Abstract
Although previous studies have implicated lymphocytes in the gut microvascular and inflammatory responses to ischemia-reperfusion (I/R), the lymphocyte population and lymphocyte-derived products that mediate these responses have not been defined. Platelet and leukocyte adhesion was measured in intestinal postcapillary venules of wild-type (WT) mice and mice genetically deficient in either CD4+ T cells (CD4−/−), CD8+ T cells (CD8−/−), B cells (B cell−/−), or interferon-γ (IFN-γ−/−) subjected to 45 min of ischemia and 4 h of reperfusion. The I/R-induced platelet and leukocyte recruitment responses were also evaluated following adoptive transfer of WT splenocytes into CD4−/−, CD8−/−, B cell−/−, and IFN-γ−/− mice. WT mice exposed to gut I/R exhibited significant increases in the adhesion of both platelets and leukocytes, compared with sham-WT mice. These blood cell adhesion responses to I/R were greatly attenuated in CD4−/−, CD8−/−, B cell−/−, and IFN-γ−/− mice. Adoptive transfer of WT splenocytes restored the WT responses to I/R in all mutants except the B cell−/− mice. These findings implicate both T and B cells and lymphocyte-derived IFN-γ as mediators of the proinflammatory and prothrombogenic phenotype assumed by intestinal microvessels after I/R.
Keywords: inflammation, microcirculation, cytokine, platelet-leukocyte adhesion
tissues exposed to ischemia and reperfusion develop an inflammatory phenotype, which is evident early after reperfusion in the microvasculature and is manifested in venules as an increased expression of endothelial cell adhesion molecules, enhanced production of reactive oxygen species, endothelial barrier dysfunction, and an increased recruitment of adherent leukocytes and platelets. Because activated, adherent neutrophils have been implicated in the microvascular dysfunction and tissue injury that results from ischemia-reperfusion (I/R), considerable effort has been devoted to defining the factors that regulate neutrophil-endothelial cell adhesion following I/R (6, 14).
A variety of cells that either circulate in blood (e.g., platelets) or normally reside in extravascular tissue (e.g., macrophages, mast cells) have been implicated in I/R-induced leukocyte-endothelial cell adhesion (6, 7, 13, 14). Recently, lymphocytes have also received attention as potential modulators of neutrophil recruitment in postischemic tissues, including liver (4, 5, 20), kidney (10, 15), and intestine (7, 16). Furthermore, monolayers of cultured endothelial cells subjected to anoxia-reoxygenation (A/R) exhibit an increased adhesivity to neutrophils, which is enhanced by the presence of T lymphocytes (11, 12). This T cell-mediated exaggeration of the A/R-induced neutrophil-endothelial cell adhesion is prevented when direct contact between the cocultured endothelial cells and T cells is prevented, suggesting that soluble factors produced by the T cells contribute to the response (12).
Studies of the intestinal microcirculation have revealed that severe combined immunodeficient (SCID) mice, which lack both T and B lymphocytes, exhibit an attenuated recruitment of adherent neutrophils and a blunted vascular permeability response following I/R (16). A similar attenuation of platelet adhesion has been noted in postischemic intestinal venules (7) of SCID mice. Although these studies indicate that T lymphocytes or B lymphocytes or both influence the recruitment of neutrophils and platelets in the postischemic intestinal microcirculation, the specific lymphocyte populations that underlie the I/R-induced blood cell recruitment have not been defined, nor has it been determined whether a lymphocyte-derived product contributes to this response. Studies in other tissues have revealed variable roles for T and B cells as well as CD4+ and CD8+ T cells in the microvascular dysfunction and tissue injury responses to I/R (2, 4, 8, 19). Similarly, interferon-γ (IFN-γ) and other lymphocyte-derived cytokines have exhibited variable contributions in different models of I/R (1, 19). Hence the overall objective of the present study was to more precisely define the contribution of different lymphocyte populations as well as lymphocyte-derived IFN-γ to the recruitment of leukocytes and platelets in intestinal venules after I/R. Our findings implicate CD4+ and CD8+ T cells as well as B lymphocytes as modulators of the I/R-induced blood cell recruitment response. T cell-derived IFN-γ also plays an important role in this response.
MATERIALS AND METHODS
Animals.
Wild-type (WT) C57/BL6J (n = 16), CD4+ T lymphocyte-deficient (CD4−/−, n = 16), CD8+ T cell-deficient (CD8−/−, n = 16), B lymphocyte-deficient (B cell−/−, n = 13), and IFN-γ-deficient (IFN-γ−/−, n = 13) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The mice were maintained on a 12:12-h light-dark cycle under pathogen-free conditions, in which they had ad libitum access to a standard diet and water until reaching the desired age (8–10 wk) and/or weight (20–25 g). All procedures using animals were reviewed and approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center and were performed according to the criteria outlined by the National Institutes of Health.
Surgical procedure.
The procedures used to evaluate blood cell adhesion in the murine intestinal microvasculature have been described previously (7). Briefly, the animals were anesthetized subcutaneously with a mixture of ketamine and xylazine at doses of 100 and 5 mg/kg, respectively. Following an upper midline abdominal incision, the superior mesenteric artery (SMA) was isolated and clamped for a period of 45 min with a microbulldog clamp. In sham control mice, the SMA was isolated without clamping and was exposed to the same procedure as mice with SMA occlusion. Upon release of the clamp, the abdominal incision was closed with silk sutures and the mice were allowed to recover from anesthesia for a period of 5 h. All mice received the analgesic carprophen (5 mg/kg sc) for relief of pain and discomfort from the surgical procedure.
Platelet preparation.
Approximately 0.9 ml of blood was collected from WT mice through a carotid artery cannula (polyethylene tubing, PE-10) into a polypropylene tube containing 0.1 ml of acid-dextrose buffer (Sigma; St. Louis, MO). The tube was centrifuged at 1,200 rpm for 8 min. The supernatant was transferred into another polypropylene tube and centrifuged again at 1,200 rpm for 3 min to remove any residual red blood cells (RBCs). This supernatant was transferred to another new tube and centrifuged at 3,000 rpm for 10 min to separate the platelets from the plasma. The platelets were suspended in phosphate-buffered saline (PBS), counted, and divided into aliquots that contained 1–1.5 million platelets. Before injection into recipient mice, the platelets were incubated for 10 min with the fluorochrome carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, Eugene, OR) at a final concentration of 90 μM. The platelet suspension was then centrifuged at 3,000 rpm for 10 min. The pellet was resuspended in ∼250 μl PBS and then protected from light until injection into mice. (7)
Splenocyte preparation.
Whole splenocyte suspensions were generated from spleens of WT mice, as previously described (17). Splenocytes were removed by blunt dissection and scraping of freshly isolated spleens through a mesh screen (Cellector Tissue Sieve, E-C Apparatus, St. Petersburg, FL) irrigated by PBS. Splenocytes were collected by centrifugation, and RBCs were removed by hypertonic lysis. Mononuclear splenocytes were harvested by centrifugation and used as whole splenocytes in this study. The splenocytes were counted and aliquoted into samples containing at least 50 × 106 splenocytes (consisting of ∼30% T cells and 60% B cells) for intravenous injection 48 h before the experiment. The timing, route of administration, and number of splenocytes injected were based on studies previously reported from this laboratory (9).
Intravital videomicroscopy.
Approximately 5 h following the procedure for occlusion and reperfusion of the SMA, the mice were anesthetized with ketamine and xylazine as described above. The right carotid artery and the right jugular vein were cannulated with polyethylene tubing (PE-10). The sutured abdominal incision was reopened, and a loop of small intestine was carefully exteriorized and placed on a thin sheet of glass through which the bowel wall could be visualized with an inverted fluorescent microscope. After a 20-min stabilization period, the CFSE-labeled platelets were injected via the jugular vein. Five minutes thereafter, rhodamine-6G (0.02%) was administered via the jugular vein for fluorescent labeling and visualization of leukocytes.
Fluorescently labeled platelets and leukocytes were visualized with a Nikon Diaphot upright microscope equipped with a 75-W XBO xenon lamp and ×40 objective. Visualization required a Nikon filter block with an excitation filter (470–490 nm), a dichroic mirror (510 nm), and a barrier filter (520 nm). The images were viewed on a monitor (Sony Trinitron, PVM-2030, diagonal 50.6 cm) and recorded on video for offline evaluation. The microscopic images were received by a charge-coupled device (CCD) video camera (model C2400, Hamamatsu) and optimized by a CCD camera controller (model C2400, Hamamatsu). The images were then recorded on a video recorder (model BR-S601MU, JVC) for offline evaluation. A 1-min recording of a 100-μm length of four to eight intestinal venules was obtained from each mouse.
The total number of adherent leukocytes adhering in postischemic intestinal venules was determined, as well as the number of adherent leukocytes with or without attached platelets. Total platelet adhesion to the venular wall was also measured as well as the number of adherent platelets binding directly to the vessel wall (endothelial cells) or indirectly via attachment to adherent leukocytes. An adherent leukocyte or platelet was defined as cells remaining stationary on the vessel wall for ≥30 s. Blood cell adherence is expressed as the number of cells per square millimeter of venular surface, calculated from diameter and length, and assuming cylindrical vessel shape. Venular diameter was measured with a video caliper. Estimates of pseudoshear rate in venules were obtained from venular diameter (Dv) and the maximal velocity of flowing platelets (Vplt) according to the following formulation: pseudoshear rate = (Vplt/1.6)/Dv × 8 (18).
Experimental groups.
The following experimental groups were included in this study: 1) WT sham controls (n = 6), 2) WT mice exposed to gut I/R (n = 10) (as described above), 3) I/R in CD4−/− mice (n = 8), 4) I/R in CD4−/− after reconstitution with WT splenocytes (adoptive transfer) (n = 8), 5) I/R in CD8−/− mice (n = 10), 6) I/R in CD8−/− mice + adoptive transfer (n = 6), 7) I/R in B cell−/− mice (n = 7), 8) I/R in B cell−/− mice + adoptive transfer (n = 6), 9) I/R in IFN-γ−/− mice (n = 7), and 10) I/R in IFN-γ−/− mice + adoptive transfer (n = 6).
Data analysis.
Statistical analyses of the data were performed with StatView 4.5 software (Abacus Concepts, Berkeley, CA) using one-way ANOVA with Fisher's (post hoc) test. All values are reported as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
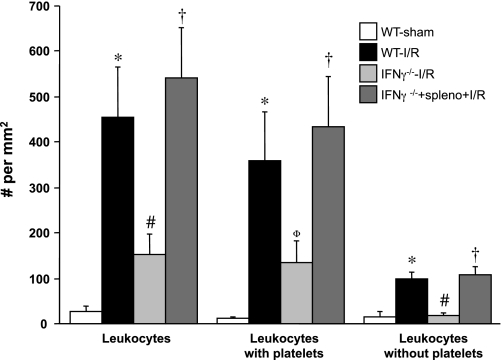
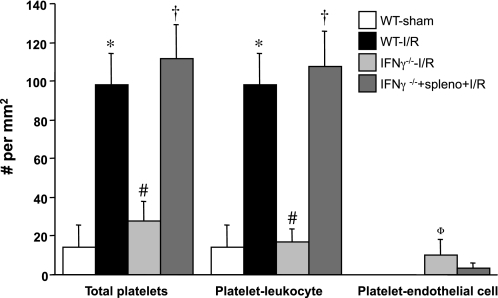
In WT I/R mice, mean arterial pressure and venular pseudoshear rate values of 67.5 ± 2.1 mmHg and 249.9 ± 15.1 s−1, respectively were recorded. No statistical differences were noted for the arterial pressure values between WT I/R and all other experimental groups. Blood platelet and neutrophil counts also did not differ between groups. A similar pattern was observed for venular wall pseudoshear rate, except that higher values were noted in CD8−/− (26%) and B cell−/− (32%) mice. As previously reported (7, 13), gut I/R leads to significant increases (16- and 6.6-fold, respectively) in the number of adherent leukocytes (Fig. 1) and platelets (Fig. 2) in postcapillary venules of WT mice, compared with sham controls. Most of the leukocytes lining the walls of postischemic intestinal venules have one or more platelets attached, with a very small proportion of the leukocytes that are platelet free. Similarly, most of the platelets accumulating in venules after I/R are bound to adherent leukocytes, with only a minor fraction of platelets binding directly to venular endothelium.
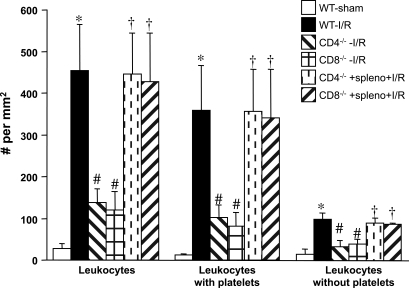
Fig. 1.
Effects of gut ischemia-reperfusion (I/R) on leukocyte adhesion in intestinal venules of wild-type (WT-I/R), CD4+ T cell-deficient (CD4−/−-I/R), CD8+ T cell-deficient (CD8−/−-I/R), as well as CD4−/− and CD8−/− mice after adoptive transfer with WT splenocytes (spleno). The I/R-induced adhesion responses in WT-sham mice are also shown. *P < 0.05 compared with WT-sham mice, #P < 0.05 relative to WT-I/R mice, †P < 0.05 compared with corresponding mutant mouse without splenocyte transfer.
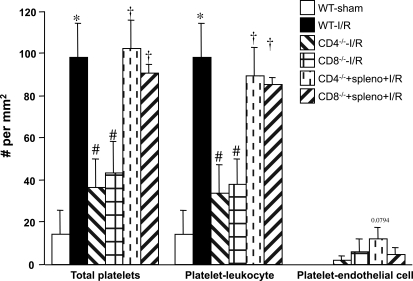
Fig. 2.
Effects of gut I/R on platelet adhesion in intestinal venules of WT-I/R, CD4−/−-I/R, CD8−/−-I/R, as well as CD4−/− and CD8−/− mice after adoptive transfer with WT splenocytes. The I/R-induced adhesion responses in WT-sham mice are also shown. *P < 0.05 compared with WT-sham mice, #P < 0.05 relative to WT-I/R mice, †P < 0.05 compared with corresponding mutant mouse without splenocyte transfer.
Figures 1 and 2 also compare the I/R-induced leukocyte and platelet adhesion responses in WT mice to the responses elicited in CD4+-T cell-deficient (CD4−/−) and CD8+-T cell-deficient (CD8−/−) mice exposed to the same I/R protocol. The data reveal that a deficiency in either T lymphocyte population results in greatly attenuated I/R-induced leukocyte and platelet adhesion responses. The relative contributions of platelet-leukocyte and platelet-endothelial adhesion to the total numbers of adherent leukocytes and platelets after I/R were not altered (compared with WT mice) in the CD4−/− and CD8−/− mice. CD4−/− and CD8−/− mice that received WT splenocytes 48 h prior to the induction of gut I/R exhibited leukocyte and platelet adhesion responses that were similar to those noted in WT I/R mice.
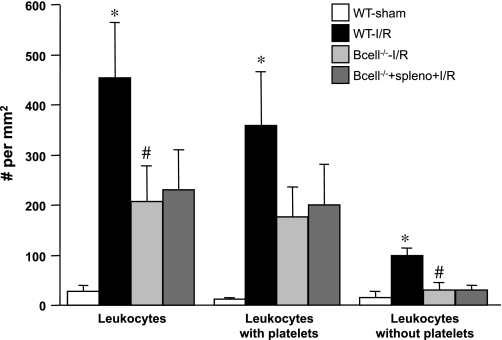
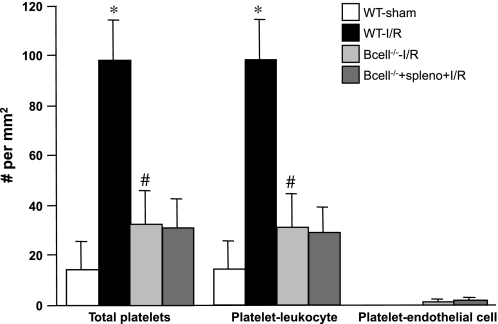
The effects of B lymphocyte deficiency (B cell−/−) on the gut I/R-induced changes in leukocyte and platelet adhesion are shown in Figs. 3 and 4. B cell−/− mice subjected to gut I/R exhibited attenuated leukocyte and platelet recruitment responses. As noted with the CD4−/− and CD8−/− mice, the relative contributions of platelet-leukocyte and platelet-endothelial adhesion to the total numbers of adherent leukocytes and platelets were not altered after I/R in B cell−/− mice (compared with WT mice). However, in B cell−/− mice, the adoptive transfer of splenocytes did not restore the I/R-induced leukocyte and platelet adhesion responses that are observed in WT mice and in splenocyte-reconstituted CD4−/− and CD8−/− mice (Figs. 1 and 2).
Fig. 3.
Role of B lymphocytes in gut I/R-induced recruitment of adherent leukocytes. The adhesion responses in venules of WT-sham and WT-I/R mice are compared with those observed in B cell-deficient (B cell−/−) with or without adoptive transfer of splenocytes. *P < 0.05 compared with WT-sham mice, #P < 0.05 relative to WT-I/R mice.
Fig. 4.
Role of B lymphocytes in gut I/R-induced recruitment of adherent platelets. The adhesion responses in venules of WT-sham and WT-I/R mice are compared with those observed in B cell−/− mice with or without adoptive transfer of splenocytes. *P < 0.05 compared with WT-sham mice, #P < 0.05 relative to WT-I/R mice.
Figures 5 and 6 summarize the gut I/R-induced blood cell adhesion responses in IFN-γ-deficient (IFN-γ−/−) mice and in IFN-γ−/− after adoptive transfer with WT splenocytes. Both the leukocyte and platelet adhesion responses to gut I/R were significantly attenuated in IFN-γ−/− mice. Adoptive transfer of WT splenocytes in IFN-γ−/− mice fully restored the leukocyte and platelet adhesion responses that were evident in WT mice exposed to gut I/R.
Fig. 5.
Responses of leukocyte adhesion in intestinal venules after I/R in interferon-γ-deficient (IFN-γ−/−) mice and in IFN-γ−/− following adoptive transfer of WT splenocytes. *P < 0.05 compared with WT-sham mice, #P < 0.05 relative to WT-I/R mice, †P < 0.05 compared with IFN-γ−/− mice without splenocyte transfer; ΦP = 0.07 compared with WT-I/R.
Fig. 6.
Responses of platelet adhesion in intestinal venules after I/R in IFN-γ-deficient (IFN-γ−/−) mice and in IFN-γ−/− following adoptive transfer of WT splenocytes. *P < 0.05 compared with WT-sham mice, #P < 0.05 relative to WT-I/R mice, †P < 0.05 compared with IFN-γ−/− mice without splenocyte transfer. ΦP = 0.056 compared with WT-I/R.
DISCUSSION
Both neutrophils and platelets are known to accumulate in postischemic tissues, where they contribute to the microvascular dysfunction and tissue injury that often accompanies ischemia and reperfusion (6, 14). The time course and intensity of neutrophils and platelet recruitment in postischemic microvessels appear to be regulated by a variety of chemical and cellular mediators. Although lymphocytes have been previously shown to modulate the recruitment of neutrophils and the subsequent neutrophil-mediated microvascular dysfunction associated with gut I/R (16), the identity of the lymphocyte subpopulation involved and the lymphocyte-derived products that mediate these responses have not been previously determined. In this study, we provide evidence that implicates both CD4+ and CD8+ T cells, B cells, as well as T cell-derived IFN-γ as key modulators of the leukocyte and platelet adhesion responses elicited by I/R in the gut microvasculature.
One of the major objectives of the present study was to determine whether T lymphocytes, B lymphocytes, or both cell populations modulate the blood cell recruitment responses elicited by gut I/R. Although previous efforts to define the contributions of these two lymphocyte populations to the pathophysiology of ischemia-reperfusion injury in other organs have revealed different outcomes, most reports indicate a dominant role for T lymphocytes. In the liver, it appears that T cells (not B cells) mediate the neutrophil infiltration and hepatocellular injury elicited by hepatic I/R (20). A similar pattern of protection by T cells (and not B cells) has been reported for the liver microcirculation after gut I/R (8). In postischemic brain venules, T cell deficiency blunts the neutrophil and platelet adhesion responses whereas B cell deficiency does not (19). However, both B cell-deficient mice as well as T cell-deficient mice show significant protection against the tubular damage and renal dysfunction that accompanies I/R in the kidney (2, 3). Furthermore, the protection afforded to the kidneys by B cell deficiency is not reversed by adoptive transfer of B cells from WT donor mice, but some reversal was noted after transfer of WT serum into the B cell−/− mice, suggesting a role for a soluble mediator, such as an antibody (2). In the present study, we observed that a deficiency of either B cells or T cells effectively blunts the recruitment of both leukocytes and platelets into the postischemic intestinal venules. Our finding that B cell deficiency affords protection whereas the adoptive transfer of splenocytes does not reverse this response is consistent with previous observations in the kidney and suggests that a circulating soluble factor, perhaps an antibody, accounts for the B cell involvement revealed in our study (1).
Another objective of the present study was to determine the relative contributions of CD4+ and CD8+ T cells to the blood cell recruitment responses elicited by gut I/R. We observed that mice deficient in either CD4+ and CD8+ T lymphocytes are very effective in attenuating the blood cell recruitment responses compared with those observed in WT mice after I/R. A similar dependency of blood cell recruitment on both CD4+ and CD8+ T cells has been demonstrated in the microvasculature of hypercholesterolemic mice (17). Furthermore, we found that reconstitution of the T cell populations by adoptive transfer of splenocytes restored the robust I/R-induced leukocyte and platelet adhesion responses normally seen in WT mice. In most other tissues studied to date, including brain, liver, and kidney, CD4+ T cells appear to have a prominent role in mediating the neutrophil infiltration induced by I/R (10, 19, 20). We have previously reported a similar predominance of CD4+ T cells in mediating the liver microvascular dysfunction induced by gut I/R, although some of the microvascular alterations, including leukocyte accumulation, exhibited a significant dependence on CD8+ T cells (8). Our observation that a deficiency of either T cell subpopulation is equally effective in blunting the blood cell recruitment responses suggests the existence of a mechanism whereby CD4+ T cells and CD8+ T cells are coupled in series to regulate leukocyte and platelet recruitment in the postischemic gut vasculature. Alternatively, it is possible that both cell populations must be present to generate sufficient levels of one or more soluble mediators that will elicit the proadhesive phenotype in the postischemic microvasculature.
IFN-γ, a cytokine produced by all CD8+ T cells and the Th1 subset of CD4+ T cells, has been implicated in other T cell-dependent models of I/R injury, including brain and kidney (1, 19). Our finding that IFN-γ−/− mice exhibit an attenuated blood cell recruitment response compared with WT mice supports a role for this cytokine in the pathogenesis of gut I/R. Furthermore, our study provides two lines of evidence that the IFN-γ that mediates the I/R-induced adhesion responses is derived from T cells: 1) the attenuation of blood cell adhesion seen in IFN-γ mice is similar to that noted in T cell-deficient mice, and 2) adoptive transfer of splenocytes from WT mice into IFN-γ−/− recipients fully restores the proadhesive phenotype in the postischemic gut microvasculature. CD4+ T cells appear to be the major source of IFN-γ that mediates renal I/R injury inasmuch as adoptive transfer of wild-type CD4+ T cells restores the injury phenotype whereas CD4+ T cells from IFN-γ−/− do not (1). The T cell-dependent recruitment of leukocytes and platelets in brain microvessels after ischemic stroke also appears to involve IFN-γ, although in this model T lymphocytes do not appear to be the major source of IFN-γ (19).
In conclusion, the results of the present study support a role for both B and T cells (both CD4+ and CD8+) in the modulation of I/R-induced leukocyte and platelet adhesion in intestinal venules. Lymphocyte-derived IFN-γ also appears to contribute to the development of a proadhesive phenotype in the postischemic intestinal vasculature. The pattern of I/R-induced adhesion responses observed in the gut following genetic or immunological manipulation of specific lymphocyte populations and IFN-γ is more similar to those previously described for kidney than other tissues such as liver and brain. The immunological basis for the different contributions of T cells and T cell-derived IFN-γ to the pathophysiology of I/R injury in various tissues remains unclear and warrants further investigation.
GRANTS
This research was supported by a grant from the National Institutes of Diabetes and Digestive and Kidney Diseases (R01 DK65649).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O'Donnell MP, Rabb H. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol 171: 3210–3215, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Burne-Taney MJ, Yokota-Ikeda N, Rabb H. Effects of combined T- and B-cell deficiency on murine ischemia reperfusion injury. Am J Transplant 5: 1186–1193, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 289: G969–G976, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell CC, Tschoep J, Lentsch AB. Lymphocyte function during hepatic ischemia/reperfusion injury. J Leukoc Biol 82: 457–464, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 190: 255–266, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Cooper D, Russell J, Chitman KD, Williams MC, Wolf RE, Granger DN. Leukocyte dependence of platelet adhesion in postcapillary venules. Am J Physiol Heart Circ Physiol 286: H1895–H1900, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Horie Y, Wolf R, Chervenak RP, Jennings SR, Granger DN. T-lymphocytes contribute to hepatic leukostasis and hypoxic stress induced by gut ischemia-reperfusion. Microcirculation 6: 267–280, 1999. [PubMed] [Google Scholar]
- 9.Horie Y, Chervenak RP, Wolf R, Gerritsen ME, Anderson DC, Granger DN. Lymphocytes mediate TNFalpha-induced endothelial cell adhesion molecule expression: studies on SCID, and RAG-1 mutant mice. J Immunol 159: 5053–5062, 1997. [PubMed] [Google Scholar]
- 10.Huang Y, Rabb H, Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol 248: 4–11, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichikawa H, Flores S, Kvietys PR, Wolf RE, Yoshikawa T, Granger DN, Aw TY. Molecular mechanisms of anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Circ Res 81: 922–931, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Kokura S, Wolf RE, Yoshikawa T, Granger DN, Aw TY. T-lymphocyte-derived tumor necrosis factor exacerbates anoxia-reoxygenation-induced neutrophil-endothelial cell adhesion. Circ Res 86: 205–213, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Massberg S, Enders G, Leiderer R, Eisenmenger S, Vestweber D, Krombach F, Messmer K. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood 92: 507–515, 1998. [PubMed] [Google Scholar]
- 14.Menger MD, Vollmar B. Pathomechanisms of ischemia-reperfusion injury as the basis for novel preventive strategies: is it time for the introduction of pleiotropic compounds? Transplant Proc 39: 485–488, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Rabb H, Daniels F, O'Donnell M, Haq M, Saba SR, Keane W, Tang WW. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 279: F525–F531, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Shigematsu T, Wolf RE, Granger DN. T-lymphocytes modulate the microvascular and inflammatory responses to intestinal ischemia-reperfusion. Microcirculation 9: 99–109, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Stokes KY, Clanton EC, Bowles KS, Fuseler JW, Chervenak D, Chervenak R, Jennings SR, Granger DN. The role of T-lymphocytes in hypercholesterolemia-induced leukocyte-endothelial interactions. Microcirculation 9: 407–417, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Tsujikawa A, Kirya J, Yamashiro K, Nonaka A, Nishijima K, Honda Y, Ogura Y. Interactions between blood cells and retinal endothelium in endotoxic sepsis. Hypertension 36: 250–258, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 113: 2105–2112, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4+ T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest 100: 279–289, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]