Abstract
Cytosolic NADP+-dependent isocitrate dehydrogenase (IDPc) synthesizes reduced NADP (NADPH), which is an essential cofactor for the generation of reduced glutathione (GSH), the most abundant and important antioxidant in mammalian cells. We investigated the role of IDPc in kidney ischemia-reperfusion (I/R) in mice. The activity and expression of IDPc were highest in the cortex, modest in the outer medulla, and lowest in the inner medulla. NADPH levels were greatest in the cortex. IDPc expression in the S1 and S2 segments of proximal tubules was higher than in the S3 segment, which is much more susceptible to I/R. IDPc protein was also highly expressed in the mitochondrion-rich intercalated cells of the collecting duct. IDPc activity was 10- to 30-fold higher than the activity of glucose-6-phosphate dehydrogenase, another producer of cytosolic NADPH, in various kidney regions. This study identifies that IDPc may be the primary source of NADPH in the kidney. I/R significantly reduced IDPc expression and activity and NADPH production and increased the ratio of oxidized glutathione to total glutathione [GSSG/(GSH+GSSG)], resulting in kidney dysfunction, tubular cell damage, and lipid peroxidation. In LLC-PK1 cells, upregulation of IDPc by IDPc gene transfer protected the cells against hydrogen peroxide, enhancing NADPH production, inhibiting the increase of GSSG/(GSH+GSSG), and reducing lipid peroxidation. IDPc downregulation by small interference RNA treatment presented results contrasting with the upregulation. In conclusion, these results demonstrate that IDPc is expressed differentially along tubules in patterns that may contribute to differences in susceptibility to injury, is a major enzyme in cytosolic NADPH generation in kidney, and is downregulated with I/R.
Keywords: reduced nicotinamide adenine dinucleotide phosphate, glutathione, proximal tubular cell, acute kidney injury, oxidative stress, ROS
cytosolic nadp+-dependent isocitrate dehydrogenase (IDPc) is a member of the isocitrate dehydrogenases (ICDHs) that catalyze oxidative decarboxylation of isocitrate into α-ketoglutarate (42). The family of ICDHs is classified on the basis of their cofactors and intracellular localization: IDPc encoded by Idh1 gene, mitochondrial NADP+-dependent ICDH encoded by Idh2 gene, and mitochondrial NAD+-dependent ICDH encoded by Idh3 gene (16, 23). IDPc is responsible for production of reduced NADP (NADPH) in cytoplasm (37). NADPH is an essential cofactor for the maintenance of glutathione in its reduced state (GSH) (45). GSH is the most abundant low-molecular-mass thiol in mammalian cells and plays an important role as an antioxidant in the oxidative stress defense system (5, 43). In addition, NADPH is used to convert not only oxidized glutathione (GSSG) to GSH but also oxidized thioredoxin to reduced thioredoxin, which also plays a role in the antioxidant system (10, 44). Recently we found that induction of IDPc protects cells against oxidative stress and ultraviolet radiation stress in fibroblasts (17, 21) and that IDPc mRNA is highly expressed in the liver and kidney (26). These observations suggest that IDPc may be involved in oxidative stress-related states such as ischemia-reperfusion (I/R) injury.
I/R markedly increases the production of reactive oxygen species (ROS), such as superoxide anions, hydrogen peroxide (H2O2), hydroxyl radicals, and peroxynitrite, to levels above the normal scavenging capacity of the organ (15, 19). The abnormal excessive generation of ROS causes lipid peroxidation, disruption of the cellular cytoskeleton and integrity, and DNA breakdown, leading to cell damage (9, 20). Thus antioxidant agents have been developed to treat I/R-related diseases, and data suggest that treatment with antioxidant agents and activation of antioxidant enzymes ameliorate I/R injury (13, 25, 28). However, the role of IDPc in I/R injury, the pathogenesis of which is associated with ROS stress, has not yet been reported in any organ.
I/R results in acute kidney injury (AKI), which has high mortality and morbidity, and effective therapeutics against this disorder have not yet been developed (7). We found that IDPc is a major enzyme for cytosolic NADPH production, and its expression and activity differ in various kidney regions. Expression is low in the S3 segment of the proximal tubule, which is particularly sensitive to I/R-induced injury. Furthermore, I/R reduces the activity and expression of IDPc, decreasing NADPH production. Upregulation of IDPc expression in cultured kidney epithelial cells decreases cell susceptibility to oxidative stress, and downregulation of IDPc expression increases cell susceptibility. These results indicate that IDPc is a critical enzyme in the pathogenesis of I/R injury to kidney epithelial cells.
MATERIALS AND METHODS
Animal preparation.
Experiments were performed in 10-wk-old male C57BL/6 mice. Mice were allowed free access to water and standard mouse chow. In all cases, studies were reviewed and approved by the Kyungpook National University Institutional Animal Care and Use Committee. Each animal group consisted of at least six mice. Kidney ischemia was carried out as described previously (33). Mice were anesthetized with pentobarbital sodium (60 mg/kg body wt ip) and then subjected to either 30 min of bilateral renal ischemia or sham operation. Kidneys were either perfusion fixed in 4% paraformaldehyde, 75 mM l-lysine, 10 mM sodium periodate (PLP; Sigma, St. Louis, MO) for histological study or snap-frozen in liquid nitrogen for biochemical study. For histological studies, kidneys fixed in PLP were washed with phosphate-buffered saline (PBS) three times for 5 min each, embedded in oxytetracycline compound (Sakura FineTek, Torrance, CA) at −20°C or in paraffin at room temperature, and then cut into 4-μm cryosections and 2-μm paraffin sections with a cryotome (CM1850; Leica) and a microtome (RM2165; Leica, Bensheim, Germany), respectively.
Plasma creatinine and blood urea nitrogen concentration.
Seventy microliters of blood was taken from the retrobulbar vein plexus at the times indicated in Fig. 4. Plasma creatinine concentration was measured with a Beckman Analyzer II (Beckman). Blood urea nitrogen (BUN) concentration was measured with a BUN assay kit (ASAN PHARM, Gyeonggi-do, Korea) according to the manufacturer's protocol.
Periodic acid Schiff stain.
Two-μm paraffin sections were stained with periodic acid Schiff (PAS) according to a standard protocol.
Nitro blue tetrazolium stain.
As described previously (15, 20), kidney sections were cut with a cryotome, incubated in 1 mg/ml nitro blue tetrazolium (NBT, Sigma) in PBS for 2 h at 37°C, and then washed with PBS. Signals were obtained with a light microscope.
Measurement of lipid peroxidation and H2O2.
Thiobarbituric acid-reactive substances (TBARS) were determined as a measure of lipid peroxidation. Samples were evaluated for malondialdehyde (MDA) production with a spectrophotometric assay for TBARS (20, 40). H2O2 levels were measured with a ferric iron-sensitive dye, xylenol orange. H2O2 oxidizes iron(II) to iron(III) in the presence of sorbitol, which acts as a catalyst. Iron(III) forms a purple complex with xylenol orange as previously described (20, 40).
Cell culture.
LLC-PK1 cells, a porcine tubular epithelial cell line, were grown in phenol red-free DMEM containing 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA) at 37°C in an incubator with a 5% CO2 atmosphere. Cells were treated with either 1 mM H2O2 or vehicle for 2 h. Cell viability was evaluated by Trypan blue exclusion assay. Apoptotic and necrotic cells were analyzed by flow cytometry (FASCAria, BD Bioscience, San Jose, CA) with an annexin V-FITC/propidium iodide (PI) detection kit (ApoScan, LS-02-100; BioBud, Seoul, Korea).
Gene and small interference RNA transfer.
LLC-PK1 cells were transfected with either the LNCX vector encoding the mouse IDPc gene (LNCX-IDPc) or with LNCX vector alone (LNCX-null) (26). Other cells were transfected with either scrambled small interference RNA (siRNA) or siRNA for IDPc (IDPc-siRNA), which were designed as follows: scrambled siRNA, 5′-CUGAUGACCUGAGUGAAUGTT-3′; IDPc-siRNA, 5′-GGACUUGGCUGCUUGCAUUTT-3′. Cells were incubated with 100 or 200 pM siRNA and 4 or 8 μg of the LNCX vectors in 5 ml of culture medium with 30 μl of Lipofectamine 2000 (Invitrogen) and 90 μl of PolyFect Reagent (Qiagen, Valencia, CA) according to the manufacturers’ protocols, respectively.
Antibodies.
An antibody against IDPc was previously generated and characterized (21, 22). Tubules were identified with antibodies against tubule-specific proteins. Descending thin limbs (DTLs) were identified with an antibody against aquaporin-1 (AQP1) (AQP-001; Alomone Labs, Jerusalem, Israel) (31). An antibody against gp330 was used to label the apical plasma membranes of proximal tubule (PT) cells (36). Distal tubules (DTs) and thick ascending limbs (TALs) were identified with an antibody against Na+-K+-Cl− cotransporter-2 (NKCC2) (NKCC21-A; Alpha Diagnostic International, San Antonio, TX) (48). Intercalated cells were identified with an antibody against vacuolar-type H+-ATPase B1/2 (H+-ATPase) (sc-21209; Santa Cruz Biotechnology, Santa Cruz, CA) (46). Principal cells in the collecting duct (CD) were identified with an antibody against AQP2 (AQP-002; Alomone Labs) (31). An antibody against Na+-K+-ATPase was used to label the tubule cells (36). For Western blot analysis, polyclonal anti-histone H1 (sc-8616; Santa Cruz Biotechnology), anti-copper-zinc superoxide dismutase (CuZnSOD) (AB1237; Chemicon, Temecula, CA), anti-manganese superoxide dismutase (MnSOD) (574596; Calbiochem, San Diego, CA), and monoclonal anti-β-actin (sc-8432; Santa Cruz Biotechnology) antibodies were used.
Immunofluorescence.
Immunofluorescence staining was performed as described previously (15, 36). Depending on the immunoreactivity of antibodies, immunofluorescence staining was carried out on microtome-cut tissue slices of paraffin-embedded tissues or cryo-cut slides of oxytetracycline compound-embedded tissues. The paraffin-embedded tissue sections were deparaffinized with xylene, rehydrated with 100%, 95%, and 80% ethanol, and then washed with PBS for 10 min each. Briefly, cryosections or deparaffinized paraffin sections were incubated in PBS containing 0.1% sodium dodecyl sulfate (SDS; Sigma) for 5 min and washed in PBS for 10 min. To unmask antigen epitopes, sections were boiled in 10 mM sodium citrate buffer (pH 6.0) for 1 min in a microwave oven, cooled at room temperature (RT) for 20 min, and then washed with PBS three times for 5 min. Sections were blocked with PBS containing 1% bovine serum albumin (blocking buffer) for 30 min at RT, incubated with polyclonal anti-IDPc antibody (1:100) in blocking buffer overnight at 4°C, and washed with PBS three times for 5 min. After that, sections were incubated with FITC-conjugated goat anti-rabbit IgG (1:100; FI-1000, Vector Laboratories, Burlingame, CA) for 60 min at RT and then washed with PBS three times for 5 min. For double staining, monoclonal anti-gp330 antibody (1:200) was repeatedly used in cryosections and monoclonal anti-Na+-K+-ATPase (1:50) and polyclonal anti-H+-ATPase (1:50) were used in paraffin sections. Polyclonal anti-NKCC2 (1:50), anti-AQP1 (1:50), or anti-AQP2 (1:200) antibody was used in serial paraffin sections. To detect cell nuclei, 4′,6-diamidino-2-phenylindole (DAPI; Sigma) was placed on sections for 1 min. Finally, cryosections and paraffin sections were mounted with Prolong Gold antifade reagent (Invitrogen) and observed under an Axioplan-2 epifluorescence microscope (Carl Zeiss, Munich, Germany). Images were collected with a digital camera (Carl Zeiss) and merged with Adobe Photoshop 7.0 software.
Western blot analysis.
Western blot analyses were performed as described previously (33). Briefly, renal tissue or cell lysates (30 μg protein/lane) were separated on 10% SDS-PAGE gels and then transferred to Immobilon membranes (Millipore, Bedford, MA). The membranes were incubated with anti-IDPc, anti-histone H1, anti-MnSOD, anti-CuZnSOD, and anti-β-actin antibodies overnight at 4°C. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies against the appropriate primary antibodies (1:4,000; PI-1000, Vector Laboratories), exposed to Western Lighting Chemiluminescence Reagent (NEL101; PerkinElmer, Boston, MA), and then developed with X-ray film. The area of each band was analyzed with LabWorks 4.5 software (UVP, Upland, CA).
Preparation of cytosolic and mitochondrial fractions.
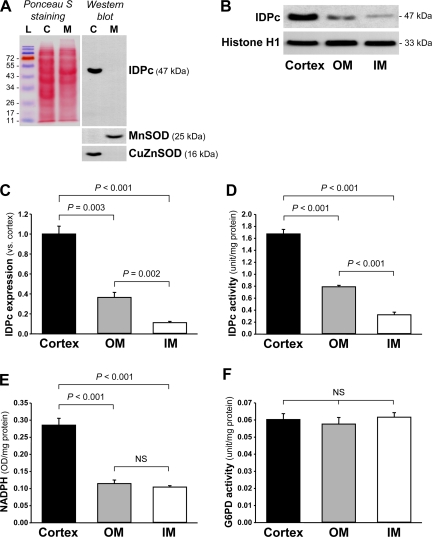
Cytosolic and mitochondrial fractions were prepared as described previously (17, 20). Briefly, frozen tissues or cells were homogenized in sucrose buffer (0.32 M sucrose, 10 mM Tris·HCl, pH 7.4; Sigma) on ice with Dounce homogenizers. The homogenate was centrifuged at 1,000 g for 5 min, and then the supernatant was centrifuged at 15,000 g for 30 min. The supernatant was the cytosolic fraction and was used to measure IDPc activity. The pellet was washed twice with sucrose buffer to collect mitochondrial pellets. The pellet was suspended in PBS containing 0.1% Triton X-100, disrupted twice with a sonicator (4710 series; Cole-Palmer, Chicago, IL) at 40% of maximum setting for 10 s, and centrifuged at 15,000 g for 30 min. The supernatant was the mitochondrial fraction and was stored at −70°C. These fractions were confirmed by Western blot analysis using antibodies against CuZnSOD for the cytosolic fraction and against MnSOD for the mitochondrial fraction (Fig. 1A).
Fig. 1.
Expression (A–C) and activity (D) of NADP+-dependent isocitrate dehydrogenase (IDPc), level of reduced NADP (NADPH; E), and activity of glucose-6-phosphate dehydrogenase (G6PD; F) in mouse kidneys. Mouse kidneys were dissected into cortex, outer medulla (OM), and inner medulla (IM) fractions. A: IDPc, manganese superoxide dismutase (MnSOD), and copper-zinc superoxide dismutase (CuZnSOD) expressions were determined by Western blot analysis using antibodies against those proteins. Ponceau S was used to detect protein bands on the membrane. L, protein ladder (SM0671; Fermentas, Hanover, MD); C, cytosol fraction; M, mitochondrial fraction. B: IDPc expression was determined by Western blot analysis using antibodies against IDPc or histone H1 as an equal loading marker. C: densities of blots were quantified with Lab Works 4.5 (n = 4). Activities of IDPc (D) and G6PD (F) and levels of NADPH (E) were determined in the kidney sections as described in materials and methods (n = 6). Results are expressed as means ± SE. NS, no significant difference.
IDPc and glucose-6-phosphate dehydrogenase activity assays.
The activities of both IDPc and glucose-6-phosphate dehydrogenase (G6PD) were measured as described previously (26, 41). Briefly, IDPc activities in the cytosolic fraction (50 μg protein) were measured in a reaction mixture containing (in mM) 40 Tris (pH 7.4), 2 NADP+, 2 MgCl2, and 50 threo-DS-isocitrate (Sigma). One unit of IDPc activity was defined as the amount of enzyme catalyzing the production of 1 μmol NADPH/min as measured by the absorbance at 340 nm at 37°C (n = 6). G6PD activities in the cytosolic fraction were measured in a reaction mixture containing (in mM) 55 Tris (pH 7.8), 3.3 MgCl2, and 4 glucose-6-phosphate, with 240 μM NADP+ (Sigma). One unit of G6PD activity was defined as the amount of enzyme catalyzing the reduction of 1 μmol NADP+/min as measured by the absorbance at 340 nm at 37°C.
Measurement of cytosolic NADPH and total NADP levels.
The cytosolic NADPH level was measured as described previously (26, 49). Briefly, NADPH in the cytosolic fraction was induced by exclusion of NADPH through heating for 30 min at 60°C in a dry heating bath. Cytosolic fractions (200 μg protein each) for NADPH and total NADP (NADPt) levels were preincubated in a reaction mixture containing (in mM) 100 Tris (pH 8.0), 2 phenazine ethosulfate, 5 ethylenediaminetetraacetic acid (EDTA), and 0.5 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), with 1.5 U of G6PD (Sigma) for 5 min at 37°C. The reactions were started by the addition of 1 mM glucose-6-phosphate. NADPH and NADPt levels were defined as the change in optical density (OD) at 570 nm for 1 min at 37°C.
Measurement of ratio of oxidized glutathione to total glutathione.
GSSG/(GSH+GSSG) was measured as described previously (1, 26). The concentration of total glutathione was determined by the rate of formation of 5-thio-2-nitrobenzoic acid. GSSG was measured by the 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB)-GSSG reductase recycling assay after removal of GSH from 2-vinylpryridine. Total glutathione and GSSG levels were defined as the change in OD at 412 nm for 1 min at 37°C.
Statistics.
Results are expressed as means ± SE. Statistical differences among groups were calculated with analysis of variance (ANOVA) followed by least significant difference post hoc comparison with the SPSS 12.0 program. Differences between groups were considered statistically significant at a P value of <0.05.
RESULTS
Expression and activity of IDPc in kidney.
The level of IDPc expression was the highest in the cortex, modest in the outer medulla (OM), and lowest in the inner medulla (IM) (Fig. 1, B and C). Consistent with the expression of IDPc, IDPc activity also was highest in the cortex, modest in the OM, and lowest in the IM (Fig. 1D). The level of cytosolic NADPH, a product of IDPc, was significantly higher in the cortex compared with the OM and the IM (Fig. 1E). Since NADPH is generated by not only IDPc but also G6PD (37), we determined the activity of G6PD. Compared with IDPc activity, G6PD activity for NADPH production was about 28-fold lower in the cortex, 13-fold lower in the OM, and 6-fold lower in the IM (Fig. 1, D and F). In contrast to IDPc activity, G6PD activities were not significantly different in the various kidney regions (Fig. 1F). These results indicate that IDPc serves as a major enzyme for cytosolic NADPH production in the kidneys.
Distribution of IDPc in mouse kidney tubules.
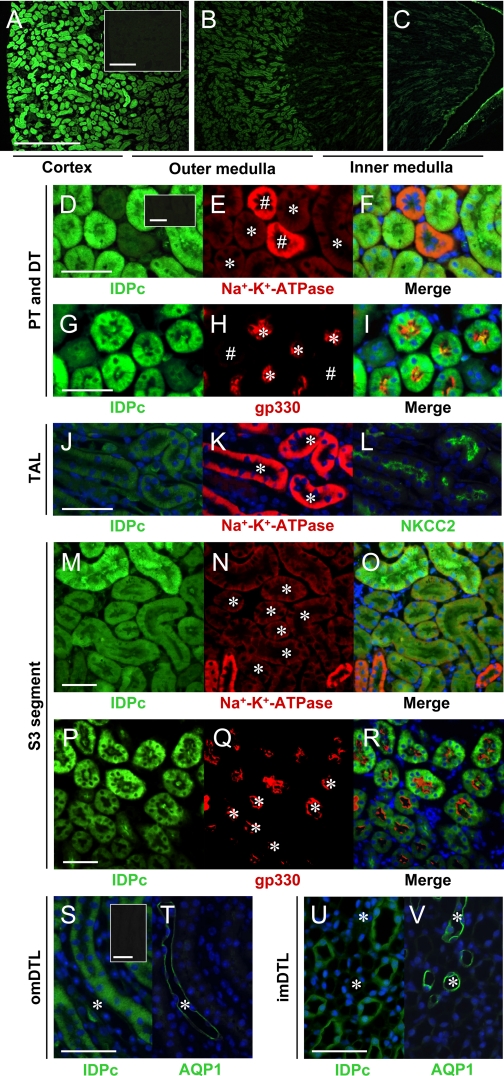
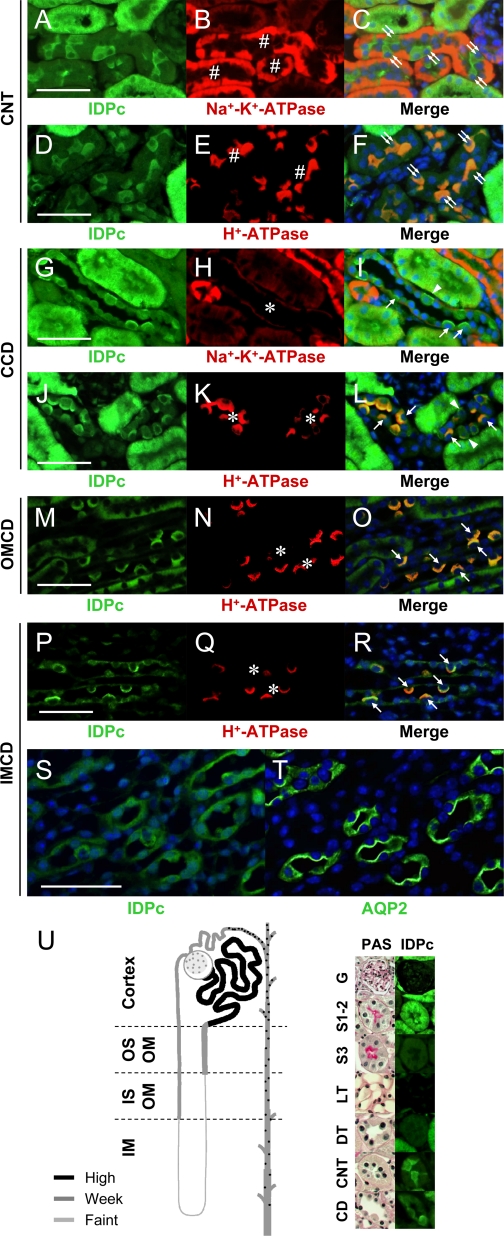
Kidney tubular cells of different nephron segments perform unique functions and are differentially susceptible to a variety of physiological and pathophysiological conditions. Consistent with the immunoblot results (Fig. 1, B and C), nephron IDPc expression was highest in the cortex, modest in the OM, and lowest in the IM (Fig. 2, A–C). IDPc was expressed in the cytoplasm of cells (Fig. 2, D–V). Expression of IDPc in the S1 and S2 segments of the PTs (Fig. 2, D–I) was greater than that in the S3 segment in the outer stripe of the OM (OSOM) (Fig. 2, M–R). The expression of IDPc in the PTs was greater than that in the DTs (Fig. 2, D–I). IDPc expression was weak in the TAL (Fig. 2, J–L) and the DTL (Fig. 2, S–V) compared with that in the PTs. IDPc was highly expressed in the intercalated cells in the connecting tubule (CNT) and the CD (Fig. 3, A–T) but weakly expressed in the CNT cells and the principal cells (Fig. 3, A–T). In the intercalated cells in the CD, IDPc was expressed in the apical or basolateral domain (Fig. 3, G–T). Kidney tubular segments were identified by immunostaining using anti-Na+-K+-ATPase (47), anti-gp330 (6), anti-NKCC2 (48), anti-AQP1 (31), anti-H+-ATPase (46), or anti-AQP2 (31) antibodies. The immunoreactivity for Na+-K+-ATPase is high in the basolateral domain of the CNT cells, moderate in the principal cells, and weak in the intercalated cells (Fig. 3, B and H) (38). H+-ATPase is expressed in the diffuse and/or apical domain of the intercalated cells (Fig. 3, E, K, N, and Q) (46). AQP2 is expressed in the apical membrane of the principal cells (Fig. 3T) (31). As summarized in Fig. 3U, the distribution of IDPc in kidney tubules differed in various tubular segments, indicating that IDPc may have different effects on oxidative stress along the tubules.
Fig. 2.
Expressions of IDPc in mouse kidney tubular epithelial cells. Kidney sections were immunostained with antibodies described in materials and methods. A–C: kidney sections were stained with anti-IDPc antibody. D–I: kidney sections were double-stained with anti-IDPc and then with anti-Na+-K+-ATPase or anti-gp330 antibodies. Pictures were taken of the cortex. IDPc is highly expressed in the cytoplasm of proximal tubule (PT, *) cells but weakly in distal tubule (DT, #) cells. J–L: serial kidney sections were mounted on the same slide for staining with anti-IDPc, anti-Na+-K+-ATPase, or anti-Na+-K+-Cl− cotransporter 2 (NKCC2) antibodies. Pictures were taken of the inner strip of the OM. IDPc expression is weak in the cytoplasm of tubules. *, Thick ascending limb (TAL). M–R: kidney sections were double-stained with anti-IDPc antibody and then with either anti-Na+-K+-ATPase or anti-gp330 antibodies. S1–2 segment cells of the PT in the cortex express IDPc to a much greater extent than S3 segment cells in the outer strip of the OM. *, S3 segment of the PT. S and T: serial kidney sections were stained with anti-IDPc and anti-AQP1 antibodies. Pictures were taken of the OM. Descending thin limb (DTL) cells faintly express IDPc in the cytoplasm. *, DTL in the OM (omDTL). U and V: serial kidney sections were stained with anti-IDPc and anti-AQP1 antibodies. Pictures were taken of the IM. Insets in A, D, S: kidney treated with secondary antibody alone. *, DTL in the IM (imDTL). D–I and M–R: pictures taken of the double staining merged with Photoshop software. Scale bars: 500 (A–C) and 50 (D–V) μm.
Fig. 3.
Expressions of IDPc in connecting tubule (CNT) and collecting duct (CD). A–L: kidney sections were double-stained with anti-IDPc and then with anti-Na+-K+-ATPase or anti-H+-ATPase antibodies. A–F: IDPc is highly expressed in the cytoplasm of intercalated cells in CNT but is weakly expressed in CNT cells. #, CNT; double arrows, intercalated cells of CNT. G–L: some intercalated cells of cortical CD (CCD) express IDPc in the apical domain (arrow), whereas some cells express IDPc in the basolateral domain (arrowhead). In principal cells, IDPc is weakly expressed in the cytoplasm. M–O: intercalated cells of outer medullar CD (OMCD) only express IDPc in the apical domain (arrow). P–R: intercalated cells of inner medullar CD (IMCD) also express IDPc in the apical domain (arrow). S and T: serial kidney sections mounted on the same slide were stained with anti-IDPc and anti-AQP2 antibodies. Principal cells in IMCD weakly express IDPc in cytoplasm. U: diagram of IDPc expression in the mouse kidney. Some kidney sections were stained with periodic acid Schiff (PAS) for morphological clarity. #, CNT; *, CD; G, glomerulus; S1–2, S1–2 segment proximal tubule; S3, S3 segment; LT, thin limb. Bars: 50 μm.
Expression and activity of IDPc in kidneys subjected to ischemia-reperfusion.
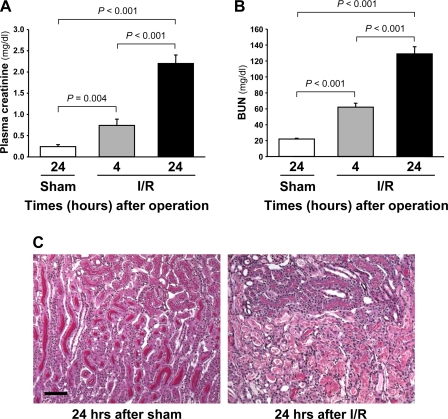
To investigate whether IDPc may play a role in I/R injury, IDPc activity and expression were determined in kidneys of mice that were subjected to 30 min of bilateral renal ischemia. Thirty minutes of ischemia resulted in significant increases of plasma creatinine and BUN concentrations 4 and 24 h after ischemia (Fig. 4, A and B). I/R resulted in deposition of tubular casts in the tubular lumens, disruption of tubular epithelial cells, and infiltration of leukocytes into the interstitium (Fig. 4C). The tubular epithelial cell damage was most severe in the S3 segments of PT in the OSOM 24 h after ischemia (Fig. 4C).
Fig. 4.
Concentrations of plasma creatinine (A) and blood urea nitrogen (BUN; B) and kidney histology (C) in mice subjected to either 30 min of bilateral ischemia or sham operation. A and B: concentrations of plasma creatinine and BUN were determined 4 and 24 h after reperfusion. C: kidney sections were PAS stained. Pictures were taken from the corticomedullary junction. Results are means ± SE (n = 6). Scale bars: 50 μm. I/R, ischemia-reperfusion.
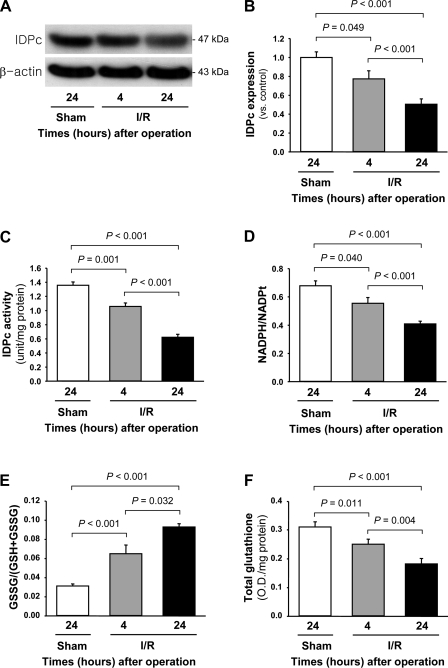
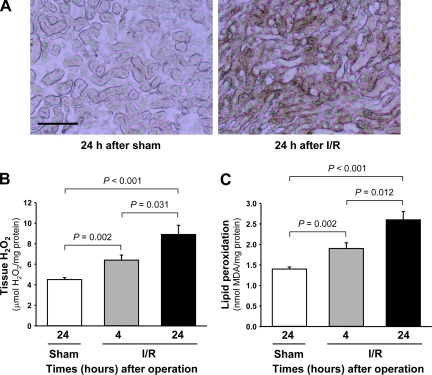
IDPc expression was significantly decreased in the kidneys 4 and 24 h after ischemia compared with that in kidneys subjected to sham operation (sham-operated controls) or those harvested 4 h after the surgery (Fig. 5, A and B). Consistent with the decrease of IDPc expression, IDPc activity in the kidney tissues was also decreased 4 and 24 h after ischemia compared with that in sham-operated control kidneys (Fig. 5C). The ratio of NADPH to total NADP (NADPH/NADPt) in the cytosolic fraction of the kidney was significantly decreased 4 and 24 h after ischemia compared with that in sham-operated control kidney (Fig. 5D). After 4 and 24 h after I/R, GSSG/(GSH+GSSG) in the cytosolic fraction of the kidney was significantly increased (Fig. 5E). Postischemic total glutathione levels in the cytosolic fraction of the kidneys were gradually decreased below normal range over time (Fig. 5F). In contrast, postischemic cytosolic GSSG levels (0.016 OD/mg protein at 4 h and 0.018 OD/mg protein at 24 h after I/R) were gradually increased above the normal range (0.011 OD/mg protein) over time (Fig. 5, E and F). These results indicate that the increases of GSSG/(GSH+GSSG) may be caused by both decreases of total glutathione and reduction of GSSG to GSH due to the decrease of IDPc activation. IDPc expression in the cortex and OM of the kidneys was significantly decreased 24 h after I/R, but that in the IM was not (Fig. 6, A and B). IDPc activity and NADPH/NADPt level were consistent with the expression of IDPc (Fig. 6, C and D). To evaluate whether I/R injury was associated with oxidative stress in our experiments, lipid peroxidation, superoxide, and tissue H2O2 levels were measured. Superoxide and H2O2 levels were significantly increased 4 and 24 h after ischemia (Fig. 7, A and B). Lipid peroxidation was significantly higher at both time points in the ischemic kidney compared with the sham-operated control kidney (Fig. 7C).
Fig. 5.
Expression (A and B) and activity (C) of IDPc, ratio of NADPH to total NADP (NADPH/NADPt; D), ratio of oxidized glutathione to total glutathione [GSSG/(GSH+GSSG); E], and level of total glutathione (F) in whole kidneys of mice subjected to either 30 min of bilateral ischemia or sham operation. Kidneys were harvested 4 and 24 h after reperfusion. A: IDPc expression was determined by Western blot analysis using anti-IDPc antibody. Antibody against β-actin was used as an equal loading marker. B: densities of blots were quantified with the Lab Works program (n = 4). IDPc activity (C), NADPH/NADPt (D), GSSG/(GSH+GSSG) (E), and the level of total glutathione (F) were determined in the kidneys as described in materials and methods. Results are means ± SE (n = 6). OD, optical density.
Fig. 6.
Expression (A and B) and activity (C) of IDPc, and NADPH/NADPt (D) in cortex, OM, and IM of kidneys of mice subjected to either 30 min of bilateral ischemia or sham operation. Kidneys were harvested 24 h after reperfusion. A: IDPc expression was determined by Western blot analysis using anti-IDPc antibody. Antibody against β-actin was used as an equal loading marker. B: densities of blots were quantified with the Lab Works program (n = 3). IDPc activity (C) and NADPH/NADPt (D) were determined in cortex, OM, and IM of kidneys as described in materials and methods. Results are means ± SE (n = 3). NS, no significant difference. *P < 0.05 vs. respective cortex; #P < 0.05 vs. respective OM.
Fig. 7.
Levels of superoxide (A), H2O2 (B), and lipid peroxidation (C) in kidneys of mice subjected to either 30 min of bilateral ischemia or sham operation. Kidneys were harvested 4 and 24 h after surgery. A: superoxide formation was determined by conversion of nitro blue tetrazolium (NBT) to formazan (dark brown color). Levels of H2O2 (B) and lipid peroxidation (C) were determined in kidneys as described in materials and methods. Results are means ± SE (n = 6).
Effect of up- and downregulation of IDPc expression against oxidative stress in cultured tubular epithelial cells.
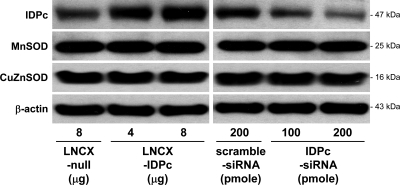
To evaluate the role of IDPc in oxidative stress, the levels of IDPc expression in LLC-PK1 cells, an established proximal tubular epithelial cell line, were increased or decreased by using the LNCX vector with IDPc (LNCX-IDPc) or the siRNA against IDPc (IDPc-siRNA), respectively; we then treated these cells with 1 mM H2O2 for 2 h. The transfection of LNCX-IDPc significantly increased IDPc expression compared with LNCX vector alone (LNCX-null) (Fig. 8). IDPc expression was significantly lower in LLC-PK1 cells transfected with IDPc-siRNA than in cells transfected with a scrambled siRNA (Fig. 8). To evaluate whether modulation of IDPc expression influenced other antioxidant enzymes, the levels of MnSOD, which is present in the mitochondria, and CuZnSOD, which is located in the cytoplasm, were determined in these transfected cells. The transfections of LNCX-IDPc or IDPc-siRNA did not influence the expression of either MnSOD or CuZnSOD compared with their respective controls (Fig. 8), indicating that expression of IDPc did not affect the expression of SOD enzymes.
Fig. 8.
Expressions of IDPc, manganese superoxide dismutase (MnSOD), and copper-zinc superoxide dismutase (CuZnSOD) in LLC-PK1 cells transfected with LNCX vector encoding mouse IDPc gene (LNCX-IDPc), LNCX vector alone (LNCX-null), small interference RNA (siRNA) against IDPc gene (IDPc-siRNA), or scrambled siRNA. Expression of IDPc, MnSOD, CuZnSOD, or β-actin as an equal protein loading marker was determined by Western blot analysis. Results are representative of experiments repeated 3 times.
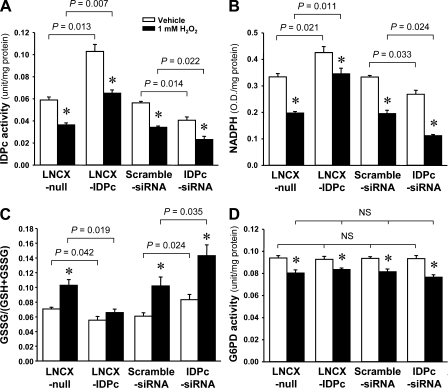
Consistent with IDPc protein expression, IDPc activity was significantly increased by LNCX-IDPc transfection compared with LNCX-null transfection (Fig. 9A). When cells were treated with 1 mM H2O2 for 2 h, IDPc activity was significantly decreased (Fig. 9A). Two hours after 1 mM H2O2 treatment, IDPc activity was significantly greater in LNCX-IDPc-transfected cells than in LNCX-null cells (Fig. 9A). IDPc activity after H2O2 treatment was significantly higher in the scrambled siRNA-transfected cells than in the IDPc-siRNA-transfected cells (Fig. 9A). NADPH levels were significantly higher in the LNCX-IDPc- and scrambled siRNA-transfected cells than in the LNCX-null- and IDPc-siRNA-transfected cells, respectively (Fig. 9B). NADPH levels after H2O2 treatment were significantly lower in LNCX-null- and IDPc-siRNA-transfected cells than in LNCX-IDPc- and scrambled siRNA-transfected cells, respectively (Fig. 9B). GSSG/(GSH+GSSG) levels were significantly lower in the LNCX-IDPc- and scrambled siRNA-transfected cells than in the LNCX-null- and IDPc-siRNA-transfected cells, respectively (Fig. 9C). GSSG/(GSH+GSSG) levels after H2O2 treatment were significantly lower in LNCX-null- and IDPc-siRNA-transfected cells than in LNCX-IDPc- and scrambled siRNA-transfected cells (Fig. 9C), indicating that IDPc regulated the ratio of antioxidant glutathione through NADPH production.
Fig. 9.
IDPc activity (A), NADPH production (B), GSSG/(GSH+GSSG) (C), and G6PD activity (D) in LLC-PK1 cells transfected with LNCX-IDPc, LNCX-null, IDPc-siRNA, or scrambled siRNA 2 h after 1 mM H2O2 treatment. LLC-PK1 cells were treated with either 1 mM H2O2 or vehicle. Two hours after treatments, IDPc and G6PD activities and NADPH and GSSG/(GSH+GSSG) levels were determined as described in materials and methods. Results are means ± SE (n = 6). *P < 0.05 vs. respective vehicle.
To evaluate whether up- or downregulation of IDPc expression influenced the activity of G6PD, which is another NADPH producer, we measured the activity of G6PD in LLC-PK1 cells transfected with LNCX-IDPc or IDPc-siRNA. Consistent with the IDPc expression shown in Fig. 8, transfection of LNCX-IDPc or IDPc-siRNA did not influence the activity of G6PD compared with their respective controls (Fig. 9D). Two hours after 1 mM H2O2 treatment, G6PD activity decreased in all experimental groups (Fig. 9D). The decreases of G6PD activities were not significantly different among the experimental groups (Fig. 9D), indicating that G6PD was not associated with IDPc expression.
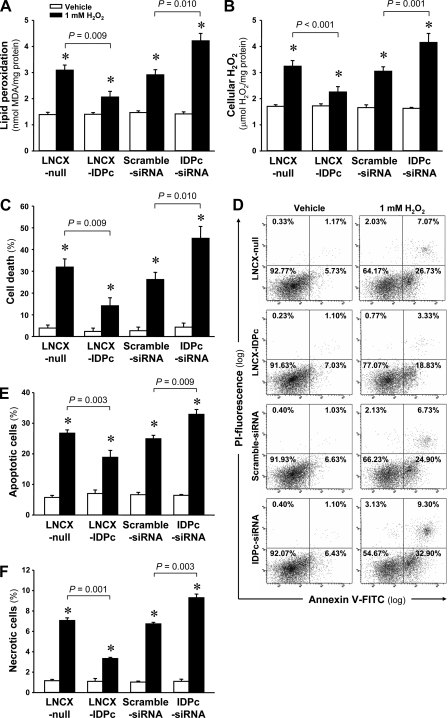
H2O2 treatment significantly increased lipid peroxidation (Fig. 10A). The increases in lipid peroxidation in the LNCX-IDPc-transfected cells were significantly less than those in LNCX-null-transfected cells (Fig. 10A). In contrast, IDPc-siRNA transfection resulted in significantly increased H2O2-induced lipid peroxidation compared with the cells transfected with scrambled siRNA (Fig. 10A). Cellular H2O2 levels significantly increased 2 h after H2O2 treatment (Fig. 10B). The increases of tissue H2O2 levels were significantly lower in LNCX-IDPc-transfected cells than those in LNCX-null-transfected cells (Fig. 10B). IDPc-siRNA transfection resulted in greater H2O2-induced lipid peroxidation compared with scrambled siRNA transfection (Fig. 10B).
Fig. 10.
Levels of lipid peroxidation (A), H2O2 production (B), cell death (C), flow cytometric analysis of annexin V-FITC/PI staining (D), and numbers of apoptotic cells (E) and necrotic cells (F) in LLC-PK1 cells transfected with LNCX-IDPc, LNCX-null, IDPc-siRNA, or scrambled siRNA 2 h after 1 mM H2O2 treatment. LLC-PK1 cells were treated with either 1 mM H2O2 or vehicle. Two hours after treatments, levels of lipid peroxidation (A) and H2O2 production (B) and cell death (C) were determined as described in materials and methods. Apoptotic cell (E) and necrotic cell (F) numbers were determined by flow cytometric analysis using annexin V-FITC positive/PI-negative cells and annexin V-FITC positive/PI-positive cells, respectively (D). Results are means ± SE (n = 6). *P < 0.05 vs. respective vehicle.
When cell viabilities were evaluated by Trypan blue exclusion, 1 mM H2O2 treatment significantly increased cell death (Fig. 10C). LNCX-IDPc transfection protected the cells against H2O2-induced cell death (Fig. 10C). In contrast, IDPc-siRNA transfection resulted in enhanced susceptibility to injury with H2O2 compared with scrambled siRNA-transfected cells (Fig. 10C). When apoptotic and necrotic cells were analyzed by flow cytometry with annexin V-FITC/PI staining, H2O2 treatment significantly induced both apoptosis and necrosis (Fig. 10, D–F). The increase of apoptosis in the LNCX-IDPc-transfected cells was significantly less than that in LNCX-null-transfected cells (Fig. 10E). In contrast, IDPc-siRNA transfection resulted in significantly increased H2O2-induced apoptosis compared with the cells transfected with scrambled siRNA (Fig. 10E). Consistent with the pattern of apoptosis, necrotic cells were significantly increased by LNCX-null and IDPc-siRNA transfection compared with LNCX-IDPc and scrambled siRNA transfection, respectively (Fig. 10F). These data indicate that IDPc expression and activation affect cell viability during oxidative stress through regulation of the cellular antioxidant capacity.
DISCUSSION
In this study we demonstrated that IDPc expression varies in different kidney regions and in different parts of the nephron. Kidney I/R decreases the activity and expression of IDPc, NADPH levels, and GSH levels. The levels of IDPc expression positively correlate with kidney epithelial cell resistance to oxidative stress. NADPH is used to convert not only GSSG to GSH, which is the most abundant antioxidant in mammalian cells, but also oxidized thioredoxin to reduced thioredoxin, which also plays a role in the antioxidant system (10, 44). At least four enzymes produce NADPH: G6PD, the key regulatory enzyme of the pentose phosphate pathway; phosphogluconate dehydrogenase; malate dehydrogenase; and ICDH (12). G6PD has long been regarded as the primary source of NADPH (44). The activities of these four enzymes in NADPH production differ among organs, however. Some studies have demonstrated that IDPc is responsible for more NADPH production than G6PD in liver and ovary (11, 45). In contrast, Frederiks et al. (12) reported that G6PD activity was ∼14-fold greater than that of ICDH in the adrenal gland. In this study we found that IDPc activity for NADPH production was 10–30 times higher than G6PD activity in various kidney regions, suggesting that IDPc is a major enzyme for the generation of NADPH in the kidney and plays a critical role in I/R injury. Jain and colleagues demonstrated (14) that reduction of NADPH by G6PD gene deficiency increased myocardial dysfunction after I/R in mice.
In a previous study, we demonstrated (18) that IDPc mRNA is highly expressed in the liver and kidney, suggesting that IDPc protein expression may be high in those organs. In the present study, we confirmed the presence of IDPc protein in kidney. In addition, the levels of IDPc expression differed in various kidney regions and tubules as summarized in Fig. 3U. Nephron segments of the kidneys possess remarkably different susceptibilities to I/R injury. S3 segment cells of the PT are most susceptible to I/R injury and oxidative stress (27, 35). IDPc expression was lower in the cells of S3 segments than in the cells of S1 and S2 segments. The different distribution of IDPc expression may provide an explanation for the different susceptibilities of kidney tubular cells to I/R injury. Interestingly, the pattern of IDPc expression was different in different segments of the nephron: IDPc was expressed diffusely in the cytoplasm in the PTs, thin limbs, and DT cells, whereas IDPc was expressed intensively in the apical and/or basolateral parts of the CNT and CD cells. This suggests that IDPc may play a different role in each tubular cell type and that the pattern of IDPc expression may serve as a marker to identify each tubule type. Further studies are required to fully explore the potential differential roles of IDPc in tubular segments.
I/R results in excessive ROS production and decreases of antioxidants (3, 8, 9). Recently we also reported (20) that I/R decreased the expression of antioxidant enzymes, and that administration of a MnSOD mimetic reduced renal I/R injury in mice. In the present study, I/R resulted in reduced IDPc expression and activity as early as 4 h after ischemia, when cell death and renal functional impairment were mild, as well as in later phases (24 h), when cell death and renal functional impairment were substantial (34), suggesting that the reduction of IDPc activity may be not only a result of I/R injury but also a cause. Since effective IDPc mimetics and genetically modified animals have not been developed, the effect of IDPc in animal studies could not be fully explored in this study. Thus we included in vitro studies using LLC-PK1 cells, an established epithelial cell line with characteristics of the PT, in the present investigation. Upregulation of IDPc protein increased NADPH production and decreased GSSG/(GSH+GSSG). A number of data have demonstrated that GSH reduces kidney cellular damage against oxidative stress (29, 32). In our present study, upregulation of IDPc reduced the increases of lipid peroxidation and cellular H2O2 levels and cell death including apoptosis and necrosis. Downregulation of IDPc protein presented results contrasting with the upregulation, indicating that IDPc overexpression protected cells from oxidative stress by increasing the GSH in the kidney tubular epithelial cells. In a previous study, we reported (21, 26) that IDPc upregulation in mouse embryonic fibroblasts reduced cell susceptibility to H2O2 and menadione. In a subsequent study, we found (17) that IDPc upregulation in fibroblasts reduced the cells’ susceptibility to irradiative stress. Thus IDPc expression controls the cell fate of nonepithelial cells as well as renal epithelial cells.
Numerous animal experiments have demonstrated that antioxidant treatments significantly reduce renal I/R damage (20, 25, 39). Antioxidant therapeutics have also been used in patients (2, 4). Since cellular antioxidants and antioxidant enzymes act in a concerted manner, multiple treatments using different kinds of antioxidants and antioxidant enzymes have been suggested for antioxidant therapy (24, 30). In the present study, changes in IDPc gene expression did not affect the levels of other antioxidant enzymes such as CuZnSOD and MnSOD, suggesting that treatment with an IDPc mimetic in combination with other antioxidants may exert a synergistic effect against oxidative stress-related diseases.
In conclusion, our results demonstrate that IDPc is a major enzyme for NADPH generation in kidney epithelial cells and is reduced in expression in renal I/R injury. Reduction of IDPc expression in vitro increases the susceptibility of epithelial cells to oxidant injury, whereas increased expression is protective against oxidant injury. Furthermore, reduced IDPc in S3 segment cells of the PT may contribute to that segment's increased susceptibility to I/R-induced injury. By recognizing the importance of IDPc and considering approaches to enhance cellular levels we suggest a new strategy for the development of therapeutic agents for AKI.
GRANTS
This work was supported by the Stem Cell Research Program of the Ministry of Science and Technology (M10641450001-06N4145-00110 and 2007 Korean Society of Nephrology CHOONGWAE grant to K. M. Park). J. V. Bonventre is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-39773, DK-72381, and DK-74099.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akerboom TP, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol 77: 373–382, 1981. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D, Dhawan A, Yardley-Jones A, Ioannides C, Webb J. Effect of antioxidant flavonoids and a food mutagen on lymphocytes of a thalassemia patient without chelation therapy in the Comet assay. Teratog Carcinog Mutagen 21: 165–174, 2001. [PubMed] [Google Scholar]
- 3.Aragno M, Cutrin JC, Mastrocola R, Perrelli MG, Restivo F, Poli G, Danni O, Boccuzzi G. Oxidative stress and kidney dysfunction due to ischemia/reperfusion in rat: attenuation by dehydroepiandrosterone. Kidney Int 64: 836–843, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Barquist E, Kirton O, Windsor J, Hudson-Civetta J, Lynn M, Herman M, Civetta J. The impact of antioxidant and splanchnic-directed therapy on persistent uncorrected gastric mucosal pH in the critically injured trauma patient. J Trauma 44: 355–360, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem 279: 47939–47951, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bhan AK, Schneeberger EE, Baird LG, Collins AB, Kamata K, Bradford D, Erikson ME, McCluskey RT. Studies with monoclonal antibodies against brush border antigens in Heymann nephritis. Lab Invest 53: 421–432, 1985. [PubMed] [Google Scholar]
- 7.Bonventre JV Mechanisms of ischemic acute renal failure. Kidney Int 43: 1160–1178, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Cooper AJ, Pulsinelli WA, Duffy TE. Glutathione and ascorbate during ischemia and postischemic reperfusion in rat brain. J Neurochem 35: 1242–1245, 1980. [DOI] [PubMed] [Google Scholar]
- 9.Dobashi K, Ghosh B, Orak JK, Singh I, Singh AK. Kidney ischemia-reperfusion: modulation of antioxidant defenses. Mol Cell Biochem 205: 1–11, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Flamigni F, Marmiroli S, Caldarera CM, Guarnieri C. Involvement of thiol transferase- and thioredoxin-dependent systems in the protection of “essential” thiol groups of ornithine decarboxylase. Biochem J 259: 111–115, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flint AP, Denton RM. The role of nicotinamide-adenine dinucleotide phosphate-dependent malate dehydrogenase and isocitrate dehydrogenase in the supply of reduced nicotinamide-adenine dinucleotide phosphate for steroidogenesis in the superovulated rat ovary. Biochem J 117: 73–83, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frederiks WM, Kummerlin IP, Bosch KS, Vreeling-Sindelarova H, Jonker A, Van Noorden CJ. NADPH production by the pentose phosphate pathway in the zona fasciculata of rat adrenal gland. J Histochem Cytochem 55: 975–980, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Criado FJ, Eleno N, Santos-Benito F, Valdunciel JJ, Reverte M, Lozano-Sanchez FS, Ludena MD, Gomez-Alonso A, Lopez-Novoa JM. Protective effect of exogenous nitric oxide on the renal function and inflammatory response in a model of ischemia-reperfusion. Transplantation 66: 982–990, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, Loscalzo J, Apstein CS, Liao R. Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation 109: 898–903, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Jang HS, Kim J, Park YK, Park KM. Infiltrated macrophages contribute to recovery after ischemic injury but not to ischemic preconditioning in kidneys. Transplantation 85: 447–455, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Jennings GT, Stevenson PM. A study of the control of NADP+-dependent isocitrate dehydrogenase activity during gonadotropin-induced development of the rat ovary. Eur J Biochem 198: 621–625, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Jo SH, Lee SH, Chun HS, Lee SM, Koh HJ, Lee SE, Chun JS, Park JW, Huh TL. Cellular defense against UVB-induced phototoxicity by cytosolic NADP+-dependent isocitrate dehydrogenase. Biochem Biophys Res Commun 292: 542–549, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Jo SH, Son MK, Koh HJ, Lee SM, Song IH, Kim YO, Lee YS, Jeong KS, Kim WB, Park JW, Song BJ, Huh TL. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J Biol Chem 276: 16168–16176, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation—the two keystones of ischemia/reperfusion injury. Int J Cardiol 86: 41–59, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Kil IS, Seok YM, Yang ES, Kim DK, Lim DG, Park JW, Bonventre JV, Park KM. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J Biol Chem 281: 20349–20356, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kim SY, Lee SM, Tak JK, Choi KS, Kwon TK, Park JW. Regulation of singlet oxygen-induced apoptosis by cytosolic NADP+-dependent isocitrate dehydrogenase. Mol Cell Biochem 302: 27–34, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Kim SY, Park JW. Cellular defense against singlet oxygen-induced oxidative damage by cytosolic NADP+-dependent isocitrate dehydrogenase. Free Radic Res 37: 309–316, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Koh HJ, Lee SM, Son BG, Lee SH, Ryoo ZY, Chang KT, Park JW, Park DC, Song BJ, Veech RL, Song H, Huh TL. Cytosolic NADP+-dependent isocitrate dehydrogenase plays a key role in lipid metabolism. J Biol Chem 279: 39968–39974, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Kriz J, Gowing G, Julien JP. Efficient three-drug cocktail for disease induced by mutant superoxide dismutase. Ann Neurol 53: 429–436, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Land W, Schneeberger H, Schleibner S, Illner WD, Abendroth D, Rutili G, Arfors KE, Messmer K. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation 57: 211–217, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Lee SM, Koh HJ, Park DC, Song BJ, Huh TL, Park JW. Cytosolic NADP+-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med 32: 1185–1196, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol Renal Physiol 275: F623–F631, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Lui SL, Chan LY, Zhang XH, Zhu W, Chan TM, Fung PC, Lai KN. Effect of mycophenolate mofetil on nitric oxide production and inducible nitric oxide synthase gene expression during renal ischaemia-reperfusion injury. Nephrol Dial Transplant 16: 1577–1582, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Mandel LJ, Schnellmann RG, Jacobs WR. Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest 85: 316–324, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanobashvili J, Neumayer C, Fuegl A, Punz A, Blumer R, Mittlbock M, Prager M, Polterauer P, Dobrucki LW, Huk I, Malinski T. Combined l-arginine and antioxidative vitamin treatment mollifies ischemia-reperfusion injury of skeletal muscle. J Vasc Surg 39: 868–877, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Paller MS, Patten M. Protective effects of glutathione, glycine, or alanine in an in vitro model of renal anoxia. J Am Soc Nephrol 2: 1338–1344, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem 276: 11870–11876, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Park KM, Cho HJ, Bonventre JV. Orchiectomy reduces susceptibility to renal ischemic injury: a role for heat shock proteins. Biochem Biophys Res Commun 328: 312–317, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 279: 52282–52292, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem 277: 2040–2049, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, Becker TC, Sherry AD, Newgard CB, Jensen MV. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem 281: 30593–30602, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Sabolic I, Herak-Kramberger CM, Breton S, Brown D. Na/K-ATPase in intercalated cells along the rat nephron revealed by antigen retrieval. J Am Soc Nephrol 10: 913–922, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Schmidtmann S, Muller M, von Baehr R, Precht K. Changes of antioxidative homeostasis in patients on chronic haemodialysis. Nephrol Dial Transplant 6, Suppl 3: 71–74, 1991. [PubMed] [Google Scholar]
- 40.Seok YM, Kim J, Choi KC, Yoon CH, Boo YC, Park Y, Park KM. Wen-pi-tang-Hab-Wu-ling-san attenuates kidney ischemia/reperfusion injury in mice. A role for antioxidant enzymes and heat-shock proteins. J Ethnopharmacol 112: 333–340, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Stanton RC, Seifter JL. Epidermal growth factor rapidly activates the hexose monophosphate shunt in kidney cells. Am J Physiol Cell Physiol 254: C267–C271, 1988. [DOI] [PubMed] [Google Scholar]
- 42.Sun L, Sun TT, Lavker RM. Identification of a cytosolic NADP+-dependent isocitrate dehydrogenase that is preferentially expressed in bovine corneal epithelium. A corneal epithelial crystallin. J Biol Chem 274: 17334–17341, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Sztajer H, Gamain B, Aumann KD, Slomianny C, Becker K, Brigelius-Flohe R, Flohe L. The putative glutathione peroxidase gene of Plasmodium falciparum codes for a thioredoxin peroxidase. J Biol Chem 276: 7397–7403, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Thon M, Al-Abdallah Q, Hortschansky P, Brakhage AA. The thioredoxin system of the filamentous fungus Aspergillus nidulans: impact on development and oxidative stress response. J Biol Chem 282: 27259–27269, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Veech RL, Eggleston LV, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem J 115: 609–619, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar-ATPase. Physiol Rev 84: 1263–1314, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Wetzel RK, Sweadner KJ. Immunocytochemical localization of Na-K-ATPase alpha- and gamma-subunits in rat kidney. Am J Physiol Renal Physiol 281: F531–F545, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Yang T, Huang YG, Singh I, Schnermann J, Briggs JP. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 271: F931–F939, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Zerez CR, Lee SJ, Tanaka KR. Spectrophotometric determination of oxidized and reduced pyridine nucleotides in erythrocytes using a single extraction procedure. Anal Biochem 164: 367–373, 1987. [DOI] [PubMed] [Google Scholar]