Summary
The anti-CD20, B cell-specific mAb rituximab (RTX) has been approved for treatment of non-Hodgkin's B cell lymphoma and rheumatoid arthritis. Under conditions of high B cell burden, exhaustion of the body's effector mechanisms, e.g. NK cell-mediated killing, may lead to substantial decreases in the immunotherapeutic efficacy of this mAb. Moreover, RTX treatment of patients with chronic lymphocytic leukemia and high levels of circulating B cells can lead to removal of CD20 from the cells, thus allowing them to persist and resist clearance. RTX therapy for several autoimmune diseases has proven to be effective, but in numerous instances there has been little correlation between reductions in disease activity and changes in titers of pathogenic autoantibodies. This paradox may be explained by a separate mechanism: Binding of RTX to B cells generates immune complexes which act as decoys to attract monoycte/macrophages and thus reduce their inflammatory activity in certain autoantibody-mediated diseases. Several second-generation anti-CD20 mAbs with enhanced cytotoxic action have been developed and are being tested in the clinic for treatment of cancer and autoimmune diseases. The application of these mAbs, potentially in combination with immune effector modifying drugs, may successfully address the shortcomings of current anti-CD20 immunotherapy.
Introduction to mechanisms of action
The introduction of the anti-CD20 mAb rituximab (RTX) has led to substantial advances in treatment of diseases associated with B cells. RTX is now being used with some degree of success, either alone or in combinations, for treatment of malignant and autoimmune diseases [1-4*,5*]. The key features of the cytotoxic mechanism of RTX were demonstrated in 1994; RTX promoted antibody-dependent cell-mediated cytotoxicity (ADCC) and complement–dependent cytotoxicity (CDC) of a human lymphoid cell line expressing CD20, and was found to be very effective at depleting B cells from peripheral blood and moderately effective at clearing B cells from lymph nodes and bone marrow [6]. Translation of this prescient basic science to the clinic has replicated and extended these findings. Animal models have demonstrated that infusion of RTX promotes rapid opsonization of circulating B cells followed by phagocytosis by FcγR-expressing fixed tissue macrophages in liver (and possibly spleen) [7-10]. Lefebvre al. demonstrated that properly differentiated human macrophages promote substantial phagocytic killing of RTX-opsonized chronic lymphocytic leukemia (CLL) cells [11*]. Apoptosis was proposed as a RTX cytotoxic mechanism, but in the absence of cross-linking with non-physiologic reagents, the ability of RTX to induce apoptosis is marginal [4*,7,12-16].
Thus, effective therapy with RTX, and most likely with several other anti-CD20 mAbs, is dependent on host effector functions. Therefore, an important general question must be considered: given that sufficient anti-CD20 mAb can be infused to saturate all CD20 sites on accessible B cells, will exhaustion or saturation of the body's effector functions limit therapeutic efficacy? Compelling evidence indicates that clearance by fixed tissue macrophages as well as ADCC and CDC can be overwhelmed under conditions of high tumor burden in cancer; alternatively effector functions may be compromised due to high burdens of immune complexes (IC) found in autoimmune diseases. We will examine these issues in the context of anti-CD20 therapies, but these questions may have applicability to other mAb-based immunotherapies.
Limitations to current therapy: consequences of exhaustion of effector mechanisms
NK cell-mediated ADCC can be exhausted
Since virtually all ADCC activity in peripheral blood mononuclear cells (PBMC) is mediated by NK cells [17-20**,21], it is important to determine how many target cells can be killed by an NK cell before it must “re-load” to continue its killing spree. Bhat and Watzl reported that IL-2-activated NK cells can engage and kill ∼3-4 target cells in 16 hours; after this time the cells appear to be exhausted, apparently due to substantial reductions in available perforin and granzyme B [20**]. However, IL-2 treatment restored cytotoxic activity. Bowles and Weiner found that changes in NK cell markers are well-correlated with ADCC activity [22]. Incubation of PBMC with RTX-opsonized target cells led to upregulation of CD54 and almost complete loss of FcγRIIIa (CD16) from the surface of NK cells; much of the CD16 appeared to be internalized [5*,22]. It would be interesting to determine whether treatment of these cells with IL-2 would restore CD16 levels and ADCC. Fisher et al. reported that NK cell-mediated ADCC of RTX-opsonized cells promotes up-regulation of CD107a, a marker of degranulation and presumably cell exhaustion [19]. Berdeja et al. found that ADCC may be significantly reduced due to high burdens of RTX-opsonized cells [21]. They measured in vitro ADCC of PBMC of eight patients with B cell lymphoma before and after RTX infusion. ADCC was substantially depressed one hour after infusion (6% lysis versus 38% lysis) and was not completely recovered after 24 hours. We suggest the cells were activated (and CD16 down-regulated) in vivo by interaction with RTX-opsonized B cells. When patients received infusions of IL-2 followed by leukapheresis and re-infusion of IL-2-treated lymphokine activated killer cells, RTX treatment did not promote reduction in ADCC activity, providing additional motivation for use of IL-2 to enhance and/or restore ADCC mediated by NK cells. Clinical studies such as this one will undoubtedly be repeated, and tests for NK cell activation based on down-regulation of CD16 and upregulation of CD54 and CD107a after RTX infusion may be quite informative [19,22].
Complement exhaustion and supplementation
Kennedy et al. reported that RTX infusion in CLL patients depleted complement, and suggested that treatment with fresh frozen plasma might restore RTX efficacy [23]. The case report of Klepfish et al. provided support for this idea; fresh frozen plasma was required for RTX to have therapeutic efficacy in a CLL patient [24].
Shaving of CD20 after RTX infusion
Treatment of CLL patients with 375 mg/m2 doses of RTX leads to rapid clearance of circulating RTX-opsonized B cells during the first few hours of infusion [23]. However, soon after infusion, additional malignant B cells enter the bloodstream from other compartments, and RTX and CD20 are removed (“shaved”) from these cells. The phenotype of circulating CLL B cells soon after RTX infusion is identical to that of circulating B cells before RTX treatment, except CD20 is reduced > 10-fold for days to weeks, thus severely limiting the efficacy of subsequent RTX infusions [23,25]. In vitro experiments indicate the high affinity receptor FcγRI plays a major role in this shaving reaction [26]. In addition, saturation of clearance mechanisms may lead to substantial down-regulation of the low affinity receptors FcγRII and FcγRIII involved in clearance [27**], thus favoring shaving over clearance. Li et al. established a subcutaneous xenograft mouse model with human Z138 CD20+ cells to determine whether shaving can occur at sites of tumor growth [28]. Analysis of tumors after RTX infusions provided unambiguous evidence for significant loss of CD20 from the cells. These results suggest that shaving may occur in compartments other than the bloodstream.
Recent clinical reports consistent with shaving
Laurent et al. identified CD20- mature B lymphocytes in nodular lymphoid infiltrates from bone marrow after patients with follicular lymphoma received RTX [29]. Leandro et al. found low or absent CD20 expression on immature and mature CD19+ cells in bone marrow aspirates taken after RTX therapy for rheumatoid arthritis (RA) [30]. Similarly, Teng et al. found that after RTX therapy, CD19+, CD20- B cells were present in bone marrow, and CD79a+, CD20-B cells were demonstrable in the synovium [31]. Although shaving was not considered, all three groups speculated that the decrease in B cell-associated CD20 was due to epitope masking by bound RTX. Several reagents and strategies are available to unambiguously test for B-cell bound RTX [23,32], and should allow for resolution of the question of shaving versus epitope masking. The implications of shaving occurring in tissue compartments after RTX therapy are substantial.
Improvements to current therapy
The clinical efficacy of RTX in several indications is well-documented, but limitations in its therapeutic action have also been noted [4*]. Therefore, several new anti-CD20 mAbs are being developed to more effectively harness effector functions. Clinical studies have linked RTX efficacy in B cell lymphoma treatment with polymorphisms in FcγR expressed by effector cells [4*,33]. Obviously the FcγRIIIa phenotype of patients' NK cells can not be changed to increase ADCC; rather, several second generation anti-CD20 mAbs have been engineered to increase the affinity of the Fc regions of the mAb for FcγRIIIa [34*,35*,36*]. This has been accomplished by amino acid substitutions or modification of the carbohydrate structure of the mAb. Lower concentrations of these mAbs are adequate to promote ADCC compared to concentrations needed for RTX; moreover, higher absolute levels of ADCC may be achieved. Clinical trials may reveal that these mAbs have enhanced activity compared to RTX, but the problem of exhaustion of ADCC/clearance mechanisms due to high tumor burdens is likely to remain an important issue. Parallel treatments based on use of IL-2 or infusion of IL-2-activated cells may further augment the action of these mAbs.
CDC should play an important role in the RTX cytotoxic mechanism in B cell lymphoma treatment [7,14,16,23,37]. Binding of RTX to CD20+ CLL cells promotes robust complement activation and C3b fragment deposition on cells [7,23], but the downstream goal of cytolysis may not be achieved; indeed, RTX has not been successful as a single agent in CLL treatment [4*]. The key question is how can the CDC potency of a human IgG1 mAb be increased? High affinity binding to a repeating epitope very close to the cell membrane should be advantageous, because C1q could be more effectively captured, and activated complement proteins could be more precisely concentrated onto the cell, allowing for enhanced killing.
A second generation fully human IgG1 anti-CD20 mAb, ofatumumab (OFA), appears to fit these criteria. OFA binds with high affinity to the small loop of the extracellular region of CD20 close to the membrane, and promotes enhanced CDC of B cell targets refractory to RTX [38,39*]. ADCC activity of OFA is similar to that of RTX, suggesting that interaction of RTX- or OFA-opsonized cells with FcγR-expressing effector cells is comparable. Experiments with isolated plasma and in whole blood indicate that OFA mediates much more killing of lymphoma cell lines and of primary lymphoma cells; this increased killing must be mediated by complement [38,39*]. In animal models, an initial high dose of OFA was required to deplete B cells throughout the body after which relatively low plasma concentrations of ∼5-10 ug/ml in monkeys were adequate to maintain B cell depletion [40].
In a phase 1-2 clinical trial of OFA for CLL, the median reduction in circulating B cells was 97% after the fourth infusion for patients who received a first dose of 500 mg followed by 3 weekly 2000 mg doses [41]. This very high level of clearance was found to be superior to more modest clearance observed in similar studies of RTX treatment for CLL. Moreover, the clinical response rate of CLL patients to OFA therapy was 50%, which was also noted to be greater than response rates obtained for comparable trials with RTX. Since the efficacy of clearance of OFA- or RTX-opsonized cells is expected to be similar for FcγR-dependent mechanisms, CDC should be the mechanism responsible for the greater clearance of CLL cells and enhanced clinical response observed for OFA. Both mAbs may consume complement equally, but the higher levels of activated complement proteins close to the cell membrane induced by OFA should promote greater CDC. Future experiments in clinical trials or animal models that compare these mAbs with respect to complement depletion versus CDC will allow testing of this hypothesis.
Anti-CD20 therapy for autoimmune disease
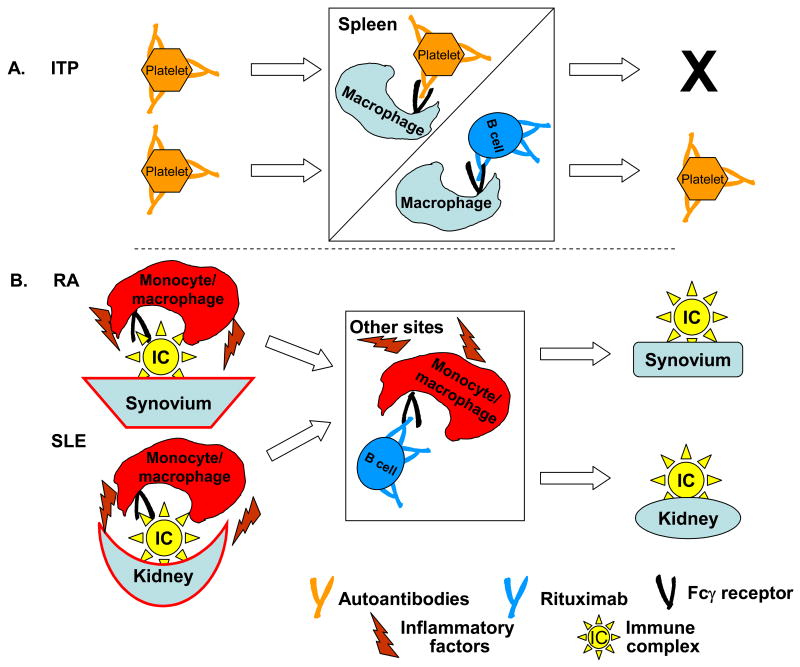
RTX was approved for RA treatment and is being examined in other autoimmune diseases [1,2,42,43]. The rationale, successfully demonstrated in acquired angiodema, is to target CD20+ precursor cells that would differentiate into autoantibody-producing plasma cells [44]. However, correlations between reductions in autoantibody levels and positive clinical responses in other autoimmune diseases have rarely been demonstrated [2,5*,43,45-47], leading us to postulate the IC decoy hypothesis (Figure 1).
Figure 1.
The Immune Complex Decoy Hypothesis [5*]. A. In ITP, autoantibodies bind to platelets, and the IgG-opsonized platelets are removed and destroyed in the spleen (and possibly in the liver) due to interaction with FcγR on fixed tissue macrophages. Infusion of RTX generates IgG-opsonized cells that bind to the macrophages, thus sparing the platelets. Modified from [5*]. B. Alternatively, in RA and in SLE respectively, immune complexes in the joints and concentrated in the synovium, or associated with the kidneys, interact with FcγR on monocyte/macrophages which initiates an inflammatory cascade, leading to the release of numerous inflammatory factors, ultimately leading to local tissue destruction. After RTX infusion, diversion of the monocyte/macrophages to other sites, due to interaction of FcγR with RTX-opsonized B cells, spares the synovium or kidney.
The IC Decoy Hypothesis: Recent findings
RTX-opsonized B cells may serve as decoys by engaging FcγR on effector cells, explaining RTX efficacy in immune thrombocytopenic purpura (ITP), in which IgG anti-platelet antibodies otherwise promote platelet clearance via FcγR on fixed-tissue macrophages [48,49]. After RTX treatment for ITP, platelet levels increase, but anti-platelet antibodies are usually unchanged; RTX infusion may lead to “diverting these cells” (liver and splenic macrophages) “from platelet phagocytosis” [49] (Figure 1A). RTX-opsonized B cells in the bloodstream or in tissues can also act as decoys to divert monocytes or macrophages from pathogenic interactions with tissue-associated IC, and reduce disease activity without affecting autoantibody levels (Figure 1B) [5*]. RTX therapy for patients with vasculitis and disease-associated antibodies to anti-proteinase-3 (anti-PR3) led to rapid clinical remissions [50], but anti-PR3 antibodies decreased slowly. RTX treatment of patients with systemic lupus erythematosus (SLE) or with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) led to rapid complete or partial remissions, but neither anti-dsDNA titers nor ANCA levels changed significantly [51].
Influence of the lifetime of antibody-producing cells
Variation in the lifetime of CD20-autoantibody-secreting cells may influence the time interval between initiation of anti-CD20 B cell-depletion therapy and reduction of autoantibody levels. Treatment of C57BL/6 mice with an anti-CD20 mAb suppressed immune responses to challenge antigens and suppressed secondary responses, but had little impact on extent serum immunoglobulin levels or on antibody-secreting cells in bone marrow, indicating CD20-antibody-secreting plasma cells are long-lived [52**]. Use of the same anti-CD20 mAb in mouse models for autoimmune diseases led to similar conclusions [53,54], thus suggesting that B cell depletion therapies may be ineffective in reducing autoantibodies in some autoimmune diseases, if cells which produce autoantibodies are long-lived CD20- plasma cells.
Saturation of clearance mechanisms
B cell depletion in an SLE mouse model with an anti-CD20 mAb was quite difficult, especially for clearing splenic B cells [55*]. The authors suggested, “a constitutive excess of IC may inhibit macrophage function, most likely in an FcγR-dependent manner”. There is substantial similarity in this SLE mouse model to “exhaustion” of clearance mechanisms discussed previously with respect to anti-CD20 mAb immunotherapy under conditions of high tumor burden.
Concluding remarks
Anti-CD20 immunotherapy is successful in the treatment of various cancers and inflammatory diseases. However, current therapy may be limited by exhaustion of immune effector mechanisms and therefore the full potential of anti-CD20 immunotherapy may not yet be fully realized. For example, high burdens of IgG-opsonized cells may deplete complement and also exhaust cellular cytotoxicity mediated by NK cells and tissue macrophages. Ineffective killing of CD20-positive cells may furthermore lead to substantial reductions in CD20 surface expression (shaving). Strategies to address these limitations include the development of novel anti-CD20 mAbs which more potently recruit effector function, and the potential combination of antibody therapy with immune modifying drugs that mobilize or replenish effector functions. Lessons learned from the strategies employed for CD20 immunotherapy may be useful for the optimization of other immunotherapeutic mAbs.
Acknowledgments
Work from the authors' laboratory described in this report was supported by The University of Virginia Cancer Support Grant (NIH) and the Commonwealth Foundation for Cancer Research.
Glossary of abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CDC
complement-dependent cytotoxicity
- CLL
chronic lymphocytic leukemia
- IC
immune complex(es)
- ITP
immune thrombocytopenic purpura
- OFA
ofatumumab
- PBMC
peripheral blood mononuclear cells
- RA
rheumatoid arthritis
- RTX
rituximab
- SLE
systemic lupus erythematosus
Footnotes
Conflict of Interest. The authors' laboratory has received research support from Genmab, the maker of ofatumumab
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ronald P. Taylor, Email: rpt@virginia.edu.
Margaret A. Lindorfer, Email: mal9e@virginia.edu.
Reference List
References and recommended reading
* of special interest
** of outstanding interest
- 1.Eisenberg R, Albert D. B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2005;2:1–8. doi: 10.1038/ncprheum0042. [DOI] [PubMed] [Google Scholar]
- 2.Sabahi R, Anolik JH. B-cell-targeted therapy for systemic lupus erythematosus. Drugs. 2006;66:1933–1948. doi: 10.2165/00003495-200666150-00004. [DOI] [PubMed] [Google Scholar]
- 3.Looney RJ, Srinivasan R, Calabrese LH. The effects of rituximab on immunocompetency in patients with autoimmune disease. Arthritis Rheum. 2008;58:5–14. doi: 10.1002/art.23171. [DOI] [PubMed] [Google Scholar]
- *4.Glennie MJ, French R, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]; This comprehensive review examines in detail the mechanisms by which RTX and other anti-CD20 mAbs mediate B cell killing. It provides a clear description of the differences between “Type I” and “Type II” anti-CD20 mAbs with respect to their binding to CD20 on the cells and their separate cytotoxic actions.
- *5.Taylor RP, Lindorfer MA. On the mechanism of action of rituximab in autoimmune disease: the immune complex decoy hypothesis. Nat Clin Pract Rheumatol. 2007;3:86–95. doi: 10.1038/ncprheum0424. [DOI] [PubMed] [Google Scholar]; This article evaluates the results of numerous clinical studies focused on the use of RTX in the treatment of autoimmune diseases. Based on analyses of these results, the Immune Complex Decoy Hypothesis is formulated and presented.
- 6.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 7.Kennedy AD, Solga MD, Schuman TA, Chi AW, Lindorfer MA, Sutherland WM, Foley PL, Taylor RP. An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by Rituximab. Blood. 2003;101:1071–1079. doi: 10.1182/blood-2002-03-0876. [DOI] [PubMed] [Google Scholar]
- 8.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 9.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. Antibody isotype-specific engagement of Fcγ receptors regulates B lymphocyte depletion during CD20 immunotherapy. J Exp Med. 2006;203:743–753. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Lefebvre ML, Krause SW, Salcedo M, Nardin A. Ex vivo-activated human macrophages kill chronic lymphocytic leukemia cells in the presence of Rituximab: mechanism of antibody-dependent cellular cytotoxicity and impact of human serum. J Immunother. 2006;29:388–397. doi: 10.1097/01.cji.0000203081.43235.d7. [DOI] [PubMed] [Google Scholar]; This paper confirms that human macrophages can indeed kill RTX-opsonized cells, principally via phagocytosis.
- 12.Congy-Jolivet N, Probst A, Watier H, Thibault G. Recombinant therapeutic monoclonal antibodies: Mechanisms of action in relation to structural and functional duality. Crit Rev Oncol Hematol. 2007;64:226–233. doi: 10.1016/j.critrevonc.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Cardarelli PM, Quinn M, Buckman D, Fang Y, Colcher D, King D, Bebbington C, Yarranton G. Binding to CD20 by anti-B1 antibody or F(ab′)2 is sufficient for induction of apoptosis in B-cell lines. Cancer Immunol Immunother. 2002;51:15–24. doi: 10.1007/s00262-001-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manches O, Lui G, Chaperot L, Gressin R, Molens JP, Jacob MC, Sotto JJ, Leroux D, Bensa JC, Plumas J. In vitro mechanisms of action of rituximab on primary non-Hodgkin's lymphomas. Blood. 2002;101:949–954. doi: 10.1182/blood-2002-02-0469. [DOI] [PubMed] [Google Scholar]
- 15.Macor P, Tripodo C, Zorzet S, Piovan E, Bossi F, Marzari R, Amadori A, Tedesco F. In vivo targeting of human neutralizing antibodies against CD55 and CD59 to lymphoma cells increases the antitumor activity of rituximab. Cancer Res. 2007;67:10556–10563. doi: 10.1158/0008-5472.CAN-07-1811. [DOI] [PubMed] [Google Scholar]
- 16.Golay J, Zaffaroni L, Vaccari T, Lazari M, Borleri G, Bernasconi S, Tedesco F, Rambaldi A, Introna M. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement mediated cell lysis. Blood. 2000;95:3900–3908. [PubMed] [Google Scholar]
- 17.Golay J, Manganini M, Facchinetti V, Gramigna R, Broady R, Borleri G, Rambaldi A, Introna M. Rituximab-mediated antibody-dependent cellular cytotoxicity against neoplastic B cells is stimulated strongly by interleukin-2. Haematologica. 2003;88:1002–1012. [PubMed] [Google Scholar]
- 18.Dall'Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, Watier H, Thibault G. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64:4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 19.Fischer L, Penack O, Gentilini C, Nogai A, Muessig A, Thiel E, Uharek L. The anti-lymphoma effect of antibody-mediated immunotherapy is based on an increased degranulation of peripheral blood natural killer (NK) cells. Exp Hematol. 2006;34:753–759. doi: 10.1016/j.exphem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- **20.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells -enhancement by therapeutic antibodies. PLoS ONE. 2007;2:1–7. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article investigates the mechanisms that limit NK cell-mediated ADCC. The results clearly indicate that killing can be exhausted at high substrate cell burden, and that IL-2 treatment appears to allow the cells to “re-cycle” and resume ADCC.
- 21.Berdeja JG, Hess A, Lucas DM, O'Donnell P, Ambinder RF, Diehl LF, Carter-Brookins D, Newton S, Flinn IW. Systemic interleukin-2 and adoptive transfer of lymphokine-activated killer cells improves antibody-dependent cellular cytotoxicity in patients with relapsed B-cell lymphoma treated with rituximab. Clin Cancer Res. 2007;13:2392–2399. doi: 10.1158/1078-0432.CCR-06-1860. [DOI] [PubMed] [Google Scholar]
- 22.Bowles JA, Weiner GJ. CD16 polymorphisms and NK activation induced by monoclonal antibody-coated target cells. J Immunol Methods. 2005;304:88–99. doi: 10.1016/j.jim.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, Densmore JJ, Williams ME, Taylor RP. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004;172:3280–3288. doi: 10.4049/jimmunol.172.5.3280. [DOI] [PubMed] [Google Scholar]
- 24.Klepfish A, Schattner A, Ghoti H, Rachmilewitz EA. Addition of fresh frozen plasma as a source of complement to rituximab in advanced chronic lymphocytic leukemia. Lancet Oncol. 2007;8:361–362. doi: 10.1016/S1470-2045(07)70106-7. [DOI] [PubMed] [Google Scholar]
- 25.Williams ME, Densmore JJ, Pawluczkowycz AW, Beum PV, Kennedy AD, Lindorfer MA, Hamil SH, Eggleton JC, Taylor RP. Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. J Immunol. 2006;177:7435–7443. doi: 10.4049/jimmunol.177.10.7435. [DOI] [PubMed] [Google Scholar]
- 26.Beum PV, Kennedy AD, Williams ME, Lindorfer MA, Taylor RP. The shaving reaction: Rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J Immunol. 2006;176:2600–2609. doi: 10.4049/jimmunol.176.4.2600. [DOI] [PubMed] [Google Scholar]
- **27.Kavai M, Szegedi G. Immune complex clearance by monocytes and macrophages in systemic lupus erythematosus. Autoimmun Rev. 2007;6:497–502. doi: 10.1016/j.autrev.2007.01.017. [DOI] [PubMed] [Google Scholar]; Although this paper does not investigate anti-CD20 mAbs, it summarizes and clarifies many of the questions concerning the activity of cells that express FcγR in SLE. This article is important in view of the increasing interest in the use of RTX in the treatment of SLE.
- 28.Li Y, Williams ME, Cousar JB, Pawluczkowycz AW, Lindorfer MA, Taylor RP. Rituximab/CD20 complexes are shaved from Z138 mantle cell lymphoma cells in intravenous and subcutaneous SCID mouse models. J Immunol. 2007;179:4263–4271. doi: 10.4049/jimmunol.179.6.4263. [DOI] [PubMed] [Google Scholar]
- 29.Laurent C, de Paiva GR, Ysebaert L, Laurent G, March M, Delsol G, Brousset P. Characterization of bone marrow lymphoid infiltrates after immunochemotherapy for follicular lymphoma. Am J Path. 2007;128:974–980. doi: 10.1309/LREBX069UXDYMBXV. [DOI] [PubMed] [Google Scholar]
- 30.Leandro MJ, Cooper N, Cambridge G, Ehrenstein MR, Edwards JCW. Bone marrow B-lineage cells in patients with rheumatoid arthritis following rituximab therapy. Rheumatism. 2007;46:29–36. doi: 10.1093/rheumatology/kel148. [DOI] [PubMed] [Google Scholar]
- 31.Teng YKO, Levarht EWN, Hashemi M, Bajema IM, Toes REM, Huizinga TWJ, van Laar JM. Immunohistochemical analysis as a means to predict responsiveness to rituximab treatment. Arthritis Rheum. 2007;56:3909–3918. doi: 10.1002/art.22967. [DOI] [PubMed] [Google Scholar]
- 32.Beum PV, Kennedy AD, Taylor RP. Three new assays for rituximab based on its immunological activity or antigenic properties: analyses of sera and plasmas of RTX-treated patients with chronic lymphocytic leukemia and other B cell lymphomas. J Immunol Methods. 2004;289:97–109. doi: 10.1016/j.jim.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Hatjiharissi E, Xu L, Santos DD, Hunter ZR, Ciccarelli BT, Verselis S, Modica M, Cao Y, Manning RJ, Leleu X, Dimmock EA, Kortsaris A, Mitsiades C, Anderson KC, Fox EA, Treon SP. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the FcγRIIIa-158 V/V and V/F polymorphism. Blood. 2007;110:2561–2564. doi: 10.1182/blood-2007-01-070656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Bowles JA, Wang SY, Link BK, Beuerlein G, Campbell MA, Marquis D, Ondek B, Wooldridge JE, Smith BJ, Breitmeyer JB, Weiner GJ. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108:2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Masuda K, Kubota T, Kaneko E, Iida S, Wakitani M, Kobayashi-Natsume Y, Kubota A, Shitara K, Nakamura K. Enhanced binding affinity for FcγRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Mol Immunol. 2007;44:3122–3131. doi: 10.1016/j.molimm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- *36.de Romeuf C, Dutertre CA, Le Garff-Tavernier M, Fournier N, Gaucher C, Glacet A, Jorieux S, Bihoreau N, Behrens CK, Beliard R, Vieillard V, Cazin B, Bourel D, Prost JF, Teillaud JL, Merle-Beral H. Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcγRIIIA/CD16. Br J Haematol. 2008;140:635–643. doi: 10.1111/j.1365-2141.2007.06974.x. [DOI] [PubMed] [Google Scholar]; These three papers demonstrate that changes in the FcγR region of anti-CD20 mAbs can enhance ADCC, due to enhanced binding to FcγRIIIa on NK cells.
- 37.Golay J, Lazzari M, Facchinetti V, Bernasconi S, Borleri G, Barbui T, Rambaldi A, Introna M. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood. 2001;98:3383–3389. doi: 10.1182/blood.v98.12.3383. [DOI] [PubMed] [Google Scholar]
- 38.Teeling J, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, Chan C, Parren PW, Hack CE, Dechant M, Valerius T, van de Winkel JG, Glennie MJ. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin's lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- *39.Teeling JL, Mackus WJM, Wiegman LJJM, van den Brakel JHN, Bees SA, French RR, van Meerten T, Ebeling S, Vink T, Slootstra JW, Parren PWHI, Glennie MJ, van de Winkel JGJ. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]; This paper (and the earlier report by the same group) describes in detail the immunochemical characteristics of OFA, including why it is capable of enhanced CDC compared to RTX.
- 40.Bleeker WK, Munk ME, Mackus WJM, van den Brakel JHN, Pluyter M, Glennie MJ, van de Winkel JGJ, Parren PWHI. Estimation of dose requirements for sustained in vivo activity of a therapeutic human anti-CD20 antibody. Br J Haematol. 2008;140:303–312. doi: 10.1111/j.1365-2141.2007.06916.x. [DOI] [PubMed] [Google Scholar]
- 41.Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, Van Oers MHJ, Wooldridge J, Kloczko J, Holowiecki J, Hellman A, Walewski J, Flensburg M, Peterson J, Robak T. Safety and efficacy of ofatumumab, a fully human monclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia. A phase I-II study. Blood. 2008;111:1094–1100. doi: 10.1182/blood-2007-09-111781. [DOI] [PubMed] [Google Scholar]
- 42.Cambridge G, Leandro MJ, Teodorescu M, Manson J, Rahman A, Isenberg DA, Edwards JC. B cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profiles. Arthritis Rheum. 2006;54:3612–3622. doi: 10.1002/art.22211. [DOI] [PubMed] [Google Scholar]
- 43.Edwards JCW, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 44.Levi M, Hack CE, Van Oers MH. Rituximab-induced elimination of acquired angioedema due to C1-inhibitor deficiency. Am J Med. 2006;119:e3–e5. doi: 10.1016/j.amjmed.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of Rituximab. Arthritis Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 46.Eisenberg R, Looney RJ. The therapeutic potential of anti-CD20: What do B-cells do? Clin Immunol. 2005;117:207–213. doi: 10.1016/j.clim.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Sfikakis PP, Boletis JN, Lionaki S, Vigklis V, Fragiadaki KG, Iniotaki A, Moutsopoulos HM. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand. Arthritis Rheum. 2005;52:501–513. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 48.Stasi R, Pagano A, Stipa E, Amadori S. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adults with chronic idiopathic thrombocytopenic purpura. Blood. 2001;98:952–957. doi: 10.1182/blood.v98.4.952. [DOI] [PubMed] [Google Scholar]
- 49.Introna M, Golay J, Barbui T. Rituximab: a new therapeutic tool for primary immune thrombocytopenic purpura? Haematologica. 2003;88:482–484. [PubMed] [Google Scholar]
- 50.Ferraro AJ, Drayson MT, Savage COS, MacLennan ICM. Levels of autoantibodies, unlike antibodies to all extrinsic antigen groups, fall following B cell depletion with Rituximab. Eur J Immunol. 2008;38:292–298. doi: 10.1002/eji.200737557. [DOI] [PubMed] [Google Scholar]
- 51.Smith KGC, Jones RB, Burns SM, Jayne DRW. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis. Arthritis Rheum. 2006;54:2970–2982. doi: 10.1002/art.22046. [DOI] [PubMed] [Google Scholar]
- **52.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]; This definitive mouse model paper shows that anti-CD20 therapy has little, if any effect on the levels of existing antibodies in mice based on previous exposure to and immunization with specific antigens because long-lived plasma cells are not depleted by anti-CD20 mAbs. The paper has important implications for our understanding of how RTX may function in the therapy of autoimmune diseases, and why individuals who receive RTX therapy are generally not at increased risk for infections.
- 53.Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, St Clair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis inducation requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 54.Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz JD, Uchida J, Fujimoto M, Matsushita T, Matsushita Y, Horikawa M, Komura K, Takehara K, Sato S, Tedder TF. B-Lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Path. 2006;169:954–966. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **55.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3359–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]; This paper investigates the mode of action of anti-CD20 mAbs in mouse models of SLE, and the results, taken in the context of the contribution by Kavai [27**], have important implications with respect to understanding the efficacy of B cell depletion therapies in human SLE.