Abstract
Aging is associated with abnormalities in kidney function, but the exact mechanisms are unknown. We examined calpains 1, 2, and 10 protein levels in kidneys from rats, mice, and humans of various ages and determined whether calpain 10 is required for cell viability. Calpain 10 protein expression decreased in the kidney, but not in the liver, of aging Fischer 344 rats, and this decrease was attenuated with caloric restriction. There was no change in calpains 1 or 2 levels in the kidney or liver in control and caloric-restricted aging rats. Aging mice also exhibited decreased calpain 10 protein levels. Calpain 10 protein and mRNA levels decreased linearly in human kidney samples with age in the absence of changes in calpains 1 or 2. Our laboratory previously found calpain 10 to be expressed in both the cytosol and mitochondria of rabbit renal proximal tubular cells (RPTC). Adenoviral-delivered shRNA to rabbit RPTC decreased mitochondrial calpain 10 expression below detectable levels by 3 days while cytosolic calpain 10 levels remained unchanged at 3 days and decreased to ∼20% of control by 5 days. Knockdown of mitochondrial calpain 10 resulted in nuclear condensation and cleaved procaspase 3, markers of apoptosis. In summary, mitochondrial calpain 10 is required for cell viability and calpain 10 levels specifically decrease in aging rat, mice, and human kidney tissues when renal function decreases, suggesting that calpain 10 is required for renal function and is a biomarker of the aging kidney.
Keywords: apoptosis, calpain 1, calpain 2, caloric restriction
aging is associated with alterations in kidney structure and decreased function (9, 14, 46). In the aging kidney there is a decrease in the number of nephrons (1) and many of the normal renal tubular absorption and secretion abilities are blunted (28, 35, 48). Structural changes include a loss of 20–25% of renal mass during aging with most of the loss involving glomeruli and tubules in the cortex (18). In addition, the aged kidney has an enhanced susceptibility to a number of stresses and the increased incidence of acute renal failure in older patients may be related to this enhanced susceptibility to pathologic or xenobiotic insults (14, 39, 41, 43, 52).
Since aging cells tend to accumulate abnormal or modified proteins, it has been suggested that the decline and death of cells are in part due to an impaired proteolytic activity or efficiency (11, 15, 25). Although this is true for some cell types, in others overactivation of proteases occurs in aging cells. For instance, there is elevated calpain, Ca2+-activated cysteine protease, and activity in cellular aging (7, 31, 34). Established examples of increased calpain activity contributing to degenerative aging are cataract formation (5, 13, 26) and Alzheimer's disease (36, 44). To date, very few studies have examined the role calpains play in the aging kidney. For example, Manya et al. (34) showed an increase in calpain 1 activation in the kidney of aged rodents; however, no other calpains were examined.
Calpains are a 15-member class of enzymes linked to the pathogenesis of numerous disease states including Alzheimer's disease, cataracts, limb-girdle muscular dystrophy, gastric carcinoma, and type II diabetes (17). Calpains also are thought to participate in the induction of cellular necrosis through interactions with the cytoskeleton in various cell types (19, 21, 29, 49, 53) and in the regulation of apoptosis through interactions with p53, AIF, Bid, Bax, and caspase 3 (2, 8, 10, 38, 40).
Only a few isoforms have been studied extensively, and little to no work has been completed on the atypical class of calpains, except for calpain 10 which has been identified as a type II diabetes susceptibility gene (20) and is important in cataract formation (30). Calpain 10 also has been suggested to play a role in the apoptotic process. Johnson and colleagues (23) reported a decrease in apoptosis in pancreatic β-cells isolated from calpain 10 knockout mice compared with wild-type cells after treatment with ryanodine, palmitate, or low glucose. Wu et al. (51), using calpain 10 antisense methodology, demonstrated a proapoptotic role for calpain 10 in rat renal interstitial fibroblasts.
Calpain 10 is a ubiquitous calpain (30) and our laboratory showed that calpain 10 is resident in kidney mitochondria of rabbits, rats, and mice (4, 16). Arrington et al. (4) identified calpain 10 as a mitochondrial calpain capable of cleaving complex 1 proteins (ND6 and NDUFV2) and inhibiting state 3 respiration after mitochondrial Ca2+ overload. We also demonstrated that mitochondrial calpain 10 may play a role in the regulation of the mitochondrial permeability transition (MPT) and that overexpression of calpain 10 leads to mitochondrial dysfunction (4). Finally, oxygen-regulated protein 150, a cytoprotective inducible endoplasmic reticulum and mitochondrial chaperone protein, is a substrate of calpain 10 (3). Because calpains have been implicated in aging, aging-associated diseases, and kidney functions decrease with age, we examined calpain 10 protein expression in aging rat, mouse, and human kidneys and determined the role of calpain 10 in cell viability.
MATERIALS AND METHODS
Tissue samples.
Male Fischer 344 rat kidney and liver samples at different ages were graciously provided by Dr. A. Parrish (Texas A&M Health Science Systems). Samples from male Fischer 344 rats [young (4–5 mo), aged-ad libitum (AL; 9, 13, 19, and 24 mo), and caloric-restricted (CR; 9, 13, 19, and 24 mo)] were snap-frozen and stored at −80°C until utilized. CR began at 10 wk at 10% until 15 wk where it was increased to 25%, and further increased to 40% beginning at 17 wk. Male C57Bl/6 mice at 5 and 16 mo of age were euthanized by CO2 asphyxiation followed by cervical dislocation and the kidneys were harvested. All animal use complied with the protocols approved by the MUSC Institutional Animal Care and Use Committee. Human kidney tissue samples used in this study were obtained from the Holling's Cancer Center Tissue Acquisition and Tumor Procurement Service at the MUSC. Tissue was obtained from fully consented patients undergoing nephrectomy for the presence of a renal neoplasm. Following routine diagnostic procedures, the pathologist identified and harvested normal kidney tissue from sites distant to margins of the renal neoplasm. Tissues were snap-frozen in liquid nitrogen and stored at −80°C. Adjacent tissues also were prepared for histological assessment to verify that normal specimens were viable and not contaminated with neoplastic cells. Tissues and corresponding demographic patient data were provided to the investigators following de-identification by Tissue Procurement personnel.
Isolation, culture, and infection of renal proximal tubules.
Isolation and culture of renal proximal tubules were performed as described previously (37). Female New Zealand White rabbits (1.5–2.5 kg) were purchased from Myrtle's Rabbitry (Thompson Station, TN). Renal proximal tubular cells (RPTC) were isolated using the iron oxide perfusion method and grown in six-well tissue culture dishes under improved conditions. The culture medium was a 1:1 mixture of DMEM/Ham's F-12 medium (without glucose, phenol red, or sodium pyruvate) supplemented with 15 mM HEPES buffer, 2.5 mM l-glutamine, 1 μM pyridoxine HCl, 15 mM sodium bicarbonate, and 6 mM lactate. Hydrocortisone (50 μM), selenium (5 ng/ml), human transferrin (5 μg/ml), bovine insulin (10 nM), and l-ascorbic acid-2-phosphate (50 μM) were added daily to fresh culture medium. On day 6, RPTC were infected with scramble-shRNA virus (Vector BioLabs; 5 × 108 PFU/ml; MOI of 10), calpain 10-shRNA virus (MOI of 10), or diluent. Samples were isolated on days 1–5 postinfection for subsequent analysis.
Mitochondrial isolation and fractionation.
RPTC mitochondria were isolated via differential centrifugation (4). Briefly, RPTC were lysed in homogenization buffer (50 mM Tris·HCl, 1 mM β-mercaptoethanol, 1 mM EDTA, 0.32 M sucrose, pH 8.0) containing protease inhibitors with brief agitation and sonicated for 10 s. The subsequent homogenate was centrifuged at 900 g for 5 min. The supernatant was centrifuged at 15,000 g for 5 min resulting in mitochondrial and cytosolic fractions. Mitochondria were resuspended in homogenization buffer for immunoblot analysis.
Reverse-transcription PCR.
Human kidney samples were homogenized with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol to extract total RNA. The mRNA was reversely transcripted to DNA using MMLV reverse transcriptase (Invitrogen) in the presence of oligo dT primers. PCR was performed using primers for calpain 1, 2, and 10. Amplification of α-GAPDH was used as internal control for normalizing PCR efficiency. Products were electrophoresed on 1.5% agarose gel and stained with ethidium bromide. Each experiment was repeated twice.
Immunoblot analysis.
Human kidney, rat kidney and liver, mouse kidney tissues, and RPTC were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated with primary antibodies to calpain 1, calpain 2, calpain 10, Hsp60 (a mitochondrial loading control), caspase 3, and GAPDH. The primary antibodies used were mouse anti-human m-calpain (domain IV; Calbiochem; 1:1,000), rabbit anti-human μ-calpain (domain III–IV; Abcam; 1:1,000), rabbit anti-rat calpain 10 (Abcam; 1:1,000), rabbit anti-human podocin (NPHS2; Abcam; 1:1,000), rabbit anti-human SGLT1 (Abcam; 1:1,000), mouse anti-human Hsp60 (Abcam; 1:1,000), and rabbit anti-human caspase 3 (Stress Signaling; 1:1,000). Antibody incubation was followed by a horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (Santa Cruz Biotechnology; 1:1,000). Immunoreactive protein was visualized by enhanced chemiluminescence (Amersham) and imaged using an Alpha Innotech imaging station.
Adenoviral construct.
Three independent siRNA sequences were obtained (Ambion) and evaluated for their ability to inhibit calpain 10 translation. One sequence produced >95% knockdown of calpain 10 expression after a 24-h transfection in HEK-293 cells. Subsequently, oligonucleotides encoding this siRNA sequence (sense: 5′-TCGAG CCGAGTGAGGTGTACATTGTTCAAGAGACAATGTACACCTCACTCGGTTA-3′, and antisense: 5′-CTAGTAACCGAGTGAGGTGTACATTGTCTCTTGAA CAATGTACACCTCACTCGG C-3′) were annealed and ligated into Ambion's pSilencer Adeno 1.0-CMV shuttle vector. Following isolation of pure plasmid, HEK-293 cells were cotransfected with the provided linearized adenoviral backbone DNA and the shuttle vector. After viral replication, HEK-293 cell lysates were harvested. Recombinant adenovirus amplification was conducted by the MUSC Viral Vector Core Facility (1 × 1010 PFU/ml).
Assessment of nuclear morphology.
Nuclear morphology was assessed as previously described (12). Briefly, RPTC were washed twice with PBS, fixed for 10 min using 10% buffered formalin/4% formaldehyde, and washed with PBS. RPTC were permabilized for 10 min in PBS/0.1% Tween 20, washed three times with ice-cold PBS, and treated for 10 min with DAPI (17 μM final concentration) at room temperature. RPTC were washed three times with ice-cold PBS. Visualization of DAPI staining was performed using a fluorescence microscope and condensed nuclei from 10 high-powered fields were counted for each treatment group.
Statistical analysis.
RPTC isolated from one rabbit represent one experiment (n = 1). The appropriate ANOVA was performed for each data set by using SigmaStat statistical software. Individual means were compared with Fisher's protected least significant difference test with P ≤ 0.05 being considered statistically significant. Linear regression was also performed using Sigma Stat statistical software.
RESULTS
Calpain protein expression in the kidney and liver of control and CR rats.
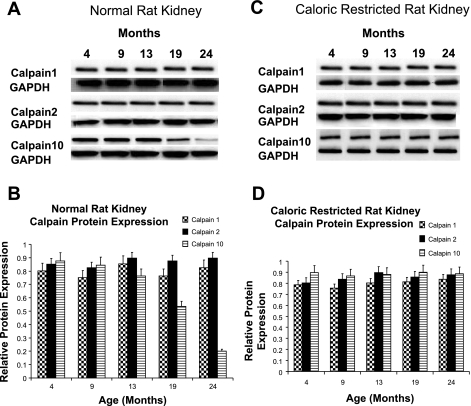
Animal models are used frequently to examine age-related alterations in the kidney. Male Fischer 344 rats develop severe renal disease over time with structural changes and proximal tubular degeneration at 24 mo that can be attenuated by life-long caloric restriction (CR) (24, 47). Therefore, we examined calpain protein expression in the aged male rat utilizing tissue samples that have previously revealed kidney dysfunction that was attenuated with CR (24). Kidney samples were taken from control and CR male Fischer 344 rats at 4, 9, 13, 19, and 24 mo of age and subjected to immunoblot analysis for calpain 1, 2, and 10. While there was no age-dependent change in calpain 1 or 2 protein expression in control or CR rats, calpain 10 protein expression decreased in control rat kidney samples at 19 mo and further decreased at 24 mo (Fig. 1). The decrease in calpain 10 protein expression was attenuated by CR, suggesting calpain 10 plays a role in the dysfunction of the aging kidney.
Fig. 1.
Calpain protein expression in normal and caloric-restricted (CR) rat kidneys. Different aged normal and CR male Fischer 344 rat kidneys (A, C) and (B, D) were subjected to immunoblot analysis for calpain 1, 2, and 10 (75 kDa) and α-GAPDH (loading control) protein expression. Densitometry was performed and relative protein expression was determined. Results are representative of 3 independent experiments.
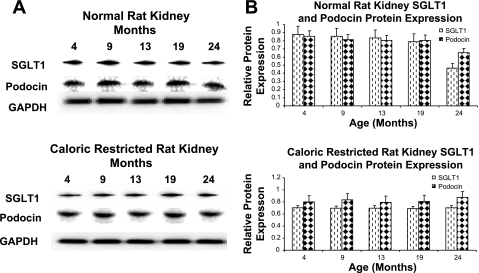
We also examined renal SGLT1, a tubular epithelial marker, and podocin, a glomeular marker, protein expression to determine whether the decrease in calpain 10 levels with age parallels tubular or glomerular content. While there was no age-dependent change in SGLT1 or podocin protein expression at 4, 9, 13, and 19 mo of age, the expression of SGLT1 and podocin decreased 41 and 28%, respectively, at 24 mo of age (Fig. 2). The decrease in SGLT1 and podocin observed at 24 mo of age was attenuated by CR.
Fig. 2.
SGLT1 and podocin protein expression in normal and CR rat kidneys. Different aged normal and CR male Fischer 344 rat kidneys (A and B) were subjected to immunoblot analysis for SGLT1, podocin, and α-GAPDH (loading control) protein expression. Densitometry was performed and relative protein expression was determined. Results are representative of 3 independent experiments.
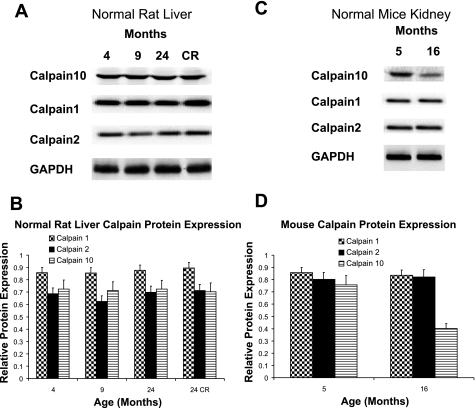
Liver samples taken from control rats at 4, 9, and 24 mo and from CR rats at 24 mo did not exhibit any changes in calpain 1, 2, or 10 protein expression, suggesting that calpain 10 is not decreased in the aging liver (Fig. 3, A and B).
Fig. 3.
Calpain protein expression in normal and CR rat livers and in mice. Different aged normal and CR male Fischer 344 rat livers (A, B) were subjected to immunoblot analysis for calpain 1, 2, and 10 (75 kDa) and α-GAPDH (loading control) protein expression. CR are caloric-restricted 24-mo-old Fischer 344 rats. C and D: kidney tissue from control 5- and 16-mo-old mice were subjected to immunoblot analysis for calpain 1, 2, and 10 (75 kDa) and α-GAPDH protein expression. Results are representative of 3 independent experiments.
Calpain protein expression in the mouse kidney.
Calpain 1, 2, and 10 levels in kidney samples taken from 5- and 16-mo-old mice were examined. Compared with 5-mo-old mice, there was a decrease in calpain 10 protein expression in 16-mo-old mice but no change in calpain 1 or 2 (Fig. 3, C and D). Thus, the age-related loss of kidney calpain 10 in mice is similar to that in the aging rat, demonstrating similarities across species.
Calpain protein expression in the human kidney.
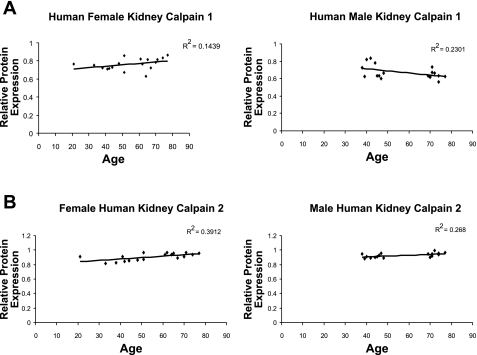
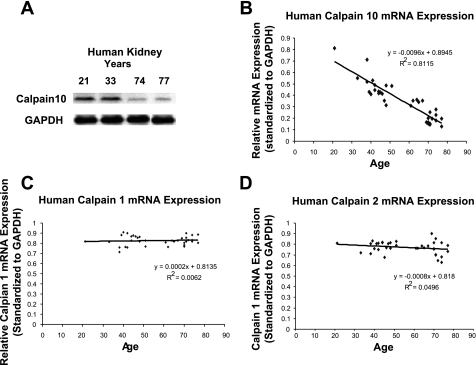
We examined calpain protein levels in human kidney samples at different ages. Human female kidney samples ranging from 21 to 77 yr old showed a linear decrease in calpain 10 protein expression with an r2 value of 0.8966 (Fig. 4). There was a similar decrease in calpain 10 protein expression with an r2 value of 0.7495 in human male kidney samples ranging from 38 to 77 yr old. Overlaying of male and female kidney samples revealed the same rate of calpain 10 loss with age between genders (data not shown). In contrast, there was no age-related change in calpain 1 or 2 protein expression in human female or male kidney samples (Fig. 5, A and B). Collectively, both human female and male kidney samples have a similar rate of loss in calpain 10 protein expression, but not calpain 1 and 2 with age, suggesting the loss of calpain 10 is specific.
Fig. 4.
Calpain 10 protein expression in male and female human kidney. Female and male human kidney samples of various ages were subjected to immunoblot analysis for calpain 10 (75 kDa) and α-GAPDH protein expression. Densitometry was performed and relative protein expression was determined.
Fig. 5.
Calpain 1 and 2 protein expression in male and female human kidney. Female and male human kidney samples of various ages were subjected to immunoblot analysis for calpain 1 and 2 and α-GAPDH protein expression. Densitometry was performed and relative protein expression was determined.
To examine whether the decrease in calpain 10 protein expression was pre- or posttranslational, we examined calpain 1, 2, and 10 mRNA expression in the human kidney. In the human kidney, there was a linear decrease in calpain 10 mRNA expression as age increased (Fig. 6, A and B). In contrast, there was no age-related change in calpain 1 or 2 mRNA expression (Fig. 6, C and D). We suggest that there is a specific loss of calpain 10 mRNA expression in the aging human kidney, which results in the age-related loss of calpain 10 protein.
Fig. 6.
Calpain mRNA expression in the human kidney. RNA was extracted from female and male human kidney samples of various ages and reverse transcription was performed using oligo(dT) primers. PCR was performed using primers for calpain 10 (A, B), calpain 1 (C), calpain 2 (D), and α-GAPDH. A: example of human kidney calpain 10 and GAPDH mRNA levels ran on an agarose gel. Densitometry was performed and relative mRNA expression was determined.
Adenovirus-mediated knockdown of calpain 10 expression.
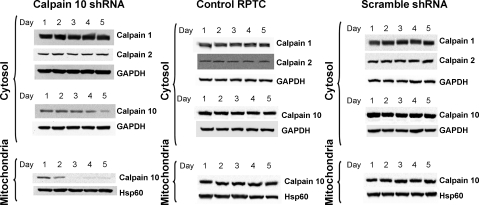
To determine whether the loss of calpain 10 results in a loss of cell viability, rabbit RPTC were infected with either scramble shRNA (scramble) or calpain 10 shRNA and incubated for a period of 5 days. Control RPTC were treated with diluent. Mitochondrial and cytosolic fractions from control, scramble, and calpain 10 shRNA-infected RPTC were isolated each day for 5 days and assayed via immunoblot analysis for calpain 10 expression. Calpain 10 levels in calpain 10 shRNA-infected cells were decreased in mitochondrial isolates on day 2 and were below detectable levels on day 3 postinfection, whereas levels in cytosolic fractions on day 3 were unchanged and decreased to ∼20% of control by day 5 (Fig. 7). Calpain 10 levels in control and scramble-infected cells remained unchanged (Fig. 7). Verification of calpain 10-specific knockdown was accomplished via immunoblot analysis of calpains 1 and 2, revealing that calpain 1 and 2 expression levels did not change among control, scramble, and calpain 10 shRNA-infected cells.
Fig. 7.
Adenovirus-mediated knockdown of calpain 10. Confluent renal proximal tubule cells (RPTC) were noninfected (control) or infected with recombinant adenovirus expressing either calpain 10 shRNA or scramble shRNA, harvested over a 5-day time course, and subfractionated into cytosolic and crude mitochondrial fractions. Resultant samples were subjected to immunoblot analysis for calpain 1, 2, and 10 protein expression. Equal protein loading was verified using α-GAPDH and α-Hsp60 antibodies for the cytosolic and mitochondrial fractions, respectively. Results are representative of 3 independent experiments.
Calpain 10 is required for RPTC viability.
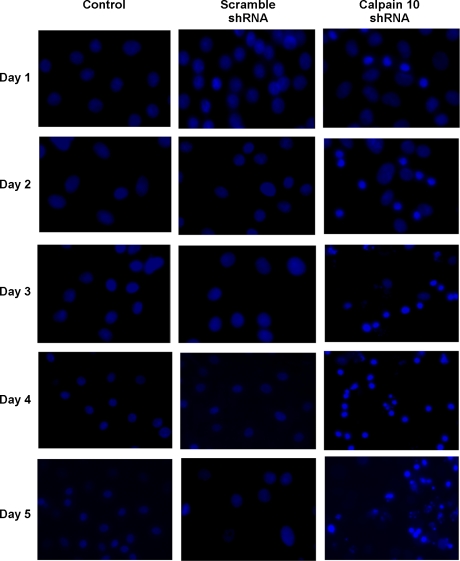
Confluent cultures of RPTC were exposed to scramble shRNA or calpain 10 shRNA and were followed for 5 days. Each treatment group was evaluated by DAPI staining for nuclear morphology and caspase 3 activation. Examination of RPTC nuclear morphology revealed condensed nuclei in calpain 10 shRNA-infected cells beginning on day 1, and continuing through the 5-day observation period (Fig. 8). The loss of calpain 10 in RPTC resulted in a decrease in cell confluency to ∼60–70% of control on day 5. Numerous nuclear aggregates were also observed on day 5 in many of the calpain 10 shRNA-infected cells (Fig. 8). Nuclear condensation was <5% in control and scramble shRNA-infected cells throughout the time period. These results support the hypothesis that the lack of calpain 10 is lethal and that the cells die by apoptosis.
Fig. 8.
Downregulation of calpain 10 induces nuclear condensation. Nuclei from control RPTC and those infected with recombinant adenovirus expressing either calpain 10 shRNA or scramble shRNA were imaged via DAPI staining over a 5-day time course. Visualization of DAPI staining was performed using a fluorescence microscope (Nikon, Melville, NY) with excitation and emission filters of 350 and 486 nm, respectively. Results are representative of 3 independent experiments.
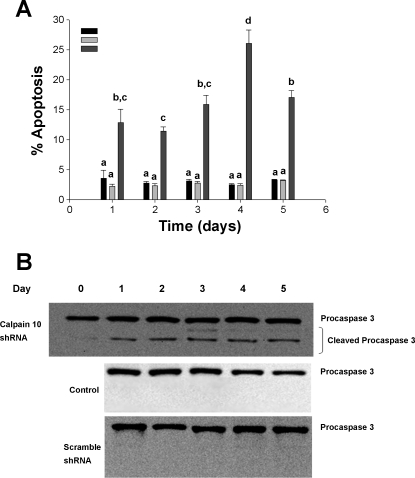
We further explored this hypothesis by measuring caspase 3 activation. Using immunoblot analysis, we observed the appearance of cleaved procaspase 3 beginning on day 1 and remained present over days 2–5 (Fig. 9). No cleaved procaspase 3 was observed in control and scramble shRNA-infected cells over the 5-day period. We suggest that RPTC calpain 10 is required for cell viability and that the loss of calpain 10 results in RPTC apoptosis.
Fig. 9.
Calpain 10 knockdown induces caspase-dependent apoptosis in RPTC. A: cells illustrated in Fig. 5 were examined for condensed nuclei, and 10 high-powered fields were counted for each treatment group. Data are expressed as means ± SE (n = 3). Means with different lettered subscripts within each group are significantly different from each other, P < 0.05. B: cytosolic fractions from control, scramble, and virus-infected RPTC were probed for total and active caspase 3 to confirm caspase-dependent apoptosis. Results are representative of 3 independent experiments.
DISCUSSION
These data reveal the novel finding that aging is associated with the selective loss of calpain 10 protein expression in the kidney of multiple species, an organ that loses function during the aging process. The loss of calpain 10 was kidney specific, with no loss of protein in the liver. In particular, male and female human kidney samples lose calpain 10 protein expression at the same linear rate with age. There was also a specific linear decrease in calpain 10 mRNA in aging human kidneys, suggesting that the decrease in calpain 10 protein expression occurs pretranslationally. Importantly, closely related family members such as calpain 1 and 2 were not altered during aging. Thus, calpain 10 is a potential biomarker of kidney aging in humans, rats, and mice. This finding is important because currently there is a lack of markers of the aging kidney.
The development of renal disease is more severe in rodent males compared with females and nutritional influences have a critical role on age-related renal dysfunction (6). Since Fischer 344 rats develop severe renal disease over time that can be attenuated by life-long CR (24, 47), any molecular change crucial for age-dependent development of renal disease should be attenuated by CR. The rat kidney samples used in these experiments were furnished by Dr. Parrish's laboratory and he observed degeneration of proximal tubules, lymphocytic infiltration, tubular dilation and degeneration, and protein casts at 24 mo, which were attenuated with CR (24). Our data revealed that the loss of calpain 10 protein expression correlates with this renal dysfunction and that CR restores calpain 10 protein levels, suggesting the loss of calpain 10 leads to renal dysfunction.
The loss of calpain 10 could be the result that the aging kidney has less nephrons or coincidentally (18). In these samples, we also observed a decrease in renal SGLT1 and podocin protein expression in 24-mo-old Fisher 344 rats, but not at earlier ages, suggesting a decrease in tubular and glomerular content in only advanced age. In agreement with our finding, a decrease in nephrin, a glomerular marker, has been reported in 24- but not 17-mo-old Fischer 344 rats (50). Thus, there is a loss of nephrons at 24 mo of age in this model. It is important to note that calpain 10 protein expression decreases before the loss of tubular and glomerular markers. Therefore, the loss of calapin 10 protein expression is independent of nephron loss and may play a role thereof.
Although it would be ideal to measure calpain 10-specific activity, there is no specific substrate for calpain 10. There are calpain substrates that measure overall calpain activity, but they are not specific and are also substrates for other proteases. In fact, increased calpain 1 activity has been reported in aging rodent kidneys (34). However, we did not observe an increase or decrease in calpain 1 protein expression. This discrepancy could be due to the lack of substrate specificity used in the calpain activity assay. While it would be desirable to study the localization of renal calpain 10 expression during aging, current calpain 10 antibodies for calpain 10 are inadequate.
Our results raised the question of whether calpain 10 is important for cell viability. Using an adenoviral delivery system to introduce calpain 10 shRNA into primary rabbit RPTC, we examined the loss of mitochondrial and cytosolic calpain 10 and cell viability over time. Calpain 10 protein expression decreased within 48 h and was undetectable in mitochondria 3 days after infection, whereas cytosolic calpain 10 levels were unchanged 3 days after infection and decreased to ∼20% of control levels by day 5. We suggest that differential rates of calpain 10 degradation exist between the two compartments. The rate differences seen between mitochondrial and cytosolic calpain 10 may be due to the increased oxidative stress experienced by mitochondrial proteins in general. For example, it is known that oxidized cytosolic proteins are degraded much faster than their nonoxidized counterparts (45). However, there is the possibility that there is a difference in protease activity in the two compartments that leads to increased degradation. Although proteases are known to be altered with age, the exact protease responsible for calpain 10 degradation is not known.
The loss of calpain 10 in RPTC resulted in a decrease in cell survival and an increase in caspase-mediated apoptosis over the 5-day period. Based on the time of initiation of apoptosis and the changes in mitochondrial and cytosolic calpain 10 levels, the loss of mitochondrial calpain 10 but not cytosolic calpain 10 was associated with apoptosis, suggesting mitochondrial-induced, caspase 3-mediated apoptosis. Although it appears there is a decrease in procaspase 3 in the control group, we never observed cleaved caspase 3 bands in any of these blots, suggesting caspase 3 is not activated in the control group. Additional studies are required to elucidate the complete mechanism of this apoptosis. The apparent discrepancy that apoptosis occurred at day 1 and mitochondrial calpain 10 levels decreased on day 2 is probably the result of the limitations of immunoblot analysis to measure small changes.
We previously overexpressed calpain 10 in NIH-3T3 fibroblasts (4). Cells expressing large levels of calpain 10 died quickly, whereas cells expressing moderately elevated levels of calpain 10 exhibited mitochondrial swelling and dysfunction (4). In this study, we show that decreased calpain 10 protein expression also results in RPTC death, supporting the importance of calpain 10 regulation in cell death. It is commonly thought that the role of calpains in pathological conditions can originate from insufficient calpain activity or excessive activation of calpain. Therefore, calpain activity can be described as a double edged sword, and with an intermediate amount of highly regulated calpain activity required for normal physiological functions, while alterations of activity in either direction can have negative consequences.
An increase in the basal rate of apoptosis has been reported in the aging rodent kidney due to a shift in balance of pro- and anti-apoptotic factors (22, 32). For example, studies of aging rats found increased expression and activation of caspases 3 and 9 in the liver, kidney, lung, and spleen (27, 41, 54). Importantly, CR inhibited the increase in caspase-mediated apoptosis in aging kidneys (27). Based on our data, we hypothesize that the loss of mitochondrial calpain 10 in aging results in increase in apoptosis that can be attenuated with CR.
In summary, we showed that calpain 10 protein levels specifically decrease in the aging kidney of multiple species and that this decrease can be attenuated with CR correlating with the preservation of kidney function and structures. In addition, we showed that the age-related decrease in calpain 10 is translationally regulated in the human kidney. Mitochondrial calpain 10 plays a significant role in renal mitochondria and cell viability which could have broad implications for multiple disease states. To date, calpain 10 has been linked to mitochondrial dysfunction, apoptosis, GLUT4 vesicle translocation, pancreatic beta-cell exocytosis, cataractogenesis, hypertriglyceridemia, and is genetically linked to type II diabetes. We think that calpain 10 may play a more global role as a crucial cell survival protein and that a decrease in calpain 10 protein expression during aging or disease results in renal cell death, dyfunctions, and/or increases the susceptibility of the kidney to an insult.
GRANTS
This study was supported by National Institutes of Health (NIH) Sciences Grants ES-012239 and GM-084147, and NIH Training Grant T32-HL-007260.
Acknowledgments
The authors thank Dr. A. Parrish for the Fisher 344 tissue samples.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adachi T, Kawamura M, Owada M, Hiramori K. Effect of age on renal functional and orthostatic vascular response in healthy men. Clin Exp Pharmacol Physiol 28: 877–880, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Altznauer F, Conus S, Cavalli A, Folkers G, Simon HU. Calpain-1 regulates Bax and subsequent Smac-dependent caspase-3 activation in neutrophil apoptosis. J Biol Chem 279: 5947–5957, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Arrington DD, Schnellmann RG. Targeting of the molecular chaperone oxygen-regulated protein 150 (ORP150) to mitochondria and its induction by cellular stress. Am J Physiol Cell Physiol 294: C641–C650, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol 291: C1159–C1171, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Azuma M, Shearer TR. Involvement of calpain in diamide-induced cataract in cultured lenses. FEBS Lett 307: 313–317, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Baylis C Age-dependent glomerular damage in the rat. Dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest 94: 1823–1829, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benuck M, Banay-Schwartz M, DeGuzman T, Lajtha A. Changes in brain protease activity in aging. J Neurochem 67: 2019–2029, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Bizat N, Hermel JM, Boyer F, Jacquard C, Creminon C, Ouary S, Escartin C, Hantraye P, Kajewski S, Brouillet E. Calpain is a major cell death effector in selective striatal degeneration induced in vivo by 3-nitropropionate: implications for Huntington's disease. J Neurosci 23: 5020–5030, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brezis M, Epstein FH. A closer look at radiocontrast-induced nephropathy. N Engl J Med 320: 179–181, 1979. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem 276: 30724–30728, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem 275: 31505–31513, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca2+-independent phospholipase A2 in cisplatin-induced renal cell apoptosis. J Pharmacol Exp Ther 308: 921–928, 2004. [DOI] [PubMed] [Google Scholar]
- 13.David LL, Wright JW, Shearer TR. Calpain II induced insolubilization of lens beta-crystallin polypeptides may induce cataract. Biochim Biophys Acta 1139: 210–216, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Fillit H, Rowe JW. The Aging Kidney. London, UK: Churchill Livingstone, 1992.
- 15.Friguet B, Bulteau AL, Chondrogianni N, Conconi M, Petropoulos I. Protein degradation by the proteasome and its implications in aging. Ann NY Acad Sci 908: 143–154, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Giguere CJ, Covington MD, Schnellmann RG. Mitochondrial calpain 10 activity and expression in the kidney of multiple species. Biochem Biophys Res Commun 366: 258–262, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 83: 731–801, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Goyal VK Changes with age in the human kidney. Exp Gerontol 17: 321–331, 1982. [DOI] [PubMed] [Google Scholar]
- 19.Harriman JF, Liu XL, Aleo MD, Machaca K, Schnellmann RG. Endoplasmic reticulum Ca2+ signaling and calpains mediate renal cell death. Cell Death Differ 9: 734–741, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Harris F, Chatfield L, Singh J, Phoenix DA. Role of calpains in diabetes mellitus: a mini review. Mol Cell Biochem 261: 161–167, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Inserte J, Garcia-Dorado D, Ruiz-Meana M, Agullo L, Pina P, Soler-Soler J. Ischemic preconditioning attenuates calpain-mediated degradation of structural proteins through a protein kinase A-dependent mechanism. Cardiovasc Res 64: 105–114, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Joaquin AM, Gollapudi S. Functional decline in aging and disease: a role for apoptosis. J Am Geriatr Soc 49: 1234–1240, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JD, Han Z, Otani K, Ye H, Zhang Y, Wu H, Horikawa Y, Misler S, Bell GI, Polonsky KS. RyR2 and calpain-10 delineate a novel apoptosis pathway in pancreatic islets. J Biol Chem 279: 24794–24802, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Jung KY, Dean D, Jiang J, Gaylor S, Griffith WH, Burghardt RC, Parrish AR. Loss of N-cadherin and alpha-catenin in the proximal tubules of aging male Fischer 344 rats. Mech Ageing Dev 125: 445–453, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Keller JN, Hanni KB, Markesbery WR. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev 113: 61–70, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Kelley MJ, David LL, Iwasaki N, Wright J, Shearer TR. α-Crystallin chaperone activity is reduced by calpain II in vitro and in selenite cataract. J Biol Chem 268: 18844–18849, 1993. [PubMed] [Google Scholar]
- 27.Lee JH, Jung KJ, Kim JW, Kim HJ, Yu BP, Chung HY. Suppression of apoptosis by calorie restriction in aged kidney. Exp Gerontol 39: 1361–1368, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Leosco D, Ferrara N, Landino P, Romano G, Sederino S, Cacciatore F, Longobardi G, Dal Canton A, Rengo F. Effects of age on the role of atrial natriuretic factor in renal adaptation to physiologic variations of dietary salt intake. J Am Soc Nephrol 7: 1045–1051, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Van Vleet T, Schnellmann RG. The role of calpain in oncotic cell death. Annu Rev Pharmacol Toxicol 44: 349–370, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Ma H, Fukiage C, Kim YH, Duncan MK, Reed NA, Shih M, Azuma M, Shearer TR. Characterization and expression of calpain 10. A novel ubiquitous calpain with nuclear localization. J Biol Chem 276: 28525–28531, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Ma H, Hata I, Shih M, Fukiage C, Nakamura Y, Azuma M, Shearer TR. Lp82 is the dominant form of calpain in young mouse lens. Exp Eye Res 68: 447–456, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Ma LJ, Nakamura S, Whitsitt JS, Marcantoni C, Davidson JM, Fogo AB. Regression of sclerosis in aging by an angiotensin inhibition-induced decrease in PAI-1. Kidney Int 58: 2425–2436, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Maddox DA, Alavi FK, Santella RN, Zawada ET Jr. Prevention of obesity-linked renal disease: age-dependent effects of dietary food restriction. Kidney Int 62: 208–219, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Manya H, Inomata M, Fujimori T, Dohmae N, Sato Y, Takio K, Nabeshima Y, Endo T. Klotho protein deficiency leads to overactivation of mu-calpain. J Biol Chem 277: 35503–35508, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Mulder WJ, Hillen HF. Renal function and renal disease in the elderly. Part I. Eur J Intern Med 12: 86–97, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Nixon RA, Saito KI, Grynspan F, Griffin WR, Katayama S, Honda T, Mohan PS, Shea TB, Beermann M. Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer's disease. Ann NY Acad Sci 747: 77–91, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Nowak G, Schnellmann RG. Improved culture conditions stimulate gluconeogenesis in primary cultures of renal proximal tubule cells. Am J Physiol Cell Physiol 268: C1053–C1061, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Pariat M, Carillo S, Molinari M, Salvat C, Debussche L, Bracco L, Milner J, Piechaczyk M. Proteolysis by calpains: a possible contribution to degradation of p53. Mol Cell Biol 17: 2806–2815, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pascual J, Orofino L, Liano F, Marcen R, Naya MT, Orte L, Ortuno J. Incidence and prognosis of acute renal failure in older patients. J Am Geriatr Soc 38: 25–30, 1990. [DOI] [PubMed] [Google Scholar]
- 40.Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem 280: 6447–6454, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Qiao X, Chen X, Wu D, Ding R, Wang J, Hong Q, Shi S, Li J, Xie Y, Lu Y, Wang Z. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci 60: 830–839, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Sabbatini M, Pisani A, Uccello F, Serio V, Seru R, Paterno R, Cianciaruso B, Fuiano G, Andreucci M. Atorvastatin improves the course of ischemic acute renal failure in aging rats. J Am Soc Nephrol 15: 901–909, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci USA 90: 2628–2632, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shringarpure R, Grune T, Davies KJ. Protein oxidation and 20S proteasome-dependent proteolysis in mammalian cells. Cell Mol Life Sci 58: 1442–1450, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva FG The aging kidney: a review. Part I. Int Urol Nephrol 37: 185–205, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Stern JS, Gades MD, Wheeldon CM, Borchers AT. Calorie restriction in obesity: prevention of kidney disease in rodents. J Nutr 131: 913S–917S, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Tiao JY, Semmens JB, Masarei JR, Lawrence-Brown MM. The effect of age on serum creatinine levels in an aging population: relevance to vascular surgery. Cardiovasc Surg 10: 445–451, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Wang CH, Chen YJ, Lee TH, Chen YS, Jawan B, Hung KS, Lu CN, Liu JK. Protective effect of MDL28170 against thioacetamide-induced acute liver failure in mice. J Biomed Sci 11: 571–578, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC. Podocyte hypertrophy, “Adaptation,” and “Decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Wu SH, Lu C, Dong L, Zhou GP, He ZG, Chen ZQ. High dose of lipoxin A4 induces apoptosis in rat renal interstitial fibroblasts. Prostaglandins Leukot Essent Fatty Acids 73: 127–137, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Yamashima T Ca2+-dependent proteases in ischemic neuronal death: a conserved “calpain-cathepsin cascade” from nematodes to primates. Cell Calcium 36: 285–293, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Zhang JH, Zhang Y, Herman B. Caspases, apoptosis and aging. Ageing Res Rev 2: 357–366, 2003. [DOI] [PubMed] [Google Scholar]