Abstract
Understanding the physiological regulation of mineral ion metabolism is essential for determining the pathomechanisms of skeletal, vascular, and renal diseases associated with an abnormal regulation of calcium and phosphate homeostasis. Normal calcium and phosphate balance is delicately maintained by endocrine factors that coordinate to influence the functions of the intestine, bone, parathyroid gland, and kidney. Under physiological conditions, the kidneys play an important role in maintaining normal mineral ion balance by fine-tuning the amount of urinary excretion of calcium and phosphate according to the body's needs. Fibroblast growth factor (FGF)23 regulates urinary phosphate excretion to maintain systemic phosphate homeostasis. The exact mode of action of the phosphaturic effects of FGF23 is not fully understood and is an intense area of research. Studies suggest, however, that FGF23, by interacting with FGF receptors, can initiate downstream signaling events and that Klotho, a transmembrane protein, facilitates the interaction of FGF23 with its receptor. FGF23 can inhibit the activities of 1-α-hydroxylase and sodium-phosphate cotransporter in the kidney to influence the overall systemic phosphate balance. This article briefly summarizes how FGF23 might coordinately regulate systemic phosphate homeostasis and how Klotho is involved in such regulation.
Keywords: vitamin D, mineral metabolism, kidney, calcification
the main functions of the kidney are to maintain water, electrolyte, and mineral ion balance and eliminate metabolic waste. Most chronic kidney diseases (CKD), without therapeutic intervention, usually progress to irreversible renal failure (45, 52, 61) and affect water, electrolyte, and mineral ion balance. Phosphorus is widely distributed in the body and has numerous essential biological functions, including contributing to bone formation, cell signaling, energy metabolism, and nucleic acid synthesis (14). More than 80% of the total phosphate in the body is present in the bone (Table 1). A very small amount of phosphate (∼0.1%) exists as inorganic phosphate (Pi) anions, either as ions or complexed with cations, such as Ca2+ and Mg2+.
Table 1.
Approximate distribution of phosphorus in a healthy 70-kg adult
| Phosphorus | |
|---|---|
| Total body content | ∼700 g |
| In bone | ∼80% |
| In viscera | ∼10.9% |
| In skeletal muscle | ∼9% |
| In extracellular fluid | ∼0.1% |
| Intracellular-to-extracellular ratio | ∼100:1 |
From Reference 14.
A significant amount of intestinal dietary phosphorus absorption partly occurs at the distal portion of the duodenum. Vitamin D helps intestinal phosphorus absorption by facilitating active uptake (6, 25). After intestinal absorption, circulating phosphorus is taken up by the cells that need it, accumulates in the bone matrix protein, and enters the kidney. About 95% of the filtered phosphate in the kidney is reabsorbed at the proximal tubule. Phosphate transport across the proximal tubule epithelial cells is mostly driven by a high extracellular sodium gradient. This gradient is thought to be maintained by membrane-associated Na+-K+-ATPase. Recently, it has been claimed that Klotho, a transmembrane protein, directly affects Na+-K+-ATPase activity to increase the Na+ gradient and drive transepithelial calcium transport in the choroid plexus and the kidney (39). It is worth mentioning that the model of Klotho activating Na+-K+-ATPase as a means to increase transepithelial calcium transport is provocative, but increasing transport of a single ion by increasing the overall workhorse of the cell is a highly unusual phenomenon. Additional studies are needed to settle this unresolved issue (47).
The molecular mechanism of renal phosphate reabsorption in the tubules is a complex process, in which sodium-phosphate (NaPi) cotransporters that are located in the luminal side of the proximal tubule epithelial cells play an important role (63). Both NaPi-2a and NaPi-2c transporters, in a sodium-dependent manner, help to recycle filtrated phosphate back into the proximal tubule epithelial cells (11, 37). Parathyroid hormone (PTH) and vitamin D actively contribute to the mineral ion transport process by influencing the biological activities of the membrane structures and transport molecules, including the NaPi and calcium-permeable transient receptor potential V (TRPV) family proteins (18, 63). For instance, PTH can suppress the reabsorption of phosphate in the proximal tubules and thus can induce an increase of urinary phosphate excretion by reducing NaPi-2a and NaPi-2c activities. PTH can also mobilize phosphorus from the bone into the circulation, possibly by enhancing osteoclastic bone resorption (62). Moreover, PTH, by inducing the renal expression of 1-α-hydroxylase, can increase the production of the active vitamin D metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D], which can enhance intestinal phosphorus absorption. A major breakthrough in the active regulation of phosphate homeostasis was achieved with the identification of a potent phosphotonin, named fibroblast growth factor (FGF)23 (1, 67).
Fibroblast Growth Factor 23
FGF23 is a protein of ∼30 kDa that is proteolytically processed to generate smaller NH2-terminal (18 kDa) and COOH-terminal (12 kDa) fragments. The NH2-terminal fragment of FGF23 contains the FGF receptor (FGFR)-binding domain. In vivo studies using synthetic peptides have indicated that neither of the processed NH2-terminal or COOH-terminal fragments of FGF23 can influence serum phosphate levels (58). FGF23 can decrease the activity of NaPi-2a and NaPi-2c cotransporters to reduce renal phosphate reabsorption and thereby can increase urinary phosphate excretion (55). Similarly, FGF23 can suppress the expression of 1-α-hydroxylase to reduce the production of the active vitamin D metabolite 1,25(OH)2D (55). Moreover, FGF23 can also induce 24-hydroxylase, which degrades 1,25(OH)2D (55). Since 1,25(OH)2D can enhance intestinal phosphate absorption, FGF23, by reducing vitamin D activities, can influence intestinal phosphate absorption (in addition to its urinary phosphate-wasting effects). The FGF23-mediated regulation of phosphate homeostasis is fairly independent of calcium homeostasis. Genetically engineered FGF23 mouse models provided convincing in vivo evidence of the phosphaturic activities of FGF23 (3, 8, 56, 59) and gave an experimental explanation of the clinical symptoms that have been noted in various human diseases, associated with the abnormal regulation of FGF23 (1, 4, 29, 57, 58).
FGF23 and Human Diseases
Human FGF23 was identified in patients as a gene associated with autosomal dominant hypophosphatemic rickets (ADHR). Gain-of-function mutations of the FGF23 gene in patients with ADHR are associated with urinary phosphate wasting, which causes rickets in the bone (1). The excessive amount of FGF23 that is produced by the tumor cells is responsible for the clinical symptoms of hypophosphatemia that are seen in patients with tumor-induced osteomalacia (TIO) (57). FGF23 has also been speculated to have a pathogenic role in patients with McCune-Albright syndrome and is believed to be responsible for hypophosphatemia in these patients (53). X-linked hypophosphatemia (XLH) is a common cause of vitamin D-resistant rickets/osteomalacia, which is caused by inactivating mutations in PHEX (a phosphate-regulating gene that is homologous to endopeptidases on the X chromosome) (19). The high level of circulating FGF23 in most patients with XLH induces excessive urinary phosphate wasting, thereby producing clinical symptoms associated with hypophosphatemia (23). Recently, mutations in dentin matrix protein-1 (DMP-1) were found in patients with autosomal recessive hypophosphatemic rickets/osteomalacia (ARHR) (33). The clinical symptoms of hypophosphatemia in patients with ARHR are mostly caused by high circulating levels of FGF23.
Patients in advanced stages of CKD have high serum levels of FGF23, partly due to decreased renal clearance of FGF23. The additional cause of increased levels of FGF23 in CKD patients may be a compensatory response to the hyperphosphatemia. Of relevance, human studies have shown that a dietary phosphorus load can increase serum FGF23 levels (2, 10). CKD patients are unable to excrete urinary phosphate, despite high serum FGF23 levels (13), and develop hyperphosphatemia, although they tend to have a lower level of 1,25(OH)2D, and suffer from hyperparathyroidism. Whether the low levels of 1,25(OH)2D and secondary hyperparathyroidism that are observed in patients with CKD are influenced by increased levels of circulating FGF23 is a complex issue. Since FGF23 has a counterregulatory effect on vitamin D (30), the elevated level of FGF23 in patients with CKD has the potential to reduce vitamin D activity; this effect may eventually facilitate the development of compensatory secondary hyperparathyroidism. Furthermore, calcitriol therapy in patients with CKD may also contribute to increased serum levels of FGF23 (42), because studies have shown that elevated serum 1,25(OH)2D can induce an increase in the serum level of FGF23 (54). The exact pathological significance of elevated serum levels of FGF23 in patients with CKD is not clear, and the question of whether increased levels of FGF23 can exert additional toxic effects, independent of other known risk factors of CKD patients (e.g., race, diabetes, hypertension, and advanced age) will need additional clinical studies.
In addition to the several hypophosphatemic diseases that are caused by an increased activity of FGF23, there are also human diseases that are caused by a reduced activity of FGF23. For instance, patients with familial tumoral calcinosis (FTC) usually develop hyperphosphatemia and ectopic calcification due to loss-of-function mutations in the FGF23 gene (4). Similarly, mutations in the GALNT3 (UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetyl-galactosaminyl transferase 3) gene are also found in patients with FTC (12). GALNT3, a Golgi-associated enzyme, can selectively O-glycosylate a furinlike convertase recognition site in FGF23, and thereby can allow secretion of intact FGF23. Mutations in the GALNT3 gene can impair O-glycosylation of the FGF23 protein; defects in glycosylation may result in rapid processing of the FGF23 protein, which may be the cause of the lower levels of the intact FGF23 protein found in patients with FTC (15). The abnormally high serum levels of phosphate in patients with FTC, due to the reduced activity of the human FGF23, resemble the significantly high serum levels of phosphate that are found in mice without Fgf23 activity [Fgf23-knockout (Fgf23−/−) mice] (8, 32). Moreover, there are strikingly similar physical, morphological, biochemical, and molecular phenotypes in Fgf23−/− and klotho-knockout (klotho−/−) mice; these similarities led to the identification of Klotho as a cofactor for FGF23 and its receptor interactions (26, 28, 48, 50).
Klotho
The Klotho protein contains a putative signal sequence at its NH2 terminus and a single transmembrane domain near its COOH terminus that is believed to anchor the protein to the membrane (39). The klotho gene encodes a type 1 membrane protein, which is predicted to be present on the cell surface of Klotho-expressing cells. The human Klotho gene has five exons and can generate two transcripts. The full-length transcript is 5.2 kb and encodes a 130-kDa membrane protein; once the short transmembrane domain is removed, this membrane form can be released into circulation. A recent study has suggested that A Disintegrin and Metalloproteinases (ADAM)-10 and ADAM-17 are capable of cleavage of Klotho from the plasma membrane, and that insulin can stimulate Klotho shedding (5). An alternative mRNA splicing event can generate another transcript, which codes for the NH2-terminal region of Klotho and creates a protein with a molecular mass of ∼65–70 kDa. Human Klotho has ∼86% homology to mouse klotho and has been mapped to chromosome 13q12 (34). Klotho expression has been detected mostly in the distal convoluted tubules of the kidney, the parathyroid gland, and the epithelium of the choroid plexus in the brain (34). The exact role of Klotho in systemic regulation of mineral ion metabolism is not clear, other than that it gives the tissue specificity for FGF23 action. The question of whether there is a functional difference between the membrane form and the secreted form of Klotho needs additional study.
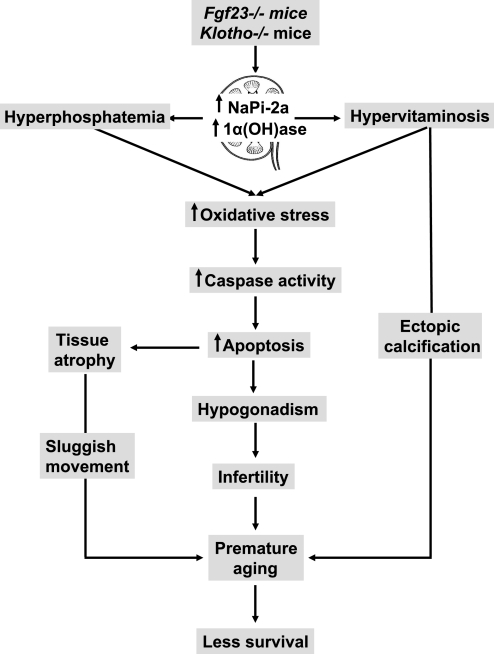
The inactivation of Klotho function in the mouse results in hyperphosphatemia and hypervitaminosis D (Fig. 1), which leads to the development of phenotypes resembling extensive premature aging-like features, including infertility and a shortened life span, in the mutant mouse (26). Interestingly, the phenotype of klotho-ablated mice resembles that of Fgf23-ablated mice (41, 48, 50); this observation led to the identification of the FGF23 signaling pathways.
Fig. 1.
Schematic diagram of possible events leading to premature aging-like phenotypes in Fgf23−/− and klotho−/− mice. The genetic inactivation of either Fgf23 or klotho results in an increased renal expression of sodium-phosphate cotransporter (NaPi)-2a and 1-α-hydroxylase [1α(OH)ase], which leads to hyperphosphatemia and hypervitaminosis D in these mutant mice. Such biochemical changes in serum phosphate and 1,25-dihydroxyvitamin D in Fgf23−/− and klotho−/− mice can induce oxidative stress to induce apoptosis and generate hypogonadism and generalized tissue atrophy. Moreover, hyperphosphatemia and hypervitaminosis D can induce vascular calcification (36, 51); the combined effect can result in premature aging-like phenotypes and early death in Fgf23−/− and klotho−/− mice.
FGF23 Signaling
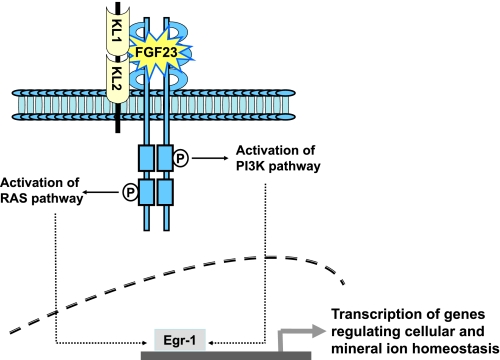
FGFs are circulatory factors that can be grouped into several subfamilies (Table 2). Most of the FGFs are able to bind to cell surface FGFRs to activate downstream signaling events with diverse biological consequences. FGF11 to FGF14, however, are unable to bind and activate FGFRs (22); these FGFs are also termed “FGF homologous factors” (43). FGF23 is a member of the FGF19 subfamily. Studies have shown that FGF23 needs Klotho as a cofactor to interact with the FGFRs (Fig. 2). FGF23 has been shown to bind to multiple FGFRs, including FGFR1c, FGFR3c, and FGFR4 (Table 3) (9, 27, 38, 64). A recent study, however, claimed that neither FGFR3 nor FGFR4 is the principal mediator of the FGF23 effects in vivo (31). Klotho appears to allow FGF23 to bind to its receptor complex with much higher affinity than to FGFR alone. Furthermore, FGF23, in the presence of Klotho, can activate downstream signaling events, as indicated by the activation of early growth response element-1 (Egr-1) and the phosphorylation of FGF receptor substrate-2a, extracellular signal-regulated kinase (ERK), p38, c-Jun NH2-terminal kinase (JNK), and AKT proteins (16, 27, 64, 68). None of these signaling phosphoproteins was detected in human proximal tubule epithelial cells treated with FGF23 without Klotho. These observations suggest that the FGF23-FGFRs interaction and subsequent signaling activities need Klotho as a cofactor (16, 35).
Table 2.
Members of FGF subfamilies
| FGF1 subfamily | FGF1 |
| FGF2 | |
| FGF4 subfamily | FGF4 |
| FGF5 | |
| FGF6 | |
| FGF7 subfamily | FGF3 |
| FGF7 | |
| FGF10 | |
| FGF22 | |
| FGF8 subfamily | FGF8 |
| FGF17 | |
| FGF18 | |
| FGF9 subfamily | FGF9 |
| FGF16 | |
| FGF20 | |
| FGF11 subfamily | FGF11 |
| FGF12 | |
| FGF13 | |
| FGF14 | |
| FGF19 subfamily | FGF19 |
| FGF21 | |
| FGF23 |
Fig. 2.
Simplified schematic diagram of fibroblast growth factor (FGF)23 signaling. FGF23, in the presence of Klotho (KL), can bind to FGF receptors with high affinity to trigger downstream signaling that induces the expression of genes regulating cellular homeostasis and mineral ion homeostasis. PI3K, phosphatidylinositol 3-kinase; Egr-1, early growth response element-1.
Table 3.
Ligand specificities of FGF receptor isoforms
| FGFR1b | FGFR1c | FGFR2b | FGFR2c | FGFR3b | FGFR3c | FGFR4 |
|---|---|---|---|---|---|---|
| FGF1 | FGF1 | FGF1 | FGF1 | FGF1 | FGF1 | FGF1 |
| FGF2 | FGF2 | FGF3 | FGF2 | FGF9 | FGF2 | FGF2 |
| FGF3 | FGF4 | FGF7 | FGF4 | FGF4 | FGF4 | |
| FGF10 | FGF5 | FGF10 | FGF6 | FGF8 | FGF6 | |
| FGF6 | FGF20 | FGF9 | FGF9 | FGF8 | ||
| FGF19 | FGF22 | FGF17 | FGF17 | FGF9 | ||
| FGF20 | FGF18 | FGF18 | FGF15 | |||
| FGF21 | FGF20 | FGF20 | FGF16 | |||
| FGF23 | FGF23 | FGF17 | ||||
| FGF18 | ||||||
| FGF19 | ||||||
| FGF21 | ||||||
| FGF23 |
Cellular, intracellular, transcellular, and pericellular mineral ion transport is achieved through a series of complex events that utilize both active and passive translocation processes. An important question that remains to be solved is how Klotho, produced in the distal tubules, can facilitate the FGF23-mediated phosphate transport process in the proximal tubules. Whether a secretory form of Klotho, released from the distal part of the tubules, can affect the functionality of the proximal part of the tubules is an important unresolved issue.
Genetic mutations in the FGF23 signaling components, such as Klotho or the FGFs, are also linked to diseases associated with altered mineral ion homeostasis and/or dysregulation of vitamin D metabolism. For example, osteoglophonic dysplasia is caused by missense mutations in the ligand-binding and transmembrane domains of FGFR1 (66). In addition, a point mutation in the human Klotho gene in a 13-year-old patient was found to be associated with ectopic calcification, despite significantly elevated serum levels of FGF23 (20); the lack of Klotho function in this patient can attenuate the ability of FGF23 to exert its phosphate-lowering effects, which can eventually lead to severe vascular and soft tissue calcifications (20). In contrast to the above-mentioned observations, however, a few recent studies have claimed that there are Klotho-independent functions of FGF23 (65).
Is Klotho Essential for FGF23 Signaling?
Although Klotho-mediated FGF23 signaling is well documented, it is not yet clear whether FGF23 may also have Klotho-independent effects. This possibility was raised by the observation that FGF23 can have effects on cells that do not express Klotho. For instance, FGF23 has been shown to suppress osteoblast differentiation and bone mineralization in fetal rat calvaria cells (65). Similarly, FGF23 was shown to exhibit weak proliferative effects on a murine bone marrow-derived pro-B cell line that was overexpressing FGFRs in the absence of Klotho (69).
Moreover, a recent study has found that Fgf23−/− mice and Fgf23−/−/NaPi-2a−/− double-knockout mice have a similar osteomalacic phenotype (less mineralization of the bone) despite opposing serum phosphate levels (high serum phosphate in Fgf23−/− mice, but low serum phosphate in Fgf23−/−/ NaPi-2a−/− mice). This result suggests that there may exist a serum phosphate-independent intrinsic effect of FGF23 on the bone (60). In fact, in vitro studies have suggested that FGF23 treatment can inhibit the mineralization of calvarial osteoblasts. Since Klotho is not expressed in osteoblasts, any effect of FGF23 on these cells would indicate that Klotho-independent effects of FGF23 exist, unless bone cells do express extremely low levels of Klotho that are undetectable with existing tools.
It is, however, necessary to mention that the injection of bioactive FGF23 protein into either klotho−/− mice or Fgf23−/−/klotho−/− double-knockout mice does not produce any obvious changes in the serum levels of phosphate (41), implying that Klotho is essential for the in vivo systemic regulation of phosphate homeostasis. Interestingly, when bioactive FGF23 protein was injected into either wild-type or Fgf23−/− mice, the serum phosphate level was significantly reduced by 12 h after the injection (41). Of course, both wild-type and Fgf23−/− mice have endogenous Klotho therefore, the exogenous injection of FGF23 can influence systemic phosphate homeostasis. On the other hand, in klotho−/− or Fgf23−/−/klotho−/− mice, in the absence of Klotho, FGF23 is unable to exert its phosphaturic effects. It is therefore likely that Klotho is essential for the FGF23-mediated systemic regulation of phosphate homeostasis. As mentioned above, the homozygous loss-of-function mutation in Klotho found in a patient with tumoral calcinosis has provided additional evidence for the dependence of FGF23 on Klotho in the regulation of phosphate and vitamin D homeostasis in humans (20).
Nonetheless, under pathological conditions in which there is a higher concentration of FGF23, it may be possible that FGF23 can exert nonspecific effects without Klotho; for example, FGF23 may be able to bind to its receptors with low affinity without Klotho (64). In this regard, the toxic effects of an extremely high serum level of FGF23 in patients with CKD, who have a reduced level of Klotho, need to be studied carefully (21, 46). Of relevance, the expression of Klotho mRNA and protein is significantly reduced in kidneys obtained from patients with CKD (24). How elevated serum levels of FGF23 might exert toxic effects in CKD patients whose tissue expression of Klotho is low is an important question, yet to be resolved.
Can FGF23 Be a Therapeutic Target?
Is anti-FGF23 therapy a feasible approach to fine-tune existing treatments for diseases associated with abnormal mineral ion metabolism? The clinical application of a controlled therapeutic inactivation of FGF23 might be of therapeutic benefit for patients with excessive urinary phosphate-wasting diseases, including ADHR, ARHR, and XLH. It is worth mentioning that the current treatment for hypophosphatemic diseases that are caused by genetic defects is mostly symptomatic, such as oral phosphate replacement. The prolonged use of such replacement can lead to the development of secondary hyperparathyroidism. Patients with CKD might benefit from the therapeutic lowering of FGF23, particularly if such an intervention could be provided in the early stages of the disease. Additional studies are needed to determine whether normalizing extremely high serum FGF23 levels in patients with late stages of CKD will reduce the various complications, including renal osteodystrophy. Elevated FGF23 levels in patients with CKD may reduce vitamin D activity by suppressing the expression of 1-α-hydroxylase. Since Klotho is an important component of FGF23 signaling, targeted manipulation of Klotho activity may also reduce the pathological effects of FGF23 and restore mineral balance. It is, however, worth mentioning that despite a number of studies proposing various roles for FGF23 in CKD patients, these studies have generated as many questions as they have answered. Whether a compensatory increased level of FGF23 in CKD patients is a protective response (in the earlier stage of the disease) or a harmful response (in the later stage of the disease) remains an unsettled issue, and any clinical interventions that target FGF23 therapeutically will need thoughtful consideration. In contrast to anti-FGF23 therapy, providing exogenous bioactive FGF23 protein might help restore the phosphate balance and reduce abnormal calcifications in patients with FTC, which is usually caused by the reduced activity of FGF23.
Concluding Remarks
Future studies will determine not only the therapeutic benefit of the manipulation of FGF23 signaling components but also its diagnostic and prognostic significance. The detection of circulatory FGF23 has become an important tool for determining the underlying causes of diseases associated with abnormal mineral ion metabolism. For instance, serum FGF23 levels can be used to differentially diagnose suspected TIO. Studies have suggested that the pretreatment serum level of FGF23 is a good predictor of the effectiveness of a vitamin D therapy in dialysis patients and might also be a useful marker for predicting the development of refractory hyperparathyroidism (40). FGF23 has been proposed to be an important biomarker of mortality in patients with early renal diseases, particularly in patients in whom the serum FGF23 level increased before the development of hyperphosphatemia (17). In light of these findings, it appears likely that FGF23 has diagnostic, prognostic, and therapeutic value.
Recent studies using mouse genetics have significantly enhanced our understanding of the in vivo regulation of phosphate homeostasis (49). The strikingly similar biochemical phenotypes of hypervitaminosis D, reduced serum PTH levels, and hyperphosphatemia in Fgf23−/− and klotho−/− single-knockout mice and Fgf23−/−/klotho−/− double-knockout mice suggest that these factors constitute a common signaling pathway (Fig. 2). These results provide compelling genetic evidence of the in vivo importance of Klotho in regulating systemic phosphate homeostasis with FGF23. Finally, it is necessary to mention that FGF23-mediated functions are mostly Klotho dependent; Klotho, however, also has numerous FGF23-independent functions, including adipogenesis (7). Our challenge will be to use these experimental observations to fine-tune the existing therapeutic options by manipulating the FGF23-Klotho axis to treat patients suffering from the complications of abnormal mineral ion metabolism (44).
GRANTS
The original research works that are cited in this paper are supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-077276 and institutional support from Harvard School of Dental Medicine.
REFERENCES
- 1.ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26: 345–348, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91: 3144–3149, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23(R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145: 5269–5279, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet 14: 385–390, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA 104: 19796–19801, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen TC, Castillo L, Korycka-Dahl M, DeLuca HF. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr 104: 1056–1060, 1974. [DOI] [PubMed] [Google Scholar]
- 7.Chihara Y, Rakugi H, Ishikawa K, Ikushima M, Maekawa Y, Ohta J, Kida I, Ogihara T. Klotho protein promotes adipocyte differentiation. Endocrinology 147: 3835–3842, 2006. [DOI] [PubMed] [Google Scholar]
- 8.DeLuca S, Sitara D, Kang K, Marsell R, Jonsson K, Taguchi T, Erben RG, Razzaque MS, Lanske B. Amelioration of the premature aging-like features of Fgf-23 knockout mice by genetically restoring the systemic actions of FGF-23. J Pathol 216: 345–355, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 16: 139–149, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90: 1519–1524, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Forster IC, Hernando N, Biber J, Murer H. Proximal tubular handling of phosphate: a molecular perspective. Kidney Int 70: 1548–1559, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Frishberg Y, Topaz O, Bergman R, Behar D, Fisher D, Gordon D, Richard G, Sprecher E. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med 83: 33–38, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Fukagawa M, Nii-Kono T, Kazama JJ. Role of fibroblast growth factor 23 in health and in chronic kidney disease. Curr Opin Nephrol Hypertens 14: 325–329, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Gaasbeek A, Meinders AE. Hypophosphatemia: an update on its etiology and treatment. Am J Med 118: 1094–1101, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Garringer HJ, Fisher C, Larsson TE, Davis SI, Koller DL, Cullen MJ, Draman MS, Conlon N, Jain A, Fedarko NS, Dasgupta B, White KE. The role of mutant UDP-N-acetyl-alpha-d-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J Clin Endocrinol Metab 91: 4037–4042, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 27: 3417–3428, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Hoenderop JG, Bindels RJ. Is vitamin D indispensable for Ca2+ homeostasis: lessons from knockout mouse models? Nephrol Dial Transplant 20: 864–867, 2005. [DOI] [PubMed] [Google Scholar]
- 19.HYP Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 11: 130–136, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 117: 2684–2691, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imel EA, Econs MJ. Fibroblast growth factor 23: roles in health and disease. J Am Soc Nephrol 16: 2565–2575, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet 20: 563–569, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348: 1656–1663, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280: 1015–1020, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Kowarski S, Schachter D. Effects of vitamin D on phosphate transport and incorporation into mucosal constituents of rat intestinal mucosa. J Biol Chem 244: 211–217, 1969. [PubMed] [Google Scholar]
- 26.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanske B, Razzaque MS. Premature aging in klotho mutant mice: cause or consequence? Ageing Res Rev 6: 73–79, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17: 1305–1315, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol 19: 2342–2350, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab 291: E38–E49, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38: 1248–1250, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242: 626–630, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Medici D, Razzaque MS, Deluca S, Rector TL, Hou B, Kang K, Goetz R, Mohammadi M, Kuro OM, Olsen BR, Lanske B. FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J Cell Biol 182: 459–465, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Memon F, El-Abbadi M, Nakatani T, Taguchi T, Lanske B, Razzaque MS. Does Fgf23-klotho activity influence vascular and soft tissue calcification through regulating mineral ion metabolism? Kidney Int 74: 566–570, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol 27: 503–515, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev 16: 107–137, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Nabeshima Y, Imura H. alpha-Klotho: a regulator that integrates calcium homeostasis. Am J Nephrol 28: 455–464, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, Gejyo F, Shigematsu T, Fukagawa M. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int 67: 1171–1178, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf 23)-mediated regulation of systemic phosphate homeostasis. FASEB J (October 3, 2008); doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed]
- 42.Nishi H, Nii-Kono T, Nakanishi S, Yamazaki Y, Yamashita T, Fukumoto S, Ikeda K, Fujimori A, Fukagawa M. Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin Pract 101: c94–c99, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Olsen SK, Garbi M, Zampieri N, Eliseenkova AV, Ornitz DM, Goldfarb M, Mohammadi M. Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J Biol Chem 278: 34226–34236, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Razzaque MS Can fibroblast growth factor 23 fine-tune therapies for diseases of abnormal mineral ion metabolism? Nat Clin Pract Endocrinol Metab 3: 788–789, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Razzaque MS Does renal ageing affect survival? Ageing Res Rev 6: 211–222, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Razzaque MS Does FGF23 toxicity influence outcome of chronic kidney disease? Nephrol Dial Transplant 24: 4–7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Razzaque MS Klotho and Na+,K+-ATPase activity: solving the calcium metabolism dilemma? Nephrol Dial Transplant 23: 459–461, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med 12: 298–305, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol 194: 1–10, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature ageing-like phenotype in fibroblast growth factor 23 null mice is a vitamin-D mediated process. FASEB J 20: 720–722, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razzaque MS, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant 20: 2032–2035, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Razzaque MS, Taguchi T. Cellular and molecular events leading to renal tubulointerstitial fibrosis. Med Electron Microsc 35: 68–80, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112: 683–692, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280: 2543–2549, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98: 6500–6505, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143: 3179–3182, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun 314: 409–414, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Sitara D, Kim S, Razzaque MS, Bergwitz C, Taguchi T, Schuler C, Erben RG, Lanske B. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet 4: e1000154, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taguchi T, Razzaque MS. The collagen-specific molecular chaperone HSP47: is there a role in fibrosis? Trends Mol Med 13: 45–53, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka S Signaling axis in osteoclast biology and therapeutic targeting in the RANKL/RANK/OPG system. Am J Nephrol 27: 466–478, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Tenenhouse HS Regulation of phosphorus homeostasis by the type IIa Na/phosphate cotransporter. Annu Rev Nutr 25: 197–214, 2005. [DOI] [PubMed] [Google Scholar]
- 64.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, Aubin JE, Maeda N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res 23: 939–948, 2008. [DOI] [PubMed] [Google Scholar]
- 66.White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, Fields J, Yu X, Shaw NJ, McLellan NJ, McKeown C, Fitzpatrick D, Yu K, Ornitz DM, Econs MJ. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet 76: 361–367, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun 277: 494–498, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, Fujita T, Kuroki R, Yamashita T, Fukumoto S, Shimada T. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res 23: 1509–1518, 2008. [DOI] [PubMed] [Google Scholar]
- 69.Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology 146: 4647–4656, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]