Abstract
It has proved to be impossible to culture epithelial cells from the gastrointestinal tract of adult animals. Researchers have had to use either cell lines derived from newborn rat small intestine or colon carcinoma cell lines that have retained some of the properties of the gastrointestinal mucosa. We have described a method for establishing conditionally immortalized cell lines from the stomach, small intestine, colon, pancreas, and liver from tissue obtained from a transgenic mouse strain carrying a temperature-sensitive mutant of the SV40 large T gene (the “Immortomouse”). This immortalizing gene has proved to be useful for establishing cell lines from a number of transgenic mice following crossbreeding of the Immortomouse with the transgenic mouse of interest. These cell lines are being used in numerous studies. In this review we describe the methods for developing such lines and list the range of cell lines that have been developed from colon, small intestine, stomach, liver, and pancreas of a number of transgenic mice.
Keywords: epithelium, intestine, mouse, tissue culture
despite years of effort in many laboratories, it has proved to be impossible to establish epithelial cell lines from the normal adult colonic epithelium of rodents. The cell lines that are widely used have been derived from the small intestine of newborn rats [RIE (2); IEC-6 and IEC-18 (9)]. The alternative approach taken by many researchers has been to use colon carcinoma cell lines that have retained properties of the colonic mucosa. There are hundreds of papers describing such studies using cell lines such as HT-29, Caco-2, T84, LoVo, etc.
We have approached the problem of establishing epithelial cell lines from the adult murine intestinal mucosa by utilizing a transgenic mouse (the “Immortomouse”) that carries a temperature-sensitive mutant of the SV40 large T gene (6). The SV40 large T gene is an immortalizing gene that binds to p53 and inhibits its role in senescence (3).
At the permissive temperature (33°C), the gene product (SV40 large T protein) is in an active conformation and will bind to p53, thus facilitating immortalization of the cells, whereas at the nonpermissive temperature (37–39°C) the conformation of the gene product changes and it will not now bind to p53 or immortalize cells.
The advantage of using a temperature-sensitive form of this gene is that the mouse has an almost normal phenotype and can be reared in standard mouse facilities and breeds well although it does develop a benign thymic hyperplasia with age that shortens the breeding life of female mice and limits the number of litters.
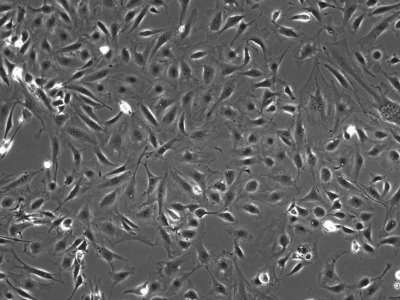
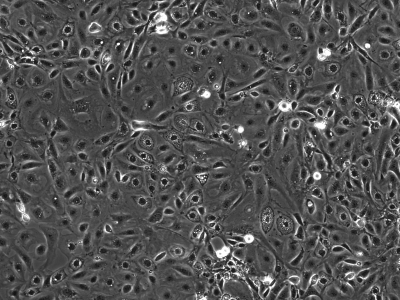
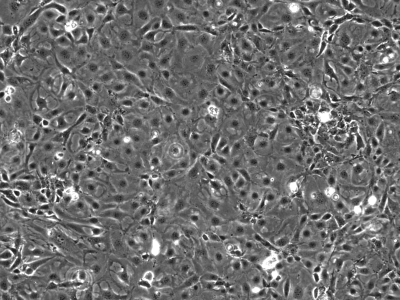
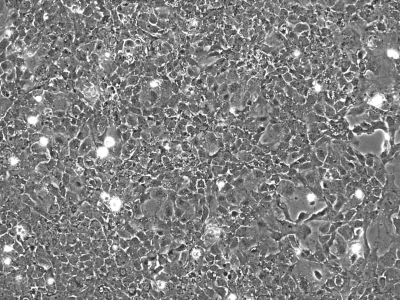
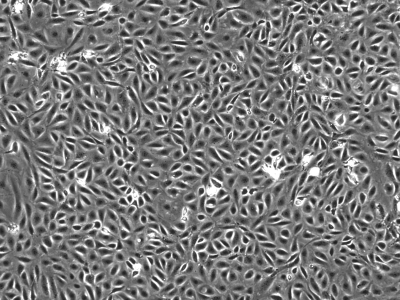
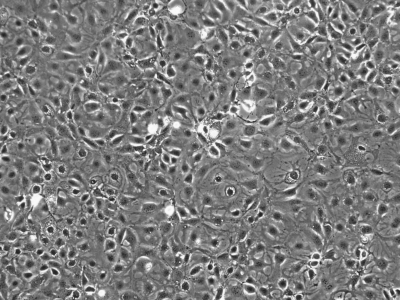
We initially utilized this mouse as a source of intestinal tissues to develop conditionally immortalized cell lines from young adult mouse colonic epithelium (YAMC; Fig. 1; Ref. 14), mouse small intestinal epithelium (MSIE; Fig. 2; Ref. 14) and Immortomouse stomach epithelium (ImSt; Fig. 3; R. H. Whitehead, unpublished results). Subsequently, epithelial cell lines have been developed from normal liver (Fig. 4; Ref. 1), and pancreas (Fig. 5) (7).
Fig. 1.
Young adult mouse colonic epithelium (YAMC) cells in monolayer culture, passage 23. Microscope magnification ×100.
Fig. 2.
Mouse small intestinal epithelium (MSIE) cells in monolayer culture, passage 20. ×100.
Fig. 3.
Immortomouse stomach epithelium (ImSt) cells in monolayer culture, passage 16. ×100.
Fig. 4.
Immortomouse liver (ImHep) cells in culture, passage 21. ×100.
Fig. 5.
Immortomouse pancreas (ImPan) cells in culture, passage 16. ×100.
The availability of this mouse has enabled us to introduce the immortalizing gene into other mouse strains by crossbreeding. Using this method we developed cell lines from the colonic epithelium of the multiple intestinal neoplasia (MIN) mouse (8) [Immortomouse × MIN colonic epithelium (IMCE); Fig. 6; Ref. 12], which carries one mutant Apc allele; the colon, stomach, liver, and pancreas of the GFP mouse (10); and the colon, stomach, and liver of the ROSA26 mouse (15).
Fig. 6.
Immortomouse × MIN colon (IMCE) cells in culture, passage 21. ×100.
The IMCE cell line has proved to be useful for studying malignant progression because, although the IMCE cell line is phenotypically normal, it has proven to be susceptible to transformation. Transfection of the cells with an activated Ras gene (4) or an activated β-catenin gene (11) induced a malignant transformation in these cells but not in the parental Immortomouse (YAMC) cell line. In a recent review, this pair of cell lines was described as the best model system available for studying malignant progression in colon cancer (5).
In this review, we describe our method for developing epithelial cell lines from the intestinal tissue of transgenic mouse strains of interest that we have bred with the Immortomouse. This method has enabled us to establish cell lines from a number of transgenic mice (see Table 1). By using this system, it should be possible to develop conditionally immortalized epithelial cell lines from the intestinal tissue of any transgenic mouse.
Table 1.
Epithelial Cell Lines Derived from the Immortomouse and from Transgenic Mice Crossed with the Immortomouse
| Immortomouse | colon (YAMC), small intestine (MSIE), stomach (InSt), liver (ImHep) |
|---|---|
| GFP-expressing | colon, stomach, liver, pancreas (ImPan) |
| MIN | colon (IMCE) |
| ROSA 26 | stomach, colonic stroma, liver |
| ADAM9−/− | colon, stomach, liver |
| ADAM17−/− | colon, stomach, SI, liver |
| Amphiregulin−/− | colon |
| COX-1−/− | colon, stomach, liver, pancreas |
| COX-2−/− | colon, stomach, SI, liver, pancreas |
| Cyclin D1 Overexpressing | stomach, liver |
| EGF-R−/− | colon, liver |
| KSR−/− | colon, stomach, SI, liver, pancreas |
| MTGR1−/− | small intestine, liver |
| MYO1a−/− | colon, stomach |
| P55−/− | colon, stomach, SI, pancreas |
| P75−/− | colon, stomach, liver |
| P53−/−× MIN | SI |
| P120−/− | stomach |
| Ptk6−/− | colon, stomach |
| Raf−/− floxed | colon |
| SMAD 3−/− | colon, liver, pancreas |
| TGFb RII (floxed) | colon, stomach, SI, liver, embryonic fibroblasts |
| Waved 2 | colon |
SI, small intestine. All of these cell lines are currently stored in liquid N2 at the Digestive Disease Research Center (DDRC) Novel Cell Line Development Core Facility, Vanderbilt University and are available by contacting: Dr. Robert H. Whitehead, Depts. of Medicine, Cancer Biology and Cell and Development Biology and Vanderbilt DDRC Novel Cell Line Core Facility, Rm. 1055E, MRB 4, Vanderbilt Univ., Nashville, TN 37232 (e-mail: robert.whitehead@vanderbilt.edu).
Although a number of cell lines from a range of abdominal tissues are listed in Table 1, it should be noted that most of the cell lines from tissues other than the colon have not been characterized beyond demonstrating that the cells are epithelial. They are included to demonstrate the potential of the technique being described. The two exceptions to this are the original cell line derived from the liver of an Immortomouse (ImHep), which was shown to stain for both albumin and α-fetoprotein (1), and a cell line derived from the Immortomouse pancreas (IMPE), which was shown to produce insulin after transfection with pdx1 and culture in the presence of glucagon-like peptide 1 (7).
MATERIALS
Reagents
Cell-stripper; catalog no. 25-056-ci, Mediatech, Herndon, VA.
Collagenase type 1; catalog no. 17100-017, GIBCO, Grand Island, NY.
Dithiothreitol (DTT); catalog no. D0632, Sigma, St. Louis, MO.
Ethylene diamine tetraacetic acid (EDTA); catalog no. ED455, Sigma.
Fetal calf serum (FCS); catalog no. sh30396.03, Hyclone, Logan, UT.
Hydrocortisone hemisuccinate; catalog no. h4881, Sigma.
Insulin [used for convenience as insulin, transferring, selenium stock solution (ITS)]; catalog no. I3146, Sigma.
LHC-9 medium; catalog no. 12680-013, Invitrogen, Carlsbad, CA.
Monoclonal anti-E-cadherin antibody; catalog no. 610181, BD, Bedford, MA.
Monoclonal anti-chromogranin A antibody; catalog no. MAB5268, Millipore, Danvers, MA.
Monoclonal anti-cytokeratin 18 antibody; catalog no. MAB3234, Millipore.
Monoclonal anti-MUC3 antibody; catalog no. RDI-MUC3abm, Research Diagnostics, Concord, MA.
Monoclonal anti-villin antibody; catalog no. MAB1639, Millipore.
Penicillin-streptomycin; catalog no. 30-002-ci, Mediatech.
Phosphate-buffered saline 0.1 M pH 7.2; catalog no. p5368, Sigma.
Primocin; catalog no. VZA-1021, Amaxa, Gaithersburg, MD. (This is an antibiotic mix designed for primary culture.)
Rat-tail collagen; catalog no. BD 354236, BD.
RPMI-1640 tissue culture medium; catalog no. 10-040-cv, Mediatech.
Sodium hypochlorite; catalog no. 3248-1, VWR, Suwanee, GA.
α-Thioglycerol (1-thioglycerol); catalog no. 88642, Sigma.
Trypsin-EDTA solution; catalog no. 23-053-ci, Mediatech.
Equipment
24-well tissue culture plates; catalog no. tp92024, TPP, Midwest Scientific, Valley Park, MO.
6-well tissue culture plates; catalog no. tp92006, TPP.
25-cm2 tissue culture flasks; catalog no. tp92026, TPP.
75-cm2 tissue culture flasks; catalog no. tp90076, TPP.
2-ml Liquid N2 storage vials; catalog no. 377224, Nunc, Roskilde, Denmark.
Lab-Tek 8-well chamber slides catalog no. 177402, Nunc.
Scienceware cloning disks; catalog no. Z374431, Sigma.
5% CO2 incubator, 100% humidity.
Liquid N2 storage freezer.
Reagent Setup
Stock solutions.
Fetal calf serum is aliquoted in 25-ml volumes and stored at −20°C.
Hydrocortisone hemisuccinate is a water-soluble form of hydrocortisone. A stock solution of 10−4 M is made in medium and filter sterilized. It is aliquoted in 5-ml amounts and stored at −80°C.
ITS is diluted with medium to a stock solution of 100 μg/ml insulin. It is filter sterilized and aliquoted in 5-ml volumes and stored at −80°C.
Murine γ-interferon is diluted according to the manufacturer's data sheet to a final concentration of 500 units/ml, filter sterilized, and aliquoted in 5-ml volumes. It is stored at −80°C. NOTE: Because there is no data on the stability of the interferon in tissue culture medium, it is added fresh to the cultures with the medium.
α-Thioglycerol is made as a stock solution of 10−3 M in PBS, filter sterilized, and stored in 5-ml aliquots at −80°C.
Tissue culture medium (final concentrations of ingredients are given).
500 ml RPMI 1640.
5% FCS.
1 μg/ml insulin (ITS suitably diluted can be used.)
10−5 M α-thioglycerol.
10−6 M hydrocortisone.
100 units/ml penicillin.
100 μg/ml streptomycin.
100 μg/ml Primocin.
10 units/ml mouse γ-interferon (reduced to 5 units/ml once the culture is established). This is added as needed. It is not added to the stock medium, because the stability of the interferon in medium is unknown.
The medium is stored at 4°C and is not used for more than 1 wk.
PROCEDURE
Mice and Breeding
Immortomice are obtained from Charles River Laboratories (Wilmington, MA). When purchased, the mice are on a CBA/CA × C57/Bl10 background. The mice were transferred to a C57/Bl6 background because most of the transgenic mice received for cell line development are on this background. Breeding of mice with both the homozygous gene deletion and the temperature-sensitive SV40 large T gene requires two rounds of breeding, first creating a heterozygous knockout mouse carrying the tsSV40 large T gene and then mating two of these heterozygous mice to obtain mice with the homozygous transgene and carrying the SV40 large T gene. The presence of the immortalizing gene is demonstrated by PCR as described previously (14). The presence of the transgene is demonstrated according to the protocol provided by the laboratory supplying the mouse. With patience, multiple mutations can be added [we have established a small intestinal cell line that is p53 −/− × MIN × Immortomouse using this method (Whitehead, unpublished results)].
Tissue Processing and Culture Method
Colon, small intestine, and stomach.
For the establishment of cell lines from these tissues, it is advantageous if the mice can be raised until they are at least 3 wk old and preferably 6–8 wk old since this allows the isolation of pure epithelial cells from the stomach and colon by a nonenzymatic method using EDTA and DTT (14).
After the mouse is euthanized according to the protocol of the Institution, the required organs are removed. The colon, small intestine, and glandular part of the stomach are opened lengthwise and the contents washed out with multiple changes of PBS. The tissues are incubated in individual screw-capped tubes in 20 ml of PBS plus 0.04% sodium hypochlorite for 15 min at room temperature in a 50-ml screw-capped centrifuge tube. The dilute hypochlorite solution is then discarded and the tissue is washed twice in sterile PBS. From this point the tissue should be considered to be sterile and all procedures and solutions should be sterile.
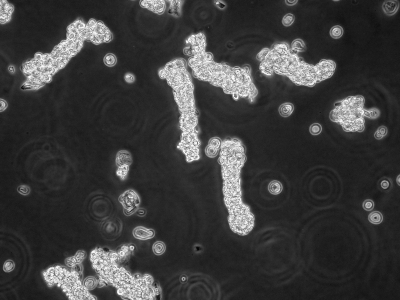
The PBS is removed and replaced with 25 ml of 3 mM EDTA/0.5 mM DTT in PBS (sterile filtered). This is incubated for 60 min at 4°C. The EDTA-DTT solution is removed and 20 ml of PBS added. The top of the screw-capped centrifuge tube is tightened and the tube is shaken as vigorously as possible for 30 s. The released crypts (Fig. 7) are removed to 15-ml centrifuge tubes and centrifuged at 400 rpm for 5 min. The shaking process can be repeated with fresh PBS but this can lead to fibroblast contamination.
Fig. 7.
Crypts liberated from adult mouse colon after EDTA-DTT extraction. ×100.
The crypt pellet is resuspended in growth medium (with freshly added γ-interferon) and plated at low density (∼100–200 crypts per well) by using a minimal volume (0.1 to 0.2 ml) of medium into the wells of 24-well plates that have been coated with rat-tail collagen. The plates are incubated at 33°C in 5% CO2 in a fully humidified incubator. After 24 h, 1 ml of medium is added gently to every well. The growth medium is replaced twice weekly.
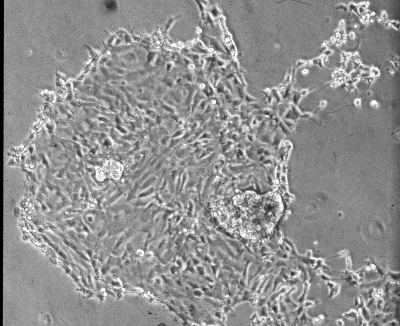
Within 24–48 h, some crypts will attach to the bottom of the wells and cell patches will begin to form (Fig. 8). Most of these cells will die out in 1–2 wk but small patches of cells will persist in occasional wells and then start to expand. The cell patches are passaged into the well of a six-well plate when the well is >50% confluent. The original well must be refed because some cells remain.
Fig. 8.
Patch of cells from an attached crypt after 24 h in culture. ×100.
Once the well of the six-well plate is confluent, the cells are passaged into a 25-cm2 flask and then into a 75-cm2 flask. The cells are stored in liquid N2 at as early a passage level as possible. The cultures are never split more than 1:2.
NOTE: Although this method yields fibroblast-free crypt suspensions from colon and stomach on most occasions, it is impossible to obtain similar cell preparations from small intestine because villi are also isolated by this procedure. The stromal cells in the villi can therefore lead to fibroblast contamination of these cultures. Although this is a potential problem, we have cultured a number of epithelial cell lines from small intestinal tissue.
Liver and pancreas.
To culture the liver and pancreas, the tissue is removed, incubated in 0.04% sodium hypochlorite for 15 min at room temperature to sterilize the surface, washed with PBS, and minced finely with sterile scissors. The tissue is incubated in a mixture of 3 units/ml collagenase and 1 unit/ml neutral protease in tissue culture medium for 90 min at 4°C with gentle shaking. The mixture is allowed to settle and the supernate removed to centrifuge tubes and centrifuged at 1,000 rpm for 5 min. The cell pellet is washed once with medium and resuspended in culture medium to which fresh γ-interferon has been added and plated in a small volume of medium in collagen-coated wells. The plates are incubated in a fully humidified 5% CO2 atmosphere at 33°C. One milliliter of medium is added after 24 h. The plates are observed regularly and medium is changed twice weekly. Any cell growth is passaged with trypsin-EDTA when the well in >50% confluent. Using this method we have established pure cultures of both hepatocytes and pancreatic epithelial cells by cloning epithelial cell patches early in the culture. For cloning, the plate is marked in the area of the epithelial patch, the medium is removed, and a sterile cloning disk that has been dipped in trypsin-EDTA solution is placed on the epithelial patch. After 5 min the disk is removed to the well of a 24-well plate and growth medium is added.
Trypsinization Procedure
The growth medium is removed and trypsin-EDTA solution is added to each well. This is then removed and the plates or flasks are incubated at room temperature until the cells begin to detach. This can be monitored with an inverted microscope. Medium is then added to the well or plate and the cells are detached by pipetting. Initially the cells from a well of a 24-well plate will be transferred to a well of a 6-well plate. Once this is confluent the cells are transferred to a 25-cm2 flask, then to a 75-cm2 flask. After this the cells are split 1:2.
Collagen Coating
The wells are coated with a solution made by diluting 1 ml of the stock collagen solution in 100 ml of sterile PBS. One milliliter of solution is pipetted into each well of multiple 24-well plates. The plates are incubated overnight at room temperature, the solution is removed, and the wells are washed once in PBS and drained well before use. The plates can be air dried in the tissue culture hood and stored at 4°C. Alternatively, collagen-coated plates can be purchased commercially.
Storage in Liquid N2
Confluent flasks are trypsinized and resuspended in culture medium containing 20% FCS and 10% DMSO at a concentration of 2 × 106 cells/ml. One-milliliter aliquots are pipetted into storage ampoules and frozen at 1°C/min for 2 h before being transferred to the liquid N2 storage facility. To recover the cells from storage, an ampoule of cells is thawed rapidly at 37°C and the cells are resuspended in 15 ml of culture medium and added to a 75-cm2 culture flask.
Cell Characterization
The cells must be checked for the genotype of interest by PCR or other applicable genotyping technique.
The cells should be checked for mycoplasmal contamination on a regular basis by using one of the commercially available testing kits. Contamination with mycoplasma can be treated with Plasmocin (Amaxa) or Mycoplasma Removal Agent (Invitrogen).
The epithelial nature of the cells must be demonstrated. Cells that have been cultured on coverslips or Lab-Tek slides are fixed in 4% paraformaldehyde for 2 h at 4°C, washed well with PBS, and stained with antibodies to keratin 18 and E-cadherin by standard immunohistochemical techniques.
In our experience, in almost all cases the cultured cells are undifferentiated and cannot be induced to differentiate under standard conditions. However, the phenotype of cells derived from colon or small intestine can be tested by staining cells that have been cultured on Lab-Tek slides with antibodies to villin (absorptive cells), mucin (goblet cells), and chromogranin A (endocrine cells) by standard immunohistochemical techniques.
NOTE: Although we have established cultures from stomach, liver, and pancreas, we have not had the resources to characterize these cells but have cultured the tissues to demonstrate the usefulness of the technique. As referred to above, the original hepatocyte culture and a pancreatic culture have both been phenotyped and shown to retain characteristics of the tissue of origin. Cultures from the stomach, liver, and pancreas should also be stained with tissue- and cell type-specific markers before use.
DISCUSSION
Once established, the conditionally immortalized epithelial cells grow readily at the permissive temperature in vitro and provide a novel means of studying the function of the specific gene of interest in vitro.
Because the cells are only conditionally immortalized, they will die over a period of 7–10 days if cultured at the nonpermissive temperature; however, there is little evidence of differentiation during this period. The most differentiation that we have achieved during growth of YAMC and MSIE cells at the nonpermissive temperature is a two- to threefold increase in the level of dipeptidyl peptidase and sucrase-isomaltase (brush border enzymes) with no evidence of morphological differentiation (Whitehead, unpublished results). In the majority of our intestinal cell lines there are no morphological changes such as the appearance of microvilli or mucin granules that would suggest that differentiation is occurring.
The one exception to this is a cell line that we established from a Ptk6-null mouse (13). The colonic epithelial cells from this mouse form hemicysts (domes) in monolayer culture indicative of vectorial fluid transport. When cultured on filters the cells form a polarized electrically resistant monolayer with good tight junction formation. When cultured in a collagen gel the cells differentiate into absorptive cells, goblet cells, and endocrine cells (13). This line should prove to be useful in both physiological studies and studies of the factors controlling differentiation in the colon.
It should be noted that the establishment of this cell line required several changes to our standard culture technique. After more than a dozen attempts to culture cells from this transgenic mouse using our standard methods we used a serum-free medium (LHC-9) and obtained both cell adhesion and slow growth. We then found that the cells did not survive standard trypsinization and had to use a nonenzymatic cell removal solution (Cell-stripper). After 20 passages, the cell line that we established had adapted to both our normal tissue culture medium and our routine trypsinization procedures without losing its novel phenotype. We have not experienced these problems with any other transgenic mouse and have not failed to establish cell lines from any other mouse using our standard techniques although it sometimes requires culture attempts from more than one mouse to obtain a cell line.
We have tested many media formulations and many additives in our attempts to establish cells lines from these tissues. Apart from our experience with the Ptk6−/− cell line described above, the medium formulation described in materials has been suitable for all of our studies.
We believe that this method based on the use of the Immortomouse offers the potential to develop epithelial cell lines from intestinal tissue of most if not all transgenic mice. The isolation technique described here yields a purified crypt population from the colon and stomach and a mixed villus and crypt population from the small intestine. Any fibroblast contamination in the cell preparation can lead to problems because the fibroblasts are also immortalized and readily overgrow the epithelial cells leading to a long and difficult cloning to establish a pure epithelial culture. This is also the problem with the culture of embryonic tissue and tissue from very young mice in which separation of the epithelial cells from the underlying stromal tissue before culture is not possible.
Of all the intestinal organs, small intestinal epithelium has proved to be the most difficult from which to establish epithelial cells lines. This is partly due to the fact that villi are always isolated with the crypt suspension. This leads to contamination of the culture with stromal cells, which can be very difficult to remove as these cells are also immortalized. Also, in our experience, small intestinal crypts do not establish as readily in culture as colonic crypts and stomach pits under these culture conditions.
There are many advantages to the use of a cell line for studies compared with the use of the whole animal. This method provides a constantly renewable source of conditionally immortalized cells that have been derived from the tissue of interest and that express the genetic phenotype of interest. This can provide the source material for studies of the function of the gene of interest in a simple controlled environment.
All of the cell lines listed in Table 1 are available for research studies. As noted above, most of these cell lines have not been characterized beyond demonstrating that they are epithelial and are listed to demonstrate the usefulness of the technique. Anyone wishing to study these cells should undertake the characterization to demonstrate the retention of organ-specific properties.
It should be noted the Ludwig Institute for Cancer Research claims intellectual property rights to the Immortomouse and to cell lines derived using this mouse and a Materials Transfer Agreement has to be signed before these cells can be shipped. We can facilitate this once a request for a cell line is received.
GRANTS
These studies were funded by the Ludwig Institute for Cancer Research, Melbourne Tumour Biology Branch and by funding to the Novel Cell Line Development Core Facility through Vanderbilt University Medical Center's Digestive Disease Research Center supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-058404.
REFERENCES
- 1.Allen KJ, Reyes R, Demmler K, Mercer JF, Williamson R, Whitehead RH. Conditionally immortalised mouse hepatocytes for use in liver gene therapy. J Gastroenterol Hepatol 15: 1325–1332, 2000. [PubMed] [Google Scholar]
- 2.Blay J, Brown KD. Characterization of an epithelioid cell line derived from rat small intestine: demonstration of cytokeratin filaments. Cell Biol Int Rep 8: 551–560, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Colby WW, Shenk T. Fragments of the Simian Virus 40 transforming gene facilitate transformation of rat embryo cells. Proc Natl Acad Sci USA 79: 5189–5193, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Abaco G, Whitehead RH, Burgess AW. Synergy between Apc and an activated ras mutation is sufficient to induce colon carcinomas. Mol Cell Biol 16: 884–891, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton JI, Hord NG. Stage matters: choosing relevant model systems to address hypotheses in diet and cancer chemoprevention research. Carcinogenesis 27: 893–902, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Jat PS, Noble MD, Ataloitis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 88: 5096–5100, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koizumi K, Doi R, Fujimoto K, Ito D, Toyoda E, Mori T, Kami K, Kawaguchi Y, Gittes GK, Imamura M. Pancreatic epithelial cells can be converted into insulin-producing cells by GLP-1 in conjunction with virus-mediated gene transfer of pdx-1. Surgery 138: 125–133, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247: 322–324, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Quaroni A Development of fetal rat intestine in organ and monolayer culture. J Cell Biol 100: 1611–1620, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takada T, Iida K, Awaji T, Itoh K, Takahashi R, Shibui A, Yoshida K, Sugano S, Tsujimoto G. Selective production of transgenic mice using green fluorescent protein as a marker. Nat Biotechnol 15: 458–461, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Wagenaar RA, Crawford HC, Matrisian LM. Stabilized β-catenin immortalizes colonic epithelial cells. Cancer Res 61: 2097–2104, 2001. [PubMed] [Google Scholar]
- 12.Whitehead RH, Joseph JL. Derivation of conditionally immortalized cell lines containing the Min mutation from the normal colonic mucosa and other tissues of F1 mouse (Immortomouse/Min). Epithelial Cell Biol 3: 119–125, 1994. [PubMed] [Google Scholar]
- 13.Whitehead RH, Robinson PS, Williams JA, Bie W, Tyner AL, Franklin JL. Conditionally immortalized colonic epithelial cell line from a Ptk6 null mouse that polarizes and differentiates in vitro. J Gastroenterol Hepatol 23: 1119–1124, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. The establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA 90: 587–591, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, William G, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta galactosidase in mouse embryos and hemopoietic cells. Proc Natl Acad Sci USA 94: 3789–3794, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]