Abstract
Oxidant stress is critically involved in various liver diseases. Superoxide formation causes c-Jun NH2-terminal kinase (JNK)- and caspase-dependent apoptosis in cultured hepatocytes. To verify these findings in vivo, male Fisher rats were treated with diquat and menadione. The oxidant stress induced by both compounds was confirmed by increased formation of glutathione disulfide and 4-hydroxynonenal protein adducts. Plasma alanine aminotransferase activities increased from 46 ± 4 U/l in controls to 955 ± 90 U/l at 6 h after diquat treatment. Hematoxylin and eosin staining of liver sections revealed large areas of necrotic cells at 3 and 6 h. DNA strandbreaks, evaluated with the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, showed clusters of TUNEL-positive cells, where the staining was predominantly cytosolic and the cells were swollen, indicating oncotic necrosis. There was no significant increase in caspase-3 activities or relevant release of DNA fragments into the cytosol at any time between 0 and 6 h after diquat treatment. Despite the activation of JNK after high doses of diquat, the JNK inhibitor SP-600125 did not protect against diquat-induced necrosis. Menadione alone did not cause liver injury, but, in combination with phorone and FeSO4, induced moderate oncotic necrosis. On the other hand, if animals were treated with galactosamine/endotoxin as positive control for apoptosis, caspase-3 activities were increased by 259%, the number of TUNEL-positive cells with apoptotic morphology was increased 103-fold, and DNA fragmentation was enhanced 6-fold. The data indicate that liver cell death initiated by diquat-induced superoxide formation in vivo is mediated predominantly by oncotic necrosis and is independent of JNK activation.
Keywords: diquat, galactosamine, endotoxin, menadione, superoxide, mechanisms of cell death, caspases
enhanced formation of reactive oxygen species (ROS; oxidant stress) has been suggested to play a role in most major liver disease processes, including alcohol-induced liver injury (1, 3, 16), hepatic ischemia-reperfusion injury (30–32), hepatic fibrosis (18, 52), acetaminophen (APAP)-induced liver failure (33, 36), iron and copper overload (8), cholestasis (21, 52), viral hepatitis (56), nonalcoholic steatohepatitis (47), and liver carcinogenesis (51). However, the mode of cell death and the intracellular signaling mechanisms induced by ROS in hepatocytes remain controversial.
In earlier studies using primary rat hepatocytes, the superoxide-generating quinone menadione caused necrosis through loss of protein sulfhydryl groups and disturbances of the intracellular Ca2+ homeostasis (17). Similarly, t-butyl hydroperoxide caused necrosis through inducing mitochondrial reactive oxygen formation, which triggered the opening of the mitochondrial membrane permeability transition (MPT) pore and collapse of the mitochondrial membrane potential (48, 49). In the human hepatoma cell line HuH-7, hydrogen peroxide (H2O2) dose-dependently caused necrosis through activation of the transcription factor activator protein-1 (AP-1) (64). However, superoxide formation with menadione or direct exposure to H2O2 induced apoptosis in the rat hepatocyte cell line RALA255 (39). Interestingly, only the H2O2-induced apoptosis was caspase dependent (39). Menadione caused apoptosis through activation of the c-Jun NH2-terminal kinase (JNK) and AP-1 in RALA255 cells (15). In contrast to these findings, only the superoxide-generating chemicals menadione and paraquat caused caspase- and JNK-dependent apoptotic cell death in primary rat hepatocytes (11, 12). H2O2 did activate neither JNK nor caspases and induced necrosis in rat hepatocytes (11). A similar observation was recently made in mouse hepatocytes (53). However, H2O2-induced necrosis involved JNK activation and was dependent on protein kinase C (53). On the other hand, menadione caused necrosis in the human hepatoma cell line HepG2 and even inhibited Fas-mediated apoptosis (54). Taken together, these data suggest that formation of superoxide and H2O2 in liver cells in vitro can trigger apoptotic and necrotic cell death through various signaling mechanisms dependent on the cell type and the experimental conditions involved. Despite these variable results, the prevailing concept favored in many reviews and primary papers appears to be that ROS triggers apoptosis (e.g., Refs. 1, 13, 57).
A number of questions remain unresolved. In particular, it is unclear why formation of superoxide, which either spontaneously or catalyzed by cellular superoxide dismutases generates H2O2, will trigger apoptosis in primary rat hepatocytes compared with the direct exposure to H2O2, which induces necrosis (11). Moreover, it remains unclear why superoxide and H2O2 induce apoptosis in a rat hepatocyte cell line but through different signaling mechanisms (39). Most importantly, none of these studies addressed the most critical question, i.e., which of these mechanisms is relevant for superoxide-mediated cell death in the intact liver in vivo. Therefore, to investigate the fundamental problem whether the enhanced formation of superoxide within hepatocytes and possibly other cell types in rat livers in vivo induces cell death through apoptosis, apoptosis deteriorating to secondary necrosis, or oncotic necrosis, we treated rats with the superoxide-generating agent diquat (59, 60) and evaluated the mode of cell death. In addition, rats were treated with menadione (10), which can cause oxidant stress but can also induce protein thiol depletion (17).
MATERIALS AND METHODS
Animals.
Male Fisher rats (190–220 g) were purchased from Harlan (Indianapolis, IN). The animals had free access to food and water. The experimental protocols, which were reviewed and approved by the Institutional Animal Care and Use Committee, followed the criteria of the University of Kansas Medical Center and the National Research Council for the Care and Use of Laboratory Animals in Research. Rats were intraperitoneally injected with diquat dibromide (0.15 mmol/kg or 0.1 mmol/kg) (generously provided by Dr. Charles V. Smith, Seattle Children's Hospital) or saline (5 ml/kg); the animals were euthanized after 1.5, 3, or 6 h. As positive control for hepatocellular apoptosis, rats were treated with a combination of galactosamine (500 mg/kg ip; Sigma, St. Louis, MO) and Salmonella enteriditis endotoxin (100 μg/kg ip; Sigma) (Gal/ET) for 6 h (22). In addition, rats were treated with menadione sodium bisulfite (150 mg/kg iv, Sigma) for up to 6 h. Some animals received also 100 mg/kg phorone (intraperitoneally, Aldrich) at 1 h before menadione and 0.36 mmol/kg ferrous sulfate (intraperitoneally, Sigma) at 15 min before menadione (59). Some of the animals treated with diquat, menadione, or phorone/iron/menadione were treated with 10 mg/kg of the JNK inhibitor SP-600125 (Calbiochem, San Diego, CA) at 1 h after the redox-cycling agents. The inhibitor was dissolved in 5% dimethyl sulfoxide in phosphate-buffered saline and administered intraperitoneally.
Experimental protocol.
At the end of the treatment period (1.5, 3, or 6 h), the animals were anesthetized with pentobarbital (50 mg/kg) and then euthanized by exsanguination. Blood was drawn from the heart into a heparinized syringe for measurement of plasma alanine aminotransferase (ALT) activities with test kit 68-B (Biotron Diagnostics, Hernet, CA). Liver samples were snap-frozen in liquid nitrogen for measurements of hepatic caspase-3 activities and DNA fragmentation. Additional liver samples were fixed in 10% phosphate-buffered formalin for histology.
Bile duct cannulation.
Animals were anesthetized with pentobarbital (50 mg/kg ip), and the common bile ducts were cannulated with polyethylene tubing (PE-10) for timed collections in microtubes. Samples were collected in 15-min intervals before and after administration of diquat or menadione.
Measurement of caspase-3 activity.
Caspase-3 activities were determined as described in detail (35). Briefly, liver samples were homogenized in 25 mM HEPES buffer (pH 7.5) containing 5 mM EDTA, 2 mM DTT, and 0.1% CHAPS. The homogenized samples were then centrifuged at 14,000 g at 4°C. Caspase-3 activity was measured using 50 μM of the fluorogenic substrate Acetyl-Asp-Glu-Val-Asp-4-methylcoumaryl-7-amide (Peptide Institute, Osaka, Japan). The samples were assayed in duplicate wells, with or without 10 μM of the pan-caspase inhibitor Z-Val-Ala-Asp-fluoromethylketone (Alexis, San Diego, CA). The kinetics of the proteolytic cleavage of the substrate were monitored every 5 min for 1 h at 30°C in a fluorescence microplate reader (Molecular Devices, Sunnyvale, CA) using an excitation wavelength of 360 nm and an emission wavelength of 460 nm.
Measurement of DNA fragmentation.
The liver samples were homogenized in an ice-cold lysis buffer (1 M Tris-HCl, pH 7.5, containing 0.5 M EDTA, 5 M NaCl, and 10% SDS). The mixture was centrifuged at 12,000 g for 10 min and measured using the supernatant. DNA fragmentation was evaluated using the Cell Death Detection ELISA (anti-histone ELISA; Roche Diagnostic, Indianapolis, IN). In this assay, the kinetics (Vmax) of product generation is a measure of DNA fragmentation. The Vmax values obtained for untreated controls (100%) are compared with those in treated groups.
GSH and GSSG measurements.
The method used was based on the Tietze assay (62). Total GSH and GSSG were measured in the liver homogenate, plasma, and bile samples. The frozen tissue was homogenized at 0°C in 3% sulfosalicylic acid (SSA) containing 0.1 mM EDTA. Each plasma and bile sample was mixed immediately with 3% SSA. These samples were then centrifuged (14,000 g) at 4°C, and GSH was measured in the diluted supernatant. GSSG levels were quantified after GSH was derivatized with 2-vinylpyridine (19). Briefly, 100 μl of the supernatant were mixed with 2 μl of 2-vinylpyridine (Sigma) and 6 μl of triethanolamine (Sigma) for 1 h at room temperature to remove GSH before the assay was initiated. Aliquots (10 μl) of the sample solutions and the standards were pipetted into a 96-well plate, and 100 μl of 0.2 M phosphate buffer containing 6.3 mM EDTA, 1.07 mM DTNB, and 0.3 mM NADPH at pH 7.4 were added to each sample, followed by the addition of 5 μl of 26.6 U/ml glutathione reductase. The plate was read several times over 2 min at 412 nm on a Molecular Devices UV-VIS Microplate reader (Molecular Devices, Sunnyvale, CA).
Measurement of liver ATP.
The liver tissue was freeze-clamped and stored in liquid nitrogen until analysis. Frozen liver samples were homogenized in 3% SSA, and ATP levels were determined in the supernatant using the ATP Determination Kit (A22066) from InVitrogen (Carlsbad, CA).
Liver histology and immunohistochemistry.
Formalin-fixed tissue samples were embedded in paraffin, and 5 μm sections were cut. Replicate sections were stained with hematoxylin and eosin for evaluation of necrosis (20, 24). The percentage of necrosis was estimated by evaluating the number of microscopic fields with necrosis compared with the entire cross section. In general, necrosis was estimated at low power (×100); questionable areas were evaluated at higher magnification (×200 or ×400). The pathologist (A. Farhood) evaluated all histological sections in a blinded fashion. Sections were also stained for 4-hydroxynonenal (4-HNE) protein adducts with the DAKO LSAB Peroxidase Kit (K684) (Dako, Carpinteria, CA), which was used according to the manufacturer's instructions. The anti-4-HNE antibody was obtained from Calbiochem (San Diego, CA) (23). For the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, liver sections were stained using the In Situ Cell Death Detection kit (Roche Molecular Biochemicals, Indianapolis, IN), according to the manufacturer's instructions. The sections were counterstained with Nuclear Fast Red (Dako) and mounted. The number of apoptotic hepatocytes was counted in 15 high-power fields (×400) and expressed as a percentage of the total cells evaluated. Apoptotic hepatocytes were identified by positive TUNEL staining and morphology (cell shrinkage, chromatin condensation and/or margination, and formation of apoptotic bodies) (20, 24).
Western blotting.
Expression levels of c-Jun and phospho-c-Jun were evaluated by Western blotting, as described in detail for liver tissue (4). The primary antibodies used were the rabbit anti-c-Jun monoclonal antibody 60A8 (Cell Signaling Technology, Danvers, MA) and the rabbit anti-phospho-c-Jun (Ser73) antibody (Cell Signaling Technology); a horseradish peroxidase-coupled anti-rabbit IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a secondary antibody. Proteins were visualized by enhanced chemiluminescence (GE Healthcare Bio-Sciences, Piscataway, NJ), according to the manufacturer's instructions.
Statistics.
Data are expressed as means ± SE. Comparison between two groups were performed with Student's t-test or one-way ANOVA followed by Bonferroni t-test for multiple groups. If the data were not normally distributed, the Mann-Whitney test was applied for comparison of two groups and the Kruskal-Wallis test (nonparametric ANOVA) followed by Dunn's multiple comparisons test for multiple groups. P < 0.05 was considered significant.
RESULTS
Oxidant stress-induced necrosis and apoptosis.
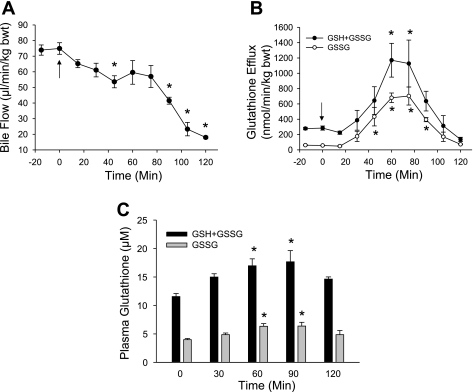
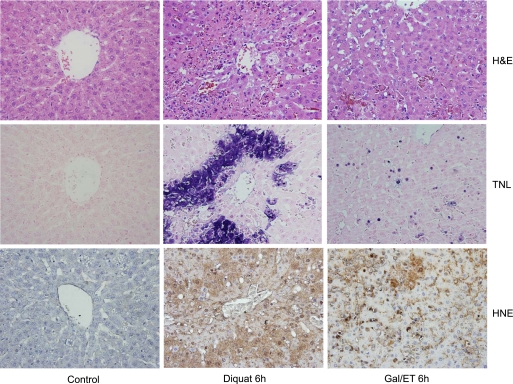
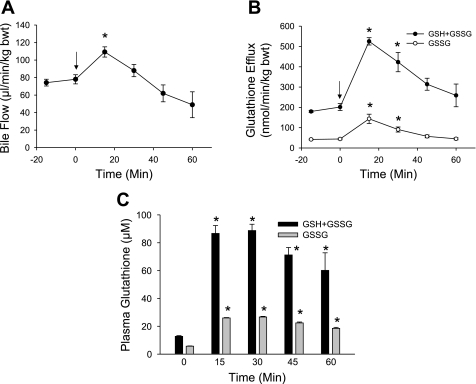
Treatment of male Fisher rats with 0.15 mmol/kg of the superoxide-generating agent diquat caused a significant oxidant stress in hepatocytes, as indicated by the 12-fold increase of the biliary GSSG efflux from a baseline of 58 nmol·min−1·kg body wt−1 before diquat to peak levels of 700 nmol·min−1·kg body wt−1 at 60–80 min after diquat administration (Fig. 1B). Bile flow was modestly reduced during the first hour, but then showed a steep decline during the second hour after diquat treatment (Fig. 1A). The plasma levels of GSSG increased by ∼60% compared with baseline values at 60 min (Fig. 1C). The diquat-induced oxidant stress resulted in progressive liver injury, as indicated by the release of ALT from hepatocytes (Fig. 2A) and histological evidence of cell necrosis at 3 and 6 h (Figs. 2B and 3). In addition, tissue ATP levels declined by 55% compared with controls at 6 h (Fig. 2C). The extensive areas of necrosis were concentrated around the central veins (Fig. 3). The areas of necrosis correlated with positive staining for 4-HNE protein adducts, an indicator of lipid peroxidation (Fig. 3). Although there were a number of TUNEL-positive cells in liver sections from diquat-treated rats, most cells showed nuclear and especially cytoplasmic staining, typically seen in cells undergoing oncotic necrosis (20, 21, 24). As a positive control for apoptosis, animals were treated with Gal/ET for 6 h (20, 22). Many individual hepatocytes showed the characteristic cell shrinkage and chromatin condensation of apoptosis and the typical TUNEL staining of apoptotic hepatocytes. In addition, increased caspase-3 activity and the leakage of small nuclear DNA fragments into the cytosol could be detected with an anti-histone ELISA at 6 h after Gal/ET (Table 1). These results are consistent with internucleosomal DNA cleavage by caspase-activated DNase (46). In contrast to these observations, there was neither an increase in hepatic caspase-3 activity, nor was there a significant increase of small DNA fragments released into the cytosol after diquat treatment. The increased numbers of TUNEL-positive cells (Table 1) were mainly cells that were swollen and had extensive cytoplasmic staining (Fig. 3).
Fig. 1.
Bile flow (A), the biliary efflux of glutathione disulfide (GSSG) and total glutathione (GSH + GSSG) (B), and plasma GSH and GSSG concentrations (C) were measured before and after intraperitoneal administration of 0.15 mmol/kg diquat (DQ) at time t = 0 min (arrow). Data represent means ± SE of n = 6 animals. *P < 0.05 (compared with baseline, t = 0).
Fig. 2.
Plasma alanine aminotransferase (ALT) activities (A), the area of necrosis (B), and liver ATP levels (C) were evaluated after intraperitoneal administration of 0.15 mmol/kg DQ. Data represent means ± SE of n = 6 animals per time point. *P < 0.05 (compared with baseline, t = 0).
Fig. 3.
Animals were treated with saline, 0.15 mmol/kg DQ, or 500 mg/kg galactosamine/0.1 mg/kg endotoxin (Gal/ET) for 6 h. Representative liver sections were stained with hematoxylin and eosin (H&E; top row), the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling [TUNEL (TNL)] assay (middle row), or a 4-hydroxynonenal (HNE) antibody (bottom row).
Table 1.
Caspase activities and DNA fragmentation after diquat and galactosamine/endotoxin
| Controls | Diquat | Gal/ET | |
|---|---|---|---|
| ALT, U/l | 46±3 | 955±91* | 3,867±158* |
| Caspase-3 activities, RFU·min−1·mg protein−1 | 23±2 | 23±1 | 59±4* |
| DNA fragmentation, %baseline | 100±3 | 127±26 | 604±4* |
| TUNEL-positive cells, cells/15 HPF | 6±1 | 35±4* | 618±79* |
Values are means ± SE of n = 6 animals per group. Fischer rats were treated with either saline (Controls), 0.15 mmol/kg diquat, or 500 mg/kg galactosamine/0.1 mg/kg endotoxin (Gal/ET) for 6 h. ALT, alanine aminotransferase; RFU, relative fluorescence unit; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; HPF, high-power field.
Role of JNK in oxidant stress-induced hepatocyte cell death.
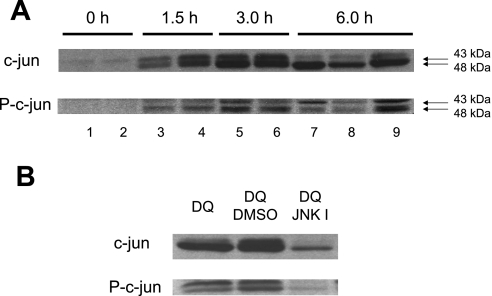
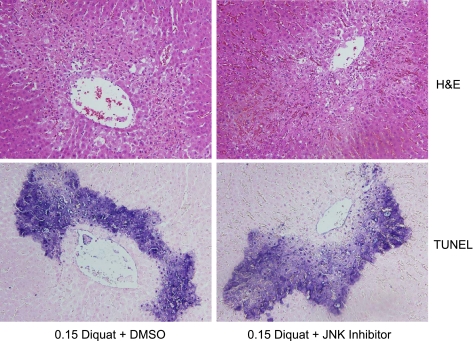
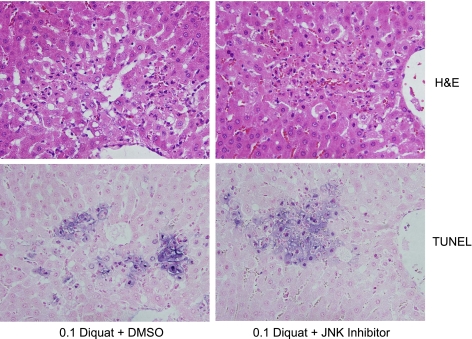
Previous observations in cultured hepatocytes suggested that JNK activation is involved in superoxide-induced apoptosis (11, 12, 44). To verify these findings in the intact liver, the levels of c-Jun and phospho-c-Jun were evaluated after diquat treatment. In control livers, only low levels of c-Jun were detectable (Fig. 4A). However, within 1.5 h after diquat administration, increased protein expression of c-Jun and its phosphorylated form were observed, suggesting rapid and prolonged JNK activation during superoxide formation in vivo (Fig. 4A). The increased c-Jun and phospho-c-Jun protein expression was effectively reduced by treatment with the JNK inhibitor SP-600125 (Fig. 4B). However, despite the efficacy of the inhibitor to prevent c-Jun expression and phosphorylation, there was no significant difference in the area of necrosis or the number of TUNEL-positive cells between vehicle- and JNK inhibitor-treated rats 6 h after diquat (Fig. 5). In addition, plasma ALT activities were also similar (Fig. 6A). These findings indicate that there is JNK activation during superoxide formation in vivo, but this effect may not be critical for the developing cell injury in the intact liver. To verify that this conclusion is also valid for a lower oxidant stress, the experiments were repeated with a dose of 0.1 mmol/kg diquat. This dose still caused liver injury, as indicated by the elevated plasma ALT activities (Fig. 6A), cell necrosis, and TUNEL-positive cells (Fig. 7). However, all parameters showed less severe injury with 0.1 mmol/kg diquat compared with the higher dose. Interestingly, there was almost no c-Jun or phospho-c-Jun expression 6 h after 0.1 mmol/kg diquat (Fig. 6B). In addition, treatment with the JNK inhibitor had no effect on any injury parameter (Figs. 6A and 7).
Fig. 4.
A: Western blot analysis of c-Jun and phospho-c-Jun (p-c-Jun) [indicator of c-Jun NH2-terminal kinase (JNK) activity] before and after treatment with 0.15 mmol/kg DQ. B: in addition to DQ (6 h), some animals were treated with 10 mg/kg of the JNK inhibitor SP-600125 or 5% DMSO/PBS 1 h after DQ. One to three representative samples are shown.
Fig. 5.
Animals were treated with 0.15 mmol/kg DQ and additionally either 5% DMSO/PBS or 10 mg/kg of the JNK inhibitor SP-600125 at 1 h after DQ. Representative liver sections were stained with H&E (top row) or the TUNEL assay (bottom row).
Fig. 6.
A: plasma ALT activities were measured 6 h after intraperitoneal administration of 0.15 or 0.10 mmol/kg DQ and, in addition, 5% DMSO/PBS (vehicle) or 10 mg/kg of the JNK inhibitor SP-600125. Data represent means ± SE of n = 4–6 animals per group. B: Western blot analysis of c-Jun and p-c-Jun (indicator of JNK) before and 6 h after treatment with DQ and SP-600125.
Fig. 7.
Animals were treated with 0.10 mmol/kg DQ and additionally either 5% DMSO/PBS or 10 mg/kg of the JNK inhibitor SP-600125 at 1 h after DQ. Representative liver sections were stained with H&E (top row) or the TUNEL assay (bottom row).
Menadione-induced oxidant stress and liver injury.
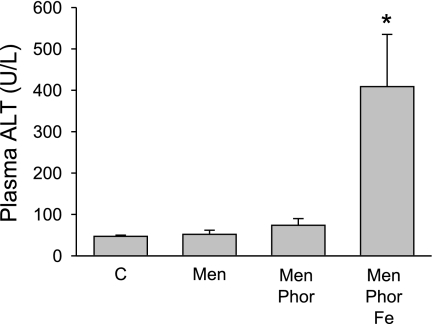
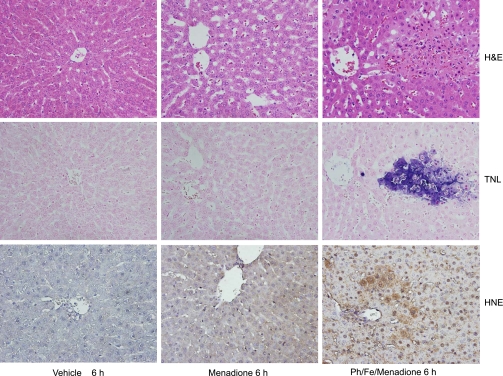
Since menadione was used in most in vitro studies on the role of superoxide-induced liver cell injury (11, 12, 39), animals were treated with a dose of 150 mg/kg menadione (10). This dose caused a mild oxidant stress in the liver, as indicated by the temporary increase of biliary GSSG efflux (Fig. 8B) and the prolonged increase of plasma GSSG levels (Fig. 8C). In addition, menadione also stimulated GSH release into plasma and bile. Despite the oxidant stress, menadione did not cause liver injury or lipid peroxidation, as indicated by the lack of 4-HNE protein adducts (Figs. 9 and 10). To increase the potential toxicity of the oxidant stress, animals were pretreated with phorone to deplete liver glutathione levels. Phorone reduced hepatic glutathione levels from 6.6 μmol/g liver to 0.8 μmol/g within 1 h. However, lowering hepatic glutathione levels by 88% did not enhance menadione-induced liver injury (Fig. 9). Therefore, a combination of iron and phorone was used (34). Under these conditions, moderate liver injury developed 6 h after menadione administration (Figs. 9 and 10). The areas of injury stained positive for 4-HNE protein adducts (Fig. 10). Histologically, the injury consisted of isolated focal areas of necrosis, where most cells stained positive with the TUNEL assay (Fig. 10). The staining pattern was similar to the one observed with diquat, i.e., mainly cytoplasmic staining of swollen cells, consistent with oncotic necrosis. There was no increase of caspase-3 activity with menadione alone or in combination with the other treatments (data not shown). Furthermore, there was no JNK activation in any of the three groups, and pretreatment with the JNK inhibitor SP-600125 had no effect on the injury 6 h after phorone-iron-menadione (data not shown).
Fig. 8.
Bile flow (A), the biliary efflux of GSSG and GSH + GSSG (B), and plasma GSH and GSSG concentrations (C) were measured before and after intravenous administration of 150 mg/kg menadione at t = 0 min (arrow). Data represent means ± SE of n = 6 animals. *P < 0.05 (compared with baseline, t = 0).
Fig. 9.
Plasma ALT activities were determined 6 h after intravenous administration of 5 ml/kg saline (C), 150 mg/kg menadione (Men) with or without 100 mg/kg phorone (Phor), and 0.36 mmol/kg ferrous iron sulfate (Fe). Data represent means ± SE of n = 4–6 animals. *P < 0.05 (compared with controls).
Fig. 10.
Animals were treated with vehicle (5 ml/kg saline) or 150 mg/kg menadione with or without 100 mg/kg phorone (Ph) and 0.36 mmol/kg Fe. Representative liver sections were stained with H&E (top row), the TUNEL assay (middle row), or a 4-HNE antibody (bottom row).
DISCUSSION
Diquat- and menadione-induced oxidant stress.
The objective of this investigation was to evaluate the mode of liver cell death during superoxide formation in vivo. We used the redox-cycling agent diquat, which can dose-dependently generate superoxide in the intact liver in vivo (59, 60) and in vitro (38). In most cell types, superoxide is metabolized by superoxide dismutases to form H2O2 and molecular oxygen. H2O2 is then effectively reduced to water by glutathione peroxidase with reducing equivalents for this reaction being provided by GSH, which is oxidized to GSSG (29). Most of the formed GSSG is immediately reduced back to GSH through glutathione reductase. However, a small percentage is exported into bile and plasma (2, 38, 42). Our laboratory previously estimated that ∼5% of the cytosolic GSSG formed is actually exported into the bile (34). Thus the increased biliary efflux of GSSG and the elevated plasma GSSG levels are reliable and specific indicators of an intracellular oxidant stress in hepatocytes (2, 38, 42). Based on the GSSG data, both diquat and menadione caused a significant oxidant stress in the liver. However, because of less side effects in vivo and potentially higher potency as a redox-cycler, it was possible to achieve higher superoxide formation with diquat than with menadione. The higher oxidant stress caused more liver injury after diquat treatment. In contrast, the moderate oxidant stress generated by menadione was unable to induce liver cell damage by itself; only depletion of the glutathione defense system, and providing iron to facilitate a Fenton-type reaction resulted in a moderate toxicity. In support of these conclusions, 4-HNE protein adducts, as indicator for lipid peroxidation, were only detected in areas undergoing necrosis after phorone-iron-menadione treatment but not after menadione alone. Overall, the data are consistent with previous observations that in vivo healthy hepatocytes are highly resistant to oxidant stress due to their effective antioxidant defense system (29, 45, 60).
In addition to formation of ROS, redox-cycling agents may cause toxicity by protein-thiol depletion. Although some quinone reagents can cause direct arylation of protein thiols (55), the well-known effect of menadione on protein thiols is caused mainly by formation of glutathione mixed disulfides (5, 41). However, the pathophysiological relevance of protein thiol loss is controversially discussed. There is evidence for a critical role of protein thiol depletion in hepatocyte cell death caused by menadione (5, 17, 55), and there is evidence to suggest that ROS formation and lipid peroxidation may be more important in vitro (11, 41). In contrast to the situation with menadione, diquat causes neither protein arylation (61) nor protein thiol depletion in vivo (58). Thus diquat toxicity is caused directly by ROS formation, iron mobilization, and lipid peroxidation in vivo (9, 59). Oxidant stress can mobilize lysosomal iron, which is critical for lipid peroxidation and the opening of the mitochondrial MPT pore opening (63). Consistent with these findings, 4-HNE protein adducts were detected in hepatocytes undergoing necrosis after diquat treatment. Therefore, diquat is the more specific reagent to generate superoxide and is better suited for experiments designed to test if extensive superoxide formation causes apoptosis in vivo.
Superoxide-induced hepatocellular necrosis.
Despite the resistance to oxidant stress, it is possible to cause hepatocellular injury by generating excessive amounts of superoxide with diquat or menadione. Under in vivo conditions, the cell death induced by superoxide formation is characterized by vacuolation and cell swelling, resulting in ALT release. In contrast, cell injury after Gal/ET, which is a well-established model of TNF-induced, caspase-mediated apoptosis (35, 43), was characterized by cell shrinkage, caspase activation, and DNA degradation assessed by two independent parameters. The anti-histone ELISA demonstrates the leakage of small DNA fragments into the cytosol, indicative of endonuclease-mediated DNA fragmentation (37). In addition, the TUNEL-positive cells showed distinct nuclear staining, characteristic of apoptotic cells (37). Although apoptotic cells generally do not release cell contents, the high ALT values indicated that some of the apoptotic hepatocytes were undergoing secondary necrosis (37, 50). In this model, this may be caused by neutrophil cytotoxicity involving ROS formation (23, 35). In contrast to these observations, diquat or menadione neither activated caspases nor showed release of small DNA fragments from the nucleus. There was TUNEL staining in most dying cells. However, these cells were enlarged (swollen) and showed not only nuclear but also cytoplasmic staining. Similar TUNEL staining patterns, which reflect mainly DNA strand breaks and large-scale DNA damage, have been shown in necrotic cells after APAP overdose and ischemia-reperfusion injury (20, 24). Taken together, based on morphological features of injured cells, caspase activation, and two methods of assessing DNA fragmentation, there are clear differences between liver cell injury induced by excessive oxidant stress and the characteristic apoptosis and secondary necrosis of the Gal/ET model. Thus these data strongly support the conclusion that diquat causes oncotic necrosis in the intact liver in vivo via mechanisms involving superoxide generation and lipid peroxidation.
Role of JNK activation in superoxide-induced cell death.
Activation of JNK was shown to be critical for superoxide-induced apoptosis in cultured rat hepatocytes (11, 12, 15). However, the exact mechanisms of the involvement of JNK in the signaling pathways of cell death remain unclear (14). A recent report in APAP toxicity suggested that JNK activation can induce mitochondrial oxidant stress and opening of the mitochondrial MPT pore (27). Since peroxide-induced oxidant stress can trigger mitochondrial reactive oxygen formation and the MPT, which caused necrotic cell death in cultured hepatocytes (48, 49), such a mechanism of action for JNK may be feasible in vitro. Our in vivo data confirmed the activation of JNK, as indicated by the phosphorylation of its substrate c-Jun. However, JNK activation was only detected after the most severe oxidant stress. A more moderate oxidant stress with a lower diquat dose or menadione did not result in JNK activation, despite significant liver injury. One caveat could be that c-Jun phosphorylation in only a limited number of hepatocytes may be difficult to detect in vivo. However, the fact that the specific JNK inhibitor SP-600125 attenuated c-Jun phosphorylation at the higher diquat dose but did not reduce injury at either the higher or lower dose suggests that JNK does not play a critical role in superoxide-mediated oncotic necrosis in vivo.
Prevalence of an apoptotic response in vitro.
Our data demonstrate that superoxide generated by different chemicals in rat liver in vivo was effectively detoxified. However, the extensive oxidant stress caused dose-dependent liver cell injury several hours later. Based on a number of specific parameters, including morphology, caspase activity, and DNA fragmentation, the mode of cell death was clearly not apoptosis but oncotic necrosis. Although there was JNK activation after treatment with a high dose of diquat, the effect did not appear to be critical for the oxidant stress-induced cell death in rat liver. These in vivo data are in clear contrast to several studies showing that superoxide-mediated cell death in cultured rat hepatocytes is mediated by apoptosis, which involves the activation of caspases and JNK (11, 12, 44). Since the investigators reporting these studies used menadione and paraquat as superoxide-generating agents, used primary rat hepatocytes or a nontransformed rat hepatocyte cell line, used a similar time frame (6 h), and used similar specific parameters to convincingly demonstrate the occurrence of apoptosis, the most relevant difference between the previous studies and our investigation is the fact that we investigated the effect of oxidant stress in vivo compared with cultured cells. Thus, under in vivo conditions, extensive superoxide formation is either effectively detoxified or, if the antioxidant defense system is overwhelmed, the cell dies by oncotic necrosis. Cultured hepatocytes can also detoxify reactive oxygen and, with excessive oxidant stress, can be killed by oncotic necrosis (17, 48, 53, 54, 64). However, a moderate oxidant stress can induce apoptosis in cultured cells (11, 12, 39, 54), an effect that is not observed in vivo. This raises the question why cultured hepatocytes are more susceptible to superoxide-induced apoptosis or why there is no apoptosis in vivo?
One possible reason for the lack of apoptotic cell death during oxidant stress in vivo might be a drop in cellular ATP levels. Indeed, our data indicate a substantially lower liver ATP content 6 h after diquat. Since this 55% drop in ATP levels reflects the average for all liver cells, it is likely that the levels in cells undergoing cell death may be even lower, i.e., low enough to prevent apoptosis. In contrast, high-glucose levels in the culture medium may allow cultured hepatocytes to maintain cellular ATP levels through enhanced glycolysis and thus allow them to undergo apoptosis. Alternatively, as has been pointed out by Halliwell (25), cells are exposed to a severe stress during the isolation procedure and culture. Many of the less hardy cells die, and only the most resistant and most adaptable cells survive (25). This stress results in a substantial modulation of the gene expression profile (6). In addition, the artificial cell culture conditions with hyperoxia for hepatocytes (21% oxygen) cause continuously enhanced mitochondrial superoxide formation (25). Thus it is not surprising that cultured cells may be more susceptible to additional insults, such as a moderate oxidant stress, and die by apoptosis (26). This hypothesis is also supported by observations with APAP toxicity. An overdose of APAP causes reactive metabolite formation, which depletes glutathione and causes mitochondrial dysfunction and oxidant stress, which ultimately leads to oncotic necrosis in vivo (24). If the metabolic activation of APAP is prevented by an inhibitor of cytochrome P-450, the livers in vivo are completely protected (28). However, if a cultured cell that lacks cytochrome P-450 is exposed to APAP, the cell does not die from necrosis but from caspase-dependent apoptosis (7). Likewise, if APAP-induced necrosis is prevented in primary hepatocytes, hepatocytes undergo apoptosis a few hours later (40). Taken together, a pattern appears to emerge that cells in culture are more prone to undergo apoptosis in response to stresses, including oxidant stress. If future studies confirm this trend, it further emphasizes the need to validate signaling mechanisms of cell injury, which have been established in cultured cells, in relevant in vivo models. Our findings with the redox-cycling agent diquat confirmed the oxidant stress-induced activation of JNK in the intact liver in vivo, but could not support the relevance of this pathway for cell death. In addition, liver cells in vivo mainly undergo oncotic necrosis in response to diquat-induced oxidant stress and not caspase-dependent apoptosis, as observed in cultured hepatocytes treated with superoxide-generating agents.
GRANTS
This investigation was supported in part by National Institutes of Health (NIH) Grants R01 DK070195 and R56 AA12916, and by grants P20 RR016475 and P20 RR 021940 from the National Center for Research Resources, a component of the NIH.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adachi M, Ishii H. Role of mitochondria in alcoholic liver injury. Free Radic Biol Med 32: 487–491, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Adams JD Jr, Lauterburg BH, Mitchell JR. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther 227: 749–754, 1983. [PubMed] [Google Scholar]
- 3.Arteel GE Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 124: 778–790, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci 58: 109–117, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bellomo G, Mirabelli F, DiMonte D, Richelmi P, Thor H, Orrenius C, Orrenius S. Formation and reduction of glutathione-protein mixed disulfides during oxidative stress. A study with isolated hepatocytes and menadione (2-methyl-1,4-naphthoquinone). Biochem Pharmacol 36: 1313–1320, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Boess F, Kamber M, Romer S, Gasser R, Muller D, Albertini S, Suter L. Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared with the in vivo liver gene expression in rats: possible implications for toxicogenomics use of in vitro systems. Toxicol Sci 73: 386–402, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Boulares AH, Zoltoski AJ, Stoica BA, Cuvillier O, Smulson ME. Acetaminophen induces a caspase-dependent and Bcl-XL sensitive apoptosis in human hepatoma cells and lymphocytes. Pharmacol Toxicol 90: 38–50, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Britton RS Metal-induced hepatotoxicity. Semin Liver Dis 16: 3–12, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Burk RF, Lawrence RA, Lane JM. Liver necrosis and lipid peroxidation in the rat as the result of paraquat and diquat administration. Effect of selenium deficiency. J Clin Invest 65: 1024–1031, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiou TJ, Zhang J, Ferrans VJ, Tzeng WF. Cardiac and renal toxicity of menadione in rat. Toxicology 124: 193–202, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol 44: 918–929, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Conde de la Rosa L, Vrenken TE, Hannivoort RA, Buist-Homan M, Havinga R, Slebos DJ, Kauffman HF, Faber KN, Jansen PL, Moshage H. Carbon monoxide blocks oxidative stress-induced hepatocyte apoptosis via inhibition of the p54 JNK isoform. Free Radic Biol Med 44: 1323–1333, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Czaja MJ Induction and regulation of hepatocyte apoptosis by oxidative stress. Antioxid Redox Signal 4: 759–767, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Czaja MJ The future of GI and liver research: editorial perspectives. III. JNK/AP-1 regulation of hepatocyte death. Am J Physiol Gastrointest Liver Physiol 284: G875–G879, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Czaja MJ, Liu H, Wang Y. Oxidant-induced hepatocyte injury from menadione is regulated by ERK and AP-1 signaling. Hepatology 37: 1405–1413, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology 43, Suppl 1: S63–S74, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Di Monte D, Bellomo G, Thor H, Nicotera P, Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys 235: 343–350, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Friedman SL Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 1: 98–105, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Griffith OW Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106: 207–212, 1980. [DOI] [PubMed] [Google Scholar]
- 20.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology 33: 397–405, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology 38: 355–363, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Gujral JS, Farhood A, Jaeschke H. Oncotic necrosis and caspase-dependent apoptosis during galactosamine-induced liver injury in rats. Toxicol Appl Pharmacol 190: 37–46, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol 287: G243–G252, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 67: 322–328, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett 540: 3–6, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Han D, Hanawa N, Saberi B, Kaplowitz N. Hydrogen peroxide and redox modulation sensitize primary mouse hepatocytes to TNF-induced apoptosis. Free Radic Biol Med 41: 627–639, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem 283: 13565–13577, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaeschke H Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther 255: 935–941, 1990. [PubMed] [Google Scholar]
- 29.Jaeschke H Mechanisms of oxidant stress-induced acute tissue injury. Proc Soc Exp Biol Med 209: 104–111, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Jaeschke H Role of reactive oxygen species in hepatic ischemia-reperfusion injury and preconditioning. J Invest Surg 16: 127–140, 2003. [PubMed] [Google Scholar]
- 31.Jaeschke H Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol 284: G15–G26, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Jaeschke H Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol 290: G1083–G1088, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 89: 31–41, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Jaeschke H, Benzick AE. Pathophysiological consequences of enhanced intracellular superoxide formation in isolated perfused rat liver. Chem Biol Interact 84: 55–68, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol 160: 3480–3486, 1998. [PubMed] [Google Scholar]
- 36.Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett 144: 279–288, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125: 1246–1257, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Jaeschke H, Smith CV, Mitchell JR. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest 81: 1240–1246, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones BE, Lo CR, Liu H, Pradhan Z, Garcia L, Srinivasan A, Valentino KL, Czaja MJ. Role of caspases and NF-kappaB signaling in hydrogen peroxide- and superoxide-induced hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol 278: G693–G699, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40: 1170–1179, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Kyle ME, Nakae D, Sakaida S, Serroni A, Farber JL. Protein thiol depletion and the killing of cultured hepatocytes by hydrogen peroxide. Biochem Pharmacol 38: 3797–3805, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Lauterburg BH, Smith CV, Hughes H, Mitchell JR. Biliary excretion of glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. J Clin Invest 73: 124–133, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol 146: 1220–1234, 1995. [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Lo CR, Czaja MJ. NF-kappaB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology 35: 772–778, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Mathews WR, Guido DM, Fisher MA, Jaeschke H. Lipid peroxidation as molecular mechanism of liver cell injury during reperfusion after ischemia. Free Radic Biol Med 16: 763–770, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H. Degradation of chromosomal DNA during apoptosis. Cell Death Differ 10: 108–116, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 37: 1202–1219, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am J Physiol Cell Physiol 272: C1286–C1294, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Nieminen AL, Saylor AK, Tesfai SA, Herman B, Lemasters JJ. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem J 307: 99–106, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature 364: 806–809, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Petersen DR Alcohol, iron-associated oxidative stress, and cancer. Alcohol 35: 243–249, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Poli G Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med 21: 49–98, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Saberi B, Shinohara M, Ybanez MD, Hanawa N, Gaarde W, Kaplowitz N, Han D. Regulation of H2O2-induced necrosis by protein kinase C and AMP-activated kinase signaling in primary cultured hepatocytes. Am J Physiol Cell Physiol 295: C50–C63, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samali A, Nordgren H, Zhivotovsky B, Peterson E, Orrenius S. A comparative study of apoptosis and necrosis in HepG2 cells: oxidant-induced caspase inactivation leads to necrosis. Biochem Biophys Res Commun 255: 6–11, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Schmieder PK, Tapper MA, Kolanczyk RC, Hammermeister DE, Sheedy BR, Denny JS. Discriminating redox cycling and arylation pathways of reactive chemical toxicity in trout hepatocytes. Toxicol Sci 72: 66–76, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Schwarz KB Oxidative stress during viral infection: a review. Free Radic Biol Med 21: 641–649, 1996. [DOI] [PubMed] [Google Scholar]
- 57.Singh R, Czaja MJ. Regulation of hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol 22, Suppl 1: S45–S48, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Smith CV Effect of BCNU pretreatment on diquat-induced oxidant stress and hepatotoxicity. Biochem Biophys Res Commun 144: 415–421, 1987. [DOI] [PubMed] [Google Scholar]
- 59.Smith CV Evidence for participation of lipid peroxidation and iron in diquat-induced hepatic necrosis in vivo. Mol Pharmacol 32: 417–422, 1987. [PubMed] [Google Scholar]
- 60.Smith CV, Hughes H, Lauterburg BH, Mitchell JR. Oxidant stress and hepatic necrosis in rats treated with diquat. J Pharmacol Exp Ther 235: 172–177, 1985. [PubMed] [Google Scholar]
- 61.Spalding DJ, Mitchell JR, Jaeschke H, Smith CV. Diquat hepatotoxicity in the Fischer-344 rat: the role of covalent binding to tissue proteins and lipids. Toxicol Appl Pharmacol 101: 319–327, 1989. [DOI] [PubMed] [Google Scholar]
- 62.Tietze F Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27: 502–522, 1969. [DOI] [PubMed] [Google Scholar]
- 63.Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology 48: 1644–1654, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Y, Bradham C, Brenner DA, Czaja MJ. Hydrogen peroxide-induced liver cell necrosis is dependent on AP-1 activation. Am J Physiol Gastrointest Liver Physiol 273: G795–G803, 1997. [DOI] [PubMed] [Google Scholar]