Abstract
We previously reported in ischemic acute kidney injury (AKI) in mice that caspase-1-mediated production of interleukin-18 (IL-18) is pathogenic and that macrophage depletion by liposome-encapsulated clodronate (LEC) is protective. Therefore, our aim was to determine whether macrophages are a source of IL-18 in ischemic AKI in mice. On immunofluorescence staining of the outer stripe of outer medulla, the number of macrophages double stained for CD11b and IL-18 was significantly increased in AKI and significantly decreased by LEC. Adoptive transfer of RAW 264.7 cells, a mouse macrophage line that constitutively expresses IL-18 mRNA, reversed the functional protection against AKI in both LEC-treated wild-type and caspase-1 −/− mice. To test whether IL-18 in macrophages is necessary to cause AKI, we adoptively transferred macrophages in which IL-18 was inhibited. Peritoneal macrophages isolated from wild-type mice, IL-18 binding protein transgenic (IL-18 BP Tg) mice, and IL-18 −/− mice were used. IL-18 BP Tg mice overexpress human IL-18 BP and exhibit decreased biological activity of IL-18. Adoptive transfer of peritoneal macrophages from wild-type as well as IL-18 BP Tg and IL-18 −/− mice reversed the functional protection against AKI in LEC-treated mice. In summary, adoptive transfer of RAW cells, that constitutively express IL-18, reverses the functional protection in macrophage-depleted wild-type and caspase-1 −/− mice with AKI. However, adoptive transfer of peritoneal macrophages in which IL-18 function was inhibited also reverses the functional protection in macrophage-depleted mice. In conclusion, IL-18 from adoptive transfer of macrophages is not sufficient to cause ischemic AKI.
Keywords: inflammation, liposomal clodronate, tissue damage, NK cells
ischemic acute kidney injury (AKI) is a life-threatening illness that continues to have a high mortality rate of 50–80% in an intensive care unit setting (14). The severity of AKI in humans has been classified according to the RIFLE (risk, injury, failure, loss of function, and end-stage kidney disease) criteria (3). The course of the illness is highly variable ranging from a transient disease lasting less than 1 wk and associated with full recovery of renal function to a disease persisting for longer than 1 mo and requiring dialysis and intensive care management. Thus, a better understanding of the pathogenesis of AKI is needed to allow interventions which would prevent the need for hemodialysis, shorten the course of AKI, and improve survival.
Potential tubular and vascular factors, as well as inflammatory processes, are involved in the pathogenesis of ischemic AKI (14). Inflammation is now believed to play a major role in the pathophysiology of ischemic AKI (4, 20). Macrophages are mediators of AKI in mice and rats. In a model of macrophage depletion using liposomal clodronate, it was demonstrated that macrophages contribute to tissue damage during acute renal allograft rejection (25) and ischemic AKI (9, 24). Gene therapy in rats expressing an NH2-terminal-truncated monocyte chemoattractant protein-1 (MCP-1) reduced macrophage infiltration and AKI (21). A key question is the mechanism of the protective effect of macrophage depletion.
We and others demonstrated that interleukin-18 (IL-18) is a mediator of ischemic AKI in mice (28, 29, 38). However, our published data demonstrate that neither neutrophils nor CD4+T cells are the source of IL-18 in ischemic AKI (18, 29). A recent study demonstrated that IL-18, derived primarily from cells of bone marrow origin, contributes to the renal damage observed during ischemic AKI in mice (38). Macrophages are well-known sources of IL-18 (26). Thus, we tested the hypothesis that macrophages are the source of IL-18 in ischemic AKI.
To determine whether macrophages are a source of IL-18 in AKI, three strategies were used: 1) double staining immunofluorescence for macrophages and IL-18 was performed in AKI and macrophage-depleted kidneys, 2) it was determined whether adoptive transfer of macrophages reverses protection against ischemic AKI in macrophage-depleted wild-type mice and in caspase-1 −/− mice, and 3) it was determined whether adoptive transfer of macrophages deficient in IL-18 function reverses the protection in macrophage-depleted mice that are protected against AKI.
MATERIALS AND METHODS
Ischemia protocol.
Male mice (C57BL/6) aged 8–10 wk were used (Jackson Laboratories, Bar Harbor, ME). Mice weighing 20–25 g were anesthetized with an intraperitoneal injection of Avertin (2,2,2-tribromoethanol: Sigma, Milwaukee, WI). A midline incision was made and both renal pedicles were clamped for 22 min with microaneurysm clamps as previously described (29). The study protocol was approved by the University of Colorado Animal Care and Use Committee. The time of ischemia was chosen to obtain a reversible model of ischemic AKI. Sham surgery consisted of the same surgical procedure except that clamps were not applied. Studies in ischemic AKI were performed at 24 h of postischemic reperfusion, unless otherwise stated. Serum creatinine was measured using quantitative colorimetric creatinine determination (QuantiChrom creatinine assay kit-DICT-500; Bioassay Systems, Hayward, CA).
IL-18 binding protein transgenic mice.
Mice transgenic for human IL-18-binding protein (IL-18 BP) isoform a have been generated and characterized by Dr. C. Dinarello at UCHSC (17). RT-PCR analysis demonstrates a high expression of human IL-18 BP in all the organs examined including kidney in the transgenic mice. IL-18 BP transgenic (Tg) mice are viable, fertile, and have no tissue or organ abnormality. The IFN-γ-inducing activity of exogenously administered IL-18 was completely neutralized in the IL-18 BP Tg mice and the mice were completely protected against hepatotoxicity induced by concavalin A. The IL-18 BP Tg mice have been backcrossed into C57BL/6 mice without a change in phenotype. The IL-18 BP Tg mouse is a critical tool in the study of the role of IL-18 in experimental animal models of acute and chronic inflammatory diseases (17).
IL-18 −/− mice.
IL-18 −/− mice on the C57BL/6 background were obtained from Jackson Laboratories. These mice were developed by Akira (1). Mice that are homozygous null for the IL-18 gene are viable, fertile, normal in size, and do not display any gross physical or behavioral abnormalities. Homozygous null mice exhibit reduced levels of IFN-γ in response to heat-killed bacteria and lipopolysaccharide.
Western blot analysis.
Macrophages were homogenized in radioimmunoprecipitation assay (RIPA) buffer plus proteinase inhibitors and immunoblotted as previously described (13). A rabbit polyclonal antibody that recognizes the COOH terminus of caspase-1 p10 of mouse origin was used (Santa Cruz Biotechnology, Santa Cruz, CA; catalog number sc-514).
RT-PCR.
Total RNA was extracted from IL-18 BP Tg and wild-type mouse kidney using the TRIzol method (Life Technologies, Gibco BRL). One microgram of total RNA was reverse-transcribed by using Invitrogen's SuperScript Synthesis System (11904–018). One-tenth of the reaction mixture was used as template for PCR amplification. The amplification profile was 94°C for 1.5 min, then 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 60 s, with a final extension of 72°C for 7 min. Products were then separated in a 1.5% agarose gel in the presence of ethidium bromide, and bands were analyzed in a UV box. GAPDH was included as an internal control. RT-PCR products were 295 bp for hIL-18BPa and 452 bp for GAPDH. Images for densitometry were analyzed using 1D Image Software (Kodak Digital Science, Rochester, NY).
IFN-γ ELISA.
A mouse IFN-γ ELISA Kit II (catalog number 558258) was used (BD Biosciences, San Diego, CA). The performance characteristics of the kit (per manufacturer's instructions) are as follows: 1) no cross reactivity with other cytokines, e.g., IL-1α, IL-1β, IL-2, IL-3, IL-5, IL-6, IL-7, IL-10, IL-12, TNF, and TNFRII, was identified; 2) the intra-assay and interassay variability was between 3 and 6%; and 3) the minimum detectable dose of IFN-γ was 0.762 pg/ml.
Immunofluorescence studies.
Kidney tissues were embedded in OCT, snap-frozen in liquid nitrogen, and stored at −80°C until sectioning. Five-micrometer cryostat sections were fixed in 70% acetone/30% methanol and prepared for immunofluorescence studies as previously described (13). Primary antibodies used were 1) a rat anti-mouse CD11b monoclonal antibody (catalog no. MCA74; Serotec, Oxford, UK) and 2) a goat polyclonal antibody against the COOH terminus of IL-18 of mouse origin (Santa Cruz Biotechnology; catalog no. sc-6179).
Macrophage depletion studies.
Liposomes (vehicle) and liposome-encapsulated clodronate (LEC) were prepared as previously described in detail (37). Clodronate was obtained from Roche Diagnostics GmbH (Mannheim, Germany). Briefly, macrophages phagocytose the liposomes resulting in the release of clodronate into the cytoplasm and death of the macrophage. Empty liposomes, not containing clodronate, and prepared under exactly the same conditions as the LEC were used as a control. Mice received a tail vein injection of 100 μl/10 g body wt of empty liposomes or LEC at 6 and 2 days before induction of ischemia by bilateral renal pedicle clamp.
Macrophage repletion studies–RAW 264.7 macrophages.
RAW 264.7 macrophages have been adoptively transferred after depletion by LEC (9). Macrophages were given 2 h before induction of ischemia. RAW 264.7 cells (108) were injected via the tail vein. Mouse RAW 264.7 cells are a mouse monocyte/macrophage cell line from ascites fluid in a male mouse in which a tumor was induced by injection of Abelson leukemia virus (A-MuLV; American Type Culture Collection). Cells are grown in DMEM high-glucose medium supplemented with Na2HCO3 (0.075%), l-glutamine, PCN/strep (1%), 0.5 mg/ml insulin, and 10% fetal bovine serum in a humidified atmosphere of 5% CO2 at 37°C.
Isolation of peritoneal macrophages.
Peritoneal macrophages were isolated from wild-type, IL-18 BP Tg mice, and IL-18 −/− mice as previously described (19). Two milliliters of Brewer thioglycolate medium were injected intraperitoneally. After 5 days, the mice were killed by decapitation. The abdomen was sterilized with 70% alcohol. With the use of sterile scissors, the abdominal skin was retracted to expose the intact peritoneal wall. Ten milliliters complete DMEM medium + 10% FCS were injected intraperitoneally. With the use of the same syringe and needle, the medium was slowly aspirated. The number of cells in an aliquot of the aspirated medium was counted with a hemacytometer. The aspirated medium was centrifuged at 400 g for 10 min at 4°C. The pellet of cells was gently resuspended in normal saline. An aliquot of cells was stained for CD11b (see results). One million cells were suspended in 0.2 ml normal saline and injected into mice via the tail vein.
Statistical analysis.
Non-normally distributed data were analyzed by the nonparametric unpaired Mann-Whitney U-test. Multiple group comparisons are performed using ANOVA with posttest according to Newman-Keuls. A P value of <0.05 was considered statistically significant. Values are expressed as means ± SE.
RESULTS
IL-18 mRNA is decreased in macrophage-depleted kidneys.
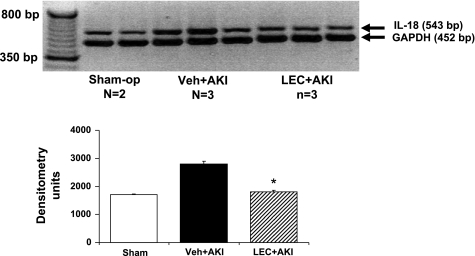
On RT-PCR IL-18 mRNA expression was increased in AKI kidneys (P < 0.05 vs. sham) and was significantly reduced in AKI kidneys depleted of macrophages with LEC (P < 0.05 vs. AKI; Fig. 1).
Fig. 1.
RT-PCR for interleukin-18 (IL-18). IL-18 mRNA expression was increased in acute kidney injury (AKI) kidneys and was significantly reduced in AKI kidneys depleted of macrophages with liposome-encapsulated clodronate (LEC). *P < 0.05 vs. vehicle (Veh) + AKI.
Macrophages double staining for CD11b and IL-18 are decreased in the kidney by LEC.
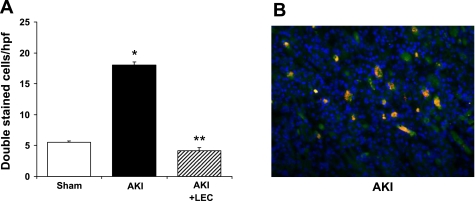
On immunofluorescence staining of the outer stripe of outer medulla, the number of cells (per HPF) double stained for CD11b and IL-18 was 5.5 in sham, 18 in AKI (P < 0.01 vs. sham and AKI + LEC), and 4.2 in AKI + LEC (Fig. 2A). A representative picture of cells double stained for CD11b and IL-18 in AKI kidneys is demonstrated in Fig. 2B.
Fig. 2.
Double staining for IL-18 and CD11b. A: in the outer stripe of the outer medulla, the number of cells double stained for IL-18 and CD11b was increased in AKI and decreased by LEC. *P < 0.01 vs. sham. **P < 0.01 vs. AKI. B: representative picture of cells double stained for CD11b and IL-18 in AKI kidneys. Double staining (orange) is composed of CD11b staining which is red, and IL-18 staining which is green.
Macrophage repletion using RAW 264.7 cells.
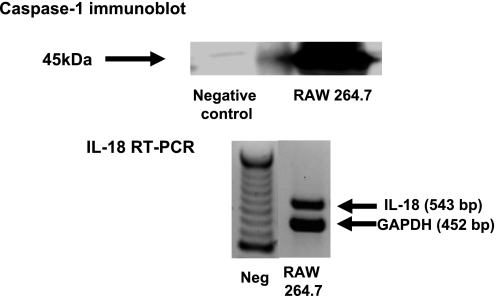
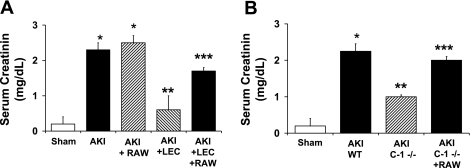
RAW 264.7 cells are a mouse monocyte/macrophage line that constitutively expresses caspase-1 protein on immunoblot and IL-18 mRNA on RT-PCR analysis (Fig. 3). RAW cells (108) were administered via tail vein before the induction of ischemia. RAW cells reversed the functional protection against AKI in LEC- treated wild-type. Serum creatinine (mg/dl) was 0.1 in sham, 2.2 in AKI + vehicle (P < 0.001 vs. sham), 2.4 in AKI + RAW cells (NS vs. AKI + vehicle), 0.6 in AKI + LEC (NS vs. sham, P < 0.01 vs. AKI + vehicle), and 1.6 in AKI + LEC + RAW (P < 0.001 vs. AKI + LEC and NS vs. AKI; Fig. 4A).
Fig. 3.
RAW 264.7 macrophages express caspase-1 protein (45 kDa) on immunoblot and IL-18 mRNA (543 bp) on RT-PCR analysis. Negative control = kidney from caspase-1 −/− mouse.
Fig. 4.
Administration of RAW 264.7 macrophages in wild-type (WT) mice (A) and caspase-1 −/− (C-1 −/−) mice (B). RAW cells were administered via tail vein before the induction of ischemia. A: RAW cells reversed the functional protection against AKI in LEC-treated WT mice. *P < 0.001 vs. sham. **P < 0.01 vs. AKI and AKI + RAW. ***P < 0.01 vs. AKI + LEC. B: RAW cells reversed the functional protection against AKI in C-1 −/− mice. *P < 0.001 vs. sham. **P < 0.01 vs. AKI WT. ***P < 0.01 vs. AKI C-1 −/− mice.
Caspase-1 converts the pro to the active forms of IL-1β and IL-18. We previously demonstrated that caspase-1 −/− mice are protected against ischemic AKI due to a lack of IL-18 (28). RAW 264.7 cells reversed the protection against AKI in caspase-1 −/− mice. Serum creatinine (mg/dl) was 0.2 in sham, 2.3 in wild-type + AKI (P < 0.05 vs. sham), 1.0 in caspase-1 −/− + AKI (P < 0.05 vs. wild-type + AKI), and 2.0 in caspase-1 −/− + AKI + RAW (NS vs. wild-type + AKI; Fig. 4B).
Characterization of peritoneal macrophages.
Attempts to inhibit IL-18 in RAW cells using siRNA were unsuccessful. Thus, peritoneal macrophages isolated from wild-type, IL-18 BP Tg mice, and IL-18-deficient −/− mice were used in further studies (5).
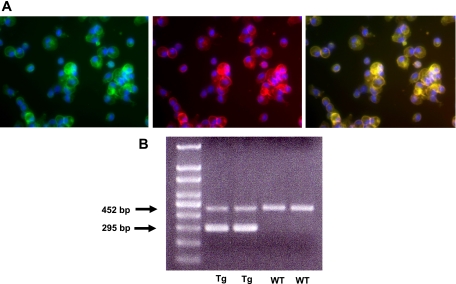
Peritoneal macrophages were isolated as described in materials and methods. Immunofluorescence staining was performed on an aliquot of the peritoneal macrophages. One hundred percent of the peritoneal macrophages stained for CD11b (Fig. 5A) demonstrating the purity of the preparation. One hundred percent of the peritoneal macrophages stained for IL-18 and double stained for both IL-18 and CD11b (Fig. 5A), demonstrating that the peritoneal macrophages contain IL-18. On RT-PCR, peritoneal macrophages from the IL-18 BP Tg mice expressed the human IL-18 BP transgene (Fig. 5B).
Fig. 5.
Peritoneal macrophages. A: peritoneal macrophages stained for both IL-18 (green) and CD11b (red) and double stained for IL-18 and CD11b (yellow) demonstrating that the peritoneal macrophages contain IL-18. B: on RT-PCR, peritoneal macrophages from the IL-18 binding protein transgenic (BP Tg) mice expressed the human IL-18 BP transgene (295 bp).
IL-18 BP interacts with IL-18 via β strands and the interaction is stabilized by strong electrostatic interactions (11). While the binding of IL-18 BP to IL-18 reduces the biological activity of IL-18 (e.g., production of IFN-γ), it does not alter detection of IL-18 protein on immunoblotting, immunohistochemistry, or ELISA assay. To confirm this, we performed the IL-18 ELISA using recombinant IL-18 with or without a large excess of recombinant IL-18 BP. IL-18 BP had no effect on the measured level of IL-18. Thus, in further studies, we measured IFN-γ as a parameter of the biological action of IL-18 in IL-18 BP Tg mice.
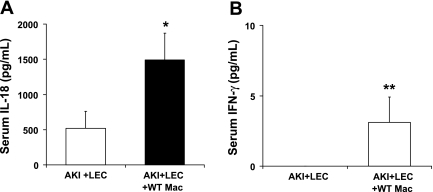
To further demonstrate that wild-type peritoneal macrophages contain IL-18 and that the IL-18 has biological activity, as measured by IFN-γ-inducing activity, mice with AKI were depleted of macrophages with LEC and then injected with wild-type peritoneal macrophages. Both IL-18 and IFN-γ blood levels at 24 h of postischemic reperfusion were increased in LEC-treated mice injected with wild-type peritoneal macrophages (Fig. 6, A and B).
Fig. 6.
WT peritoneal macrophages contain IL-18 that has biological activity, as measured by IFN-γ-inducing activity. Mice with AKI were depleted of macrophages with LEC and then injected with WT macrophages. Both IL-18 (A) and IFN-γ (B) blood levels at 24 h of postischemic reperfusion were increased in LEC-treated mice injected with WT peritoneal macrophages. *P < 0.01 vs. AKI + LEC. **P = 0.05 vs. AKI + LEC.
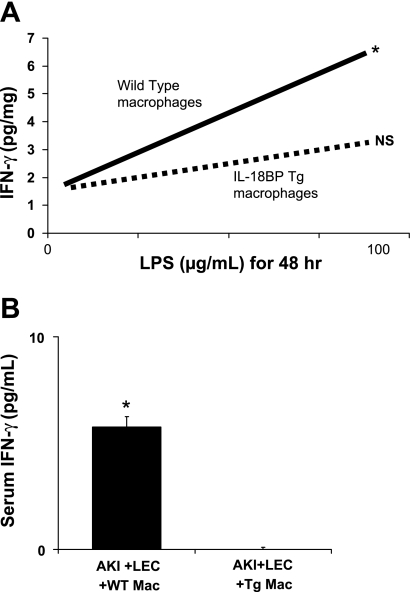
To demonstrate that peritoneal macrophages from IL-18 BP Tg mice have decreased biological activity of IL-18, IFN-γ levels were measured in wild-type and IL-18 BP Tg macrophages stimulated with 100 μg lipopolysaccharide (LPS) for 48 h. The biological function of IL-18 in the peritoneal macrophages (as indicated by IFN-γ activity after LPS stimulation) was significantly decreased in macrophages isolated from IL-18 BP Tg mice compared with wild-type mice (Fig. 7A).
Fig. 7.
Peritoneal macrophages from IL-18 BP Tg mice have decreased biological activity of IL-18. A: IFN-γ levels were measured in WT and IL-18 BP Tg macrophages stimulated with 100 μg/ml lipopolysaccharide (LPS) for 48 h. The biological function of IL-18 in the peritoneal macrophages (as indicated by IFN-γ activity after LPS stimulation) was significantly decreased in macrophages isolated from IL-18 BP Tg mice compared with WT mice. *P < 0.01 vs. 0 LPS, not significant (NS) vs. 0 LPS. B: IFN-γ levels in serum were significantly decreased in mice that received peritoneal macrophages from IL-18 BP Tg mice compared with WT mice. *P < 0.05 vs. AKI + LEC + IL-18 BP Tg macrophages (Tg Mac).
To further determine that peritoneal macrophages from IL-18 BP Tg mice have decreased biological activity of IL-18, IFN-γ levels in serum were measured in mice that received wild-type and IL-18 BP Tg macrophages. IFN-γ levels in serum were significantly decreased in mice that received peritoneal macrophages from IL-18 BP Tg mice compared with wild-type mice (Fig. 7B).
There was no difference in the macrophage marker characteristics between wild-type and IL-18 BP Tg macrophages. Suspensions of peritoneal macrophages from both wild-type and IL-18 BP Tg mice were analyzed by flow cytometry using F4/80 and CD11b staining. Eighty-two to ninety-three percent of the cells were positive for CD11b and the number of CD11b-positive cells did not differ between wild-type and IL-18 BP Tg mice. Sixty-nine to seventy-three percent of the cells were positive for F4/80 and the number of F4/80-positive cells did not differ between wild-type and IL-18 BP Tg mice.
Macrophage repletion using peritoneal macrophages from IL-18 BP Tg mice and IL-18 −/− mice.
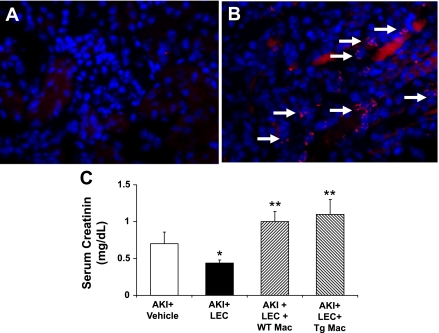
Peritoneal macrophages (one million cells) isolated from wild-type mice, IL-18 BP Tg mice, and IL-18 −/− mice were administered via the tail vein before the induction of ischemia. On immunofluorescence staining, the number of macrophages in the kidney was increased to the same degree after tail vein administration of peritoneal macrophages from wild-type, IL-18 BP Tg mice, and IL-18 −/− mice. The number of macrophages in the kidney (per high-power field) after tail vein administration was 24.3 for wild-type macrophages, 23 for IL-18 BP Tg macrophages, and 23.8 for IL-18 −/− macrophages (n = 3 per group). Thus adoptive transfer of macrophages into LEC-treated mice resulted in macrophage infiltration in the kidney and thus reconstitution of the macrophages depleted by LEC (Fig. 8, A and B).
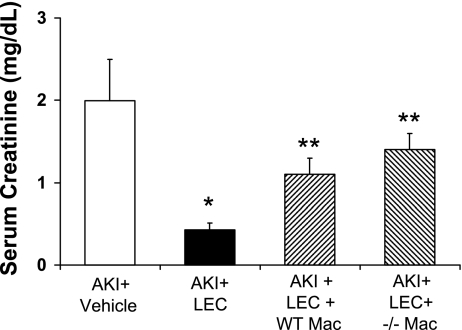
Fig. 8.
Peritoneal macrophage repletion–IL-18 BP Tg macrophages. Peritoneal macrophages isolated from WT mice were administered via tail vein before the induction of ischemia. A: in immunofluorescence staining, macrophages were not present in the AKI kidney after treatment with LEC. B: number of macrophages (arrows) in the kidney was increased after tail vein administration demonstrating that adoptive transfer of macrophages into LEC-treated mice resulted in macrophage infiltration in the kidney and thus reconstitution of the macrophages depleted by LEC. The number of macrophages in the kidney was increased to the same degree after tail vein administration of peritoneal macrophages from WT, IL-18 BP Tg mice, and IL-18 −/− mice. C: peritoneal macrophages from both WT (WT Mac) and Tg Mac mice reversed the functional protection against AKI in LEC-treated mice. *P < 0.05 vs. AKI + Veh. **P < 0.01 vs. AKI + LEC.
Both the macrophages from wild-type and IL-18 BP Tg mice reversed the functional protection against AKI in LEC-treated mice (Fig. 8C). These data suggest that IL-18 from macrophages is not the cause of kidney injury in AKI.
To confirm that IL-18 from macrophages is not the cause of kidney injury in AKI, peritoneal macrophages were isolated from IL-18 −/− mice and administered via the tail vein. Both the macrophages from wild-type and IL-18 −/− mice reversed the functional protection against AKI in LEC-treated mice (Fig. 9).
Fig. 9.
Peritoneal macrophage repletion–IL-18 −/− macrophages. Peritoneal macrophages were administered via tail vein before the induction of ischemia. Peritoneal macrophages from both WT Mac and IL-18 −/− (−/− Mac) mice reversed the functional protection against AKI in LEC-treated mice. *P < 0.01 vs. AKI + Veh. **P < 0.05 vs. AKI + LEC.
Depletion of NK cells with NK 1.1 antibody is not protective against AKI.
NK cells are a known source of IL-18 (16). Two hundred micrograms of NK 1.1 antibody have been shown to eliminate NK cell activity in mice for up to 7 days (6, 32). In our study, mice received an intraperitoneal injection (200 μg) of NK 1.1 antibody (BDPharMinogen, San Diego, CA) or vehicle (PBS) 48 h before renal pedicle clamp. Administration of NK 1.1 antibody was not functionally protective against AKI, suggesting that NK cells are not the source of injurious IL-18 in AKI. Serum creatinine (mg/dl) was 0.2 ± 0.1 in sham-operated mice, 1.8 ± 0.4 in AKI mice treated with the vehicle (P < 0.01 vs. sham), and 2.2 in AKI mice treated with the NK 1.1 antibody (not significant vs. AKI mice treated with vehicle). Blood urea nitrogen (mg/dl) was 19 ± 1 in sham-operated mice, 137 ± 22 in AKI mice treated with the vehicle (P < 0.01 vs. sham), and 148 ± 14 in AKI mice treated with the NK 1.1 antibody (not significant vs. AKI mice treated with vehicle; n = 5 per group).
DISCUSSION
IL-18 is involved in diverse functions including inflammation (innate immunity) (1), ischemic tissue injury (8), and T cell-mediated immunity (33). IL-18 is a mediator of ischemic tissue injury in the heart (36), brain (23), and kidney (28, 29, 38). IL-18 plays an important role in the activation of macrophages and NK cells (27) and is produced by macrophages and NK cells (26). The present study focuses on whether macrophages are the source of IL-18 in AKI.
Macrophage inflammation in the interstitium of the outer stripe of the outer medulla has been described early in the course of ischemic AKI in the rat (10) and mouse (21). The macrophage infiltration early in the course of ischemic AKI can produce various cytokines, chemokines, and other mediators of tissue injury. We demonstrated that the chemokine/adhesion molecule fractalkine (CX3CL1) may play a role in the entry of macrophages from the vascular compartment to the interstitium (34). Depletion of macrophages in the kidney during AKI using LEC (9, 24, 34) or genetic techniques (21) results in protection against ischemic AKI. The mechanism of the protective effect of macrophage depletion in ischemic AKI, as it relates to IL-18, is the focus of the present study.
The fact that macrophages that are present at the site of injury make IL-18 does not necessarily mean that the IL-18 is playing a role in the pathogenesis of the injury. To answer the question whether IL-18 from macrophages is contributing to the development of AKI, we determined the effect of adoptive transfer of wild-type and IL-18-deficient macrophages in macrophage-depleted mice with AKI. Our goal was to determine whether adoptive transfer of wild-type macrophages reversed the protection produced by macrophage depletion and to determine the effect of adoptive transfer of macrophages deficient in IL-18. We used peritoneal macrophages from IL-18 BP Tg mice. IL-18 BP is a soluble decoy receptor for IL-18 that inhibits the biological functions of IL-18 both in vitro and in vivo (12). Mice transgenic for human IL-18 BP isoform a (IL-18 BP Tg mice) demonstrate high expression of human IL-18 BP in macrophages and the IFN-γ-inducing activity of exogenously administered IL-18 is completely neutralized in macrophages from these mice. To confirm that IL-18 from macrophages does not play an injurious role in AKI, we demonstrated that macrophages from IL-18 BP Tg mice and IL-18-deficient mice reversed the protection against ischemic AKI to the same degree as wild-type macrophages.
What then is the mechanism of protection against AKI by macrophage depletion? Macrophages are a source of the chemokine CXCL1 (also known as IL-8 or KC). In this regard, we demonstrated that CXCL1 is increased in AKI and that macrophage depletion results in a decrease in CXCL1 (22). Studies with CXCL1 inhibition protecting against AKI confirm a possible role for CXCL1 derived from macrophages as an injurious factor in AKI (7, 30).
What is the source of injurious IL-18 in AKI? IL-18 may be activated in the proximal tubules and directly contribute to tubular injury. The role of caspase-1 and IL-18 in hypoxia-induced membrane injury of freshly isolated mouse proximal tubules in vitro was studied (15). Proximal tubules were isolated in parallel from wild-type and caspase-1-deficient −/− mice. Proximal tubules from caspase-1 −/− mice demonstrated less hypoxia-induced membrane injury. IL-18 was detected in proximal tubules by immunoblotting and ELISA. Thus, caspase-1, the activator of IL-18, has a deleterious effect on proximal tubules in vitro in the absence of inflammatory cells and vascular effects (15).
The IL-18 causing the injury may be activated in an inflammatory cell other than the macrophage. For example, NK cells are a type of lymphocyte that expresses IL-18 receptors and are activated by IL-18 (2, 35). A model of NK cell activation in injured tissues in which NK cells are recruited to sites of injury from the bloodstream has been proposed (31). However, NK cell depletion using the NK 1.1 antibody was not protective against ischemic AKI, suggesting that NK cells are not the source of injurious IL-18 in ischemic AKI.
The vascular endothelium plays a central role in the recruitment and migration of circulating effector cells into sites of inflammation. Caspase-1 and IL-18 production by the endothelium may result in the upregulation of adhesion molecules by the endothelium and recruitment of inflammatory cells from the blood vessels into the kidney. Further studies of the role on IL-18 in the endothelium will be interesting.
In summary, in ischemic AKI, 1) macrophage depletion with LEC reduces the number of macrophages double staining for CD11b and IL-18 and is protective against ischemic AKI, 2) adoptive transfer of RAW cells, which constitutively express IL-18, reverses the functional protection against AKI in LEC-treated wild-type and caspase-1−/− mice, and 3) adoptive transfer of peritoneal macrophages in which IL-18 function was inhibited is not sufficient to reverse the functional protection in LEC-treated mice, suggesting that macrophages are not the source of injurious IL-18 in ischemic AKI. The present study does not exclude the possibilities that IL-18 can activate macrophages to make other cytokines and chemokines in AKI or that tubules or endothelium are/is the source of injurious IL-18 in ischemic AKI.
GRANTS
This work was supported by National Institutes of Health Grants RO1-DK-56851 (to C. L. Edelstein), K08-DK-65022 (to S. Faubel), and an American Heart Association Grant-in-Aid (to C. L. Edelstein). D.-J. Oh was sponsored by a grant from Chung-Ang University, Department of Internal Medicine, Seoul, Korea. B. Dursun was sponsored by a grant from the Turkish Society of Nephrology.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akira S The role of IL-18 in innate immunity. Curr Opin Immunol 12: 59–63, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Badgwell B, Parihar R, Magro C, Dierksheide J, Russo T, Carson WE III. Natural killer cells contribute to the lethality of a murine model of Escherichia coli infection. Surgery 132: 205–212, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med 3: 409–413, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cailhier JF, Partolina M, Vuthoori S, Wu S, Ko K, Watson S, Savill J, Hughes J, Lang RA. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol 174: 2336–2342, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Carroll JL, Nielsen LL, Pruett SB, Mathis JM. The role of natural killer cells in adenovirus-mediated p53 gene therapy. Mol Cancer Ther 1: 49–60, 2001. [PubMed] [Google Scholar]
- 7.Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F, Noris M, Remuzzi G, Benigni A. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int 67: 1753–1761, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Daemen MA, Van't Veer C, Wolfs TG, Buurman WA. Ischemia-reperfusion-induced IFN-gamma upregulation: involvement of IL-12 and Il-18. J Immunol 162: 5506–5510, 1999. [PubMed] [Google Scholar]
- 9.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005. [DOI] [PubMed] [Google Scholar]
- 10.De Greef KE, Ysebaert DK, Dauwe S, Persy V, Vercauteren SR, Mey D, De Broe ME. Anti-B7–1 blocks mononuclear cell adherence in vasa recta after ischemia. Kidney Int 60: 1415–1427, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello CA Targeting interleukin 18 with interleukin 18 binding protein. Ann Rheum Dis 59, Suppl 1: i17–i20, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello CA Novel targets for interleukin 18 binding protein. Ann Rheum Dis 60, Suppl 3: iii18–iii24, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dursun B, He Z, Somerset H, Oh DJ, Faubel S, Edelstein CL. Caspases and calpain are independent mediators of cisplatin-induced endothelial cell necrosis. Am J Physiol Renal Physiol 291: F578–F587, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Edelstein CL, Schrier RW. Pathophysiology of ischemic acute renal injury. In: Diseases of the Kidney and Urinary Tract, edited by Schrier RW. Philadelphia, PA: Lippincott, Williams and Wilkins, 2007, p. 930–961.
- 15.Edelstein CL, Hoke TS, Somerset H, Fang W, Klein CL, Dinarello CA, Faubel S. Proximal tubules from caspase-1 deficient mice are protected against hypoxia-induced membrane injury. Nephrol Dial Transplant 22: 1052–1061, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Fabian MC, Lakey JR, Rajotte RV, Kneteman NM. The efficacy and toxicity of rapamycin in murine islet transplantation. In vitro and in vivo studies. Transplantation 56: 1137–1142, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Fantuzzi G, Banda NK, Guthridge C, Vondracek A, Kim SH, Siegmund B, Azam T, Sennello JA, Dinarello CA, Arend WP. Generation and characterization of mice transgenic for human IL-18-binding protein isoform a. J Leukoc Biol 74: 889–896, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Faubel SG, Ljubanovic D, Poole B, Dursun B, Cushing S, He Z, Gill RG, Edelstein CL. Peripheral CD4 T cell depletion is not sufficient to prevent ischemic acute renal failure. Transplantation 80: 643–649, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Fortier AH, Falk LA. Isolation of murine macrophages. In: Current Protocols in Immunology, edited by Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, and Strober W. Hoboken, NJ: John Wiley and Sons, 2001. [DOI] [PubMed]
- 20.Friedwald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int 66: 486–491, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Tomosugi N, Mukaida N, Matsushima K, Egashira K, Yokoyama H. Gene therapy expressing amino-terminal truncated monocyte chemoattractant protein-1 prevents renal ischemia-reperfusion injury. J Am Soc Nephrol 14: 1066–1071, 2003. [DOI] [PubMed] [Google Scholar]
- 22.He Z, Altmann C, Hoke TS, Ljubanovic D, Jani A, Dinarello CA, Faubel S, Edelstein CL. Interleukin-18 (IL-18) binding protein transgenic mice are protected against ischemic AKI. Am J Physiol Renal Physiol 295: F1414–F1421, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedtjarn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci 22: 5910–5919, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischemic acute renal failure in rats. Nephrol Dial Transplant 21: 1231–1239, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Jose MD, Ikezumi Y, Van Rooijen N, Atkins RC, Chadban SJ. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation 76: 1015–1022, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kanai T, Watanabe M, Okazawa A, Sato T, Hibi T. Interleukin-18 and Crohn's disease. Digestion 63, Suppl 1: 37–42, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lochner M, Forster I. Anti-interleukin-18 therapy in murine models of inflammatory bowel disease. Pathobiology 70: 164–169, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107: 1145–1152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melnikov VY, Faubel SG, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 110: 1083–1091, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol 159: 2137–2145, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moretta A Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol 2: 957–964, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Muhlen KA, Schumann J, Wittke F, Stenger S, Van Rooijen N, Van Kaer L, Tiegs G. NK cells, but not NKT cells, are involved in Pseudomonas aeruginosa exotoxin A-induced hepatotoxicity in mice. J Immunol 172: 3034–3041, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev 12: 53–72, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Oh DJ, Dursun B, He Z, Lu L, Hoke TS, Ljubanovic D, Faubel S, Edelstein CL. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol 294: F264–F271, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol 10: 259–264, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Pomerantz BJ, Reznikov LL, Harken AH, Dinarello CA. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci USA 98: 2871–2876, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: 83–93, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Craft ML, Wang P, Wyburn KR, Chen G, Ma J, Hambly B, Chadban SJ. IL-18 contributes to renal damage after ischemia-reperfusion. J Am Soc Nephrol 19: 2331–2341, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]