Abstract
Calcineurin is an important intracellular signaling molecule which can be inhibited by cyclosporin resulting in immune suppression and nephrotoxicity. Previously, we reported that homozygous loss of the α isoform of calcineurin impairs kidney development and function and mimics many features of cyclosporin nephrotoxicity. However, early lethality of null mice prevented further study of renal changes. Alternatively, we examined aged heterozygous (CnAα+/−) mice. In addition to renal dysfunction and inflammation, we find that CnAα+/− mice spontaneously develop tertiary lymphoid aggregates in the kidney, small intestine, liver, and lung. Lymphoid aggregates contain both T cells and B cells and exhibited organization suggestive of tertiary lymphoid organs (TLOs). Kidney function and TLO formation were highly correlated suggesting that this process may contribute to nephrotoxicity. Consistent with previous findings, transforming growth factor (TGF)-β is significantly increased in CnAα+/− mice. Neutralization of TGF-β attenuated TLO formation and improved kidney function. In conclusion, we report that haploinsufficiency of CnAα causes uregulation of TGF-β which contributes to chronic inflammation and formation of TLOs. While the process that leads to TLOs formation in transplant allografts is unknown, TLOs are associated with poor clinical prognosis. This study suggests that calcineurin inhibition itself may lead to TLO formation and that TGF-β may be a novel therapeutic target.
Keywords: calcineurin inhibition, nephrotoxicity, posttransplant lymphoproliferative disorder
the serine/threonine phosphatase calcineurin has been implicated in the normal development and function of a variety of systems. In addition to perhaps the best-known calcineurin action downstream of the T cell receptor (20), calcineurin has been shown to be involved in skeletal muscle and cardiac hypertrophy (3, 18), trafficking of neuronal vesicles (6, 15), and renal development (8, 12). Posttransplant drug regimens include calcineurin inhibitors to suppress the immune system but use of drugs such as cyclosporin and FK506 is frequently complicated by side effects including nephrotoxicity, hypercholesterolemia, diabetes, gingival hyperplasia, and, less often, skin cancers and posttransplant lymphoproliferative disorder. It is clear from these diverse effects that calcineurin plays substantial roles in many systems.
The catalytic subunit of calcineurin has three isoforms-α, β, and γ-which may contribute to different aspects of the enzyme's function (20). For example, loss of the β isoform leads to perturbation of T cell development within the thymus leading to fewer mature T cells in the periphery (2). In contrast, loss of the α isoform does not affect immune system development and only mildly alters T cell response to antigen stimulation (26). Interestingly, T cells of CnAα−/− mice can still be inhibited by cyclosporin (26), whereas CnAβ null mice tolerate grafts comparable to cyclosporin-treated animals (2). On the other hand, loss of the α isoform impairs nephrogenesis while kidney development in β null mice is normal (12). These results demonstrate that the α and β isoforms contribute unique functions which may have tremendous clinical significance.
Previously, we showed that loss of the α isoform is sufficient to reproduce many features of cyclosporin nephrotoxicity including matrix deposition, arterial changes, and increased transforming growth factor (TGF)-β production (11). Together, these findings suggest a state of chronic inflammation with loss of the α isoform that is associated with declining renal function in null mice. The basis for these changes is still an area of active investigation. However, in vitro data show that CnAα−/− renal fibroblasts have dramatically increased fibronectin and TGF-β production, suggesting that calcineurin is required for regulation of these factors on a cellular level (11). Interestingly, fibroblasts that lack the α isoform retain normal regulation of the calcineurin substrate NFATc. In contrast, CnAβ−/− fibroblasts have normal matrix and TGF-β expression but no longer regulate NFATc (11). These findings are consistent with dual roles for calcineurin isoforms with the β acting predominantly to regulate the transcription factor NFATc, which is of particular importance in T cells, and the α isoform contributing to normal expression of TGF-β and matrix proteins in organs including the kidney.
Further characterization of CnAα mice, however, is complicated by early lethality in the majority of null pups. To further understand the unique role of the α isoform, we examined mice expressing only one copy of the isoform (CnAα+/−) up to 1 year of age. In this study, we report that aged CnAα+/− mice display spontaneous lymphoid neogenesis in multiple organs and that these areas of lymphoproliferation organize into tertiary lymphoid organs (TLOs). Consistent with our previous work, we find that TGF-β is a key mediator of the effects attributable to loss of calcineurin Aα. TGF-β is dramatically overexpressed in aged CnAα+/− mice and neutralization of the cytokine resolved development of TLOs and improved renal function. These data illustrate a new role for calcineurin Aα and TGF-β in chronic inflammation and the initiation of lymphoid neogenesis.
MATERIALS AND METHODS
Animal Model
Calcineurin Aα (CnAα) knockout mice were created by J. Seidman as previously described (26) and provided to our laboratory. Mice were maintained and bred at the Atlanta Veterans Affairs Medical Center. All procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee. CnAα mice were created on mixed genetic background. Therefore, all experiments were carried out with littermate controls. TGF-β neutralizing antibody (100 μg/kg body wt diluted in PBS) was administered to groups of CnAα+/− mice by intraperitoneal injection three times per week from 18 to 24 wk of age. As controls, CnAα+/− and wild-type (WT) littermates were injected with PBS alone or 100 μg/ml nonspecific IgG. Whole blood and serum were collected at the time of death for analyses. Blood urea nitrogen was determined using a Reflotron analyzer (Rosche Diagnostics) and blood count and white blood cell differentials were obtained using a Beckman Coulter Counter.
Histology
Kidneys were immersed in 10% neutral buffered formalin or quick frozen in liquid nitrogen for further analyses. Immunohistochemistry and immunofluorescence were carried out as previously described (10, 12).
Cytokine Analyses
TGF-β.
Serum and kidney TGF-β levels were determined using a commercially available ELISA kit (Promega, Madison, WI) according to the manufacturer's instructions.
Cytokine array.
Cytokine array analyses were performed using a Panomics Cytokine array (Fremont, CA). Briefly, 100 μl of serum from 2 different mice of the same genotype were pooled and incubated with the provided membrane overnight. The membranes were then washed, and cytokine binding was detected using chemiluminescence. Results were analyzed by densitometry and then normalized to internal controls. Each experiment was repeated at least twice with different sets of pooled serum samples and final results were combined data for the experimental group normalized to the control group to determine the percent change.
Western Blot
Whole kidneys were minced, homogenized, and then lysed in TNESV buffer (50 mM Tris·HCl, pH 7.4, 2 mM EDTA, 1% NP-40, 100 mM NaCl, 100 mM Na orthovanadate, 100 μg/ml leupeptin, 20 μg/ml aprotonin, and 10−7 M phenylmethylsulfonyl) (5, 12). Fifty micrograms of total protein were separated by 10% SDS-PAGE, transferred to nitrocellulose, and blocked in 5% milk. Protein expression was examined by incubating membranes with 1:1,000 dilutions of specific primary antibody followed by washing and then incubation with 1:10,000 dilutions of horseradish peroxidase-conjugated secondary antibody. Proteins were detected by enhanced chemiluminescence and autoradiography. Resulting bands were semiquantitated and graphed.
Flow Cytometry
Lymphocytes were harvested from the kidney as previously described (16) and splenocytes were harvested as previously described (23). Isolated lymphocytes from either the kidney or spleen were fixed, permeabilized, and then labeled using anti-mouse CD45 and CD3, CD19, or CD11b (BD Biosciences, San Jose, CA). Flow cytometry was performed on a FACS Caliber (BD Biosciences) and results were analyzed using CellQuest software (BD Biosciences).
Statistics
All analyses were performed using GraphPad software (Prism, La Jolla, CA) using the appropriate tests as indicated. Results were considered significant if P < 0.05.
RESULTS
Mice Heterozygous for CnAα Develop TLOs
The two main isoforms of the catalytic subunit of calcineurin-α and β-are involved in diverse cellular processes and may have unique functions. In particular, the α isoform is required for normal kidney development and function (12) and CnAα−/− mice share many features with cyclosporin nephrotoxicity (11). However, early lethality in the majority of CnAα−/− mice has prevented long-term studies of the effect of the α isoform on renal function. To further understand the unique role of the α isoform, we examined mice expressing only one functional copy of the isoform (CnAα+/−) up to 1 year of age. Kidneys from WT, 6-mo-old CnAα+/−, and 1-yr-old CnAα+/− mice were collected, fixed in paraffin, stained with hematoxylin and eosin, and examined by light microscopy. WT sections displayed a normal histology (Fig. 1, A and D) while areas of focal lymphocyte infiltrations were observed in 6-mo-old animals (arrow, Fig. 1, B and E) and multiple large areas of infiltration were apparent in kidney sections from 1-yr-old animals (Fig. 1, C and E). Lesions appear first in the outer stripe of the outer medulla (OSOM), which is also the region that is most severely attenuated in a dose-dependent manner by loss of the α isoform (12). In the 1-yr samples, the OSOM appears increasingly abnormal and contains a large volume of infiltrating cells. In addition, in samples from 1-yr-old mice focal lesions are also found in the cortex. Areas of infiltration were not observed in the inner medullas in either age group. These findings suggest that reduced CnAα activity associated with haploinsufficiency results in a novel phenotype of spontaneous lymphocyte infiltration. Despite reported changes in T cell development (26), examination of CnAβ+/− and CnAβ−/− mice did not reveal a similar disorder. Moreover, CnAα/Aβ double heterozygous mice were comparable to CnAα+/−, suggesting that loss of one copy of the β isoform is not additive to loss of the α allele (data not shown).
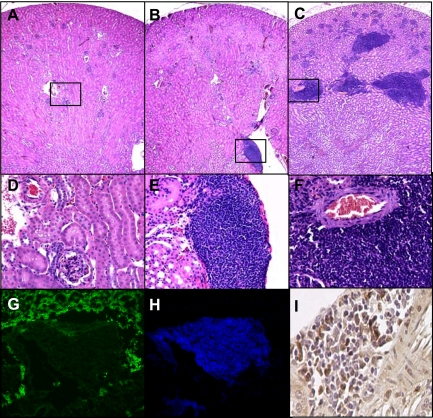
Fig. 1.
Infiltration of lymphocytes in kidneys of aged CnAα+/− mice corresponds to declining renal function. Kidney sections were obtained from wild-type (WT; A and D), CnAα+/− at 6 mo of age (B and E), and CnAα+/− at 1 yr of age (C and F). Sections were stained with hematoxylin and eosin (H&E) and examined by light microscopy. Original magnification was ×4 (A–C) and ×20 (D–F). Data shown are representative of at least 12 mice per group. Frozen kidney sections from 1-yr-old CnAα+/− mice were examined by immunofluorescence with antibodies that recognize E-cadherin (G) and CD45 (H). Images shown were obtained by confocal microscopy; original magnification was ×40. I: proliferation of infiltrating lymphocytes was assessed by detection of proliferating cell nuclear antigen (PCNA) expression in renal sections by immunohistochemistry. Data shown are representative PCNA expression in a renal section from a 1-yr-old CnAα+/− mouse. Original magnification was ×40. Data shown in G–I are representative of at least 6 mice per group.
Next, infiltrating cells were confirmed to be lymphocytes by double immunofluorescence labeling of kidney sections with E-cadherin and CD45 to identify epithelial cells and lymphocytes, respectively. A representative area of dense cellular infiltration stained only with CD45 while the surrounding tissue was positive for E-cadherin (Fig. 1, G and H). In addition, similar areas were found to contain numerous proliferating cell nuclear antigen (PCNA)-positive cells, suggesting that the infiltrating lymphocytes are actively proliferating (Fig. 1I).
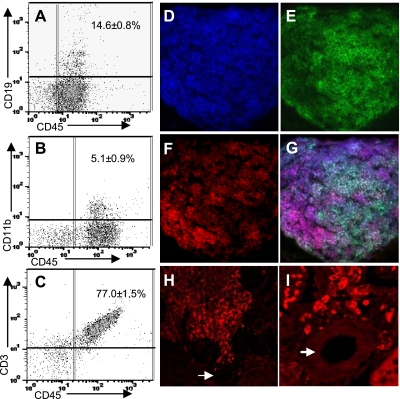
To further characterize the type of immune cells present in the kidneys of CnAα+/− mice, lymphocytes were isolated from whole kidneys of 1-yr-old mice and then labeled with CD45 along with CD19, CD11b, and CD3 to identify B cells, polymorphonuclear (PMN) leukocytes/macrophages, and T cells, respectively. Labeled cells were then analyzed by flow cytometry and quantitated. Fifteen percent of the whole kidney immune cells were B cells, 5% PMN/macrophages, and 77% were T cells (Fig. 2, A–C). Triple immunofluorescence staining and confocal microscopy were used to further characterize a typical focal lymphoproliferative site. The entire lesion again demonstrated uniform expression of CD45 (Fig. 2D). The area contained both B220-positive cells (Fig. 2E) and CD3-positive lymphocytes (Fig. 2F). A merged image indicates that the lymphocytes are generally arranged with a core of B cells surrounded by T cells at the periphery of the aggregate (Fig. 2G). This pattern indicates the formation of an organized lymphoid structure and is consistent with a TLO.
Fig. 2.
Areas of lymphoid proliferation are organized into tertiary lymphoid organs (TLOs). Lymphocytes were harvested from CnAα+/− kidneys as described (16), cells were labeled with CD45 along with CD3 (A), CD19 (B), or CD11b (C), and then quantitated by flow cytometry. Graphs shown are representative of 4 mice with means ± SE of the combined group indicated in each panel. D–G: single lymphoid aggregate was examined by immunofluorescence and confocal imaging following triple staining with CD45 (D), B220 (E), and CD3 (F). All 3 images are shown merged in G. Original magnification was ×60. Data shown are representative of at least 3 different animals. H–I: expression of the lymphatic marker, LYVE-1, was examined in CnAα+/− kidney sections by immunofluorescence. H: typical result from 4 different mice at ×20 magnification. Arrow indicates a renal vessel. I: same area as H at ×40 magnification showing the perivascular region.
TLOs have been described to form in response to chronic inflammation and arise from cells that migrate out of the vasculature and commence expression of lymphatic cell markers (21). Therefore, we examined expression of the lymphatic marker LYVE-1 (17) in CnA kidney sections by immunofluorescence. Figure 2H shows that LYVE-1-expressing cells can be identified in areas of TLO formation. Moreover, the highest level of expression occurs in cells closest to a vessel (arrow) and declines further away. A higher magnification confirms that LYVE-1-positive cells are located in close proximity with the vasculature (Fig. 2I).
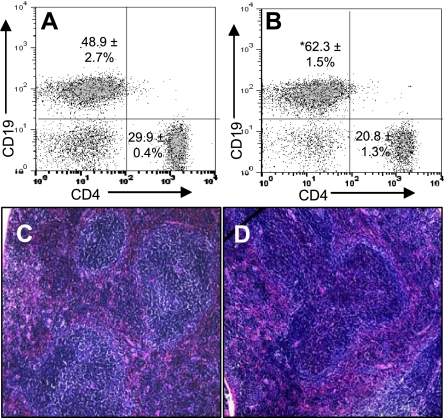
A key distinguishing feature of TLO formation is the expansion of B cells (21). Therefore, to further characterize lymphocyte changes in aged CnAα+/− mice, spleens were harvested from 1-yr-old WT mice and CnAα+/− littermates and the distribution of CD19-positive and CD3-positive cells was measured (Fig. 3, A and B). There was a statistically significant increase in B cells from 48.9 ± 2.7 to 62.3 ± 1.5% and a corresponding decrease in T cells (which did not reach statistical significance). Histological examination of spleens from WT and CnAα+/− mice supported the expansion of B cells with haploinsufficiency of CnAα. Figure 3, C and D, demonstrates changes in the architecture of the follicles in CnAα+/− spleen sections compared with WT littermates. Finally, whole blood was collected and circulating granulocytes, monocytes, and total lymphocytes were counted in CnAα+/− and WT littermate mice; there were no differences between the two groups (data not shown), suggesting that changes in lymphocytes do not involve peripheral cells.
Fig. 3.
Evidence of B cell expansion in CnAα+/− spleens. Spleenic lymphocytes were isolated from WT (A) and aged CnAα+/− (B) animals and CD19/CD3-positive cells were quantitated. Graphs shown are representative of 4 mice with means ± SE of the combined group indicated in each panel. *P < 0.05 compared with WT, Student's t-test. C–D: spleens from WT (shown in C) and CnAα+/− mice (D) were examined by light microscopy following H&E staining. Data shown are representative of at least 8 animals per genotype. Original magnification was ×20.
Development of TLOs Coincides with Declining Renal Function and Involves Multiple Organs
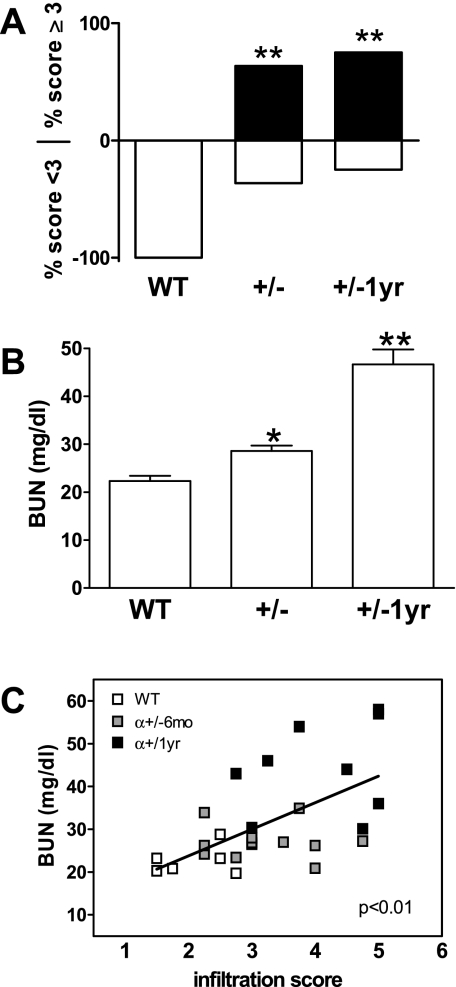
The frequency of TLO formation in CnAα+/− mice was assessed by first establishing a scoring system from 1 to 5 and then blindly rating multiple tissue sections from CnAα+/− and WT littermate animals (Supplemental Data 1; the online version of this article contains supplemental data). This system was employed to score the degree of infiltration and the frequency of TLOs at 6 mo and 1 yr of age. Scores of 1 or 2 corresponded to minimal presence of lymphocytes in a diffuse pattern. A score of 3 was used in the case of frequent but diffuse lymphocyte infiltration while scores of 4 or 5 indicated at least 1 organized TLO or multiple TLOs, respectively. Figure 4A shows that the phenotype is highly penetrate with 62% of 6-mo-old animals receiving a score of “severe” infiltration (≥3) at 6 mo of age and a further increase to 75% by 1 yr. While lymphocytes were commonly observed in normal WT kidneys, all WT animals received a score of less than 3.
Fig. 4.
Quantitation of lymphoproliferative disorder and renal function. A: infiltration of lymphocytes was quantitated by 2 blinded reviewers scoring each section on a scale from 1–5. Data shown are the percentage of each group that received a score <3 or ≥3. **P < 0.01, Fisher's exact test. B: blood urea nitrogen (BUN) levels were determined for WT and CnAα+/− mice at 6 mo and 1 yr of age. Data shown are means of 6 WT and 12 CnAα+/− mice at each age. **P < 0.01, ANOVA. C: BUN and infiltration scores were compared by linear regression for 6 WT (open squares) and 12 CnAα+/− mice at each age (gray squares are 6-mo-old mice and filled squares are 1-yr-old mice). r2 = 0.307 and P < 0.01.
Loss of the α isoform is known to impair renal function (11, 12). Therefore, we examined renal function in 6-mo- and 1-yr-old CnAα+/− mice and determined whether there was a correlation to development of TLOs. Figure 4B shows that there is a small but significant increase in blood urea nitrogen (BUN) at 6 mo (*P < 0.05, ANOVA) and a substantial increase in BUN at 1 yr in CnAα+/− mice (**P < 0.01, ANOVA), suggesting that renal function progressively declines. Interestingly, when BUN values were compared with lymphocyte infiltration scores for each animal, there was a significant correlation as determined by linear regression (r2 = 0.307, P < 0.01), indicating that impaired renal function and development of TLOs may be related (Fig. 4C).
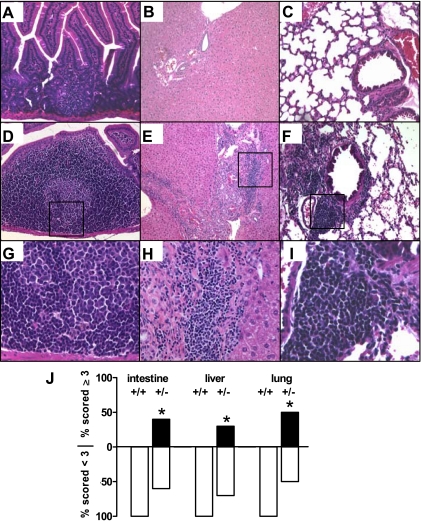
We next sought to determine whether development of TLOs was restricted to the kidney or involved other organs. Figure 5 shows that abnormal lymphocyte aggregations in CnAα+/− mice are wide-spread and can be found in expansion of existing isolated lymphoid follicles in the small intestine (Fig. 5A compared with and D and G), as well as in the liver (Fig. 5B compared with E and H), and lung (Fig. 5C compared with F and I) of 6-mo-old WT and CnAα+/− mice. The same scoring and quantification method as described for the kidney was employed and Fig. 5J shows that there is a significant increase in the percentage of animals demonstrating development of TLOs (score ≥3) in the intestine, liver, and lung at 1 yr of age (*P < 0.05, χ2 analysis). CD45 and PCNA staining was again performed and confirmed that the observed areas of infiltration are proliferating lymphocytes (data not shown).
Fig. 5.
Multiple organs in aged CnAα+/− mice show evidence of lymphoid neogenesis and TLO formation. Small intestine (A, D, and G), liver (B, E, and H), and lung (C, F, and I) samples were obtained from 6-mo-old WT mice (A–C) and CnAα+/− littermates (D–F and G–I). Sections were stained with H&E and examined by light microscopy. Original magnification was ×20. Data shown are representative of sections from at least 12 different mice. J: lymphocyte infiltration was scored by 2 blinded reviewers. Data shown are the percentage of WT and CnAα+/− mice that received a score of <3 or ≥3. *P < 0.05, χ2 analysis.
Formation of TLOs in CnAα+/− Mice Is Associated with Upregulation of TGF-β and Matrix Accumulation
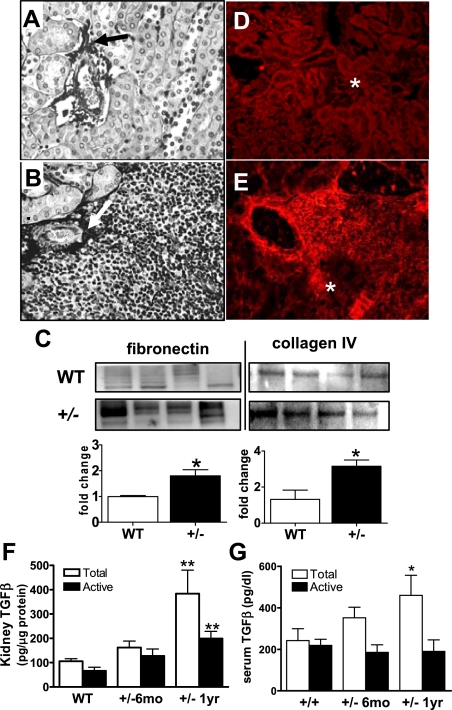
TLOs have been suggested to occur in the kidney as a result of chronic inflammation and accumulation of matrix deposition (21). Previously, we showed that homozygous loss of CnAα results in increased matrix production and overexpression of TGF-β in vivo and in cultured fibroblasts in vitro (11). We therefore examined both matrix deposition and TGF-β expression in kidneys from 1-yr-old CnAα+/− mice. Matrix accumulation was examined by silver stain and demonstrated areas of dense matrix accumulation in perivascular regions associated with lymphoid neogenesis in CnAα+/− samples (Fig. 6, B compared with A). More specifically, Western blotting of whole kidney lysates revealed significant increases in both fibronectin and collagen IV in CnAα+/− samples compared with WT littermates (Fig. 6C). Next, Fig. 6E shows that sections from CnAα+/− kidneys demonstrated areas of strong TGF-β expression in conjunction with infiltrating immune cells; these features are not present in WT sections (Fig. 6D). Moreover, total TGF-β expression is also slightly elevated in kidney lysates from 6-mo-old CnAα+/− mice and is significantly increased at 1 yr (**P < 0.01, ANOVA; Fig. 6F). Active TGF-β follows a similar trend and reaches significance in 1-yr-old animals. Both increased matrix depositions and TGF-β expression were confirmed in other organs (data not shown). Similarly, serum samples of CnAα+/− mice show increased levels of TGF-β over time (Fig. 6G). At 6 mo, there was a slight increase in total TGF-β and at 1 yr a significant upregulation (**P < 0.01, ANOVA). In contrast to kidney values, active TGF-β remained unchanged in aged CnAα+/− mice.
Fig. 6.
Increased matrix deposition and transforming growth factor (TGF)-β expression with loss of CnAα+/−. Matrix deposition was visualized by silver stain in kidney sections from WT (A) and CnAα+/− mice (B). The arrow indicates an area of increased deposition in the perivascular region. Data shown are representative of at least 6 different animals. C: specific matrix proteins were quantified by Western blotting of kidney lysates from 4 WT and 4 CnAα+/− mice. Results for fibronectin and collagen IV were semiquantitated and graphed. *P < 0.05, Student's t-test. TGF-β expression was examined in frozen kidney sections from WT (D) and CnAα+/− animals (E) by immunofluorescence with a specific antibody. Data shown are representative of at least 6 different animals. Glomeruli (*) are indicated as positive controls for TGF-β expression. F: proteins were extracted from frozen kidney samples in WT, 6-mo-, and 1-yr-old CnAα+/− mice and then total and active TGF-β levels were measured by ELISA. Data shown are means ± SE of 6–10 mice per group (**P < 0.01, 2-way ANOVA). G: serum was collected from WT, 6-mo-, and 1-yr-old CnAα+/− mice and then total and active TGF-β levels were measured by ELISA. Data shown are means ± SE of 6–10 mice per group (*P < 0.05, 2-way ANOVA).
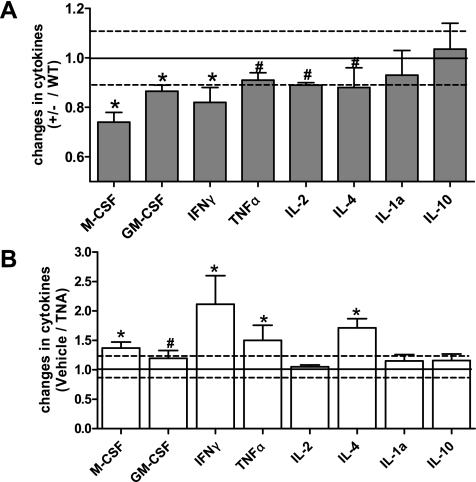
Data from aged CnAα+/− mice suggest that there is a state of chronic inflammation perhaps stemming from increased TGF-β and matrix accumulation in tissues. Therefore, to determine whether the increase in TGF-β seen in CnAα+/− mice contributed to the formation of TLOs or to declining renal function, 100 μg/kg body wt of anti-TGF-β neutralizing antibody was administered to CnAα+/− mice from 18 to 24 wk of age. To first examine the efficacy of TGF-β neutralization, serum samples from untreated CnAα+/− mice were collected, analyzed using a Panomics Cytokine Array, and results were compared with data obtained from WT mice. The results are summarized in Fig. 7A and indicate that a number of cytokines are altered with partial loss of CnAα. In particular, there is a greater than 10% decrease in M-CSF, GM-CSF, and IFN-γ. TNF-α, IL-2, and IL-4 are slightly decreased compared with WT (between 1 and 10%), whereas IL-1a and IL-10 are unchanged. Next, serum samples from anti-TGF-β neutralizing antibody (TNA)-treated CnAα+/− mice were obtained, similarly analyzed, and compared with vehicle-treated CnAα+/− animals. Figure 7B shows that neutralization of TGF-β reversed many of the trends observed in Fig. 7A and resulted in increases in M-CSF, IFN-γ, TNF-α, and IL-4.
Fig. 7.
Cytokine changes in CnAα+/− mice are reversed by TGF-β neutralizing antibody (TNA). A: expression of a panel of cytokines was determined for 6 WT and 6 CnAα+/− mice. Data shown are means ± SE of CnAα+/− values normalized by WT. *Mean change >10%. #Mean change 5–10%. B: expression of a panel of cytokines was examined in 6 vehicle-treated and 6 TNA-treated CnAα+/− animals. Data shown are means ± SE of TNA-treated mice normalized by the mean of vehicle-treated samples. * >10% change. # 1–10% change.
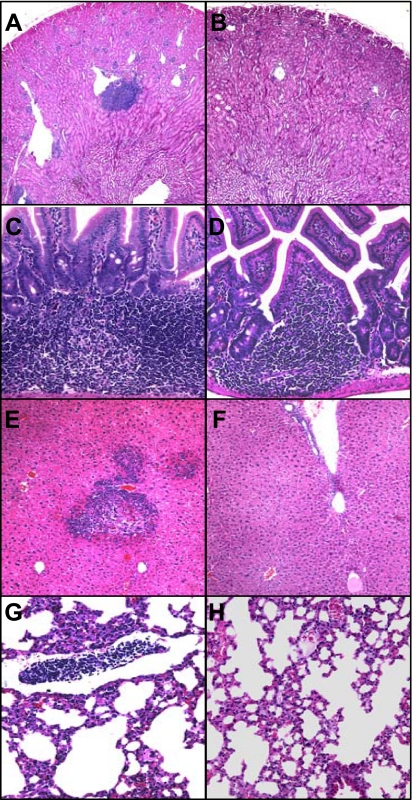
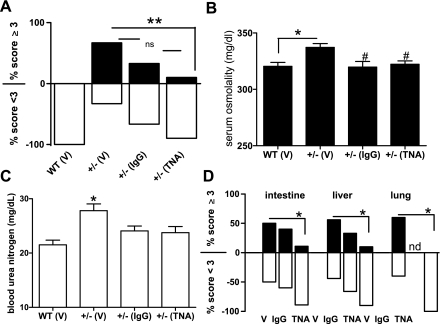
Next, TLO formation and kidney function were assessed in 24-wk-old TNA-treated CnAα+/− mice and compared with both vehicle-treated controls (PBS) and to nonspecific IgG-treated controls. TNA treatment decreased the appearance of TLOs in the kidney (Fig. 8, A and B), intestine (Fig. 8, C and D), liver (Fig. 8, E and F), and lung (Fig. 8, G and H). Furthermore, Fig. 9A shows that TNA treatment significantly reduced TLO formation in CnAα+/− mice from 67% with vehicle treatment to only 10% with TNA treatment (**P < 0.01, χ2 analysis). IgG treatment also reduced TLO formation but the change was not significant compared with vehicle treatment alone. Both TNA treatment and IgG treatment improved renal function in CnAα+/− mice. Figure 9B shows that BUN values in both treatment groups were no longer significantly different compared with WT mice (*P < 0.05, ANOVA). Similarly, serum osmolality was reduced with both TNA and IgG treatments (*P < 0.05 compared with WT; #P < 0.05 compared with vehicle-treated +/− mice, ANOVA; Fig. 9C). Finally, TLO formation was also significantly decreased in the liver, intestine, and lung with TNA treatment (Fig. 9D).
Fig. 8.
Appearance of TLOs is decreased with TNA treatment. CnAα+/− mice were treated with vehicle or neutralizing TGF-β antibody from 16–24 wk of age and then TLO formation was examined in the kidney (A and B), intestine (C and D), liver (E and F), and lung (G and H). Data shown in A, C, E, and G are vehicle-treated mice and are representative of at least 10 mice. Data shown in B, D, F, and H are TNA-treated and are representative of at least 10 mice. Original magnification in A and B was ×40; C–H were ×10.
Fig. 9.
TLO formation is reduced and renal function is improved by neutralizing TGF-β antibody. A: kidney sections from WT mice treated with vehicle (V; PBS) and CnAα+/− mice treated with vehicle (PBS), nonspecific IgG, or TNA were examined and lymphocyte infiltration scores by blinded reviewers. Data shown are percentage of 6 WT and 10 CnAα+/− mice in each group that received a score <3 or ≥3. **P < 0.05, Fisher's exact test. B: serum was collected and BUN values were measured for WT and CnAα+/− mice treated with vehicle (PBS), IgG, or TNA. Data shown are means ± SE of 6 WT mice and 10 CnAα+/− mice per treatment group. *P < 0.05 ANOVA. C: serum osmolality was measured for WT and CnAα+/− mice treated with vehicle (PBS), IgG, or TNA. Data shown are means ± SE of 6 WT mice and 10 CnAα+/− mice per treatment group. *P < 0.05 ANOVA. D: lymphocyte infiltration was scored by 2 blinded reviewers in intestine, liver, and lung sections. Data shown are percentage of vehicle-treated, IgG-treated, and TNA-treated CnAα+/− mice that received a score of <3 or ≥3. *P < 0.05, χ2 analysis.
DISCUSSION
Previously, we reported that homozygous loss of the α isoform of the catalytic subunit of calcineurin leads to defects in renal development and function (12) and is sufficient to reproduce many features of cyclosporin nephrotoxicity (11). Together, these data indicated an essential role for the α isoform in the kidney. However, early lethality of homozygous null mice prevented further study of renal function over time. As an alternative experimental approach, we examined heterozygous mice at 6 mo and 1 yr of age and found a progressive decline in renal function and increased TGF-β expression. In addition to these observations, which are in keeping with our reports of null mice, we also report an unexpected finding of lymphoid neogenesis that was present in multiple organs including the kidney, liver, small intestine, and lung. Characterization of these lesions identified a semiorganized structure containing central B cells and peripheral T cells, reminiscent of organized lymphoid follicles. This organization and the identification of LYVE-1-expressing lymphatic endothelial cells suggest that CnAα+/− mice are a novel model of spontaneous TLO development. Combined with our previous reports, these data suggest a model whereby loss of calcineurin α activity leads to increased TGF-β production and fibrosis. These changes provoke a local inflammatory response which, over time, leads to formation of TLOs coincident with a progressive decline in renal function.
Several limitations to the study should be noted. First, renal function was an important outcome but measuring renal function in rodent can be difficult. Traditional indexes including BUN may be of only limited value. Additional experiments are necessary in the future to fully characterize renal changes in this model. Second, CnAα+/− mice lack only one isoform of the catalytic subunit of calcineurin and, by all indications, still express half of the normal levels of α as well as the β isoform. While our previous work indicates that loss of the α isoform may recapitulate many features of calcineurin inhibitor nephrotoxicity and haploinsufficiency is likely to be a more realistic representation of pharmacological enzyme inhibition, cyclosporin and FK506 inhibit both the α and β isoforms of calcineurin. As such, results from this model can only be cautiously applied to clinical changes due to calcineurin inhibitors.
Our data provide several important insights. First, although the significance of TLOs to the progression or resolution of chronic inflammatory processes is still largely unknown, CnAα+/− mice offer a spontaneous model of TLO and, therefore, an opportunity to better understand the mechanisms involved. Furthermore, the presence of TLOs in this model indicates TLOs formation in transplant allografts may be an additional manifestation of calcineurin inhibitor toxicities. Second, the sequence of events suggested by the data in this study points to a novel role of TGF-β and fibrosis in the initiation of TLOs. Moreover, the phenotype of CnAα+/− mice and the central role of TGF-β in this model suggest that regulation of TGF-β is an essential function of calcineurin.
The formation of TLOs in organs including the kidney has been recognized to coincide with chronic inflammatory processes. In a recent review of the topic, Segerer and Schlöndorff (21) propose a model whereby local inflammatory mediators are recruited following an injury such as ischemia, matrix deposition, or release of inflammatory cytokines. This initial recruitment is followed by an accumulation of lymphatic and dendritic cells in the tubulointerstitium. Finally, local interaction between resident cells and infiltrating lymphoid cells promotes the organization of TLOs. This model is supported by data from the kidney and other organs and is also consistent with CnAα+/− mice as reported in the present study. Although lacking an acute injury, it is likely that haploinsufficiency of CnAα leads to chronic ischemia and/or accumulation of matrix deposition either of which may serve as the “injury” signal that promotes TLO formation. The primary role of TGF-β identified in this study points to the latter as a likely initiating event, although we cannot rule out the former.
In addition to spontaneous TLO formation, this study resulted in another equally novel finding: upregulation of TGF-β production in renal and, presumably, other organs is sufficient to induce TLO formation and may be one of the most critical consequences of downregulation of CnAα. Our data demonstrate the importance of TGF-β upregulation in the formation of TLOs as neutralization of the cytokine in mice from 16–24 wk of age dramatically reverses the phenotype. Histological analyses show reductions in TLOs in all organs surveyed and a corresponding improvement in renal function. The effects of a nonspecific IgG suggested mild improvements but did not reach the significance of blocking TGF-β specifically. The implication of TGF-β as an initiating event for lymphoid infiltration and organization in the kidney suggests a complex role for the cytokine. While this action is in contrast to well-known immunosuppressive effects of TGF-β, it is likely that TLO formation in CnAα+/− mice is an indirect effect of intraorgan inflammation. Second, regulation of TGF-β is a central role of CnAα. Support for this idea can be found in numerous reports that demonstrate upregulation of TGF-β following inhibition of calcineurin with cyclosporin in human allograft studies (4, 7, 19), in animal models (9, 22), and in in vitro experiments (24, 25). These findings suggest a link between TGF-β and calcineurin but cannot exclude the possibility that TGF-β is altered as a result of an indirect effect of cyclosporin such as ischemia. However, Wolf et al. (25) reported that cyclosporin treatment of cultured renal epithelial cells resulted in increased TGF-β message and protein. Similarly, our previous data showed that renal fibroblasts derived from CnAα−/− mice overexpress both TGF-β and fibronectin (11). Reexpression of the α isoform restored normal levels of both. Taken together, it is reasonable to conclude that loss of CnAα activity or expression directly leads to increased TGF-β expression. The associated matrix deposition and cytokine changes we report suggest that this event initiates the inflammatory response that precedes TLO formation.
While the sequence of events leading to TLO formation seems plausibly explained by Segerer and Schlöndorff's model, it is less clear what role TLOs play in the resolution or progression of an inflammatory processes. One theory is the danger signal hypothesis whereby local aggregates of lymphoid cells recruit additional factors to promote removal of damaged tissue. This is the primary role of secondary lymphoid organs such as the tonsils and Peyer's patch in the intestine. There is also, however, evidence that the presence of TLOs may contribute to pathological processes. For example, TLOs in cardiac and renal allografts are predictive of poor transplant outcomes (1, 13, 14). More specifically, Baddoura et al. (1) reported that a retrospective survey of murine cardiac allografts found that almost 25% showed TLO formation, the majority of which were associated with chronic as opposed to acute rejection. This suggests that, unlike secondary lymphoid organs, TLO formation may either be directly detrimental to organ function or may be indicative of an alloimmune response. The present study adds an important caveat to the understanding of TLO formation and allograft survival in that calcineurin inhibition itself may be sufficient to induce both chronic inflammation and TLO formation. This is significant for two reasons. First, since resolution of the underlying inflammatory cause via neutralization of TGF-β also blocked TLO formation, it is reasonable to conclude that TLOs are a result of an underlying injurious process rather than the cause. Second, it is likely that TLOs represent an additional aspect of calcineurin inhibitor toxicity and, as such, are the result of over immune suppression as opposed to insufficient immune suppression.
In summary, we report a novel phenotype of TLO formation and nephrotoxicity with partial loss of the α isoform of calcineurin. These defects appear to be secondary to dsyregulation of TGF-β as neutralization of the cytokine resolves both development of TLOs and improves renal function. There is growing evidence that the isoforms of calcineurin function in separate pathways and contribute to unique aspects of calcineurin action. Our data suggest that one critical role of the α isoform is to regulate expression of TGF-β. TLO formation downstream of TGF-β-mediated inflammation is a novel pathway and the significance of this finding to other areas including transplant rejection remains to be understood.
GRANTS
This work was supported the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-066422 (to J. L. Gooch) and the Department of Veterans Affairs (J. L. Gooch).
Supplementary Material
Acknowledgments
The authors thank D. Smith and J. Herskowitz for technical contributions and S. Speck, M. Okusa, and K. Kokko for helpful discussion.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Baddoura FK, Nasr IW, Wrobel B, Li Q, Ruddle NH, Lakkis FG. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant 5: 510–516, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bueno OF, Brandt EB, Rothenberg ME, Molkentin JD. Defective T cell development and function in calcineurin Abeta-deficient mice. Proc Natl Acad Sci USA 99: 9398–9403, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in calcineurin Abeta-deficient mice. Proc Natl Acad Sci USA 99: 4586–4591, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citterio F, Pozzetto U, Romagnoli J, Tondolo E, Silvestri P, Nanni G, Castagneto M. Plasma levels of transforming growth factor-beta1 in renal transplant recipients receiving different immunosuppressive regimens. Transplant Proc 36: 698–699, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cobbs SL, Gooch JL. NFATc is required for TGF-beta-mediated transcriptional regulation of fibronectin. Biochem Biophys Res Commun 362: 288–294, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cousin MA Synaptic vesicle endocytosis: calcium works overtime in the nerve terminal. Mol Neurobiol 22: 115–128, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Cuhaci B, Kumar MS, Bloom RD, Pratt B, Haussman G, Laskow DA, Alidoost M, Grotkowski C, Cahill K, Butani L, Sturgill BC, Pankewycz OG. Transforming growth factor-beta levels in human allograft chronic fibrosis correlate with rate of decline in renal function. Transplantation 68: 785–790, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Gooch JL An emerging role for calcineurin Aα in the development and function of the kidney. Am J Physiol Renal Physiol 290: F769–F776, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol 284: F144–F154, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Gooch JL, Pergola PE, Guler RL, Abboud HE, Barnes JL. Differential expression of calcineurin A isoforms in the diabetic kidney. J Am Soc Nephrol 15: 1421–1429, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Gooch JL, Roberts BR, Cobbs SL, Tumlin JA. Loss of the alpha isoform of calcineurin is sufficient to induce nephrotoxicity and altered expression of TGF-beta. Transplantation 83: 439–447, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Gooch JL, Toro JJ, Guler RL, Barnes JL. Calcineurin A-alpha but not A-beta is required for normal kidney development and function. Am J Pathol 165: 1755–1765, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heller F, Lindenmeyer MT, Cohen CD, Brandt U, Draganovici D, Fischereder M, Kretzler M, Anders HJ, Sitter T, Mosberger I, Kerjaschki D, Regele H, Schlondorff D, Segerer S. The contribution of B cells to renal interstitial inflammation. Am J Pathol 170: 457–468, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, Laakkonen P, Petrova T, Langer B, Raab I. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 15: 603–612, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Kumashiro S, Lu YF, Tomizawa K, Matsushita M, Wei FY, Matsui H. Regulation of synaptic vesicle recycling by calcineurin in different vesicle pools. Neurosci Res 51: 435–443, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Liersch R, Nay F, Lu L, Detmar M. Induction of lymphatic endothelial cell differentiation in embryoid bodies. Blood 107: 1214–1216, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molkentin J, Lu J, Antos C, Markham B, Richardson J, Robbins J, Grant S, Olsen E. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pribylova-Hribova P, Kotsch K, Lodererova A, Viklicky O, Vitko S, Volk HD, Lacha J. TGF-beta1 mRNA upregulation influences chronic renal allograft dysfunction. Kidney Int 69: 1872–1879, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev 80: 1483–1521, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Segerer S, Schlondorff D. B cells and tertiary lymphoid organs in renal inflammation. Kidney Int 73: 533–537, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Shihab F, Bennett W, Tanner A, Andoh T. Angiotensin II blockade decreases TGF-beta1 and matrix proteins in cyclosporine nephropathy. Kidney Int 52: 660–673, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Stapler D, Lee ED, Selvaraj SA, Evans AG, Kean LS, Speck SH, Larsen CP, Gangappa S. Expansion of effector memory TCR Vbeta4+ CD8+ T cells is associated with latent infection-mediated resistance to transplantation tolerance. J Immunol 180: 3190–3200, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Waiser J, Dell K, Bohler T, Dogu E, Gaedeke J, Budde K, Neumayer HH. Cyclosporine A upregulates the expression of TGF-beta1 and its receptors type I and type II in rat mesangial cells. Nephrol Dial Transplant 17: 1568–1577, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Wolf G, Thaiss F, Stahl RA. Cyclosporine stimulates expression of transforming growth factor-beta in renal cells. Possible mechanism of cyclosporines antiproliferative effects. Transplantation 60: 237–241, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Zhang BW, Zimmer G, Chen J, Ladd D, Li E, Alt FW, Wiederrecht G, Cryan J, O'Neill EA, Seidman CE, Abbas AK, Seidman JG. T cell responses in calcineurin A alpha-deficient mice. J Exp Med 183: 413–420, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.