lakewood, NJ) for 20 min at room temperature. Fresh DMEM with 10% fetal bovine serum was added to inactivate the collagenase/dispase. The tissue fragments were pipetted up and down several times to further dissociate the cells. Dissociated cells and tissue fragments were plated in T75 flasks and cultured for 2 days. Nonadherent cells and debris were removed by being washed, and fresh culture media was added. MF were cultured until confluency and then subcultured and used for experiments between passages 3 and 8. This preparation protocol and number of passages used is consistent with other published studies using murine MF cultures (51). As expected, cultured cells were positive for SMA by immunofluorescent staining and Western blot and positive for vimentin expression by Western blot, with typical MF morphology (Fig. 1C). MF cultures also demonstrated negative expression of desmin and cytokeratin by Western blot and no expression of CD11b by immunofluorescent staining and flow cytometry, showing lack of contamination of cultures with smooth muscle cells, epithelial cells, and monocytes/dendritic cells, respectively (data not shown). No consistent differences in the parameters measured were noted between MF of ileal origin compared with colonic MF, and thus cells from either tissue source were used for the present studies. Variability in absolute quantities of COX-2, PGE2, IL-6, and KC were observed in MF prepared from different mice although the qualitative patterns of LPS-induced expression were similar in different MF cultures. Therefore, all experiments were repeated in intestinal MF cultures established from at least three different mice.
Fig. 1.
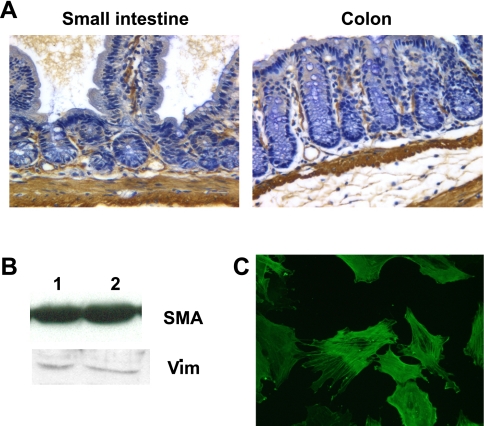
Cultured intestinal cells express phenotypic markers of intestinal myofibroblasts (MF). A: immunohistochemical staining for α-smooth muscle actin (SMA) in murine small intestine and colon shows expression throughout the muscularis propria and in subepithelial MF in the lamina propria. B: Western blots of 20 μg of total cell lysates of intestinal MF cultures show expression of α-SMA and vimentin (Vim). Lanes 1 and 2 indicated 2 independently established MF cultures. C: immunofluorescent staining of cultured intestinal MF with an antibody to α-SMA.
Treatment of MF with LPS.
Cells were seeded at ∼75% confluency (8 × 104/well) in six-well plates and allowed to adhere overnight. The next day cells were treated with LPS (20 ng/ml) for times indicated in various experiments. In some experiments, inhibitors of NF-κB, p38 MAPK, or PI3-kinase signaling were added at the same time as LPS. Culture supernatants were collected at times outlined in various experiments and frozen at −20°C for subsequent analysis of secreted PGE2, IL-6, KC, and IL-10. For analysis of intracellular proteins, cells were washed in PBS, lysed and collected by scraping in Laemmli buffer, and stored at −20°C until analysis by Western blot. For analysis of RNA expression, cells were washed in PBS, lysed in TRIzol reagent (Invitrogen, Carlsbad, CA), and frozen at −20°C until subsequent analysis.
Infection of MF with adenoviral vectors.
MF were cultured to ∼90% confluency and then switched to serum-free media (Opti-MEM; GIBCO, Gaithersburg, MD) for 8 h. Adenovirus vectors expressing either dominant negative IκB (AdIκB) or green fluorescent protein (GFP) (AdGFP) molecules were added at a multiplicity of infection of 50 and incubated overnight. The adenoviral vectors, developed at the Viral Vector Core, Center for Gastrointestinal Biology and Disease at the University of North Carolina, were generously provided by Dr. Christian Jobin, University of North Carolina, and were characterized and described previously (18). After infection, cells were washed and treated with LPS for 1 h for RNA experiments or 24 h for assessment of secreted molecules and COX-2 expression using the methods described above.
Western blotting.
The concentration of proteins in whole cell lysates was determined by standard methods using the Bio-Rad protein quantification assay (Bio-Rad Laboratories, Hercules, CA). A portion (20 μg) of protein from each sample was loaded onto a 10% SDS-polyacrylamide gel, subjected to electrophoresis, and transferred to a nitrocellulose membrane. Western blotting was performed according to previously described protocols (20) with the use of the indicated primary antibodies at 1:1,000 dilutions. Specific immunoreactivity was detected using the Western Lightning chemiluminescence reagent (Perkin Elmer, Wellesley, MA).
RNA extraction and semiquantitative RT-PCR.
Total RNA was isolated from MF using the TRIzol reagent (Invitrogen) according to the protocol supplied by the manufacturer. Total RNA concentrations were determined by the absorbance at 260 nm, and the quality of each sample was verified before further use by inspection of the 18S and 28S ribosomal bands on an ethidium bromide-stained agarose gel. A portion (2 μg) of each sample was reverse transcribed into cDNA, and semiquantitative PCR was performed as previously described (20). The primer sequences used were as follows: TLR4, 5′-TACTCGAGTCAGAATGAGGACTGG-3′ sense, 5′-TTCGAGGCTTTTCCATCCAATAGG-3′ antisense; MD-2, 5′CTGAATCTGAGAAGCAACAGTGG-3′ sense, 5′-CAGTCTCTCCTTTCAGAGCTCTGA-3′ antisense; CD14, 5′GCAAAAGCCAGAGTTCCTGAC-3′ sense, 5′-GAGTTGTGACTGGCCCAGTCAGC-3′ antisense; COX-2, 5′-GCAAATCCTTGCTGTTCCAATC-3′ sense, 5′-GGAGAAGGCTTCCCAGCTTTTG-3′ antisense; IL-6, 5′-ATGAAGTTCCTCTCTGCAAGAGACT-3′ sense, 5′-CACTAGGTTTGCCGAGTAGATCTC-3′ antisense; KC, 5′-TTCTCTGTGCAGCGCTGCTG-3′ sense, 5′-GGAGCTTCAGGGTCAAGGCAA-3′ antisense; and GAPDH, 5′-GTGTTCCTACCCCCAATGTG-3′ sense, 5′-TGTGAGGGAGATGCTCAGTG-3′ antisense. The amplified products were 427 base pairs (bp) (TLR4), 293 bp (MD-2), 264 bp (CD14), 335 bp (COX-2), 637 bp (IL-6), 222 bp (KC), and 396 bp (GAPDH).
ELISAs.
The concentrations of PGE2, IL-6, KC, and IL-10 in culture supernatants from control and LPS-treated MF were assessed by sandwich ELISAs, performed by the ImmunoTechnologies core facility of the Center for Gastrointestinal Biology and Disease at the University of North Carolina. Samples were diluted to ensure detectability within the standard curve of each assay. ELISA kits were purchased from Assay Designs, Ann Arbor, MI (PGE2); BioSource, Carlsbad, CA (IL-6, IL-10); and R&D Systems (KC). The detection limits for the assays were as follows: PGE, 39 pg/ml; IL-6, 3 pg/ml; KC, 2 pg/ml; and IL-10, 13 pg/ml.
Statistics.
Data are presented as means ± SD for individual experiments or means ± SE for pooled experiments, as indicated in each figure legend. An unpaired t-test for samples with unequal variances was performed to compare treatments to control values. A P value of <0.05 was considered significant in all experiments.
RESULTS
Murine intestinal MF express TLR4 and are responsive to LPS.
As expected, immunohistochemical staining of murine small intestine and colon revealed α-SMA expression in smooth muscle cells throughout the muscularis propria. Expression was also evident in subepithelial and pericryptal areas of the mucosa (Fig. 1A), where MF have previously been shown to reside (reviewed in Ref. 41). Intestinal MF cultures established from murine small intestine or colon were tested to verify expression of MF markers including α-SMA and vimentin. Western blot analysis of cell lysates from MF cultures confirmed that these cells are α-SMA positive and vimentin positive (Fig. 1B). Immunofluorescent staining of MF for α-SMA showed uniform staining of stress fibers within the cells (Fig. 1C).
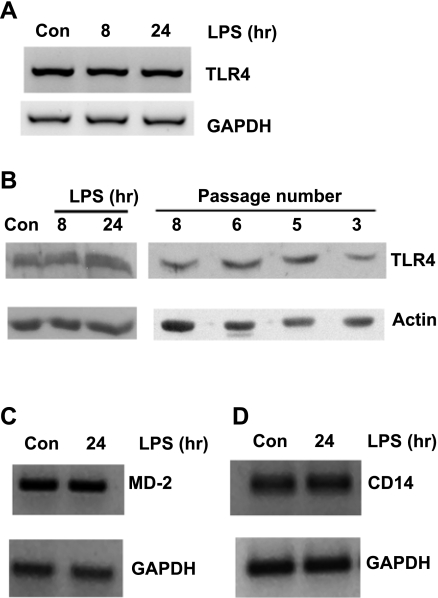
Intestinal MF expressed TLR4, MD-2, and CD14 at the mRNA level as determined by semiquantitative RT-PCR (Fig. 2, A, C, and D). No alterations in TLR4, MD-2, or CD14 mRNA expression were evident after LPS stimulation. TLR4 protein expression was also detected in whole MF cell lysates and was not changed by LPS treatment (Fig. 2B). No consistent differences were observed in TLR4 protein levels in MF of different passage numbers (Fig. 2B) or in MF of ileal vs. colonic origin (data not shown).
Fig. 2.
Intestinal MF express Toll-like receptor (TLR)4 and MD-2. Intestinal MF were treated with 20 ng/ml LPS for the indicated times or left untreated as controls (Con). A: 2 μg total RNA was reverse transcribed into cDNA, and TLR4 mRNA was assessed by PCR. GAPDH is shown as a control. The images shown are representative of 3 independent experiments. B: Western blots of 20 μg of total cell lysates show TLR4 expression in untreated and LPS-treated cells (left) or untreated MF of different passage numbers (right). Actin is shown as a loading control. Blots shown are representative of 6 (left) or 1 (right) independent experiments. C and D: 2 μg total RNA was reverse transcribed into cDNA, and MD-2 (C) and CD14 (D) mRNA were assessed by PCR. GAPDH is shown as a control. The images shown are representative of 2 independent experiments.
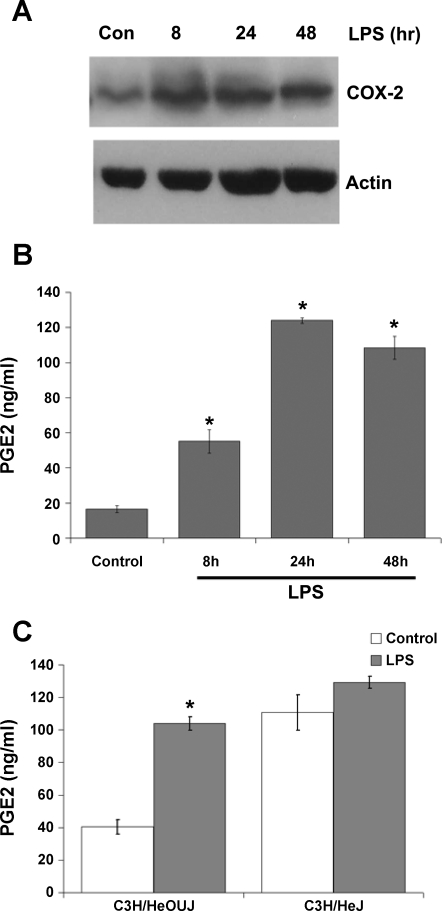
MF were treated with LPS for 8, 24, and 48 h, and the expression of COX-2, a well-described LPS-responsive gene product, was analyzed by Western blot. As shown in Fig. 3A, intestinal MF typically expressed low but detectable levels of COX-2 constitutively. LPS upregulated COX-2 protein expression by 8 h of treatment, and levels remained elevated after 48 h of LPS treatment (Fig. 3A). COX-1 was constitutively expressed, as determined by Western blot analysis in preliminary experiments, but was not regulated by LPS (data not shown). Culture supernatants were then analyzed for secretion of PGE2, a major arachidonic acid metabolite produced by enzymatic action of COX-1 or COX-2. Intestinal MF secreted moderate levels of PGE2 constitutively (Fig. 3B), which is in agreement with the expression of COX-1 and COX-2 detected in unstimulated control cells. PGE2 levels in the culture supernatants were significantly elevated after 8 h of LPS exposure and reached peak levels following 24 h of LPS treatment (Fig. 3B). The role of TLR4 in LPS-induced PGE2 secretion was further tested by stimulation of MF derived from LPS-resistant, TLR4-defective C3H/HeJ mice vs. MF from LPS-sensitive syngeneic C3H/HeOUJ mice housed in SPF conditions. Consistent with observations in MF derived from germ-free wild-type mice of other strains, induction of PGE2 was observed in SPF C3H/HeOUJ-derived MF, whereas only a small and not statistically significant increase in PGE2 secretion was detected in C3H/HeJ-derived MF (Fig. 3C). Interestingly, MF from C3H/HeJ mice secreted very high levels of PGE2 even in the absence of any LPS stimulation (Fig. 3C).
Fig. 3.
LPS induces cyclooxegenase-2 (COX-2) expression and prostaglandin E2 (PGE2) secretion in intestinal MF. Cells were treated with 20 ng/ml LPS for the indicated times or left untreated (Con). A: COX-2 expression was detected by Western blotting of total cell lysates. Actin is shown as a loading control. Blots shown are representative of 4 independent experiments. B: secreted PGE2 was measured by ELISA in supernatants from MF treated as indicated; control cells were incubated in media alone. Data are presented as means ± SD and are representative of 4 independent experiments. *P < 0.05 vs. control. C: MF derived from LPS-resistant C3H/HeJ or wild-type syngeneic C3H/HeOUJ mice were stimulated with LPS for 24 h, and secreted PGE2 was measured in supernatants as in B. Data show the means ± SD of duplicate samples, each assayed in duplicate. *P < 0.05 vs. control of the same cell type.
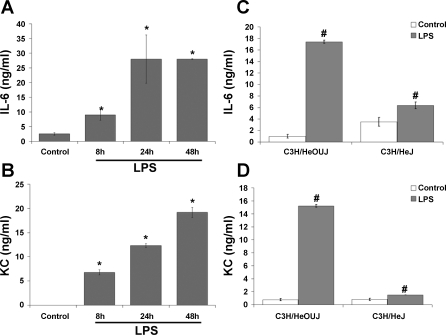
We also measured the presence of IL-6, a major proinflammatory cytokine, and KC, a member of the CXC chemokine family with potent neutrophil chemoattractant ability that functions as an IL-8 homolog in mice (27). IL-6 was secreted into the culture supernatants at low levels constitutively by intestinal MF (Fig. 4A). LPS induced a significant increase in IL-6 secretion by 8 h, with a further maximal increase evident at 24 h. KC was undetectable in unstimulated control MF supernatants but was strongly induced by LPS treatment (Fig. 4B). KC expression continued to increase through 48 h of LPS treatment. In MF from C3H/HeJ mice, a small but significant increase in IL-6 and KC secretion was observed in LPS-treated cells compared with untreated control C3H/HeJ cells (Fig. 4, C and D). This indicates that the LPS used may have low levels of impurities that activate other receptors. However, the induction of IL-6 and KC observed in C3H/HeJ-derived cells was considerably less than that observed in MF from C3H/HeOUJ mice, indicating that the majority of IL-6 and KC induction observed in these experiments is mediated by TLR4.
Fig. 4.
LPS induces secretion of IL-6 and keratinocyte-derived chemokines (KC) in intestinal MF. Cells were treated as in Fig. 2. Supernatants were collected and analyzed by ELISA for IL-6 (A) and KC (B). Data represent means ± SD and are representative of 4 independent experiments. *P < 0.05 vs. control. C and D: IL-6 and KC secretion, respectively, after 24 h of LPS stimulation in MF derived from LPS-resistant C3H/HeJ or wild-type syngeneic C3H/HeOUJ mice. #P < 0.05 vs. control of the same cell type.
The expression of the immunoregulatory cytokine IL-10 after LPS stimulation was also analyzed. Secreted IL-10 was not detectable by ELISA in any of the culture supernatants tested, and IL-10 mRNA was not detected by RT-PCR of total RNA from control or LPS-treated MF (data not shown).
Multiple intracellular signaling pathways are activated in MF treated with LPS.
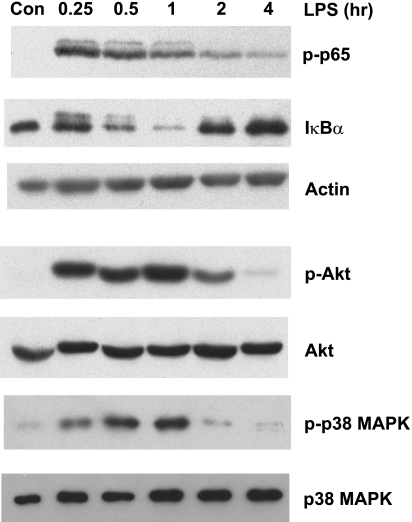
We next sought to determine which signaling pathways were activated by LPS treatment in MF. Cells exposed to LPS for 15 min to 4 h were analyzed by Western blot for activation of the NF-κB, p38 MAPK, and PI3-kinase pathways. As shown in Fig. 5, top, strong phosphorylation of the p65 (RelA) subunit of NF-κB was detected in MF after 15 min of LPS stimulation. Phosphorylated p65 was still detectable after 4 h although levels were decreased relative to earlier time points. Phosphorylated p65 was accompanied by degradation of Iκ-Bα, maximal at 1 h, and then restored to higher than baseline levels by 2–4 h (Fig. 5). In a separate control experiment, we confirmed the specificity of the LPS used and the requirement for TLR4 in these experiments by incubating MF isolated from TLR4-defective C3H/HeJ mice with ultrapure LPS for 30 min. Phosphorylated NF-κB p65 was not detected in C3H/HeJ MF stimulated with ultrapure LPS (data not shown).
Fig. 5.
Multiple intracellular signaling pathways are induced by LPS treatment of intestinal MF. Cells were treated with 20 ng/ml LPS for the indicated times or with media alone (Con). Total cell lysates were analyzed by SDS-PAGE and Western blotting for the phosphorylated p65 subunit of NF-κB (p-p65) and Iκ-Bα to assess activation of NF-κB signaling. Actin is shown as a loading control. The same samples were also analyzed for phosphorylated Akt (p-Akt) and total Akt and for phosphorylated p38 MAPK (p-p38 MAPK) and total p38 MAPK. Blots shown are representative of 4 independent experiments.
Activation of the PI3-kinase pathway was detected by the phosphorylation of Akt, a downstream target of PI3-kinase. Phosphorylated Akt was detectable at peak levels after 15 min of LPS treatment and was reduced to near control levels by 4 h of LPS exposure (Fig. 5, middle). Total Akt expression was not changed by LPS treatment in this time course. Finally, activation of the p38 MAPK pathway by LPS was detected by the presence of phosphorylated p38 MAPK 15 min after LPS stimulation (Fig. 5, bottom). Peak levels of phosphorylated p38 MAPK were detected at 30–60 min of LPS stimulation, followed by rapid recovery to basal expression levels. Total levels of p38 MAPK were not altered by LPS treatment. Taken together, these data show that LPS causes rapid but transient activation of multiple signaling pathways with similar kinetics in intestinal MF. Activation was evident by 15 min and resolved by 2–4 h after LPS stimulation.
Inhibition of intracellular signaling in MF attenuates IL-6 and KC mRNA expression.
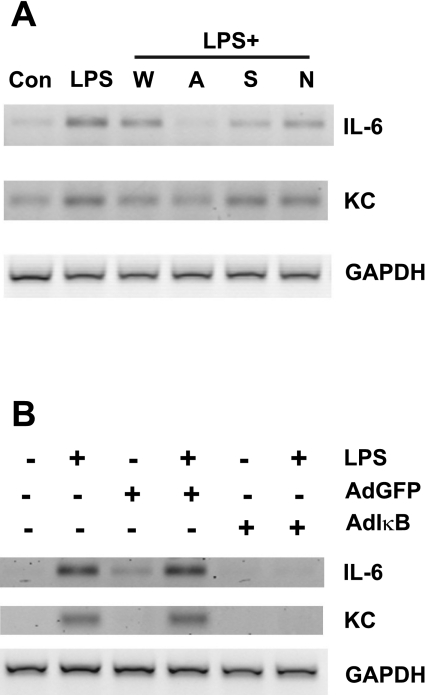
To determine whether transcription of IL-6 and KC is induced by LPS in intestinal MF, cells were treated with LPS and mRNA was analyzed by semiquantitative RT-PCR. Induction of mRNAs for IL-6 and KC was detectable after 1 h of LPS treatment (Fig. 6A). To determine the relative contributions of the NF-κB, p38 MAPK, and PI3-kinase pathways to LPS-mediated alterations in gene expression in MF, cells were incubated with LPS plus specific inhibitors of each of these pathways. Wortmannin inhibits the catalytic activity of PI3-kinase, and Akt inhibitor IV is a specific inhibitor of Akt that targets its ATP binding site and prevents activity. We used the specific inhibitor SB239063 to target p38 MAPK activity. To inhibit NF-κB signaling, we used a cell-permeable NEMO-binding domain binding peptide that disrupts the interaction of the NEMO protein/IKK-γ with IKK-α and IKK-β, thus preventing NF-κB phosphorylation and activation. Addition of wortmannin, Akt inhibitor IV, SB239063, or the NEMO-binding domain binding peptide reduced the LPS-induced increases in IL-6 and KC expression although the effects of Akt inhibitor IV were most pronounced for IL-6 mRNA (Fig. 6A). Inhibition of LPS-induced KC mRNA expression was minimal in cells coincubated with LPS and SB239063 or NEMO-binding domain binding peptide. The relatively small effect of the NEMO-binding domain peptide was somewhat unexpected since NF-κB signaling is a major pathway downstream of TLR4. Therefore, to further address the effects of inhibition of the NF-κB pathway on expression of IL-6 and KC mRNAs, we utilized a dominant negative approach with an adenovirus vector that expressed a dominant negative Iκ-Bα (AdIκB). An adenovirus vector containing GFP (AdGFP) served as a negative control. Cells infected with AdGFP or AdIκB were treated with LPS as indicated, and mRNA expression was assessed after 1 h. Infection of MF with AdIκB abolished LPS-dependent induction of IL-6 and KC mRNA expression (Fig. 6B). No effect of infection with AdGFP on LPS-dependent induction of IL-6 or KC mRNA was detectable by RT-PCR. Results for COX-2 mRNA expression after 1 h of LPS stimulation in the presence or absence of pathway-specific inhibitors or the dominant negative AdIκB were inconsistent across experiments (data not shown).
Fig. 6.
Inhibition of intracellular signaling attenuates gene expression. Expression of IL-6 and KC mRNAs was assessed by semiquantitative RT-PCR. GAPDH is shown as a loading control. A: cells were treated with LPS in the presence of wortmannin (W), Akt inhibitor IV (A), SB239063 (S), or NF-κB-essential modulator (NEMO)-binding domain binding peptide (N). Control cells were incubated with media alone. Images shown are representative of 4 independent experiments. B: cells were infected with adenovirus expressing dominant negative IκB (AdIκB) or green fluorescent protein (GFP) (AdGFP) and treated with 20 ng/ml LPS as indicated. Images shown are representative of 2 independent experiments.
Inhibition of downstream signaling pathways reduces LPS-induced secretion of proinflammatory mediators.
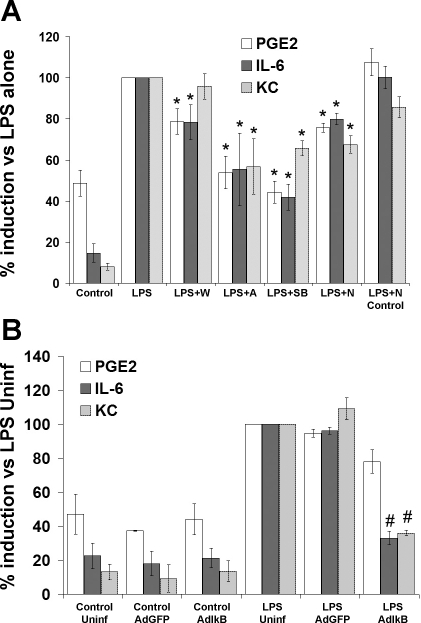
The contribution of the NF-κB, PI3-kinase, and p38 MAPK pathways to PGE2, IL-6, and KC secretion was also determined. Because the absolute concentrations of LPS-induced PGE2, IL-6, and KC secretion varied among different MF cell cultures, the data were normalized to the values for LPS alone. This allowed us to pool the results from multiple experiments to show the mean inhibition that resulted from incubation with various inhibitors. Wortmannin reduced LPS-induced PGE2 production and IL-6 secretion although KC secretion was not significantly different from the value in MF stimulated with LPS alone (Fig. 7A). However, addition of Akt inhibitor IV together with LPS had a stronger effect to diminish LPS-induced PGE2, IL-6, and KC secretion (Fig. 7A). Addition of SB239063 in the presence of LPS caused significant decreases in PGE2, IL-6, and KC secretion (Fig. 7, A and B). Addition of the NEMO-binding domain binding peptide resulted in small but significant reductions in LPS-induced PGE2, IL-6, and KC secretion (Fig. 7A). The negative control mutated NEMO-binding domain binding peptide did not significantly alter PGE2, IL-6, or KC secretion.
Fig. 7.
Inhibition of intracellular signaling pathways attenuates LPS-induced PGE2, IL-6, and KC secretion in intestinal MF. A: MF were treated with LPS in the presence of wortmannin (LPS + W), Akt inhibitor IV (LPS + A), SB239063 (LPS + SB), or NEMO-binding domain binding peptide (LPS + N). Control cells were incubated with media alone. After 24 h, culture supernatants were collected and analyzed by ELISA for PGE2, IL-6, and KC. B: MF were infected with AdIκB or with AdGFP as a control. 8 h later, cells were treated with LPS or media. After 24 h, culture supernatants were collected and analyzed by ELISA for PGE2, IL-6, and KC. Data were normalized to the values in MF treated with LPS for each individual experiment in A and to uninfected cells treated with LPS (LPS Uninf) for each experiment in B. The data from 4–6 independent experiments were then pooled for analysis. Data are represented as means ± SE. *P < 0.05 vs. LPS alone. #P < 0.05 vs. LPS + AdGFP.
To further address the effects of inhibition of the NF-κB pathway on expression of COX-2 and secretion of PGE2, IL-6, and KC, we infected MF with either the dominant negative AdIκB or AdGFP adenovirus vectors. No differences in cell appearance or adherence to the culture plates were noted after infection with AdGFP or AdIκB (data not shown). LPS-induced IL-6 and KC secretion were significantly reduced in AdIκB-infected MF compared with uninfected MF or MF infected with AdGFP (Fig. 7B). A trend toward a reduction in LPS-induced PGE2 secretion was observed in AdIκB-infected MF although this did not reach statistical significance compared with PGE2 levels in AdGFP-infected cells (P = 0.087). The quantitative differences in effects of the NEMO-binding domain binding peptide vs. adenoviral infection with the dominant negative IκB are likely attributable to more complete inhibition of the NF-κB signaling pathway in the adenoviral infection model system. No significant changes in basal PGE2, IL-6, or KC secretion were detected in adenovirus-infected cells without LPS stimulation. Taken together, the data using pharmacological or molecular inhibitors indicate that each of the signaling pathways assessed in these experiments is involved in LPS-induced IL-6 and KC secretion in intestinal MF, with the PI3-kinase/Akt and p38 MAPK pathways more involved in PGE2 regulation than the NF-κB pathway.
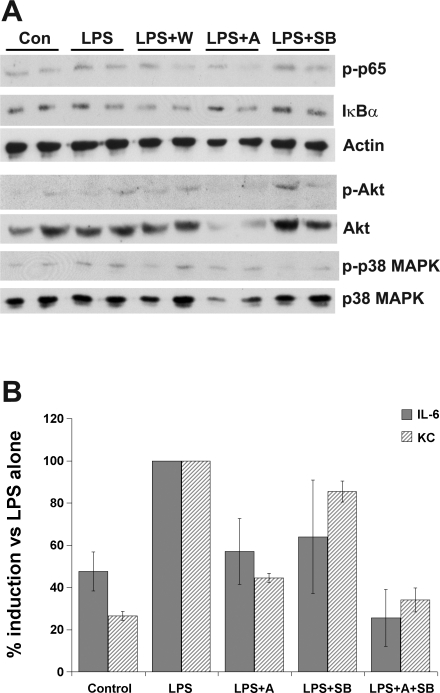
The activation of the NF-κB, PI3-kinase, and p38 MAPK pathways in response to LPS may be independent or may involve interactions between molecules in these pathways. Thus, to assess whether activation of NF-κB in MF requires activation of the PI3-kinase or p38 MAPK pathways, cells treated for 30 min with LPS in the presence of inhibitors of the PI3-kinase or p38 MAPK were analyzed by Western blotting, as shown in Fig. 8A. Phosphorylated NF-κB p65 was detected in LPS-stimulated cells and was not consistently reduced in cells coincubated with inhibitors of PI3-kinase or p38 MAPK pathway activation. Phosphorylation of Akt was not altered by coincubation of MF with LPS and SB239063 but was reduced in the presence of Akt inhibitor IV. Total Akt was also reduced in cells treated with Akt inhibitor IV. Interestingly, phosphorylated Akt could still be detected in MF treated with LPS and wortmannin, indicating that some wortmannin-independent phosphorylation of Akt may occur in LPS-treated MF. Incubation of cells with LPS and SB239063 abolished phosphorylation of p38 MAPK, but inhibition of the PI3-kinase pathway with wortmannin did not alter p38 MAPK phosphorylation. Interestingly, cells incubated with Akt inhibitor IV showed a small reduction in p38 MAPK phosphorylation accompanied by a reduction in total p38 MAPK.
Fig. 8.
Independent activation of phosphoinositide 3 (PI3)-kinase and p38 MAPK pathways by LPS stimulation. A: cells were treated with media alone (Con), LPS alone (LPS), or in the presence of wortmannin (LPS + W), Akt inhibitor IV (LPS + A), or SB239063 (LPS + SB). Total cell lysates were analyzed in duplicate by SDS-PAGE and Western blotting with antibodies as described in Fig. 5. Blots are representative of 1–3 independent experiments. Actin is included as a control for protein loading for the phospho-NF-κB p65 and Iκ-Bα blots, whereas total Akt and total p38 MAPK blots are shown to demonstrate protein loading for the p-Akt and p-p38 MAPK blots, respectively. B: MF were treated with LPS alone or in the presence of Akt inhibitor IV, SB239063, or both inhibitors. Supernatants were collected after 24 h and analyzed by ELISA for IL-6 and KC. Data represent the means ± SD of duplicate samples, each assayed in duplicate, and are representative of 3 independent experiments.
Since similar levels of inhibition of LPS-induced cytokine secretion were observed when Akt or p38 MAPK inhibitors were used, we performed additional experiments to determine whether simultaneous inhibition of both Akt and p38 MAPK further reduced LPS-induced IL-6 and KC secretion. As shown in Fig. 8B, coincubation of MF with both Akt inhibitor IV and SB239063 reduced LPS-induced IL-6 and KC secretion to levels near or below those seen in unstimulated control cells, consistent with an additive effect of Akt and p38 MAPK signaling, as well as involvement of other pathways such as the NF-κB pathway in LPS-induced production of IL-6 and KC.
DISCUSSION
The role of intestinal MF in intestinal injury, inflammation, and repair is not completely understood although attention has been focused on MF as mediators of fibrosis associated with inflammation (42, 53). The proximity of intestinal subepithelial MF to the epithelial layer and lamina propria immune cells supports the concept that these cells may be important mediators of repair and regulation of inflammation after the mucosal barrier is breached. Our studies extend previous studies in primary human MF (36, 45, 57) by showing that murine intestinal MF secrete proinflammatory mediators after stimulation with a prototypic bacterial product, LPS, which activates multiple signaling pathways. Our studies were done with MF isolated from the ileum or colon of germ-free 129/SvEv mice. We cannot rule out the possibility that TLR4 expression and LPS responsiveness is different in these cells than in intestinal MF from mice that are colonized with normal microbiota. However, MF derived from SPF C3H/HeOUJ mice showed that LPS-induced PGE2, IL-6, and KC secretion at levels within the range of those seen in MF derived from germ-free wild-type 129/SvEv mice (Figs. 3, 4). In addition, a preliminary experiment indicated that NF-κB phosphorylation and IκB degradation occurred in a similar time frame in MF derived from SPF mice as in our germ-free-derived cells (data not shown), supporting the concept that LPS responsiveness is similar in MF from colonized vs. germ-free mice.
The concept that intestinal MF affect proliferation, differentiation, and function of neighboring epithelial cells has considerable experimental support (39). MF may also interact with adjacent mucosal immune cells. In support of this, unstimulated primary human intestinal fibroblasts that were α-SMA positive increased survival of mucosal T cells, an effect abolished when IL-10 activity was blocked (22). We were not able to detect IL-10 mRNA or protein in our murine MF cultures, either in unstimulated cells or cells stimulated with LPS. In addition, blockade of IL-10 signaling with an IL-10 receptor-blocking antibody did not alter LPS-induced PGE2, IL-6, or KC secretion in preliminary experiments (data not shown). This discrepancy in IL-10 production may be due to differences in species or differences in the phenotype of the primary cell populations used in our studies vs. the studies by Ina et al. (22). However, in our hands, unstimulated and LPS-stimulated MF secreted high levels of PGE2, which can also have antiapoptotic effects on lymphocytes (38).
Our data showing LPS-induced secretion of PGE2, IL-6, and KC in murine intestinal MF support the hypothesis that MF may play a role in innate immune responses in the intestine and contribute to epithelial repair and fibrosis. Prostaglandins, particularly PGE2, have been demonstrated to promote epithelial proliferation and barrier function (34) as well as regulate differentiation and activation of innate and adaptive immune cells (4, 30). Intestinal MF are major producers of PGE2 (Fig. 3 and Ref. 41), and it is possible that MF-derived PGE2 acts through autocrine or paracrine mechanisms to influence production of growth factors and cytokines by intestinal MF or other intestinal cell types although we did not directly assess this in our studies. IL-6 inhibits apoptosis in activated T cells (3), stimulates differentiation of TH17 cells (26), and induces expression of the adhesion molecule ICAM-1 on intestinal epithelial cells (55). IL-6 produced by subepithelial MF in response to LPS may thus have proinflammatory paracrine effects on multiple neighboring cell types, including epithelial cells and lamina propria T cells.
Chemokines also play a major role in the pathogenesis of intestinal inflammation. For example, mice that are deficient in TLR4 expression have increased susceptibility to acute colitis induced by dextran sulfate sodium (DSS), accompanied by a decreased infiltration of neutrophils into the mucosa and decreased secretion of the neutrophil-attracting chemokine macrophage inflammatory protein-2 by macrophages (16). Our findings that LPS induces KC, which has potent neutrophil chemotactic properties (6), in intestinal MF support the hypothesis that TLR4-mediated effects on cell types other than macrophages may also promote neutrophil infiltration into the mucosa. The importance of TLR-mediated signaling in epithelial homeostasis, repair, and inflammation is underscored by enhanced acute DSS colitis and reduced induction of IL-6 and KC in MyD88-deficient mice (43). This suggests that commensal bacterial-induced TLR-mediated signaling is important in protecting against chemical disruptions of the epithelial barrier. However, these studies do not differentiate responses to TLR ligand-induced signaling in epithelial cells vs. MF or immune cells in the lamina propria. Since intestinal subepithelial MF have well-documented effects to promote intestinal epithelial cell proliferation and restitution (41, 47), we speculate that MF function and/or MF-epithelial interactions may also be altered in TLR4- or MyD88-deficient mice. Interestingly in this regard, intraperitoneal LPS administration protects mice from radiation-induced enteritis and causes upregulation of COX-2 expression in subepithelial MF in the small intestine, as well as epithelial cells in the villus, but not the crypt (44).
TLR4 activation initiates a complex array of downstream intracellular signaling, beginning with recruitment of one or more adaptor proteins such as MyD88, Toll-interleukin 1 receptor domain containing adaptor protein Mal, TRIF, or TRAM (reviewed in Ref. 1). The NF-κB signaling pathway mediates many effects of TLR4; activation of NF-κB regulates transcription of multiple target genes including IL-6, KC, and COX-2. TLR4 activation also leads to activation of MAPK kinase 6, which phosphorylates p38 MAPK and JNK (54). Activated p38 MAPK can enhance NF-κB activity (23) and have effects independent of NF-κB (37). The PI3-kinase pathway is also activated by LPS, but the data regarding whether this pathway exerts positive or negative regulation on downstream TLR4 targets are mixed (15, 29). The relative contributions of and interactions between these signaling pathways in LPS-induced gene expression are complex and vary in different cells and experimental conditions. In our experiments, activation of the NF-κB signaling pathway was still evident in LPS-stimulated MF treated with inhibitors of Akt or p38 MAPK. In addition, phosphorylation of Akt and p38 MAPK were detectable in the presence of LPS + SB239063 or LPS + Akt inhibitor, respectively, supporting the concept that each pathway is activated independently in LPS-stimulated MF, at least to some extent. Interestingly, we consistently observed a decrease in total Akt with use of the Akt inhibitor IV in these experiments and, to a lesser extent, a small decrease in total p38 MAPK. We found in pilot experiments that a 10 μM concentration of Akt inhibitor IV (10-fold higher than the dose used for the experiments reported herein) was toxic to MF by 2–3 days of culture (data not shown). Cell death was not apparent at 2–3 days with the 1 μM concentration used for the experiments shown, nor did incubation with Akt inhibitor IV abolish transcription or translation in these cells since GAPDH mRNA, actin protein, and secreted IL-6 and KC were still detectable (Figs. 6, 7, and 8). However, it is possible that the reduced total Akt and total p38 MAPK concentrations following Akt inhibitor IV treatment reflects a nonspecific toxic effect on cells that is not reflected by detectable decreases in levels of more abundant proteins. We cannot rule out the possibility that Akt inhibitor IV affects the stability or degradation of Akt or p38 MAPK.
In our experiments, LPS-induced NF-κB, p38 MAPK, and PI3-kinase activation each contributed to some extent to the induction of PGE2, IL-6, and KC secretion. Interestingly, wortmannin did not significantly inhibit LPS-induced KC secretion although specific inhibition of Akt did inhibit KC secretion. Akt phosphorylation was observed in cells treated with LPS + wortmannin. It is possible that the dose of wortmannin used was not sufficient to fully block PI3-kinase activity in this cell type. Alternatively, some degree of PI3-kinase-independent Akt activation may occur in MF stimulated with LPS, as has been reported in neurons stimulated with dopamine (7). None of the selective inhibitors used completely blocked PGE2, IL-6, or KC secretion, suggesting that each pathway mediates a component of LPS-induced signaling in intestinal MF. We acknowledge that pharmacological inhibitors are not completely selective and can incompletely block a pathway. Therefore, we used a molecular approach to selectively inhibit NF-κB, which was more efficient (60% inhibition of KC and IL-6) than the NEMO-binding domain binding peptide (20–30% inhibition).
PGE2 was reduced to a greater degree by inhibiting p38 MAPK than by inhibiting NF-κB signaling. This is in contrast to a report demonstrating similar levels of inhibition of IL-1-induced COX-2 expression by inhibiting p38 MAPK or by inhibiting NF-κB (33). However, in another recent study, inhibition of NF-κB in rat intestinal epithelial cells did not alter LPS-induced COX-2 expression, whereas blockade of p38 MAPK substantially reduced COX-2 expression (17). PGE2 secretion was also inhibited in intestinal MF when PI3-kinase signaling was blocked by wortmannin or Akt inhibitor IV. This is consistent with induction of COX-2 by PI3-kinase signaling, which positively regulated NF-κB activation in macrophages (28). In the present study, LPS induced COX-2 protein expression and PGE2 secretion although effects on mRNA expression were variable at the time point we analyzed. The COX-2 promoter includes multiple regulatory elements, including binding sites for NF-κB, C/EBP-β, and Jun/ATF, as well as a cyclic AMP response element (32). These binding sites exhibited synergistic effects, indicating that multiple signaling pathways may converge to maximally induce COX-2 transcription. NF-κB activation induces transcription of COX-2 (33), and p38 MAPK activation induces transcription and stabilization of COX-2 mRNA independent of NF-κB activity (17). Taken together with our data, this demonstrates that COX-2 regulation is influenced by a wide variety of signaling pathways, and data from one cell type or stimulus type cannot necessarily be extrapolated to other cell types and stimuli.
Regulation of IL-6 secretion is also complex and involves multiple signaling pathways, which vary between cell types. In human monocytes, LPS-induced IL-6 secretion was not altered in the presence of a p38 MAPK inhibitor (21). In contrast, induction of IL-6 by LPS treatment of murine Kupffer cells was reduced when p38 MAPK activation was inhibited (52), consistent with our data showing that IL-6 mRNA levels and secretion are reduced in LPS-treated intestinal MF. LPS-stimulated PI3-kinase activation resulted in NF-κB activation and subsequent IL-6 secretion in endothelial cells (29), in agreement with our data supporting positive regulation of IL-6 secretion by PI3-kinase activation in response to LPS. Notably, the authors of that study demonstrated reduction but not complete abolishment of IL-6 secretion when PI3-kinase activity was blocked with a dominant negative construct (29). Our data also suggest that LPS-induced IL-6 secretion in MF is not completely abolished by blockade of individual NF-κB, p38 MAPK, or PI3-kinase signaling pathways, supporting the concept that all three pathways are independently involved in maximal induction of IL-6 by LPS in these cells.
Regulation of KC by TLR signaling in macrophages has recently been shown to require MyD88, and the KC promoter contains multiple potential NF-κB binding sites (11). LPS can regulate KC by inducing transcription (9) and by stabilizing KC transcripts in a p38 MAPK-dependent mechanism (10). To our knowledge, the contribution of the PI3-kinase/Akt pathway to LPS-induced KC secretion is not well defined. Interestingly, serum KC levels, as well as serum IL-6 levels, were increased in PI3-kinase-deficient mice exposed to the TLR5 agonist flagellin (56). Notably, in the same study, blockade of PI3-kinase activity had no effect on flagellin-induced activation of the NF-κB pathway in cultured intestinal epithelial cells (56), indicating that PI3-kinase effects in response to TLR activation are not mediated through NF-κB. In intestinal MF, LPS-induced KC mRNA levels and secretion were decreased by blockade of NF-κB and p38 MAPK and by a pharmacological inhibitor of Akt. Akt inhibition did not abolish LPS-induced NF-κB activation, suggesting that Akt has effects on KC production that are not mediated through NF-κB. The further reduction in KC induction observed when both Akt and p38 MAPK were inhibited provide evidence that these signaling molecules may act through independent pathways to regulate LPS-induced KC secretion in MF.
In conclusion, our data confirm that MF derived from murine intestine and colon express TLR4 and that LPS induces COX-2, PGE2, IL-6, and KC expression in these cells. The combined NF-κB, p38MAPK, and PI3-kinase pathways contribute to maximal induction of PGE2, IL-6, and KC, with each signaling pathway having variable relative contributions to different mediators. On the basis of these studies, we conclude that intestinal MF innate responses to luminal commensal bacteria or bacterial products may promote tissue injury and recruitment of inflammatory cells while simultaneously stimulating epithelial repair in a paracrine manner.
GRANTS
The National Gnotobiotic Rodent Resource Center is supported by UPHS grant P40 RR018603. The CGIBD is supported by NIH P30 DK34987. K. Walton was supported by the SPIRE program at the University of North Carolina, NIH grant GM00678. This work is also funded by NIH grants RO1 DK53347 and P40 RR018603 to R. Sartor.
Acknowledgments
The authors thank Ms. Rosemary Link at the Immunoassay Core of the Center for Gastrointestinal Biology and Disease (CGIBD) at the University of North Carolina for assistance with ELISA assays and Ms. Maureen Bower at the National Gnotobiotic Rodent Resource Center and the Gnotobiotic Core of the CGIBD at the University of North Carolina for animal care. The authors also thank Dr. Arianne Theiss (University of North Carolina) for assistance with the protocol for murine intestinal myofibroblast cultures, Dr. P. Kay Lund and Dr. James Simmons (University of North Carolina) for generously sharing SPF mouse-derived myofibroblasts for pilot experiments, and Dr. Jonathan Hansen (University of North Carolina) for useful discussions during manuscript preparation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129: 969–984, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schurmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med 6: 583–588, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc'h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 175: 1483–1490, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Beltinger J, McKaig BC, Makh S, Stack WA, Hawkey CJ, Mahida YR. Human colonic subepithelial myofibroblasts modulate transepithelial resistance and secretory response. Am J Physiol Cell Physiol 277: C271–C279, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Bozic CR, Kolakowski LF Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol 154: 6048–6057, 1995. [PubMed] [Google Scholar]
- 7.Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci 22: 8911–8921, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol 164: 966–972, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Dai Y, Datta S, Novotny M, Hamilton TA. TGFbeta inhibits LPS-induced chemokine mRNA stabilization. Blood 102: 1178–1185, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Datta S, Biswas R, Novotny M, Pavicic PG Jr, Herjan T, Mandal P, Hamilton TA. Tristetraprolin regulates CXCL1 (KC) mRNA stability. J Immunol 180: 2545–2552, 2008. [DOI] [PubMed] [Google Scholar]
- 11.De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol 180: 4308–4315, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Eijkelkamp N, Heijnen CJ, Lucas A, Premont RT, Elsenbruch S, Schedlowski M, Kavelaars A. G protein-coupled receptor kinase 6 controls chronicity and severity of dextran sodium sulphate-induced colitis in mice. Gut 56: 847–854, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13780–13785, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritsch C, Swietlicki EA, Lefebvre O, Kedinger M, Iordanov H, Levin MS, Rubin DC. Epimorphin expression in intestinal myofibroblasts induces epithelial morphogenesis. J Clin Invest 110: 1629–1641, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol 3: 875–881, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol 288: G1055–G1065, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Grishin AV, Wang J, Potoka DA, Hackam DJ, Upperman JS, Boyle P, Zamora R, Ford HR. Lipopolysaccharide induces cyclooxygenase-2 in intestinal epithelium via a noncanonical p38 MAPK pathway. J Immunol 176: 580–588, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Haller D, Russo MP, Sartor RB, Jobin C. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem 277: 38168–38178, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol 165: 618–622, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells. Blood 105: 689–696, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Horwood NJ, Page TH, McDaid JP, Palmer CD, Campbell J, Mahon T, Brennan FM, Webster D, Foxwell BM. Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol 176: 3635–3641, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Ina K, Kusugami K, Kawano Y, Nishiwaki T, Wen Z, Musso A, West GA, Ohta M, Goto H, Fiocchi C. Intestinal fibroblast-derived IL-10 increases survival of mucosal T cells by inhibiting growth factor deprivation- and Fas-mediated apoptosis. J Immunol 175: 2000–2009, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Jijon H, Allard B, Jobin C. NF-kappaB inducing kinase activates NF-kappaB transcriptional activity independently of IkappaB kinase gamma through a p38 MAPK-dependent RelA phosphorylation pathway. Cell Signal 16: 1023–1032, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Jupp J, Hillier K, Elliott DH, Fine DR, Bateman AC, Johnson PA, Cazaly AM, Penrose JF, Sampson AP. Colonic expression of leukotriene-pathway enzymes in inflammatory bowel diseases. Inflamm Bowel Dis 13: 537–546, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Karrasch T, Steinbrecher KA, Allard B, Baldwin AS, Jobin C. Wound-induced p38MAPK-dependent histone H3 phosphorylation correlates with increased COX-2 expression in enterocytes. J Cell Physiol 207: 809–815, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA 104: 12099–12104, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol 155: 2158–2164, 1995. [PubMed] [Google Scholar]
- 28.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem 278: 37041–37051, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Tupper JC, Bannerman DD, Winn RK, Rhodes CJ, Harlan JM. Phosphoinositide 3 kinase mediates Toll-like receptor 4-induced activation of NF-kappa B in endothelial cells. Infect Immun 71: 4414–4420, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIlroy A, Caron G, Blanchard S, Fremaux I, Duluc D, Delneste Y, Chevailler A, Jeannin P. Histamine and prostaglandin E up-regulate the production of Th2-attracting chemokines (CCL17 and CCL22) and down-regulate IFN-gamma-induced CXCL10 production by immature human dendritic cells. Immunology 117: 507–516, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKaig BC, Makh SS, Hawkey CJ, Podolsky DK, Mahida YR. Normal human colonic subepithelial myofibroblasts enhance epithelial migration (restitution) via TGF-beta3. Am J Physiol Gastrointest Liver Physiol 276: G1087–G1093, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Mestre JR, Mackrell PJ, Rivadeneira DE, Stapleton PP, Tanabe T, Daly JM. Redundancy in the signaling pathways and promoter elements regulating cyclooxygenase-2 gene expression in endotoxin-treated macrophage/monocytic cells. J Biol Chem 276: 3977–3982, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Mifflin RC, Saada JI, Di Mari JF, Adegboyega PA, Valentich JD, Powell DW. Regulation of COX-2 expression in human intestinal myofibroblasts: mechanisms of IL-1-mediated induction. Am J Physiol Cell Physiol 282: C824–C834, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Moeser AJ, Haskell MM, Shifflett DE, Little D, Schultz BD, Blikslager AT. ClC-2 chloride secretion mediates prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Gastroenterology 127: 802–815, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Mueller C, Macpherson AJ. Layers of mutualism with commensal bacteria protect us from intestinal inflammation. Gut 55: 276–284, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otte JM, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology 124: 1866–1878, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis—CREB and NF-kappaB as key regulators. Immunity 23: 319–329, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Porter BO, Malek TR. Prostaglandin E2 inhibits T cell activation-induced apoptosis and Fas-mediated cellular cytotoxicity by blockade of Fas-ligand induction. Eur J Immunol 29: 2360–2365, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors. I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol 289: G2–G7, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol 277: C1–C9, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol 277: C183–C201, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Pucilowska JB, McNaughton KK, Mohapatra NK, Hoyt EC, Zimmermann EM, Sartor RB, Lund PK. IGF-I and procollagen alpha1(I) are coexpressed in a subset of mesenchymal cells in active Crohn's disease. Am J Physiol Gastrointest Liver Physiol 279: G1307–G1322, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Riehl TE, Newberry RD, Lorenz RG, Stenson WF. TNFR1 mediates the radioprotective effects of lipopolysaccharide in the mouse intestine. Am J Physiol Gastrointest Liver Physiol 286: G166–G173, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Rogler G, Gelbmann CM, Vogl D, Brunner M, Scholmerich J, Falk W, Andus T, Brand K. Differential activation of cytokine secretion in primary human colonic fibroblast/myofibroblast cultures. Scand J Gastroenterol 36: 389–398, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Sartor RB Microbial influences in inflammatory bowel diseases. Gastroenterology 134: 577–594, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Shao J, Sheng GG, Mifflin RC, Powell DW, Sheng H. Roles of myofibroblasts in prostaglandin E2-stimulated intestinal epithelial proliferation and angiogenesis. Cancer Res 66: 846–855, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Shattuck-Brandt RL, Varilek GW, Radhika A, Yang F, Washington MK, DuBois RN. Cyclooxygenase 2 expression is increased in the stroma of colon carcinomas from IL-10(-/-) mice. Gastroenterology 118: 337–345, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11: 443–451, 1999. [DOI] [PubMed] [Google Scholar]
- 50.te Velde AA, de Kort F, Sterrenburg E, Pronk I, ten Kate FJ, Hommes DW, van Deventer SJ. Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease. Inflamm Bowel Dis 13: 325–330, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem 280: 36099–36109, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Thobe BM, Frink M, Hildebrand F, Schwacha MG, Hubbard WJ, Choudhry MA, Chaudry IH. The role of MAPK in Kupffer cell toll-like receptor (TLR) 2-, TLR4-, and TLR9-mediated signaling following trauma-hemorrhage. J Cell Physiol 210: 667–675, 2007. [DOI] [PubMed] [Google Scholar]
- 53.van Tol EA, Holt L, Li FL, Kong FM, Rippe R, Yamauchi M, Pucilowska J, Lund PK, Sartor RB. Bacterial cell wall polymers promote intestinal fibrosis by direct stimulation of myofibroblasts. Am J Physiol Gastrointest Liver Physiol 277: G245–G255, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412: 346–351, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV. IL-6 induces NF-kappa B activation in the intestinal epithelia. J Immunol 171: 3194–3201, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Yu Y, Nagai S, Wu H, Neish AS, Koyasu S, Gewirtz AT. TLR5-mediated phosphoinositide 3-kinase activation negatively regulates flagellin-induced proinflammatory gene expression. J Immunol 176: 6194–6201, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z, Andoh A, Inatomi O, Bamba S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-17 and lipopolysaccharides synergistically induce cyclooxygenase-2 expression in human intestinal myofibroblasts. J Gastroenterol Hepatol 20: 619–627, 2005. [DOI] [PubMed] [Google Scholar]