Abstract
The actions of cholecystokinin (CCK) on gastrointestinal functions occur mainly via paracrine effects on peripheral sensory vagal fibers, which engage vago-vagal brain stem circuits to convey effector responses back to the gastrointestinal tract. Recent evidence suggests, however, that CCK also affects brain stem structures directly. Many electrophysiological studies, including our own, have shown that brain stem vagal circuits are excited by sulfated CCK (CCK-8s) directly, and we have further demonstrated that CCK-8s induces a remarkable degree of plasticity in GABAergic brain stem synapses. In the present study, we used fasted, anesthetized Sprague-Dawley rats to investigate the effects of brain stem administration of CCK-8s on gastric tone before and after activation of the esophageal-gastric reflex. CCK-8s microinjected in the dorsal vagal complex (DVC) or applied on the floor of the fourth ventricle induced an immediate and transient decrease in gastric tone. Upon recovery of gastric tone to baseline values, the gastric relaxation induced by esophageal distension was attenuated or even reversed. The effects of CCK-8s were antagonized by vagotomy or fourth ventricular, but not intravenous, administration of the CCK-A antagonist lorglumide, suggesting a central, not peripheral, site of action. The gastric relaxation induced by DVC microinjection of CCK-8s was unaffected by pretreatment with systemic bethanecol but was completely blocked by NG-nitro-l-arginine methyl ester, suggesting a nitrergic mechanism of action. These data suggest that 1) brain stem application of CCK-8s induces a vagally mediated gastric relaxation; 2) the CCK-8s-induced gastric relaxation is mediated via activation of nonadrenergic, noncholinergic pathways; and 3) CCK-8s reverses the esophageal-gastric reflex transiently.
Keywords: vago-vagal reflexes, gastric reflexes
esophageal distension has long been recognized to elicit a robust and reflexive gastric relaxation (21). Early models of gastrointestinal vago-vagal reflexes presented a framework to explain many of the features of the brain stem circuits devoted to gastric reflex control, whereby stimulation of sensory vagal afferent fibers activates second-order neurons of the nucleus tractus solitarii (NTS). Subsequently, these second-order neurons modulate the output of preganglionic neurons in the dorsal motor nucleus of the vagus (DMV) that, in turn, control gastric functions to complete the vago-vagal loop (reviewed in Ref. 72).
The esophageal-gastric reflex (or receptive relaxation) decreases gastric tone and allows swallowed food to be transported to the stomach with a minimal increase in intragastric pressure. Neurophysiological studies by Jean first showed the association of neurons of the caudal brain stem with esophageal function (reviewed in Refs. 8, 36). We showed recently (54) that the esophageal-gastric reflex requires intact vagal connections between the esophagus, the brain stem, and the stomach. In its simplest model, the esophageal-gastric reflex, as with other gastrointestinal vago-vagal reflexes, presumes that activation of any given vagal afferent fiber elicits the same stereotypical efferent response, i.e., esophageal distension always induces gastric relaxation. Our recent in vitro electrophysiological studies (14, 15, 17, 18), however, revealed a high degree of plasticity in the brain stem circuits involved in vago-vagal modulation of gastric functions. Circulating hormones and neuromodulators, such as thyrotropin releasing hormone and cholecystokinin (CCK), play fundamental roles in the plasticity and modulation of these circuits (14–16).
CCK is secreted from endocrine cells in the small intestine in response to nutrients such as proteins or fat (46, 53, 62). This postprandial release of CCK activates vagally mediated feedback control of gastrointestinal functions such as stimulation of pancreatic exocrine secretion, inhibition of gastric emptying, acid secretion, and food intake (27, 39, 46, 53, 62). While it is clear that most of the effects of endogenous CCK are mediated via a paracrine activation of the peripheral endings of vagal sensory fibers, mounting evidence has shown that other sites of action should be considered. Behavioral, biochemical, and electrophysiological studies have shown that a relevant portion of the gastrointestinal effects of sulfated CCK (CCK-8s) are due to direct actions at the level of brain stem vagal circuits. In fact, CCK-8s acts on vagal afferent fibers that synapse on NTS neurons as well as at the level of subpopulations of NTS and DMV neurons (4, 6, 7, 9, 12, 13, 48, 51, 71, 74, 75, 79). These studies suggest that functional CCK-A receptors are present on the membrane of subgroups of NTS and gastric-projecting DMV neurons. It is possible that these CCK-A receptors are physiologically relevant in the mechanism of action of CCK in the central nervous system (CNS). Despite the low circulating concentrations of gut-derived CCK, we showed recently (74) that endogenously released CCK is capable of increasing pancreatic exocrine secretion via a vagally dependent action. These studies indicate that the gastrointestinal effects of CCK-8s are not limited to a paracrine activation of the peripheral terminal of vagal afferent (sensory) fibers, and direct effects on brain stem circuits must be considered. It is also possible that the source of CCK acting on vagal brain stem circuits is a consequence of the activation of brain stem circuits that trigger the synaptic release of locally produced CCK. In fact, NTS neurons containing CCK-immunoreactive as well as CCKergic fibers have been described (32, 40).
In the search for the mechanism of action of CCK, immunohistochemical studies by Sayegh and Ritter (55) showed that intraperitoneal administration of CCK-8s increases c-Fos expression in nitric oxide synthase-immunoreactive myenteric neurons of the upper small intestine. When this finding is combined with the electrophysiological reports showing that the majority (48) or all (79) of DMV neurons responding to CCK-8s are excited, we can hypothesize that CCK-8s-induced gastric relaxation occurs as a consequence of activation of a nonadrenergic, noncholinergic (NANC) postganglionic pathway. Surprisingly, this hypothesis has not been investigated.
The aims of the present study were therefore to use an anesthetized rat model to investigate 1) whether application of CCK-8s in the brain stem induces a vagally mediated gastric relaxation through activation of CCK-A receptors; 2) the CCK-8s-induced gastric relaxation is mediated by a postganglionic NANC pathway; and 3) the esophageal-gastric reflex is modulated by brain stem application of CCK-8s.
METHODS
All procedures followed National Institutes of Health guidelines for the use of animals in research and were performed under the approval of the Pennington Biomedical Research Center Institutional Animal Care and Use Committee.
Experiments were performed on male Sprague-Dawley rats (n = 55) weighing 175–250 g (Harlan, Indianapolis, IN). Animals were maintained in a temperature-controlled room on a 12:12-h light-dark cycle with unrestricted access to food and water. Rats were fasted overnight (with water ad libitum) before the experimental procedures.
On the day of the experiment, rats were anesthetized deeply with thiobutabarbitol (Inactin, Sigma; 100–150 mg/kg ip). Dexamethasone (1 mg/kg sc) was administered to prevent cerebral edema. Rats were then intubated with a tracheal catheter to maintain an open airway, a jugular catheter was inserted for the intravenous delivery of drugs, and a laparotomy was performed. A 6 × 8-mm encapsulated miniature strain gauge (RB Products, Minneapolis, MN) was aligned with the circular smooth muscle fibers and sutured to the gastric corpus. The strain gauge leads were exteriorized before closure of the abdominal incision. The strain gauge signal was amplified (QuantaMetrics EXP CLSG-2, Newton, PA) and recorded on a polygraph (model 79, Grass, Quincy, MA) or on a computer using Axotape software (Axon Instruments, Union City, CA).
Esophageal distension balloons were fabricated from biomedical silicone tubing (ID 0.7 mm, OD 1.7 mm) that had been sealed at one end with dental impression material (Reprosil Heavy Body, Dentsply, Milford, DE) and stretched such that filling with 100 μl of water induced a distension of 3 mm in diameter over a 5-mm length. This stimulus has been reported to exert a transmural esophageal pressure of 14–18 mmHg (54), a range sufficient to stimulate low-threshold vagal mechanoreceptors but not spinal nociceptors (57). The esophageal balloon was placed ∼1 cm oral to the esophageal hiatus. The location of the balloon was verified both before closing the midline incision and at the end of the experimental procedures by briefly inflating the balloon and visually noting the position in the esophagus.
After surgical instrumentation, animals were placed in a stereotaxic frame and rectal temperature was monitored and maintained at 37 ± 1°C (TCAT 2LV, Physitemp Instruments, Clifton, NJ). After a midline incision and removal of the overlying neck musculature, the head of the animal was oriented such that the floor of the fourth ventricle was exposed and the brain stem surface was horizontally oriented in a manner that prevented washout of the solution applied. The pial membrane overlying the vagal trigone was dissected, and the exposed tissues were covered with a warm, saline-infused cotton patch. After 1 h of stabilization, 10 min of baseline motility was recorded before any experimental manipulation. The esophageal distension balloon was filled with 100 μl of water for 1 min and then released. Three esophageal distensions were produced, each separated by a 30-min rest period.
A glass micropipette (30- to 40-μm tip diameter) was directed into the dorsal vagal complex (DVC) with the aid of a micromanipulator under microscopic guidance (0.1–0.3 mm mediolateral, 0.1–0.3 mm rostral to calamus scriptorius and 0.5–0.7 mm dorsoventral to the surface of the medulla) for drug delivery. The volume of the ejectate was measured via a calibrated monocular microscope mounted on the stereotaxic frame and directed at the meniscus inside the micropipette. Vehicle (PBS, in mM: 147.6 NaCl, 83.3 NaH2PO4, 12.9 KH2PO4) or CCK-8s (450 pmol in 60 nl PBS; solution was inspected visually to confirm that CCK-8s was completely dissolved before microinjection) was applied by pressure ejection; the application usually required ∼1 min.
At the end of the experiment, the animals were killed via injection of an overdose of pentobarbital sodium and perfused with PBS followed by a solution of 4% paraformaldehyde in PBS. The brain stem was extracted and fixed overnight in 4% paraformaldehyde, 20% sucrose in PBS. After several rinses in PBS the brain stem was frozen and sliced at 50-μm thickness, and alternate slices were visualized on a Nikon E400 microscope for identification of the site of injection or stained with cresyl violet for identification of anatomic markers.
In another series of experiments, the continually produced excess cerebrospinal fluid was gently wicked away with a cotton patch, 2 μl of PBS was applied with a Hamilton syringe to the surface of the fourth ventricle at the level of the obex, and baseline motility and tone signals were acquired for 30 min. After the observation period, three esophageal distensions were elicited as described above. At the conclusion of the control observation period, a second 2-μl application of either PBS or CCK-8s (0.15, 1.5 or 15 nmol; Sigma) was made as described above. These higher CCK-8s doses were necessary to account for the dilution occurring as a consequence of mixing the solution with the cerebrospinal fluid that is continually produced. The basal strain gauge output was observed for any change for 10 min after the second infusion. When the effects of CCK-8s on the esophageal-gastric relaxation reflex were measured, esophageal distension was elicited upon return of gastric tone to baseline values, usually within 10 min.
Further groups of animals were prepared as for the control experiments. In one group, before apposition of the gastric strain gauge, the subdiaphragmatic posterior vagus was sectioned and a ligature of silk suture was gently placed around the left cervical vagus as it passed alongside the internal carotid artery. The ligature was exteriorized through a length of PE-240 tubing for later transection of the vagus nerve. The esophageal-gastric reflex was elicited as described above, followed by brain stem application of 15 nmol of CCK-8s. After a 30-min observation period, the ligature was withdrawn, severing the remaining vagal outflow to the stomach. The absence of the esophageal-gastric reflex was confirmed, and a second application of 15 nmol of CCK-8s was made. A second group of animals was instrumented for experimental blockade of exogenous CCK-8s effects by application of 40 nmol of the CCK-A receptor antagonist lorglumide (Sigma). Lorglumide was applied to the medullary surface 3 min before a second application of a solution containing both lorglumide and 15 nmol CCK-8s. In the third group of rats, the same dose of lorglumide was injected intravenously 3 min before administration of CCK-8s on the surface of the fourth ventricle.
To study the vagal pathway(s) involved in the gastric relaxation induced by brain stem microinjection of CCK-8s, baseline gastric motility was increased by systemic (iv) administration of the muscarinic agonist bethanecol (50 μg/kg bolus followed by continuous iv infusion with 20 μg·kg−1·h−1 for 20 min). CCK-8s (450 pmol) was microinjected into the DVC 2–5 min after intravenous administration of bethanecol. To study the contribution of the NANC component of the vagal efferents involved in the gastric relaxation, the same animals were administered the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 10 mg/kg iv bolus injection) after bethanecol administration. CCK-8s was injected into the DVC as described above. Drug doses were chosen based on previously published data (67–69, 74) or were extrapolated from in vitro stud ies (4, 7).
Data analysis and statistics.
Individual strain gauges were calibrated with a 1-g weight applied externally before and after the experimental procedures. Changes in baseline gastric signal were determined by comparing the maximal reduction of the signal after drug application relative to the 5-min average of the signal immediately before drug application. Gastric relaxation evoked by distension of the esophageal balloon was measured by determining the change in signal from the strain gauge before and during the 1-min-long inflation of the balloon. The effect of CCK-8s was normalized to pretreatment values of esophagus-induced gastric relaxation.
Data are expressed as means ± SE and were evaluated by comparing the change in response between pre- and posttreatment values within each group by ANOVA and paired t-test (SPSS, Chicago, IL). In all instances, significance was presumed when P < 0.05.
RESULTS
In fasted rats, neither microinjection of PBS into the DVC (n = 7) nor application of PBS to the floor of the fourth ventricle (n = 30) altered either gastric motility or tone (P > 0.05).
Application of CCK-8s in DVC induces a vagally mediated decrease in gastric tone.
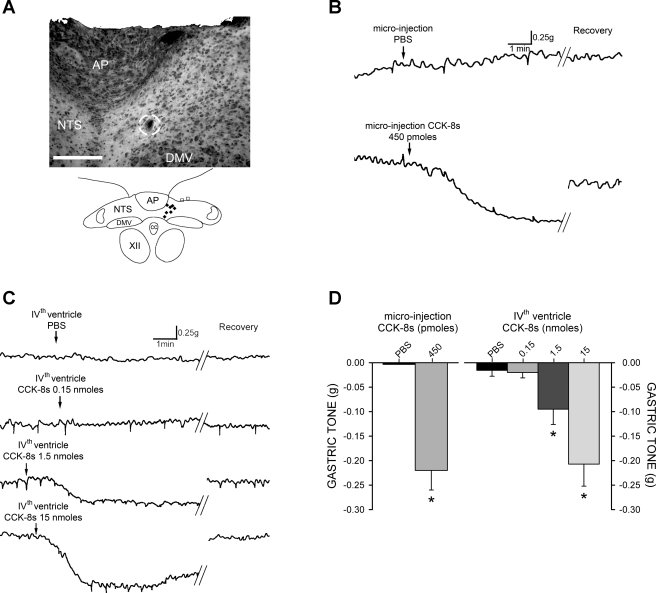
In naive rats, microinjection of 450 pmol of CCK-8s in the DVC induced a decrease in gastric tone of −0.21 ± 0.04 g (n = 7, P < 0.05; Fig. 1). The gastric tone returned to baseline values within 5.70 ± 1.34 min. Similarly, application of a single dose of CCK-8s to the floor of the fourth ventricle induced a dose-dependent (0.15–15 nmol) decrease in gastric tone (Fig. 1). With each animal serving as its own control, application of 0.15 nmol of CCK-8s reduced gastric tone by −0.02 ± 0.01 g (n = 6, P > 0.05). Application of 1.5 nmol of CCK-8s reduced the baseline signal by −0.1 ± 0.03 g (n = 6, P < 0.05); administration of the highest dose of CCK-8s (15 nmol, n = 6) decreased gastric tone by −0.21 ± 0.04 g (P < 0.05).
Fig. 1.
Application of sulfated cholecystokinin (CCK-8s) in the dorsal vagal complex (DVC) induces a vagally mediated decrease in gastric tone. A: representative 50-μm-thick photomicrograph (top) of brain stem injection site with the region of injection circled and schematic representation (bottom) of effective injection areas (⧫) and injections without a response (□). AP, area postrema; NTS, nucleus tractus solitarii; DMV, dorsal motor nucleus of the vagus; CC, central canal; XII, hypoglossus. Bar: 250 μm. B: representative original polygraph records of gastric motility and tone from fasted animals after microinjection of PBS or 450 pmol of CCK-8s. C: original polygraph records from fasted animals after 4th ventricle application of 2 μl of PBS or CCK-8s demonstrating dose-dependent relaxation of the stomach. D: graphic summary of dose-dependent gastric relaxation induced by CCK-8s. *P < 0.05.
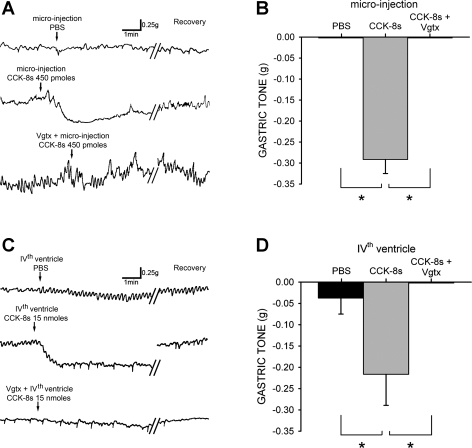
As in naive rats, application of PBS to the floor of the fourth ventricle did not alter gastric tone in prevagotomized rats. In three rats, application of 15 nmol of CCK-8s produced a −0.22 ± 0.07 g relaxation. Upon return of gastric tone to baseline values, we conducted a complete vagotomy. After 10-min stabilization and recovery, reapplication of 15 nmol of CCK-8s on the floor of the fourth ventricle no longer decreased gastric tone (P < 0.05; Fig. 2).
Fig. 2.
Gastroinhibition induced by centrally applied CCK-8s is vagally mediated. A: original trace from fasted animal in which posterior branch of gastric vagus was transected subdiaphragmatically before recording and suture ligature was loosely affixed around anterior vagus branch. Oblique lines indicate 5- to 10-min interval for recovery. After microinjection of PBS or 450 pmol of CCK-8s (top and middle, respectively) ligature was withdrawn, completing vagotomy (Vgtx). Gastric relaxation produced by subsequent application of CCK-8s (450 pmol) was abolished by vagotomy (bottom). B: graphic summary of gastric relaxation induced by CCK-8s microinjection before and after complete vagotomy. C: application of PBS or 15 nmol CCK-8s to floor of 4th ventricle (top and middle, respectively) produced results similar to that observed after microinjection. Vagotomy also abolished the response to 4th ventricle application of CCK-8s (15 nmol) (bottom). D: graphic summary of gastric relaxation induced by 4th ventricle CCK-8s before and after complete vagotomy. *P < 0.05.
These data indicate that the gastric relaxation resulting from microinjection of CCK-8s in the DVC or application of CCK-8s on the floor of the fourth ventricle is mediated via activation of vagal pathways originating in the brain stem.
To further confirm that the actions of CCK-8s were restricted to the activation of brain stem circuits, we pretreated the animals with the selective CCK-A receptor antagonist lorglumide, either applied on the floor of the fourth ventricle or administered systemically at the same dose. If the effects of CCK-8s were antagonized by lorglumide administered to the floor of the fourth ventricle as well as when injected intravenously, we would conclude that the effects of brain stem CCK-8s may have been determined by its diffusion to areas outside the brain stem. Conversely, if brain stem, but not systemic, lorglumide antagonized the gastric relaxation induced by brain stem application of CCK-8s, then we could reasonably conclude that the gastroinhibitory effects of CCK-8s were mediated by activation of CCK-A receptors located in the brain stem itself.
In six rats, 40 nmol of lorglumide was applied to the floor of the fourth ventricle. Lorglumide per se did not alter gastric tone but reduced the gastric relaxation induced by subsequent reapplication of 15 nmol of CCK-8s to 8.39 ± 18.28% of the initial response (P < 0.05; Fig. 3).
Fig. 3.
Gastroinhibition induced by CCK-8s is mediated by activation of brain stem CCK-A receptors. A: top: 4th ventricle application of 15 nmol of CCK-8s induces marked decrease in gastric tone. After recovery to baseline tone values, 40 nmol of lorglumide was applied to floor of 4th ventricle. Reapplication of 15 nmol of CCK-8s no longer had any effect on gastric tone. Oblique lines indicate 5- to 10-min interval. Bottom: after marked decrease, and recovery, in gastric tone after 15 nmol of CCK-8s, 40 nmol of lorglumide was injected intravenously. Reapplication of 15 nmol CCK-8s still decreased gastric tone. B: graphic summary of the decrease in gastric tone induced by CCK-8s in rats before and after application of lorglumide (Lorg) on either floor of the 4th ventricle or systemically (iv). Data are expressed as % of relaxation response compared with initial CCK-8s application (i.e., lorglumide alone did not evoke gastric relaxation, while application of CCK-8s after iv lorglumide evoked gastric relaxation nearly identical to that obtained after application of CCK-8s). *P < 0.05.
Conversely, in another group of rats, 40 nmol of lorglumide was administered intravenously 5 min before medullary application of 15 nmol of CCK-8s (n = 5). In these rats, the decrease in gastric tone following CCK-8s was 92.6 ± 10.83% of the initial response to CCK-8s (P > 0.05; Fig. 3).
These data indicate that the CCK-8s-induced gastroinhibition is mediated by CCK-A receptors located in the brain stem, and confirm our data showing that CCK-A receptors are not tonically active in the DVC circuits controlling gastric motility (7, 79).
Gastroinhibitory actions of CCK-8s are mediated by activation of NANC pathways.
These experiments were designed on the premise that gastroinhibition is mediated by either a withdrawal of cholinergic tone or an increase in NANC activity (reviewed recently in Ref. 72). To test whether the gastroinhibition is mediated by withdrawal of cholinergic tone, peripheral muscarinic receptors are maximally activated via intravenous administration of the nonselective cholinergic muscarinic agonist bethanecol (30, 42, 58, 59). Under these conditions, if DVC microinjection of CCK-8s no longer induces gastroinhibition, this implies that the pathway used is “overcome” by the maximal receptor activation induced by bethanecol. This approach is preferable to the use of atropine to antagonize or negate the effects of withdrawal of cholinergic inputs since atropine itself induces such a marked decrease in gastric tone and motility that an additional decrease would potentially not be detected. In contrast, if gastric motility and/or tone are still decreased after DVC microinjection of CCK-8s in bethanecol-treated rats, this would suggest involvement of mechanisms other than the withdrawal of the cholinergic muscarinic pathway, possibly activation of the NANC pathway, portions of which can be blocked by systemic administration of the nitric oxide synthase inhibitor l-NAME.
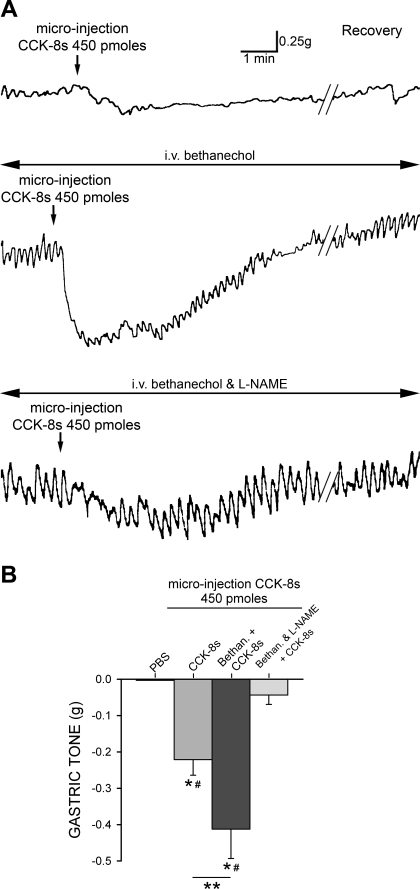
In seven rats, microinjection of 450 pmol of CCK-8s in the DVC induced a −0.22 ± 0.04 g decrease in gastric tone (P < 0.05). Upon recovery from the gastroinhibition, baseline gastric motility was increased by systemic (iv) administration of bethanecol (50 μg/kg bolus followed by continuous iv infusion with 20 μg·kg−1·h−1 for 20 min). Upon stabilization of motility and tone, usually 2–5 min after intravenous administration of bethanecol, 450 pmol of CCK-8s (n = 4) was micropressure injected into the DVC. Under these conditions, microinjection of CCK-8s still induced a −0.41 ± 0.08 g decrease in gastric tone (P < 0.05 vs. untreated rats; Fig. 4). After recovery from the gastroinhibition produced by CCK microinjection, nitrergic-mediated gastroinhibition was blocked by systemic administration of l-NAME (10 mg/kg bolus iv injection; Refs. 67–69) coadministered with bethanecol. The gastroinhibition induced by DVC microinjection of 450 pmol of CCK-8s was completely abolished by pretreatment with l-NAME (P < 0.05 vs. CCK + bethanechol-treated rats; Fig. 4).
Fig. 4.
Gastroinhibitory actions of CCK-8s are mediated by activation of nonadrenergic, noncholinergic (NANC) pathways. A: top: microinjection of CCK-8s induced gastric relaxation. Middle: gastroinhibitory action following microinjection of CCK-8s remained during continual infusion of the promotility, cholinergic agonist bethanechol (20 μg·kg−1·h−1 iv). Bottom: continual infusion of bethanechol coupled with blockade of NANC neurotransmission by bolus injection of nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 10 mg/kg iv) abolished gastroinhibition induced by microinjection of CCK-8s. B: graphic summary of gastric relaxation produced by PBS, CCK-8s alone, CCK-8s during continual intravenous bethanechol (Bethan), and CCK-8s after bolus of l-NAME and continual intravenous bethanechol. *P < 0.05 vs. PBS, #P < 0.05 vs. Bethan and l-NAME, **P < 0.05 vs. CCK-8s.
These data indicate that the gastroinhibition induced by administration of CCK-8s in the DVC is mediated by activation of the NANC pathway.
DVC application of CCK-8s reverses esophageal-gastric reflex.
In fasted naive rats, esophageal distension decreased gastric tone by 0.12 ± 0.01 g (n = 32; Fig. 5). With each animal serving as its own control, the esophageal distension-induced gastroinhibition was not affected by fourth ventricle application of PBS (n = 7, P > 0.05; Fig. 5) but was blocked completely by vagotomy (n = 6; data not shown).
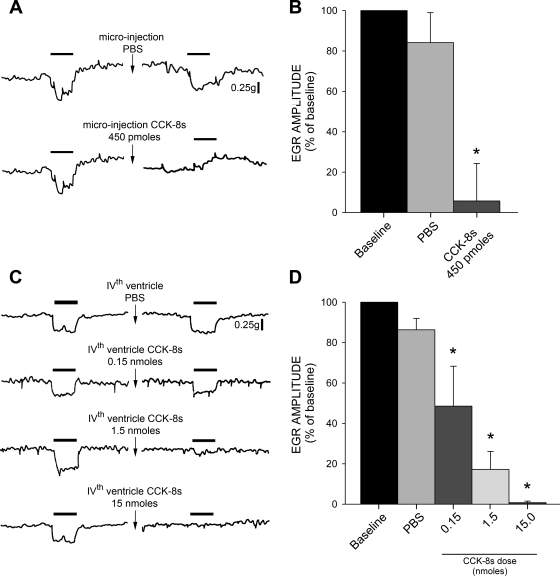
Fig. 5.
Gastroinhibition induced by esophageal distension is reduced by CCK-8s. A: esophageal distension (1 min; solid bar) induced gastric relaxation that was unaffected by PBS microinjection (top) but was attenuated by CCK-8s (450 pmol) (bottom). Break in trace indicates 5- to 10-min recovery interval, during which gastric tone was permitted to recover from the CCK-8s-mediated decrease in gastric tone (see Fig. 1). B: graphic summary of attenuation of esophagus-induced decrease in gastric tone induced by CCK-8s microinjection. EGR, esophageal-gastric reflex. C: original traces of dose-dependent inhibition of EGR following a single 4th ventricle application of CCK-8s. D: graphic summary of attenuation of esophagus-induced decrease in gastric tone induced by 4th ventricle application of CCK-8s. *P < 0.05 vs. baseline EGR.
The gastric relaxation induced by esophageal distension was reduced by a single microinjection of 450 pmol of CCK-8s in the DVC. To prevent the confounder of the gastroinhibition that is observed after microinjection of CCK-8s in the DVC, esophageal distension was elicited on return of gastric tone to baseline values and 2–5 min of stable baseline (i.e., within 10 min after CCK-8s administration). Application of 450 pmol of CCK-8s reduced the esophageal-gastric relaxation to 13.7 ± 19.4% of control (n = 7, P < 0.05; Fig. 5).
Similarly, the gastric relaxation induced by esophageal distension was reduced, in a dose-dependent manner, by a single fourth ventricle application of CCK-8s (0.15, 1.5, or 15 nmol). Esophageal distension was elicited on return of gastric tone to baseline values and a stabilization period of 2–5 min. Application of 0.15 nmol of CCK-8s reduced the esophageal-gastric relaxation to 48.5 ± 19.8% of control (n = 6; P < 0.05); 1.5 nmol CCK-8s reduced the esophageal-gastric relaxation to 17.2 ± 8.9% of control (n = 6; P < 0.05); while the highest dose of CCK-8s (15 nmol) reduced esophageal-gastric relaxation to 0.75 ± 0.8% of control (n = 5, P < 0.05; Fig. 5).
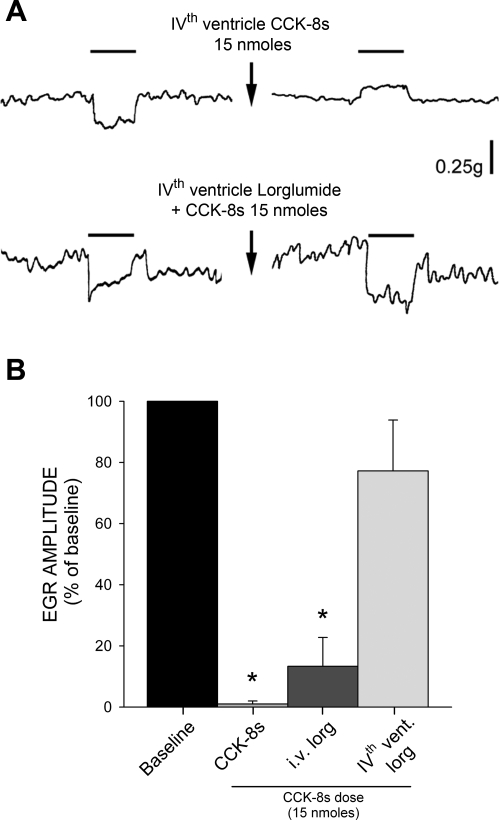
Pharmacological blockade of brain stem CCK-A receptors with lorglumide (40 nmol) did not have any effect on the amplitude of the esophagus-induced gastric relaxation per se (n = 5, P > 0.05; data not shown) but attenuated the gastric relaxation induced by fourth ventricle application of 15 nmol of CCK-8s to 77.2 ± 16.64% of baseline value (n = 5; P < 0.05 vs. CCK-8s alone, P > 0.05 vs. baseline; Fig. 6). Conversely, medullary application of 15 nmol of CCK-8s still reversed the esophagus-induced gastric distension after pretreatment with intravenous lorglumide (40 nmol, n = 4; Fig. 6).
Fig. 6.
CCK-8s mediated reduction of EGR is via activation of brain stem CCK-A receptors. A: original trace showing example in which 4th ventricle CCK-8s (15 nmol) induced an actual reversal in EGR (top). Pretreatment with 40 nmol lorglumide applied to floor of 4th ventricle prevented CCK-8s-induced attenuation of EGR (bottom). Break in trace indicates 5- to 10-min interval. During this period (and only in experiment represented in top trace) there was a CCK-8s-mediated decrease in gastric tone (see Fig. 1). B: graphic summary of decrease in EGR induced by CCK-8s in rats before and after application of lorglumide either systemically (iv; original traces not shown) or on floor of 4th ventricle. *P < 0.05 vs. baseline EGR.
These data indicate that DVC application of CCK-8s reverses the esophageal-gastric reflex via activation of medullary CCK-A receptors, and further confirm our data showing that CCK-A receptors are not tonically active in the DVC (7, 79).
DISCUSSION
The data presented in this study demonstrate that 1) activation of CCK-A receptors in the DVC induces a vagally mediated gastric relaxation; 2) CCK-8s-induced gastric relaxation is mediated by a postganglionic NANC pathway; and 3) the esophageal-gastric reflex is modulated by brain stem application of CCK-8s. Although the endogenous source of CCK in the DVC has not been elucidated, our results support a possible physiologically relevant hormonal or neuromodulator role of CCK to activate brain stem vagal neurons devoted to the control of gastric motility.
Previous clinical and experimental studies have demonstrated that systemic administration of CCK-8s decreases gastric tone (49, 56, 63, 70). The well-accepted mechanism of CCK-induced gastroinhibition suggests that CCK-8s activates C-type vagal afferent fibers via a paracrine mechanism, since capsaicin attenuates, or even abolishes, the effects of systemic CCK-8s (34, 50, 61). Several studies, however, have reported direct effects of CCK-8s on brain stem circuits (4, 6, 7, 13, 48, 79). Our data imply that at least some of the effects of CCK-8s on gastric tone and the modulation of the esophageal-gastric reflex may be due to a direct effect of CCK-8s on brain stem neurocircuitry. In fact, if the effects of CCK-8s are exclusively paracrine, then our observed reductions in basal gastric tone and esophageal-gastric reflex would be dependent on centrally applied CCK-8s diffusing into the systemic circulation.
The postprandial plasma concentration of CCK is in the low picomolar range (60), which would not be sufficient to excite brain stem NTS and/or DMV neurons. Portions of the DVC, however, have a “leaky” blood-brain barrier (2, 5, 25, 28), and several groups have provided functional evidence of a possible hormonal action of CCK to affect vagal neurons directly. More specifically, Hommer and colleagues (35) showed that systemic administration of CCK activates a NTS-nigral pathway, which is interrupted by lesion of the medulla but not of vagal afferent pathways. Similarly, the short-term satiety effects of CCK are antagonized by the blood-brain barrier-permeant CCK-A antagonist devazepide, but not by A-70104, a selective CCK-A antagonist that does not cross the blood-brain barrier (52). Furthermore, we showed that systemic injection of CCK-8s at low concentrations phosphorylates (i.e., activates) CCK-A receptors in the DVC (79) or induces c-Fos expression in the DVC of vagally deafferented rats (6). Furthermore, we have shown (74) that intraduodenal perfusion with the CCK-releasing protein casein increases pancreatic exocrine secretion via a central, vagally mediated action, thus indicating a nonparacrine effect of endogenous CCK. It should be pointed out, however, that the effects of CCK on vagal brain stem neurons may also be due to CCK acting as a neurotransmitter or neuromodulator. Indeed, CCK-containing neural projections originating within the NTS itself, the hypothalamus, and/or the amygdala are present throughout the rostro-caudal extent of the DMV (40, 43, 76).
It is thus very likely that the actions of CCK-8s are not limited to paracrine effects on peripheral vagal afferent fibers but that other sites of action, including the second-order neurons of the NTS or vagal motoneurons of the DMV, should be taken into account. Indeed, several groups have reported direct effects of CCK-8s on brain stem circuits (4, 6, 7, 13, 48, 79). Our data imply that at least some of the modulatory effects of CCK-8s on gastric tone and the esophageal-gastric reflex may be due to actions on brain stem circuits. It is also possible that the effects of CCK-8s on brain stem neurons differ from the action exerted at the level of vagal afferent fibers. This would not be sufficient to explain the gastric relaxation obtained upon brain stem application of CCK-8s, however, suggesting that other unidentified factors may be also involved in this effect of CCK-8s.
To further confirm that our observed actions of brain stem application of CCK-8s were restricted to the activation of brain stem circuits, we pretreated the animals with the selective CCK-A receptor antagonist lorglumide, either applied to the floor of the fourth ventricle or administered systemically. Our data clearly show that the effects of brain stem applications of CCK-8s are antagonized by lorglumide applied to the floor of the fourth ventricle but not by its systemic administration. These data thus support our hypothesis that our observed actions of CCK-8s were restricted to the activation of brain stem circuits.
Previous reports (70) have suggested that gastric smooth muscle relaxation in response to intravenous CCK-8s occurs via the activation of both vagal and splanchnic neural circuits. Our data demonstrate that the effects of brain stem application of CCK-8s are exclusively vagally mediated, since the gastric responses evoked by central application of CCK-8s were abolished after complete vagotomy. In addition, we demonstrated previously that the range of esophageal pressure utilized in these studies (14–18 mmHg) does not activate nociceptive circuitry. In fact, vagal afferent signaling conveys mostly physiological information, while nociceptive signaling has been shown to be relayed through splanchnic and, ultimately, spinal circuits (reviewed in Ref. 29).
In the present study we confirm previous data showing that brain stem application of CCK-8s evokes an immediate, short-term gastric relaxation via direct activation of CCK-A receptors. We extend previous observations by reporting that the CCK-8s-induced gastric relaxation is mediated by activation of a NANC pathway.
Vagally mediated gastroinhibition is mediated by either a withdrawal of cholinergic tone or an increase in NANC activity (reviewed recently in Ref. 72). Much of the vagal NANC inhibitory effect on the stomach is mediated by the release of nitric oxide onto gastric smooth muscle (1, 10, 24, 37, 41, 44, 45, 68). Perfusion with CCK-8s induces an excitation in most neurons, including identified gastrointestinal-projecting DMV neurons (4, 7, 13, 23, 38, 47, 48, 65, 66, 75, 79). The excitatory effects of CCK-8s on vagal motoneurons imply that the gastroinhibition induced by brain stem apposition of CCK-8s is likely due to activation of the NANC vagal pathway rather than withdrawal of the vagal cholinergic pathway. Indeed, our data show that the CCK-8s-induced gastroinhibition was still present after supramaximal stimulation of peripheral muscarinic receptors via systemic administration of the specific, but nonselective, muscarinic agonist bethanecol. Our hypothesis, i.e., that the gastroinhibitory effects observed upon brain stem CCK-8s activation are mediated by activation of a vagal NANC pathway, was confirmed by the complete antagonism of the gastric relaxation after pretreatment with the nitric oxide synthase inhibitor l-NAME. Indeed, immunohistochemical studies by Sayegh and Ritter (55) showed that systemic CCK-8s activates nitric oxide synthase-positive myenteric neurons of the upper small intestine. The persistent gastroinhibition following CCK microinjection in the presence of systemic bethanecol does not necessarily exclude the possibility that the paracrine effects of CCK are mediated via withdrawal of vagal cholinergic tone (20); it rather suggests that the gastroinhibitory effects of brain stem CCK are mediated by activation of postganglionic neurons that are part of the NANC pathway.
Our demonstration of a short-term gastric relaxation only serves to illuminate the effects of CCK on the efferent limb of the vago-vagal reflex arc. To test the effects of CCK on the integration of a complete reflex loop, we tested the effects of brain stem application of CCK-8s on the modulation of the esophageal-gastric reflex. The reduction in the esophagus-induced gastric relaxation was observed after gastric tone recovered to pre-CCK values and revealed a longer-lasting modulation of this particular vago-vagal reflex function.
CCK has been demonstrated to alter tone of the lower esophageal sphincter (LES) in canine and human studies (11, 31). As enteroendocrine cells have not been identified at the level of the esophagus, this suggests that any esophageal effects of CCK are due to hormonelike actions of the peptide on local afferent fibers and/or central esophageal neurocircuitry. Both possibilities may hold true for systemic administration of CCK; however, given the localized application of CCK to the brain stem the latter seems more likely in our preparation.
CCK increases the frequency of transient LES relaxations (TLESRs) (Ref. 11, reviewed in Ref. 31) and has been proposed to be a causative factor in esophageal-gastric reflux (78). The abolition of the esophageal-gastric reflex by CCK-8s may be a consequence of TLESRs, although this possibility is unlikely since the balloon distension of the esophagus in our experimental procedures lasted sixfold longer than the criterion for TLESRs described by Holloway et al. (33). One could argue that in our experiments CCK may have relaxed the esophagus directly, such that balloon distension was below the threshold necessary for activation of stretch receptors. From our data, however, we conclude that this scenario is unlikely since we occasionally observed a reversal of the esophageal-gastric reflex in response to application of the highest dose of CCK-8s (i.e., a contraction of the stomach after esophageal distension). This suggests that, after CCK-8s application, esophageal tone was sufficient for balloon inflation to activate mechanosensitive afferent fibers.
The prolonged alteration of the esophageal-gastric reflex also provides evidence that CCK-8s induces a more sustained modulation of the vagal circuits responsible for the gastric relaxation induced by esophageal distension. In addition to the immediate effect of CCK-8s on gastric tone, our data suggest that CCK-8s acts at the level of the brain stem to modulate gastric functions over a longer period. An additional conclusion to be drawn from our results is that activation of the esophageal-gastric vago-vagal reflex does not induce static, stereotyped responses but rather is open to prolonged modulation by hormones such as CCK.
Since our data demonstrate that the effects on the esophageal-gastric reflex are present even after the immediate gastric relaxation observed in response to medullary application of CCK-8s has recovered, it is possible that the gastric responses to CCK are temporally distinct. For example, it is possible that the immediate effect of CCK is a gastric relaxation mediated via activation of vagal afferent fibers and subsequent engagement of vagal effector circuits. Later, however, after an elevation in plasma CCK levels, CCK may act directly at the level of the brain stem either to prolong the gastric relaxation or to modulate/alter the gain of the esophageal-gastric reflex by inducing short-term plasticity within the brain stem circuits. Indeed, a theoretical framework supporting this hypothesis has been presented recently by our group (14, 16), showing that μ-opioid receptor trafficking in brain stem vagal circuits induced by CCK-8s lasts for ∼1 h after its removal (14).
Many studies of gastric motility and tone have been performed with intragastric balloons either alone (as a pressure transducer) or in conjunction with barostat control devices. Methods dependent on the placement of a balloon within the gastric lumen, however, are not without limitations (reviewed recently in Ref. 73). Gastric tone can be affected by the mechanical distortion induced by the balloon, which can, per se, activate vagal afferent fibers and mask pharmacological manipulations. Instead, we used small force transducers to record gastric circular muscle contraction, a method that is well established in similar studies and well within the range of sensitivity of the miniaturized strain gauge (26, 42).
Behaviorally, meals are terminated before the physical limit of the stomach is reached. The cessation of a meal is also induced by circulating hormones, such as CCK, released as a consequence of meal ingestion (19, 22, 51, 77). Indeed, an increase in the volume of ingesta is observed when food is diluted with a noncaloric medium (reviewed in Ref. 64). Exogenously administered CCK reduces meal size and results in the early appearance of a behavioral satiety sequence: the cessation of eating, the initiation of grooming sequences and exploration for a brief time, and then sleep (3). The behavioral repertoire of this satiety sequence strongly indicates that it is a CNS-mediated effect.
Delayed gastric emptying due to gastric distension is generally associated with satiety, but the delayed gastric emptying may itself reduce the secretion of CCK and blunt satiation. CCK acting at the level of the fourth ventricle may circumvent this negative loop and provide an additional mechanism to reduce ingestion. For example, by antagonizing the esophageal-gastric reflex, CCK may reinforce satiation. After a meal, when the stomach is full and CCK has been released in sufficient quantity, esophageal stimulation (e.g., further food ingestion) would not induce any further gastric relaxation, resulting in an unpleasant increase in gastric pressure and making further food ingestion less likely.
In conclusion, the present experiments have replicated earlier findings demonstrating an effect of medullary CCK-8s on basal gastric tone and extended those observations to show that medullary CCK-8s induces a longer-lasting modulation of brain stem vagal circuits. These data suggest that, beyond a paracrine mechanism of action, circulating CCK may be part of a larger, more sustained, integration of gastric reflex function at the level of the brain stem. This CCK-mediated effect at the level of the brain stem may produce functional plasticity within the medullary neurocircuitry controlling gastric function that serves to prolong postprandial satiety. Pathophysiological changes in the responsiveness of vagal neurocircuitry to CCK may be one contributing factor to the postprandial onset of dyspepsia.
GRANTS
This work was supported by National Institutes of Health Grants DK-56373 and DK-55530 (R. A. Travagli) and NINDS 49177 (G. M. Holmes).
Acknowledgments
We thank Cesare M. Travagli for support and encouragement, Dr. Kirsteen N. Browning for comments on earlier versions of the manuscript, and Emily Q. Creekmore for surgical and technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abrahamsson H Non-adrenergic non-cholinergic nervous control of gastrointestinal motility patterns. Arch Int Pharmacodyn 280: 50–61, 1986. [PubMed] [Google Scholar]
- 2.Ambalavanar R, Morris R. Fluoro-Gold injected either subcutaneously or intravascularly results in extensive retrograde labelling of CNS neurones having axons terminating outside the blood-brain barrier. Brain Res 505: 171–175, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol 89: 784–790, 1975. [DOI] [PubMed] [Google Scholar]
- 4.Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 25: 3578–3585, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banks WA The blood-brain barrier as a regulatory interface in the gut-brain axes. Physiol Behav 89: 472–476, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Baptista V, Browning KN, Travagli RA. Effects of cholecystokinin-8s in the nucleus tractus solitarius of vagally deafferented rats. Am J Physiol Regul Integr Comp Physiol 292: R1092–R1100, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baptista V, Zheng Z, Coleman FH, Rogers RC, Travagli RA. Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis. J Neurophysiol 94: 2763–2771, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blessing WW The Lower Brainstem and Bodily Homeostasis. Oxford: Oxford Univ. Press, 1997.
- 9.Blevins JE, Stanley BG, Reidelberger RD. Brain regions where cholecystokinin suppresses feeding in rats. Brain Res 860: 1–10, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Boeckxstaens GE, Pelckmans PA, Bogers J, Bult H, De Man JG, Oosterbosch L, Herman AG, Van Maercke YM. Release of nitric oxide upon stimulation of noadrenergic noncholinergic nerves in the rat gastric fundus. J Pharmacol Exp Ther 256: 441–447, 1991. [PubMed] [Google Scholar]
- 11.Boulant J, Fioramonti J, Dapoigny M, Bommelaer G, Bueno L. Cholecystokinin and nitric oxide in transient lower esophageal sphincter relaxation to gastric distention in dogs. Gastroenterology 107: 1059–1066, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Branchereau P, Bohme GA, Champagnat J, Morin-Surun MP, Durieux C, Blanchard JC, Roques BP, Denavit-Saubie M. CholecystokininA and cholecystokininB receptors in neurons of the brainstem solitary complex of the rat: pharmacological identification. J Pharmacol Exp Ther 260: 1433–1440, 1992. [PubMed] [Google Scholar]
- 13.Branchereau P, Champagnat J, Denavit-Saubie M. Cholecystokinin-gated currents in neurons of the rat solitary complex in vitro. J Neurophysiol 70: 2584–2595, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci 24: 9344–9352, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol 531: 425–435, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Browning KN, Travagli RA. Short-term receptor trafficking in the dorsal vagal complex: an overview. Auton Neurosci 126–127: 2–8, 2006. [DOI] [PMC free article] [PubMed]
- 17.Browning KN, Travagli RA. Functional organization of presynaptic metabotropic glutamate receptors in vagal brainstem circuits. J Neurosci 27: 8979–8988, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Browning KN, Zheng Z, Gettys TW, Travagli RA. Vagal afferent control of opioidergic effects in rat brainstem circuits. J Physiol 575: 761–776, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchan AMJ Nutrient tasting and signaling mechanisms in the gut. III. Endocrine cell recognition of luminal nutrients. Am J Physiol Gastrointest Liver Physiol 277: G1103–G1107, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Bucinskaite V, Kurosawa M, Lundeberg T. Exogenous cholecystokinin-8 reduces vagal efferent nerve activity in rats through CCKA receptors. Br J Pharmacol 129: 1649–1654, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannon WB, Leib CW. The receptive relaxation of the stomach. Am J Physiol 29: 267–273, 1911. [Google Scholar]
- 22.Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal satiety signals. Annu Rev Physiol 70: 239–255, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Cox CL, Huguenard JR, Prince DA. Cholecystokinin depolarizes rat thalamic reticular neurons by suppressing a K+ conductance. J Neurophysiol 74: 990–1000, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature 351: 477–479, 1991. [DOI] [PubMed] [Google Scholar]
- 25.Duvernoy HM, Risold PY. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev 56: 119–147, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda H, Tsuchida D, Koda K, Miyazaki M, Pappas TN, Takahashi T. Impaired gastric motor activity after abdominal surgery in rats. Neurogastroenterol Motil 17: 245–250, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Geary N Endocrine controls of eating: CCK, leptin, and ghrelin. Physiol Behav 81: 719–733, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 259: R1131–R1138, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Grundy D Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut 51, Suppl 1: i2–i5, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermann GE, Travagli RA, Rogers RC. Esophageal-gastric relaxation reflex in the rat: dual control of peripheral nitrergic and cholinergic transmission. Am J Physiol Regul Integr Comp Physiol 290: R1570–R1576, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch DP, Tytgat GNJ, Boeckxstaens GE. Transient lower oesophageal sphincter relaxations—a pharmacological target for gastro-oesophageal reflux disease? Aliment Pharmacol Ther 16: 17–26, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Hokfelt T, Cortes R, Schalling M, Ceccatelli S, Pelto-Huikko M, Persson H, Villar MJ. Distribution patterns of CCK and CCK mRNA in some neuronal and non-neuronal tissues. Neuropeptides 19, Suppl: 31–43, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Holloway RH, Penagini R, Ireland AC. Criteria for objective definition of transient lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 268: G128–G133, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Holzer P Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43: 143–201, 1991. [PubMed] [Google Scholar]
- 35.Hommer DW, Palkovits M, Crawley JN, Paul SM, Skirboll LR. Cholecystokinin-induced excitation in the substantia nigra: evidence for peripheral and central components. J Neurosci 5: 1387–1392, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jean A Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Kim CD, Goyal RK, Mashimo H. Neuronal NOS provides nitrergic inhibitory neurotransmitter in mouse lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 277: G280–G284, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Knoper SR, Meehan AG, Purnyn S, Coggan JS, Anthony TL, Kreulen DL. CCKA receptors mediate slow depolarizations in cultured mammalian sympathetic neurons. Eur J Pharmacol 232: 65–69, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Konturek SJ, Zabielski R, Konturek JW, Czarnecki J. Neuroendocrinology of the pancreas; role of brain-gut axis in pancreatic secretion. Eur J Pharmacol 481: 1–14, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Kubota Y, Takagi H, Morishima Y, Kaway Y, Smith AD. Relationship between catecholaminergic neurons and cholecystokinin-containing neurons in the caudal part of the dorsomedial medulla oblongata of the rat: light and electron microscopic observations by the mirror technique. Brain Res 370: 343–348, 1986. [DOI] [PubMed] [Google Scholar]
- 41.Lefebvre RA, Hasrat J, Gobert A. Influence of NG-nitro-l-arginine methyl ester on vagally induced gastric relaxation in the anaesthetized rat. Br J Pharmacol 105: 315–320, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol 543: 135–146, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maley BE Immunohistochemical localization of neuropeptides and neurotransmitters in the nucleus solitarius. Chem Senses 21: 367–376, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Meulemans AL, Eelen JG, Schuurkes JA. NO mediates gastric relaxation after brief vagal stimulation in anesthetized dogs. Am J Physiol Gastrointest Liver Physiol 269: G255–G261, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Meulemans AL, Helsen LF, Schuurkes AJ. Role of NO in vagally-mediated relaxations of guinea-pig stomach. Naunyn Schmiedebergs Arch Pharmacol 347: 225–230, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Moran TH, Kinzig KP. Gastrointestinal satiety signals. II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol 286: G183–G188, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Peters JH, Ritter RC, Simasko SM. Leptin and CCK modulate complementary background conductances to depolarize cultured nodose neurons. Am J Physiol Cell Physiol 290: C427–C432, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Plata-Salaman CR, Fukuda A, Oomura Y, Minami T. Effects of sulphated cholecystokinin octapeptide (CCK-8) on the dorsal motor nucleus of the vagus. Brain Res Bull 21: 839–842, 1988. [DOI] [PubMed] [Google Scholar]
- 49.Raybould HE, Roberts ME, Dockray GJ. Reflex decreases in intragastric pressure in response to cholecystokinin in rats. Am J Physiol Gastrointest Liver Physiol 253: G165–G170, 1987. [DOI] [PubMed] [Google Scholar]
- 50.Raybould HE, Tache Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol Gastrointest Liver Physiol 255: G242–G246, 1988. [DOI] [PubMed] [Google Scholar]
- 51.Reidelberger RD Cholecystokinin and control of food intake. J Nutr 124: 1327S–1333S, 1994. [DOI] [PubMed] [Google Scholar]
- 52.Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol 286: R1005–R1012, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Ritter RC Gastrointestinal mechanisms of satiation for food. Physiol Behav 81: 249–273, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol 514: 369–383, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sayegh AI, Ritter RC. Cholecystokinin activates specific enteric neurons in the rat small intestine. Peptides 24: 237–244, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz GJ, Moran TH, White WO, Ladenheim EE. Relationships between gastric motility and gastric vagal afferent responses to CCK and GRP in rats differ. Am J Physiol Regul Integr Comp Physiol 272: R1725–R1733, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol 61: 1001–1010, 1989. [DOI] [PubMed] [Google Scholar]
- 58.Shi M, Jones AR, Ferreira M Jr, Sahibzada N, Gillis RA, Verbalis JG. Glucose does not activate nonadrenergic, noncholinergic (NANC) inhibitory neurons in the rat stomach. Am J Physiol Regul Integr Comp Physiol 288: R742–R750, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Shi M, Jones AR, Niedringhaus MS, Pearson RJ, Biehl AM, Ferreira M Jr, Sahibzada N, Verbalis JG, Gillis RA. Glucose acts in the CNS to regulate gastric motility during hypoglycemia. Am J Physiol Regul Integr Comp Physiol 285: R1192–R1202, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Soudah HC, Lu Y, Hasler WL, Owyang C. Cholecystokinin at physiological levels evokes pancreatic enzyme secretion via a cholinergic pathway. Am J Physiol Gastrointest Liver Physiol 263: G102–G107, 1992. [DOI] [PubMed] [Google Scholar]
- 61.South EH, Ritter RC. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides 9: 601–612, 1988. [DOI] [PubMed] [Google Scholar]
- 62.Stanley S, Wynne K, McGowan B, Bloom S. Hormonal regulation of food intake. Physiol Rev 85: 1131–1158, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Straathof JWA, Mearadji B, Lamers CBHW, Masclee AAM. The effect of CCK on proximal gastric motor function in humans. Am J Physiol Gastrointest Liver Physiol 274: G939–G944, 1998. [DOI] [PubMed] [Google Scholar]
- 64.Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology 128: 175–191, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Sun K, Ferguson AV. Cholecystokinin activates area postrema neurons in rat brain slices. Am J Physiol Regul Integr Comp Physiol 272: R1625–R1630, 1997. [DOI] [PubMed] [Google Scholar]
- 66.Sutton GM, Patterson LM, Berthoud HR. Extracellular signal-regulated kinase 1/2 signaling pathway in solitary nucleus mediates cholecystokinin-induced suppression of food intake in rats. J Neurosci 24: 10240–10247, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi T, Owyang C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J Physiol 484: 481–492, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accomodation reflex in rats. J Physiol 504: 479–488, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi T, Owyang C. Regional differences in the nitrergic innervation between the proximal and the distal colon in rats. Gastroenterology 115: 1504–1512, 1998. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi T, Owyang C. Mechanism of cholecystokinin-induced relaxation of the rat stomach. J Auton Nerv Syst 75: 123–130, 1999. [DOI] [PubMed] [Google Scholar]
- 71.Talman WT, Andreasen K, Calvin J, Eversmann-Johanns S. Cholecystokinin in nucleus tractus solitarii modulates tonic and phasic gastric pressure. Am J Physiol Regul Integr Comp Physiol 261: R217–R222, 1991. [DOI] [PubMed] [Google Scholar]
- 72.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tutuian R, Vos R, Karamanolis G, Tack J. An audit of technical pitfalls of gastric barostat testing in dyspepsia. Neurogastroenterol Motil 20: 113–118, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Viard E, Zheng Z, Wan S, Travagli RA. Vagally mediated, nonparacrine effects of cholecystokinin-8s on rat pancreatic exocrine secretion. Am J Physiol Gastrointest Liver Physiol 293: G493–G500, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Wan S, Coleman FH, Travagli RA. Cholecystokinin-8s excites identified rat pancreatic-projecting vagal motoneurons. Am J Physiol Gastrointest Liver Physiol 293: G484–G492, 2007. [DOI] [PubMed] [Google Scholar]
- 76.Wang ZJ, Rao ZR, Shi JW. Tyrosine hydroxylase-, neurotensin-, or cholecystokinin-containing neurons in the nucleus tractus solitarii send projection fibers to the nucleus accumbens in the rat. Brain Res 578: 347–350, 1992. [DOI] [PubMed] [Google Scholar]
- 77.Woods SC Gastrointestinal satiety signals. I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol 286: G7–G13, 2004. [DOI] [PubMed] [Google Scholar]
- 78.Zerbib F, Bruley D, V, Scarpignato C, Leray V, D'Amato M, Roze C, Galmiche JP. Endogenous cholecystokinin in postprandial lower esophageal sphincter function and fundic tone in humans. Am J Physiol Gastrointest Liver Physiol 275: G1266–G1273, 1998. [DOI] [PubMed] [Google Scholar]
- 79.Zheng Z, Lewis MW, Travagli RA. In vitro analysis of the effects of cholecystokinin on rat brain stem motoneurons. Am J Physiol Gastrointest Liver Physiol 288: G1066–G1073, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]