Abstract
Detrusor smooth muscle (DSM) hypertrophy induced by partial bladder outlet obstruction (PBOO) is associated with changes in the NH2-terminal myosin heavy chain isoform from predominantly SM-B to SM-A, alteration in the Ca2+ sensitization pathway, and the contractile characteristics from phasic to tonic in rabbits. We utilized the SM-B knockout (KO) mouse to determine whether a shift from SM-B to SM-A without PBOO is associated with changes in the signal transduction pathway mediated via PKC and CPI-17, which keeps the myosin phosphorylation (MLC20) level high by inhibiting the myosin phosphatase. DSM strips from SM-B KO mice generated more force in response to electrical field stimulation, KCl, carbachol, and phorbol 12,13-dibutyrate than that of age-matched wild-type mice. There was no difference in the ED50 for carbachol but the maximum response was greater for the SM-B KO mice. DSM from SM-B KO mice revealed increased mass and hypertrophy. The KO mice also showed an overexpression of PKC-α, increased levels of phospho-CPI-17, and an elevated level of IP3 and DAG upon stimulation with carbachol. Two-dimensional gel electrophoresis revealed an increased level of MLC20 phosphorylation in response to carbachol. Together, these changes may be responsible for the higher level of force generation and maintenance by the DSM from the SM-B KO bladders. In conclusion, our data show that ablation of SM-B is associated with alteration of PKC-mediated signal transduction and CPI-17-mediated Ca2+ sensitization pathway that regulate smooth muscle contraction. Interestingly, similar changes are also present in PBOO-induced DSM compensatory response in the rabbit model in which SM-B is downregulated.
Keywords: smooth muscle contraction, alternative splicing, CPI-17, light chain phosphorylation
myosin ii is the major component of the thick filament in smooth muscles from all sources. Smooth muscle myosin II is regulated by alternative splicing at both 3′ and 5′ end of the myosin heavy chain (MHC) pre-mRNA producing COOH-terminal (SM1 and SM2) and NH2-terminal(SM-A and SM-B) MHC isoforms, respectively. Alternative splicing at the 5′ end inserts a 21-nt insertion that encodes a seven amino acid sequence in the NH2-terminal head region of the myosin near the ATP-binding site (3, 26). Studies have shown that smooth muscle myosin containing the SM-B isoform (with the 7-amino acid insert) have a higher actin-activated ATPase activity and a higher shortening velocity compared with those smooth muscle which predominantly consist of SM-A, lacking the seven amino acid insert (14, 26, 29). Studies have also shown that smooth muscles containing primarily this high-velocity isoform (SM-B), such as the urinary bladder, tend to be more phasic while those containing primarily SM-A such as aorta and esophageal sphincter tend to be more tonic (14, 26, 38, 47). Thus, the SM-B myosin isoform is present in phasic smooth muscles, although other characteristics such as the membrane properties are different (12, 47). The tonic and phasic properties of smooth muscle and the predominance of various SM isoforms are highly tissue specific and dependent on the function of the organ in which they are present. The functional role of these isoforms is demonstrated in the lower urinary tract where urinary bladder (containing predominantly SM-B) (15) requires a rapid and phasic contraction in the bladder body to initiate bladder emptying while the more tonic urethral smooth muscle (containing more of the SM-A isoform) (8) helps to facilitate urine storage during the filling phase (51, 52).
Changes in the myosin isoforms in hypertrophy associated with various disease processes have been reported in cardiac (30, 37), skeletal (19, 45), and smooth muscle (15, 50). Partial bladder outlet obstruction (PBOO)-induced hypertrophy in both rabbits and mice causes a shift in the NH2-terminal MHC isoform from predominantly SM-B to SM-A (2, 15). Concomitant with a decrease in SM-B (the high ATPase isoform) and an increase in SM-A (low ATPase isoform), there is a decrease in maximum shortening velocity and the hypertrophied detrusor smooth muscle (DSM) reveals contractile characteristics typical of tonic smooth muscle compared with the phasic contraction shown by normal DSM (46). In addition, PBOO-induced DSM hypertrophy also shows an upregulation of Rho-kinase (5) which is implicated in calcium sensitization of muscle contraction and an increase in the resting myosin light chain (MLC20) phosphorylation level (8, 10, 44).
In addition to the myosin NH2-terminal isoforms playing a role in controlling the actin-activated myosin ATPase activity, the Ca2+/calmodulin-dependant phosphorylation of the myosin regulatory light chain (MLC20) regulates the actin-activated myosin ATPase activity of smooth muscle myosin (9, 20, 40) and cross bridge cycling in smooth muscles (7, 13, 24). The level of myosin phosphorylation is also regulated under certain conditions without an elevation of cytosolic Ca2+ by the small GTPase Rho-activated kinase, which regulates myosin light chain phosphatase activity (MLCP), through the membrane-activated second messenger systems such as Rho A (11, 42, 43). The regulation of MLCP is complex, involving a cascade of reactions including second messengers and downstream effectors of membrane and receptor activation. The availability of an SM-B knockout model in which the smooth muscle MHC B isoform is ablated offers a unique opportunity to study the effect of alteration of myosin isoforms on the pathways that regulate contraction in smooth muscle in the absence of bladder outlet obstruction. This study utilized the SM-B knockout mouse to determine whether a shift in the NH2-terminal MHC isoform from SM-B to SM-A without PBOO is associated with changes in the signal transduction pathway mediated via the PKC and CPI-17, a pathway that lowers the myosin phosphatase activity and keeps the myosin LC20 at a high level of phosphorylation, thus maintaining the smooth muscle tone.
MATERIALS AND METHODS
SM-B null mice.
The method for producing the SM-B null mice has been previously described (4). Mice were killed by exposure to CO2 in accordance with the ethical treatment of animals under a protocol approved by the University of Pennsylvania Institutional Animal Care and Use Committee. The mice used for these studies were F2 generation males and females between the ages of 20 and 24 wk old.
Tissue preparation.
DSM strips were obtained from male and female mice. The bladder was surgically removed at the level of the bladder neck and placed into Tyrodes buffer (125 mM NaCl, 2.7 mM KCl, 23.8 mM NaHCO3, 0.5 mM MgCl2·6H2O, 0.4 mM NaH2PO4·H2O, 1.8 mM CaCl2, and 5.5 mM dextrose). The urothelial layer was gently removed by scraping the surface of the open bladder with a sharp scalpel and the rest of the bladder, comprised primarily of smooth muscle tissue, was equilibrated at 37°C in 95% O2-5% CO2. Samples of smooth muscle tissue were obtained from the bladder body (region extending proximally above the ureteral opening to the bladder).
Force measurements.
Strips of DSM were suspended longitudinally in 15 ml of Tyrodes buffer at 37°C as previously described (30). After a 30-min equilibration, the length of optimal force development (Lo) was determined by increasing the length of each strip in 1.5-mm increments until maximal contractile force to electrical field stimulation (EFS; 70 V, 32 Hz, 1-ms duration) was achieved. After determination of Lo, the bath was replaced with fresh Tyrode's buffer for 15 min to allow stabilization of the muscle at the resting level. Longitudinal strips of bladder (∼1 × 4 mm, 10 mg) were prepared for depolarization with high-KCl solution (125 mM) to evaluate tonic and phasic properties (41). The tissue was then washed three times (10 min each) with Tyrodes buffer and a carbachol concentration-response curve (0–25 μM) was carried out to determine the effect of cholinergic stimulation on the muscle strips. Once again, the strips were washed, as previously described, and 3 μM PDBu was added to evaluate the effect of PKC activation in the smooth muscle. A final wash and addition of high KCl (125 mM) establish that the potency of the muscle strips has not declined disproportionately during the course of the experiment. In separate experiments, we used the specific PKC inhibitor, Bisindolylmaleimide-1 (Bis-1; 10 μM), and the Rho-kinase inhibitor, Y27632 (20 μM), to reverse the contractions caused by PDBu. These inhibitors were also preincubated with muscle strips for 15 min before activation with FS (32 Hz) or carbachol (10 μM) to determine their inhibitory effects. The force was standardized to the cross-sectional area of the muscle strips.
Estimation of myosin light chain phosphorylation.
Analysis to determine the level of myosin light chain phosphorylation in muscle strips was described previously (22). Strips were frozen rapidly at rest or at different levels of force by snapping with clamps previously chilled in dry ice-acetone slurry followed by immersion in dry ice-acetone slurry for 30 s and stored in liquid nitrogen. Strips of bladder smooth muscle were also stimulated with carbachol (25 μM) and rapidly snap-frozen at 100% maximum force and stored in liquid nitrogen. Frozen muscle strips immersed in liquid nitrogen were ground to a fine powder using a prechilled mortar and pestle. The powder was then added to a mixture of dry ice-acetone to inactivate any residual endogenous kinases and phosphatases remaining after the first treatment in the dry ice-acetone slurry. This mixture was left at room temperature for 30 min until all of the dry ice had evaporated. The sample in acetone was then centrifuged (8,000 g for 10 min) and the acetone was removed. The pellet was mixed with isoelectric focusing (IEF) sample buffer [50 μl/10 mg tissue containing 9.5 M urea, 1.6% ampholyte (pH 5–7), 0.4% ampholyte (pH 3–10), 2% NP40, and 5% β-mercaptoethanol] and homogenized using a mini-electric homogenizer. After centrifugation, ∼50 μl of the supernatant were then applied to IEF cylindrical gels (1 × 65 mm) and IEF at 350 V was carried out overnight. Gels were then subjected to SDS-PAGE (14%) and stained as previously described (22). Spots corresponding to 20 kDa were identified as smooth muscle LC20 by Western blotting using antibody specific to 20-kDa myosin light chain. The phosphorylated and unphosphorylated LC20 were scanned and analyzed using a Bio-Rad GS-700 imaging densitometer and using a 2D-PAGE Molecular Analyst Software program (Bio-Rad, Hercules, CA).
Measurement of inositol 1,4,5-triphosphate and total IP.
To measure 1,4,5-triphosphate (IP3), we prelabeled murine bladders with [3H] inositol, treated the tissue with LiCl (10 mM) and carbachol (0.03 mM), and rapidly froze the tissue in liquid N2. The tissue was then extracted with trichloroacetic acid and the inositol phosphates were separated by Dowex chromatography (28). The radioactivity was measured in a Packard 1500 Tri-Carb liquid scintillation counter and the results were normalized as disintegrations per minute (DPMs) per 15 mg bladder tissue.
Measurement of diacylglycerol.
Diacylglycerol (DAG) is extracted and measured according to the method of Bligh and Dyer (6). Briefly, DAG is extracted with a 1:1 mixture of chloroform/methanol for 1 to 2 h on ice. Next, 0.2 M KCl is added to the mixture with vortexing to break the phase and allow the chloroform to separate from the methanol. The mixture is centrifuged briefly which aids the separation and the lower chloroform phase is transferred to a new test tube. The sample is then dried under nitrogen and the lipid extract made soluble in octyl-β-glucoside/cardiolipin solution. The DAG in the sample is then assayed using DAG kinase in the presence of [γ-32P]ATP, further separated by thin layer chromatography (TLC) and the radioactivity is measured in a scintillation counter.
SDS-PAGE and Western blot analysis.
Proteins were separated by electrophoresis of denatured protein on SDS-polyacrylamide gels (7.5%). Protein amounts used for quantitation as well as for the Western blots were in the linear range for absorbance. Identical gels were run and transferred to an Immobilon-P membrane (Millipore, Bedford, MA) overnight at 30 V (Bio-Rad mini-transfer unit) in buffer (25 mM Tris, 192 mM glycine, 0.005% SDS, and 0.056% β-mercaptoethanol). After being blocked with 5% milk for 1 h, the membrane was incubated with a 1:10,000 dilution of primary antibody for PKC-α (Sigma) and 1:3,000 dilution for CPI-17 and phospho-CPI-17 (Upstate), 1:1,000 dilution of primary antibody for ROK-α, 1:1,000 dilution for ROK-β, and 1:2,000 dilution for RhoA (Cytoskeleton) for 1 h at room temperature, washed three times with 10 ml PBS containing 0.05% Tween 20 (PBST), and further incubated with corresponding secondary antibody (goat anti-mouse IgG; 1:4,000) or (donkey anti-rabbit; 1:4,000) for 1 h at room temperature. All antibody solutions were diluted in PBST and membranes were washed thoroughly with PBST between incubations. Target proteins were detected using enhanced chemiluminescence kit from Amersham Life Sciences. The amount of the target proteins was determined by scanning densitometry using Bio-Rad GS-800 Calibrated Densitometer and Quantity 1 software (Bio-Rad).
RESULTS
Bladder weight.
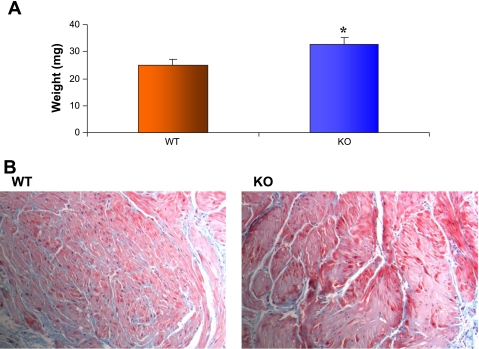
Figure 1A shows the bladder weights for wild-type (WT; n = 10) and SM-B knockout (KO; n = 10) bladders. The mean bladder weights in milligrams for age- and weight-matched mice were 25 ± 0.074 and 33.0 ± 0.09 for WT and SM-B KO mice, respectively (P < 0.05). Figure 1B shows the histologic appearance of a representative Masson's trichrome-stained cross section (5 μm) of the bladder strips used for physiological studies. The amount of the bladder smooth muscle layer relative to interstitial connective tissue, respectively, was greater for the SM-B KO (72 vs. 28%) compared with WT (61 vs. 39%) bladder, as determined using imaging software (ImagePro) to quantify the area of smooth muscle and collagen in the histological sections.
Fig. 1.
A: mean bladder weights for wild-type (WT; mean = 25 ± 0.074) and SM-B knockout (KO; mean = 33.0 ± 0.09), n = 10, P < 0.05. The average body weight of WT and KO mice was similar. B: representative photomicrographs (×40) showing 5-μm cross sections stained with Masson's trichrome for both WT and SM-B KO bladders. A much higher density of muscle fibers can be observed in the SM-B KO compared with the WT bladders with significantly less intermuscular connective tissue matrix in the KO.
Force.
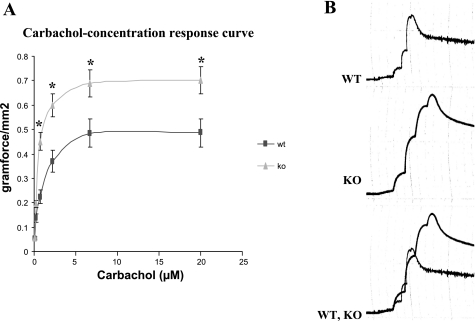
Figure 2 shows the carbachol concentration-response curve for WT and KO mice (n = 4). There was no difference in the ED50 (0.7 ± 0.059 and 0.68 ± 0.065 for WT and KO, respectively). However, the maximum response (Emax) for the KO bladder (0.71 ± 0.12 g/mm2) was significantly higher than that for the WT (0.47 ± 0.059 g/mm2), P < 0.05. Figure 2B shows representative tracings of the raw data for WT, KO, and when superimposed.
Fig. 2.
A: summary data for the carbachol concentration-response curve for WT (n = 5) and SM-B KO (n = 5). The force data for each point represent the mean and SE of strips of bladder smooth muscle. There was no difference in the ED50 but the Emax of 0.47 ± 0.059 g/mm2 for the WT was significantly less than that of the SM-B KO (0.71 ± 0.12) g/mm2, P < 0.05. B: representative tracings of the raw data for WT, SM-B KO, and when superimposed, respectively.
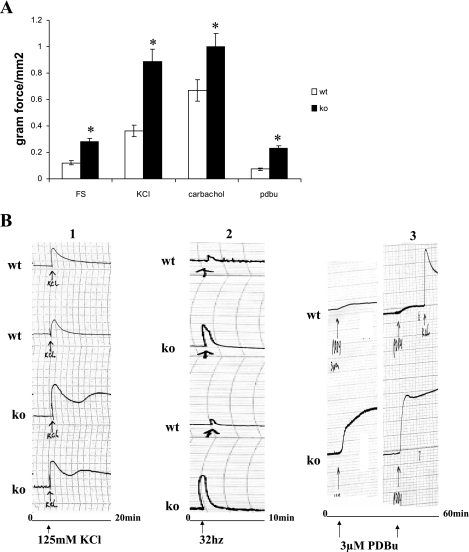
Figure 3A shows the responses to EFS (70 V, 32 Hz, 1-ms duration), KCl (125 mM), PDBu (3 μM), and carbachol. The data indicate that the KO bladder strips produce significantly higher levels of force in response to all forms of stimulation (n = 4; P < 0.05). Representative tracings of the summary data in Fig. 3A are shown in Fig. 3B. It is noteworthy that the plateau phase of the force curve is higher for the KO bladder strips than the WT bladders for all forms of activation, indicating that the force is maintained at a higher level as a percentage of maximum force for the KO bladder strips compared with WT.
Fig. 3.
A: maximal response to electrical field stimulation (EFS; 70 V, 32 Hz, 1-ms duration), KCl (125 mM), PDBU (3 μM), and carbachol (20 μM). In all cases, the SM-B KO bladder strips responded with higher force than the WT bladders, P < 0.05. B: representative tracings of the summary data in A for KCl, EFS, and PDBu. Note the higher levels of force maintenance in the SM-B KO bladders compared with WT.
Table 1 shows the effects of the PKC inhibitor, Bis-1, and the Rho-kinase inhibitor, Y27632, on contractile function of WT and SM-B KO bladders. Bis-1 caused 100% relaxation of the contraction in response to PDBu for both WT and SM-B KO bladders. Preincubation of muscle strips with Bis-1 caused a less dramatic but significant reduction in force generation in response to FS and carbachol. While the Rho-kinase inhibitor, Y27632, seems to cause a decline in force levels in WT bladders in response to all stimulations, this decline was not significant. This was also true for SM-B KO bladders except for the response to carbachol which was significantly inhibited by Y27632.
Table 1.
Effect of Bis-1 (10 μM) and Y27632 (20 μM) on contraction induced by FS (32 Hz), carbachol (10 μM), and PDBu (3 μM) in WT and SM-B KO bladders
|
FS |
Carbachol
|
PDBu
|
|
|---|---|---|---|
| Force, g | Force, g | Force, g | |
| WT | 1.13±0.15 | 1.1±0.53 | 0.16±0.07 |
| SM-B KO | 2.03±0.27* | 1.62±0.08* | 0.26±0.04* |
| WT+BIS-1 | 0.82±0.08 | 0.74±0.03* | 0.02±0.015* |
| SM-B KO+BIS-1 | 1.82±0.38 | 0.74±0.08† | 0.01±0.006† |
| WT+Y27632 | 0.90±0.22 | 0.87±0.21 | 0.11±0.05 |
| SM-B KO+Y27632 | 1.71±0.31 | 1.01±0.29† | 0.21±0.03 |
Values are means ± SE, n = 5, P < 0.05. FS, field stimulation; WT, wild-type; KO, knockout.
Significantly different from WT.
Significantly different from SM-B KO.
PKC-α, CPI-17, and Phospo-CPI-17 at the protein level.
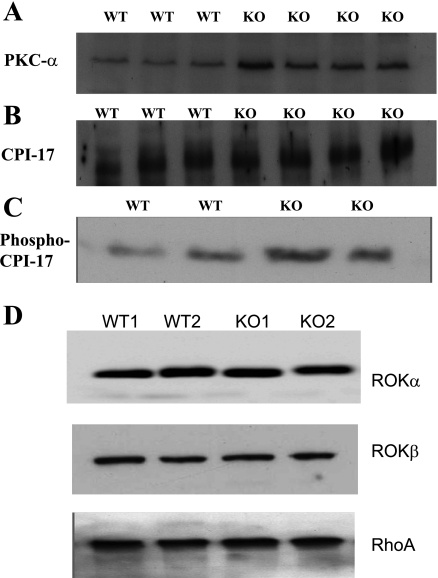
Since the amount of myosin affects the number of cross bridges that can be formed in a muscle, we estimated the myosin content in the DSM from WT and SM-B KO mice. Western blot analysis revealed no significant difference (data not shown). Force maintenance in the DSM from PBOO is associated with overexpression and overactivity of Rho-kinase (5); however, the expression of Rho-kinase and RhoA in the DSM from SM-B null mouse bladders is not different from that of bladders from WT mice (Fig. 4D). Since CPI-17 has been shown to contribute to the calcium sensitization pathway (17), we analyzed the expression of CPI-17 and PKC which is important for the phosphorylation of CPI-17 in the DSM from the SM-B null bladders. Figure 4 shows the Western blot for PKC-α (A), CPI-17 (B), and Phospho-CPI-17 (C) at the resting level after loading 20 μg of protein for each sample. The loading level was determined by doing protein assays of the extracted proteins and loading 20 μg which was within the linear range of the protein standard curve. Scanning densitometry of the bands reveals a significant, P < 0.05, upregulation of PKC-α in the SM-B KO bladders [8.87 ± 0.34 optical density units (ODU) compared with 5.0 ± 0.43 ODU for WT]. There was no difference in the other PKC isoforms (PKC, ɛ and θ) tested; however, the amount of phosphorylated CPI-17 was significantly elevated for SM-B KO bladders as determined from scanning densitometry of the Western blots made using antibody specific to phospho-CPI-17 (6.03 ± 0.9 ODUs, compared with 3.30 ± 0.53 ODUs for WT), P < 0.05.
Fig. 4.
Western blot for PKC-α (A), CPI-17 (B), and Phospho-CPI-17 (C). PKC-α was significantly elevated in SM-B KO bladders (8.87 ± 0.34) ODUs compared with WT bladders (5.0 ± 0.43) ODUs, P < 0.05. There was no difference in the total CPI-17 level (B); however, the level of phospho-CPI-17 (C) was higher in the SM-B KO mice (5.04 ± 0.95) compared with the WT mice (2.74 ± 0.78) ODUs, n = 4, P < 0.05. D: results for ROKα, ROKβ, and RhoA. The data reveal no significant difference between WT and SM-B KO bladders.
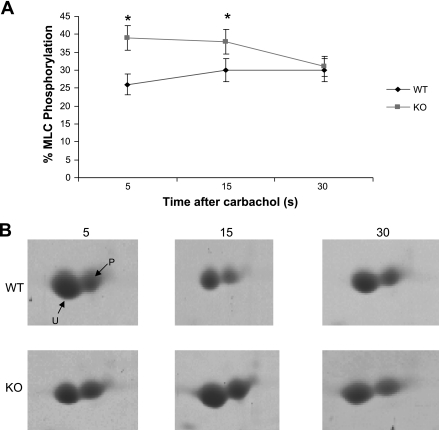
MLC phosphorylation.
A time course of phosphorylation at 5, 15, and 30 s after activation with 20 μM carbachol is shown in Fig. 5A. Figure 5B shows representative two-dimensional gels of the unphosphorylated and phosphorylated MLC20 at each time point. The level of MLC20 phosphorylation at each time point (n = 4) was 26.4 ± 2.9, 30.0 ± 3.2, and 30.0 ± 3.5 for WT bladders and 39 ± 5.1, 38 ± 4.4, and 31 ± 3.3 for bladders from SM-B null mice, P < 0.05 for time points at 5.0 and 15 s, respectively. There was no significant difference at 30 s. There was also no difference in the level of MLC20 phosphorylation between WT (19.96 ± 2.7%) and SM-B (22.02 ± 3.4%) null bladders under resting conditions, n = 4, P = 0.061.
Fig. 5.
A: time course of MLC20 phosphorylation at 5, 15, and 30 s after activation with 20 μM carbachol. The mean level of phosphorylation at each time point was 26.4 ± 2.9, 30.0 ± 3.2, and 30.0 ± 3.5%, respectively, for WT bladders and 39 ± 5.1, 38 ± 4.4, and 31 ± 3.3% for SM-B KO bladders, P < 0.05, n = 4. B: representative 2-dimensional gels showing unphosphorylated and phosphorylated MLC20 at each time point for WT and SM-B KO bladders, respectively.
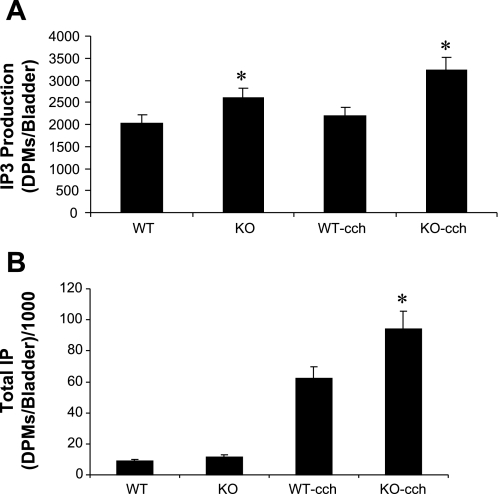
Total IP, IP3 production, and DAG.
Since our data show that PKC-mediated signaling is involved as shown from the activation of the DSM muscle strips with PDBU, we analyzed the IP3 production, one of the downstream effects of PKC stimulation. Figure 6A shows the results (n = 4) for total IP3 production at rest (L0) and when stimulated with 25 μM carbachol. The IP3 levels were significantly higher in the KO bladders both at rest and when stimulated with carbachol (25 μM), P < 0.05. Figure 6B shows the results for total IP production under stimulated and unstimulated conditions. There was no significant difference between the WT and KO under unstimulated conditions while carbachol (25 μM) caused a significant (P < 0.05) increase in total IP production in the SM-B KO bladders. The level of DAG was significantly elevated in the KO bladders (1,869 ± 94.1 ODUs compared with 1,397 ± 128 for WT, P < 0.05).
Fig. 6.
A: total IP3 production at rest (L0) and when stimulated with 25 μM carbachol. The IP3 levels were higher in the SM-B KO bladders both at rest and when activated by carbachol, P < 0.05. B: results for total IP production. There was no difference in the IP production in the absence of stimulation while carbachol (25 μM) caused an increase in the SM-B KO compared with the WT, P < 0.05.
DISCUSSION
The role of the NH2-terminal smooth muscle myosin isoforms, SM-A and SM-B, in the regulation of smooth muscle myosin ATPase activity and shortening velocity has been well-documented (4, 14, 26). These studies established a compelling relationship between the presence of the SM-B isoform, which contains a 7-amino acid insert in the head region of the SMMHC close to the ATP binding site, and the high actin-activated Mg-ATPase activity and shortening velocity of the smooth muscle in which it is present. Smooth muscles lacking the insert (SM-B) contain myosin with low ATPase activity and low velocity of shortening. Thus, it has been demonstrated that the regulation of smooth muscle function is highly dependent on the nature and composition of the myosin isoforms present within the smooth muscle bundles.
Increasingly, however, a link has been established between the regulation of smooth muscle function and other pathways such as RhoA/Rho-kinase and PKC-CPI-17 (23, 39, 43). Regulation through these pathways has been implicated in the calcium sensitization of smooth muscle, an increase in tone without a corresponding rise in intracellular calcium (18, 42). Thus, it is clear that the absence or presence of specific myosin isoforms may play a role in the regulation of tone and contraction through these pathways also. In this study, we report that bladders in which the SM-B myosin has been knocked out (bladders containing only SM-A) show an elevated plateau phase in response to KCl stimulation (Fig. 3B) and peak contraction, an increase in mass, and a heightened contractile response to the PKC activator, PDBu, compared with WT mice. An increased level of tone is typically present in smooth muscle containing high levels of SM-A such as aorta, urethra, esophageal sphincter, and corpus cavernosum smooth muscle (16, 22, 47). However, the mechanism responsible for high levels of tone and force maintenance in smooth muscle has not been fully understood. Previous studies on bladder tissue from the SM-B KO mouse indicated that the insert can affect the cooperativity between myosin heads and modulate the kinetics of crossbridge cycling (25).
One of the changes in the contractile proteins associated with obstruction-induced bladder hypertrophy is a shift in the NH2-terminal isoform from SM-B to SM-A (15). Thus, the SM-B KO mouse provides a unique opportunity to investigate the changes in the signal transduction regulation and pathophysiology of the smooth muscle following a switch in the myosin from SM-B to SM-A in the absence of PBOO. Our initial observations reveal that the bladders from SM-B null mice were significantly heavier than bladders from WT mice and that the DSM strips from SM-B null produced higher levels of force when normalized for cross-sectional area. A representative Trichrome stain shows the difference in cross-sectional area of smooth muscle relative to collagen (Fig. 1B). This indicates that the bladder wall from SM-B null mice may indeed have more smooth muscle relative to collagen than that of WT bladders which may contribute to the higher force produced by the DSM from SM-B null bladders when normalized for cross-sectional area. Of particular interest was the effect of EFS, carbachol, and PKC activator. These three forms of smooth muscle activation converge at a common intersection in the signal transduction pathway that involves the activation of PKC. EFS has been shown to release neurotransmitters such as acetylcholine from nerve terminals which can then bind cholinergic receptors on the smooth muscle cell membrane, while carbachol can directly bind to, and activate these receptors when added to a bath solution bathing the SM strip. These G protein-coupled receptors when activated lead to phosphoinositide turnover with the release of the phosphoinositide hydrolysis products IP3 and DAG. IP3 has been shown to release calcium from intracellular Ca2+ stores while DAG can directly activate PKC. Thus, both of these hydrolytic products of phosphoinositide can further amplify contraction in smooth muscle (21, 48). To probe the enhanced force seen in the SM from SM-B null bladders, we used the synthetic PKC activator PDBu to stimulate the smooth muscle in our physiological studies. PDBu is a well-known activator of PKC signaling and contraction in a variety of smooth muscles (1, 12, 33, 53). Our results indicate a remarkable difference with bladders from SM-B null, recording substantially higher force levels than WT bladders when corrected for cross-sectional area. To gain additional insight into the remarkable contractile response and high level of force of DSM from bladders from SM-B null (containing only SM-A) to both carbachol and PDBu, we studied PKC-α expression, CPI-17, and phospho-CPI-17 at the protein level. Carbachol can activate PKC indirectly through G protein-coupled receptors leading to DAG formation (31, 36), while PDBu can directly activate PKC as indicated previously. Our data indicate significant upregulation of PKC-α and phospho-CPI-17 but no change in the total CPI-17 expression level. CPI-17 is a substrate for PKC and when phosphorylated becomes active and inhibits MLCP. Thus, an increased level of phospho-CPI-17 can further inhibit MLCP leading to a shift toward continued and persistent MLC20 phosphorylation and force in the absence of a rise in intracellular calcium (18, 49). This phenomenon is referred to as calcium sensitization and may further explain the high maximum force and force maintenance seen in the KO bladders. Interestingly, a high level of force maintenance and upregulation of Rho-kinase have also been reported in rabbit bladders subjected to PBOO. Rho-kinase has also been shown to phosphorylate the regulatory subunit of phosphatase (MYPT) and can also inhibit MLCP activity. Rho-kinase expression was similar in SM-B KO and WT detrusor (Fig. 4D). In addition, the human myometrium undergoes hypertrophic changes during pregnancy (34). Studies by Ozaki et al. (35) also reveal a heightened contractile response of the pregnant myometria to PDBu and upregulation of PKC-β and CPI-17 at the mRNA level. PKC-β expression in the DSM was similar for SM-B KO and WT.
As indicated in a recent paper by Eto et al. (17), CPI-17 phosphorylation induced conformational changes in CPI-17, making it a potent inhibitor of MLCP. Thus, phosphorylated CPI-17 caused increased inhibition of MLCP activity (27). The inhibited MLCP is unable to dephosphorylate the MLC which is required for relaxation of the smooth muscle, and as a consequence, this may promote higher levels of force. Since our data showed elevated levels of phospho-CPI-17, we studied the MLC20 phosphorylation level both under resting and stimulated conditions in WT and SM-B KO bladders. While the data showed no difference in the resting phosphorylation level, the SM-B KO bladders showed a significant increase in MLC20 phosphorylation compared with WT bladders after activation with carbachol. This increased phosphorylation was persistent at 5 and 15 s after activation and coincided at 30 s. This suggests that the higher phosphorylation levels in the SM-B KO bladders may contribute to the elevated force levels seen in these bladders due to increased number of activated cross bridges. Prior studies from this lab reveal that after PBOO, force levels were maintained at a high level compared with unobstructed bladders, and this increased force was accompanied by higher levels of MLC20 phosphorylation in the PBOO bladders (46). In conclusion, our data show that ablation of SM-B is associated with smooth muscle hypertrophy, alteration of PKC-mediated signal transduction, and CPI-17-mediated Ca2+ sensitization pathway that regulate smooth muscle contraction. Interestingly, similar changes are present also in PBOO-induced DSM compensatory response in the rabbit model in which SM-B is downregulated. Thus, there appears to be a cross-talk between the smooth muscle myosin isoforms and the signal transduction pathway in the SM-B KO bladders which contribute to higher force levels in these tissues.
GRANTS
This work was supported by George O'Brien Urology Research Center Grant P50 DK-52620.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahn SC, Kim YC, Kim SJ, So I, Kim KW. Dual roles of phorbol 12,13-dibutyrate in the regulation of guinea-pig gastric contraction. J Smooth Muscle Res 33: 11–22, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Austin JC, Chacko SK, DiSanto M, Canning DA, Zderic SA. A male murine model of partial bladder outlet obstruction reveals changes in detrusor morphology. J Urol 172: 1524–1528, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Babij P, Periasamy M. Myosin heavy chain isoform diversity in smooth muscle is produced by differential RNA processing. J Mol Biol 210: 673–679, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Babu GJ, Loukianov E, Loukianova T, Pyne GJ, Huke S, Osol G, Low RB, Paul RJ, Periasamy M. Loss of SM-B myosin affects muscle shortening velocity and maximal force development. Nat Cell Biol 3: 1025–1029, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Bing W, Chang S, Hypolite JA, DiSanto ME, Zderic SA, Rolf L, Wein AJ, Chako S. Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho-kinase. Am J Physiol Renal Physiol 285: F990–F997, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959. [DOI] [PubMed] [Google Scholar]
- 7.Butler TM, Siegman MJ. Chemical energetics of contraction in mammalian smooth muscle. Fed Proc 41: 204–208, 1982. [PubMed] [Google Scholar]
- 8.Chacko S, Chang S, Hypolite J, DiSanto M, Wein A. Alteration of contractile and regulatory proteins following partial bladder outlet obstruction. Scand J Urol Nephrol Suppl 215: 26–36, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Chacko S, Conti MA, Adelstein RS. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc Natl Acad Sci USA 74: 129–133, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chacko S, DiSanto M, Wang Z, Zderic SA, Wein AJ. Contractile protein changes in urinary bladder smooth muscle during obstruction-induced hypertrophy. Scand J Urol Nephrol Suppl 184: 67–76, 1997. [PubMed] [Google Scholar]
- 11.Chakder S, Sarma DN, Rattan S. Mechanism of internal anal sphincter smooth muscle relaxation by phorbol 12,13-dibutyrate. Am J Physiol Gastrointest Liver Physiol 280: G1341–G1350, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Chang S, Hypolite JA, Changolkar A, Wein AJ, Chacko S, DiSanto ME. Increased contractility of diabetic rabbit corpora smooth muscle in response to endothelin is mediated via Rho-kinase beta. Int J Impot Res 15: 53–62, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science 211: 495–497, 1981. [DOI] [PubMed] [Google Scholar]
- 14.DiSanto ME, Cox RH, Wang Z, Chacko S. NH2-terminal-inserted myosin II heavy chain is expressed in smooth muscle of small muscular arteries. Am J Physiol Cell Physiol 272: C1532–C1542, 1997. [DOI] [PubMed] [Google Scholar]
- 15.DiSanto ME, Stein R, Chang S, Hypolite JA, Zheng Y, Zderic S, Wein AJ, Chacko S. Alteration in expression of myosin isoforms in detrusor smooth muscle following bladder outlet obstruction. Am J Physiol Cell Physiol 285: C1397–C1410, 2003. [DOI] [PubMed] [Google Scholar]
- 16.DiSanto ME, Wang Z, Menon C, Zheng Y, Chacko T, Hypolite J, Broderick G, Wein AJ, Chacko S. Expression of myosin isoforms in smooth muscle cells in the corpus cavernosum penis. Am J Physiol Cell Physiol 275: C976–C987, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Eto M, Kitazawa T, Matsuzawa F, Aikawa S, Kirkbride JA, Isozumi N, Nishimura Y, Brautigan DL, Ohki SY. Phosphorylation-induced conformational switching of CPI-17 produces a potent myosin phosphatase inhibitor. Structure 15: 1591–1602, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldspink G Gene expression in skeletal muscle. Biochem Soc Trans 30: 285–290, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Gong MC, Gorenne I, Read P, Jia T, Nakamoto RK, Somlyo AV, Somlyo AP. Regulation by GDI of RhoA/Rho-kinase-induced Ca2+ sensitization of smooth muscle myosin II. Am J Physiol Cell Physiol 281: C257–C269, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Gorecka A, Aksoy MO, Hartshorne DJ. The effect of phosphorylation of gizzard myosin on actin activation. Biochem Biophys Res Commun 71: 325–331, 1976. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Mahavadi S, Sriwai W, Hu W, Murthy KS. Gi-coupled receptors mediate phosphorylation of CPI-17 and MLC20 via preferential activation of the PI3K/ILK pathway. Biochem J 396: 193–200, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hypolite JA, DiSanto ME, Zheng YM, Chang SH, Wein AJ, Chacko S. Regional variation in myosin isoforms and phosphorylation at the resting tone in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C254–C264, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Ikebe M, Brozovich FV. Protein kinase C increases force and slows relaxation in smooth muscle: evidence for regulation of the myosin light chain phosphatase. Biochem Biophys Res Commun 225: 370–376, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol 25: 593–620, 1985. [DOI] [PubMed] [Google Scholar]
- 25.Karagiannis P, Babu GJ, Periasamy M, Brozovich F. The smooth muscle myosin seven amino acid heavy chain insert's kinetic role in the crossbridge cycle for mouse bladder. J Physiol 547: 2, 463–473, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem 268: 12848–12854, 1993. [PubMed] [Google Scholar]
- 27.Kitazawa T, Onodera C, Taneike T. Potentiation of motilin-induced contraction by nitric oxide synthase inhibition in the isolated chicken gastrointestinal tract. Neurogastroenterol Motil 14: 3–13, 2002. [DOI] [PubMed] [Google Scholar]
- 28.LaBelle EF, Murray BM. Differences in inositol phosphate production in rat tail artery and thoracic aorta. J Cell Physiol 144: 391–400, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Lauzon AM, Tyska MJ, Rovner AS, Freyzon Y, Warshaw DM, Trybus KM. A 7-amino-acid insert in the heavy chain nucleotide binding loop alters the kinetics of smooth muscle myosin in the laser trap. J Muscle Res Cell Motil 19: 825–837, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Levin RM, Shofer FS, Wein AJ. Cholinergic, adrenergic, and purinergic response of sequential strips of rabbit urinary bladder. J Pharmacol Exp Ther 212: 536–540, 1980. [PubMed] [Google Scholar]
- 31.Liles WC, Hunter DD, Meier KE, Nathanson NM. Activation of protein kinase C induces rapid internalization and subsequent degradation of muscarinic acetylcholine receptors in neuroblastoma cells. J Biol Chem 261: 5307–5313, 1986. [PubMed] [Google Scholar]
- 32.Litten RZ, Martin BJ, Low RB, Alpert NR. Altered myosin isozyme patterns from pressure-overloaded and thyrotoxic hypertrophied rabbit hearts. Circ Res 50: 856–864, 1982. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto T, Nishiyama M, Kobayashi T, Kasuya Y, Kamata K. Effect of phorbol 12,13-dibutyrate on smooth muscle tone in rat stomach fundus. J Smooth Muscle Res 41: 107–116, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Ono M, Maruyama T, Masuda H, Kajitani T, Nagashima T, Arase T, Ito M, Ohta K, Uchida H, Asada H, Yoshimura Y, Okano H, Matsuzaki Y. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci USA 104: 18700–18705, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozaki H, Yasuda K, Kim YS, Egawa M, Kanzaki H, Nakazawa H, Hori M, Seto M, Karaki H. Possible role of the protein kinase C/CPI-17 pathway in the augmented contraction of human myometrium after gestation. Br J Pharmacol 140: 1303–1312, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raffaniello RD, Raufman JP. Carbachol activates protein kinase C in dispersed gastric chief cells. Biochem J 300: 21–24, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaub MC, Hefti MA, Zuellig RA, Morano I. Modulation of contractility in human cardiac hypertrophy by myosin essential light chain isoforms. Cardiovasc Res 37: 381–404, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood JJ, Eddinger TJ. Shortening velocity and myosin heavy- and light-chain isoform mRNA in rabbit arterial smooth muscle cells. Am J Physiol Cell Physiol 282: C1093–C1102, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Sobieszek A, Small JV. Regulation of the actin-myosin interaction in vertebrate smooth muscle: activation via a myosin light-chain kinase and the effect of tropomyosin. J Mol Biol 112: 559–576, 1977. [DOI] [PubMed] [Google Scholar]
- 40.Somlyo AP, Himpens B. Cell calcium and its regulation in smooth muscle. FASEB J 3: 2266–2276, 1989. [DOI] [PubMed] [Google Scholar]
- 41.Somlyo AP, Somlyo AV. Vascular smooth muscle. I. Normal structure, pathology, biochemistry, and biophysics. Pharmacol Rev 20: 197–272, 1968. [PubMed] [Google Scholar]
- 42.Somlyo AP, Somlyo AV. Signal transduction by G proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522: 177–185, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somlyo AP, Wu X, Walker LA, Somlyo AV. Pharmacomechanical coupling: the role of calcium, G proteins, kinases and phosphatases. Rev Physiol Biochem Pharmacol 134: 201–234, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Stanton MC, Clement M, Macarak EJ, Zderic SA, Moreland RS. Partial bladder outlet obstruction alters Ca2+ sensitivity of force, but not of MLC phosphorylation, in bladder smooth muscle. Am J Physiol Renal Physiol 285: F703–F710, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Stone J, Brannon T, Haddad F, Qin A, Baldwin KM. Adaptive responses of hypertrophying skeletal muscle to endurance training. J Appl Physiol 81: 665–672, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Su X, Stein R, Stanton MC, Zderic S, Moreland RS. Effect of partial outlet obstruction on rabbit urinary bladder smooth muscle function. Am J Physiol Renal Physiol 284: F644–F652, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Szymanski PT, Chacko TK, Rovner AS, Goyal RK. Differences in contractile protein content and isoforms in phasic and tonic smooth muscles. Am J Physiol Cell Physiol 275: C684–C692, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Tugba Durlu-Kandilci N, Brading AF. Intracellular calcium stores in beta-escin skinned rat and guinea-pig bladders. Eur J Pharmacol 566: 172–180, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389: 990–994, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Wang ZE, Gopalakurup SK, Levin RM, Chacko S. Expression of smooth muscle myosin isoforms in urinary bladder smooth muscle during hypertrophy and regression. Lab Invest 73: 244–251, 1995. [PubMed] [Google Scholar]
- 51.Wein AJ, Levin RM, Barrett DM. Voiding function: relevant anatomy, physiology, pharmacology. In: Adult and Pediatric Urology, 2nd ed., edited by Gillenwater JY, Grayhack JT, Howards SS, Duckett JD. Philadelphia, PA: Mosby Year Book, 1991, p. 933–999.
- 52.Wein AJ, Raezer DM. Physiology of micturition. In: Clinical Neurourology, edited by Krane RJ, Siroky MB. Boston, MA: Little Brown, 1979, p. 1–33.
- 53.Xu SF, Collins MA, Chang KJ. Phorbol esters induce oscillatory contractions of intestinal smooth muscles. Eur J Pharmacol 201: 215–222, 1991. [DOI] [PubMed] [Google Scholar]