Abstract
Contraceptive hormones, most commonly prescribed as oral contraceptives (OC), are a widely utilized method to prevent ovulation, implantation and therefore pregnancy. The Women’s Health Initiative demonstrated cardiovascular risk linked to menopausal hormone therapy among women without pre-existing cardiovascular disease, prompting review of the safety, efficacy and side effects of other forms of hormone therapy. A variety of basic science, animal and human data suggest that contraceptive hormones have anti-atheromatous effects, however relatively less is known regarding the impact on atherosclerosis, thrombosis, vasomotion and arrhythmogenesis. Newer generation OC formulations currently in use indicate no increased myocardial infarction (MI) risk for current users, but a persistent increased risk of venous thrombo-embolism (VTE). There are no cardiovascular data available for the newest generation contraceptive hormone formulations, including those that contain newer progestins that lower blood pressure, as well as the non-oral routes (topical and vaginal). Current guidelines indicate that, as with all medication, contraceptive hormones should be selected and initiated by weighing risks and benefits for the individual patient. Women 35 years and older should be assessed for cardiovascular risk factors including hypertension, smoking, diabetes, nephropathy and other vascular diseases including migraines, prior to use. Existing data are mixed with regard to possible protection from OC for atherosclerosis and cardiovascular events; longer-term cardiovascular follow-up of menopausal women with regard to prior OC use, including subgroup information regarding adequacy of ovulatory cycling, the presence of hyperandrogenic conditions, and the presence of prothrombotic genetic disorders is needed to address this important issue.
Keywords: Hormones, Contraception, Cardiovascular Disease
Introduction
In the United States, hormone therapy delivered as oral contraceptives (OC) is one of the most commonly prescribed birth control methods, used by 11.6 million or 19% of women (1). Since their introduction in the 1960s, OC have been used by approximately 80% of US women at some point in their life to block ovulation, implantation, and therefore pregnancy (2). The simplicity of the available regimens, low frequency of side effects, and relative safety compared to pregnancy (3) have resulted in widespread use.
Observational studies demonstrate that young women have a relatively lower age-adjusted risk of cardiovascular disease compared to men. Cardiovascular risk rises following menopause (4), suggesting that endogenous reproductive hormones may play a protective role. We and others have further demonstrated that disruption of ovulatory cycling, indicated by estrogen deficiency and hypothalamic dysfunction (5), or irregular menstrual cycling (6,7) in premenopausal women is associated with an increased risk of coronary atherosclerosis and adverse cardiovascular events, respectively. The concept that premenopausal contraceptive hormone use may be protective for atherosclerosis is appealing.
Conversely, recently published data on mortality from cardiovascular disease has shown that since the year 2000, mortality rates have increased in women between the ages of 35 and 44 years compared to decreases in all other age groups (4). Increased rates of obesity and smoking, and declines in physical activity are prevalent in this group of young women (8). Also coincident in this age group was an increased OC use during the same decades, from 4% to 17% (1,2). In part because OC are effective and safe for contraception, and because premenopausal women are at relatively lower cardiovasculoar risk than the general public, there has been relatively little specific study devoted to evaluating links between contraceptive hormone use and cardiovascular disease.
Data from the Women’s Health Initiative that demonstrated an increased cardiovascular risk with menopausal hormone therapy use among women without pre-existing cardiovascular disease (9-11), has prompted review of risks and benefits of other forms of hormone therapy for women. This review outlines the physiology and mechanisms of cardiovascular action of contraceptive hormones, particularly those found in OC. It includes basic science, animal and human clinical studies that address contraceptive hormone use and cardiovascular disease. We also review the current guidelines for contraceptive hormone use in women with elevated cardiovascular risk.
Estrogen and Progesterone Physiology
Endogenous estrogen is produced by the ovaries in the form of 17β-estradiol, which acts at two estrogen receptors, ERα and ERβ, with equal binding affinity (12-14). There are two known pathways triggered by estrogen activation of these receptors, commonly referred to as the genomic and non-genomic pathways. The genomic pathway occurs through ligand binding, in which estrogen as a steroid passes through the lipid membrane and binds receptors located in the nucleus, which either activates or suppresses gene transcriptions. The non-genomic pathway is a rapid activation of the receptor located at the cell membrane and causes a release of intracellular messengers such as nitric oxide, calcium or kinases. For example, the non-genomic pathway results in activation of nitric oxide synthase to cause acute arterial vasodilation (15,16).
Endogenous progesterone blood levels rise each month from the corpus leutem after ovulation and remain high during the luteal menstrual phase to inhibit ovulation, and eventually drop at the time of menstruation (17). Progestins are the synthetic form of the hormone progesterone derived from 19-nortestosterone, 17-OH progesterone derivatives or 19-norprogesterone (18). Bio-identical progesterone is used for menopausal therapy but not for contraception. There are many types of progestins, each differing in their potency and affinity to the progesterone, estrogen, and androgen receptors. Levonogestrel and norethindrone directly bind to the receptor while desogestrol needs to be actively converted in the body before being bioavailable (17). The newer progestins, including gestodene, desogestrel and norgestimate, are selective in that they have little androgenic effect while inhibiting ovulation and endometrial hypertrophy. The newest contraceptive and menopausal hormone formulations include combinations of estrogen and drospirenone, where the progestin is derived from spironolactone and has antiandrogenic and diuretic properties (19).
Contraceptive Hormones – Initiation and Evolution
Contraceptive hormones were first introduced in the 1960s as oral OC that simulated a state of pregnancy by causing high hormonal blood levels that suppressed ovulation and implantation. The “Pill” was developed to be cyclical, with a 28-day cycle of three weeks of continuous combined fixed dose estrogen and progestin followed by one week of sham pills. This design induced hormonal withdrawal bleeding to simulate the monthly menses and reassure women of the absence of pregnancy.
There have been three main evolutions in OC development, including changes to: 1) the dose and types of hormones used; 2) the formulation timing and dosing; and 3) the delivery method. Doses of contraceptive hormones have decreased considerably since the 1960’s, with initial OC containing relatively high doses of both estrogen and progestin. While first generation estrogen doses started at 150 mcg, second generation dosages decreased to 50 mcg, and current generation doses are now even lower, ranging from 20-35 mcg of ethinyl estradiol (EE)(20). Contemporary OC remain at fairly high estrogen doses in contrast to menopausal hormone therapy, which typically contains one-tenth the dose or the equivalent of 2.5-5 mcg of EE.
Contraceptive hormone formulation timing and dosing also varies. Table 1 outlines current hormonal contraceptive formulations available in the United States. Monophasic dosing consists of doses that do not vary throughout the entire month, while in tricyclic dosing, the progestin portion of the contraceptive hormone increases each week to mimic the natural hormonal cycling in a woman. While many OC are still taken for 21 days with a 7 day sham pill or no treatment phase, continuous dosing formulations of OC which produce 4 menses per year, and a continuous monophasic low-dose formulation that is taken 365 days per year with virtually absent menses have been approved (21).
Table 1.
Overview of Hormonal Contraception Formulations Available in United States 2008
| Oral Triphasic Formulation | ||
|---|---|---|
| Estrogen/ Progestin* | Dose | Brand/Trade Name |
| ethinyl estradiol/desogestrel | 25 mcg/0.1,0.125,0.15 mg | Cyclessa |
| ethinyl estradiol/levonorgestrel | 30 mcg/0.05mg, 40mcg/0.075mg, 30mcg/0.125mg | Enpresse, Trivora |
| ethinyl estradiol/norgestimate | 25 mcg/0.18,0.215,0.25 mg | Ortho Tri-Cyclen Lo |
| 35 mcg/0.18,0.215,0.25 mg | Ortho Tri-Cyclen, Tri-Previfem, Tri-Sprintec, TriNessa | |
| ethinyl estradiol/norethindrone | 35 mcg/0.5,0.75,1 mg | Necon 7/7/7, Ortho-Novum 77/7/7 Aranelle, Tri-Norinyl |
| 35 mcg/0.5,1,0.5 mg | ||
| Oral Monophasic Formulation | ||
|---|---|---|
| Estrogen/ Progestin† | Dose | Brand/Trade Name |
| ethinyl estradiol/levonorgestrel | 20 mcg/0.09 mg | Lybrel |
| 20 mcg/0.1 mg | Alesse, Aviane, Lutera | |
| 30 mcg/0.15mg | Jolessa, Levora, Nordette, Portia, Quasense, Seasonale§ | |
| 30mcg/0.15mg, 10mcg/0mg | Seasonique§ | |
| ethinyl estradiol/ desogestrel | 30 mcg/0.15 mg | Apri, Desogen, Reclipsen, |
| ethinyl estradiol/norethindrone | 20 mcg/1 mg‡ | Junel 21 1/20, Loestrin 21 1/20, Loestrin 24 Fe 1/20‡, Microgestin 1/20, Microgestin Fe 1/20 |
| 30 mcg/1.5 mg | Junel 21 1.5/30, Loestrin 21 1.5/30, Loestrin Fe 1.5/30, Microgestin 1.5/30, Microgestin Fe 1.5/30 | |
| 35 mcg/0.4 mg | Balziva, Femcom Fe, Ovcon35 | |
| 35 mcg/0.5 mg | Brevicon, Modicon, Necon 0.5/35 | |
| 35 mcg/1 mg | Necon 1/35, Norinyl 1/35, Ortho-Novum 1/35 | |
| 50 mcg/1 mg | Necon 1/50, Ovcon 50 | |
| ethinyl estradiol/norgestrel | 30 mcg/0.3 mg | Cryselle, Lo/Ovral, Low-Ogestrel |
| ethinyl estradiol/norgestimate | 35 mcg/0.25 mg | MonoNessa, Ortho-Cyclen, Previfem, Sprintec |
| mestranol/norethindrone | 50 mcg/1 mg | Norinyl 1/50 |
| ethinyl estradiol/drospirenone | 20 mcg/3 mg‡ | Yaz |
| 30 mcg/3 mg | Ocella, Yasmin | |
| ethinyl estradiol/ethynodiol | 35 mcg/1 mg | Kelnor, Zovia 1/35 |
| 50 mcg/1 mg | Zovia 1/50 | |
| ethinyl estradiol/desogestrel | 30mcg/0.15 mg | Ortho-Cept |
| Non-Oral Combined Formulations | ||
|---|---|---|
| Transdermal Estrogen/Progestin | Dose | Brand/Trade Name |
| ethinyl estradiol/norelgestromin | 20mcg/0.15mg/day patch | Ortho Evra |
| Vaginal Ring Estrogen/Progestin | Dose | Brand/Trade Name |
|---|---|---|
| ethinyl estradiol/etonogestrel vaginal | 15mcg/0.12mg/day vaginal ring | NuvaRing |
| Progestin only | ||
|---|---|---|
| Oral Progestin only | Dose | Brand/Trade Name |
| norethindrone | 0.35 mg | Camilla, Errin, Jolivette, Nor-QD, OrthoMicronor, Ovrette |
| Progestin injection | Dose | Brand/Trade Name |
|---|---|---|
| medroxyprogesterone acetate | 150mg, intramuscular, every 3 months | Depo-Provera |
| 104mg, subcutaneous, every 3 months | Depo-SubQ Provera | |
| Progestin releasing IUD | Dose | Brand/Trade Name |
|---|---|---|
| levonorgestrel | 52 mg IUD, daily release 20mcg | Mirena |
21 active tablets and 7 placebo, active tablets divided into 7 tablet doses as indicated.
21 active tablets and 7 placebo, active tablets are all same dose
24 active tablets and 4 placeb
91-day extended formulation available with 84 consecutive active tablets and 7 placebo or 10mcg estradiol
OC are classified into generations (first, second, and third), depending upon their introduction into the US market, and vary according to their dose of estrogen and type of progestin used. The first generation OC used progestins called ‘estranes,’ such as norethindrone, norethindrone-acetate, or ethynodiol diacetate. This generation of OC contained 2-5 times the dose of estrogens and up to 10 times the dose of progestins compared to later generations (22). All subsequent generation OC contained ≤ 50 mcg estrogen and varied by the type of progestin used. The second generation used progestins called ‘gonanes,’ which are more potent and allowed use of lower doses to produce an anovulatory effect. Examples include levonorgestrel (LNG) or norgestimate. Third generation OC are also gonane progestins, such as desogestrol or gestondene, and have reduced androgenic and metabolic side effects. Most recently available are two non-testosterone derived progestins, chlormadinone acetate and drospirenone, which may lead to a fourth generation classification. Drospirenone is an aldosterone antagonist with anti-androgenic and diuretic effects (19).
Contraceptive hormones also vary according to the method of delivery, and now include non-oral routes such as the combined estrogen/progestin transdermal patch and vaginal ring. The transdermal patch or vaginal ring is worn continuously for 21 days and removed for 7 days and delivers a continuous estrogen and progestin formulation. Both of these methods avoid first-pass metabolism in the liver, provide continuous hormone dosing, and simplify compliance (23-25).
Mechanisms of Estrogen and Progestin Action on the Cardiovascular System
Estrogen receptors are found throughout the body in essentially all tissues in both women and men, and play an important role in health and disease. In animal models, estrogen administration directly prevents atherosclerosis (12). Specific pathways to the cardiovascular system include activation of the ERα receptor on endothelial and myocardial cells that has antioxidant effects and improved endothelial cell injury recovery (12). Estrogen receptors in the cardiovascular system modulate a rapid vasodilatory response via nitric oxide, and also have long-term effects via the genomic pathway by increasing endothelial-cell growth and inhibiting smooth muscle cell proliferation. Estrogen reduces low density lipoprotein cholesterol (LDL-C) oxidation and binding, platelet aggregation, and increases cyclooxygenase-2 activity (12). There is relatively less known regarding cardiovascular actions of progesterone and progestins.
Lipoproteins
Estrogen also affects the cardiovascular system indirectly through its impact on cardiovascular risk factors such as the lipid profile. OC alter the lipid profile via the genomic pathway, in which ER alterations affect hepatic apolipoprotein upregulation (12,26,27). Studies in premenopausal women using OC have shown a dose-related response in the lipid profile. Women using a 20 mcg EE/100 mcg levonorgestrel OC demonstrated reductions in high density lipoprotein cholesterol (HDL-C) and small increases in LDL-C and triglycerides, in contrast to a 30 mcg EE/150 mcg levonorgestrel OC (28,29). The amount of lipid alteration also depends on the delivery route, where transdermal contraceptive hormone delivery is relatively less potent compared to oral (12). Barkfeldt et al (30) conducted a randomized, double-blind study that evaluated the effects of lipid metabolism on 98 women who received two different types of progestin-only pills, desogestrel 75μg/day or levonorgestrel 30μg/day. There were minimal changes seen to the lipid profile with decreased levels of HDL-C, its subfractions, and the apolipoproteins apolipoprotein-I and II. No differences were observed between the two formulations despite the higher progestin dose found in desogestrel, including no changes in LDL-C or apolipoprotein B (30).
Blood Pressure
Most studies on blood pressure in normotensive women have shown an increase in blood pressure associated with OC use (31). A review of two studies found an increase in systolic blood pressure by 7-8 mmHg on average compared with those not using OC (32,33). The newer progestins such as drospirenone, with anti-mineralocorticoid diuretic effect, produce lower blood pressure. In a study of 120 women randomized to drospirenone/EE or levonogestrel/EE, the drospirenone group demonstrated a mean decrease in the systolic blood pressure (from 107.4 to 103.5 mm Hg), and had a statistically significant lower group mean blood pressure compared to the levonorgestrel group (34). Another study of 80 healthy women randomized into groups of 3 mg of drospirenone combined with 30μg, 20μg, or 15μg doses EE found that systolic blood pressure at six months fell by a range of 1-4 mm Hg across the groups, compared to an elevation of blood pressure of 4 mmHg in the control group of levonorgestrel/EE (35). Additionally, body weight fell by a range of 0.8-1.7 kg in the groups receiving the drospirenone compared to an increase in the levonorgestrel/EE group by 0.7 kg.
Glucose Tolerance and Diabetes Mellitus
Contraceptive hormones can also impact glucose tolerance and diabetes mellitus. Oelkers et al (35) studied glucose levels in 80 healthy women who received 3 mg of drospirenone combined with 30μg, 20μg, or 15μg doses of EE compared to levoneorgestrel/30μg EE. Each woman performed oral glucose tolerance tests at pretreatment and at the end of the six-month OC cycle. On treatment fasting glucose was unchanged for all groups, but the area under the curve for the glucose tolerance increased for all formulations. Although not statistically significant between groups, the drospirenone/30μg EE group had a 19% worsening of glucose tolerance (35). Available evidence with the earlier generation OC demonstrates no apparent worsening of established diabetes (36,37).
Novel Risk Factors
Estrogen use elevates inflammatory markers such as C-reactive protein used in menopausal women (38,39), although it is unclear if this is a specific adverse cardiovascular effect or a nonspecific upregulation of hepatic protein synthesis. Elevations in highly sensitivity C-reactive protein have also been found in third generation OC users containing desogestrel or gestodene. A case-control study of healthy women found high risk levels of high sensitivity C-reactive protein (3-10 mg/L) in 27% of OC users compared to 8.5% of non-OC users (OR 4.04, 95% CI, 1.99-8.18)(40). There is little known regarding hormonal contraception use and other novel risk factors such as homocysteine, uric acid, and other inflammatory markers.
There are additional hormonal pathways that may impact cardiovascular disease. The dose of EE in OC sustains relatively higher blood levels of estrogen than the ovaries in women with normal ovulatory cycling, and ensures adequate estrogen levels in women with ovulatory dysfunction/estrogen deficiency. Prior work demonstrates that up to 33% of premenopausal women can have ovulatory dysfunction and estrogen deficiency, and that this is associated with an increased osteoporosis risk (41). Recent work from the Nurses Health Study has documented a positive association between history of irregular menstrual cycling and adverse cardiovascular events (6), suggesting that ovulatory dysfunction and relatively low estrogen levels may also elevate cardiovascular risk. Contraceptive hormones also suppress ovarian androgens and raise sex hormone binding globulin, thus reducing the free fraction of plasma testosterone. This is a useful mechanism of action of OC in women with polycystic ovary syndrome and hyperandrogenemia, a condition that may be associated with elevated cardiovascular risk (44). Finally, contraceptive hormones appear to blunt the adverse adreno-corticol stress response in primates, which might also offer indirect protection from atherosclerosis via neuroendodrine pathways (42).
Thrombosis
Estrogen has known pro-thrombotic effects and elevates cardiovascular venous thrombo-embolism (VTE) risk by increasing prothrombin and decreasing antithrombin III (14). In a large match case-control study, Sidney et al (43) found that OC use with less than 50 mcg EE was correlated with a four times higher risk of VTE as compared to nonusers (95% CI, 2.77-4.00). Jick et al (44) studied the risk of nonfatal VTE in a case control study of low dose estrogen < 35 mcg plus second generation (levonrgestrel) or third generation (desogestrel or gestodene) progestins and found that after adjusting for smoking and BMI, third generation progestins had a twofold higher risk ratio compared to second generation progestins for nonfatal VTE. It was also noted that the increased risk associated with newer OC formulations was seen in the women who used OC for less than 6 months as compared to longer periods of time, although the difference was not statistically significant.
Coronary Vasomotion
Numerous clinical observations support the role of these reproductive hormones on regulation of vasomotor tone. Migraine headaches, Raynauds and Prinzmetal’s angina are more common in women than man, and can vary according to endogenous or exogenous reproductive hormones (45,46). While animal and human work demonstrates that low endogenous estrogen levels exacerbate endothelial dysfunction (47,48), and that estrogen replacement abolishes this effect (47,49,50), the data are mixed with regard to whether long-term estrogen therapy maintains or improves coronary or peripheral endothelial function in humans (50). Even less is known regarding progesterone, progestins and androgens. Primate study has demonstratesd a coronary vasoconstrictive effect with medroxyprogesterone that was not apparent with progesterone (51,52). More clinical work is needed.
Arrhythmogenesis
Women face a life-long higher risk of sudden cardiac death associated with electrocardiographic QT prolongation compared to men (53), and this is particularly apparent in the post-adolescence years. Androgens have been demonstrated to blunt QT-prolongation in response to quinidine (54), in contrast to estrogens which modify the expression of potassium channels (55). Other investigators have demonstrated that the 9 month post-partum period has a significantly increased risk of cardiac events among women with long QT genotype carriers (56). In healthy postmenopausal women, hormone replacement therapy with estrogen alone usually produces a prolongation of QT interval, while estrogen plus progesterone had no significant effects on QT interval but reduces QT dispersion, however there are conflicting data reported (57,58). Further work is needed to understand the basis of gender differences in ventricular repolarization and arrhythmogenic etiologies of cardiac death. In particular, no study has been directed at the impact of contraceptive hormones and susceptibility to drug-induced QT interval prolongation and drug-induced arrhythmia that is relatively more prevalent in women.
Figure 1 depicts the known mechanisms whereby contraceptive hormones impact the cardiovascular system including effects on atherosclerosis, thrombosis, vasomotion and arrhythmogenesis.
Figure 1.
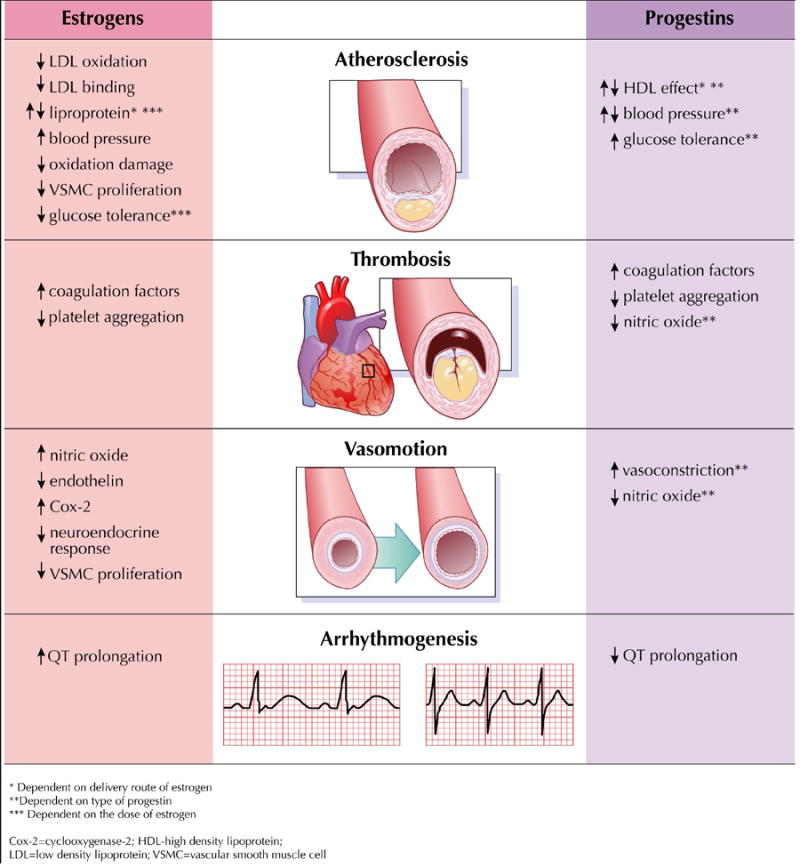
Impact of Hormonal Contraception on Mechanisms of Cardiovascular Disease. Estrogens and progestins individual effects on atherosclerosis, thrombosis, vasomotion and arrhythmogenesis. (12,14,28-37,42,51,52,76,78).
Contraceptive Hormone Use and Cardiovascular Disease
Animal Studies
Stress-induced interruption of the hypothalamic signaling of the ovary, resulting in anovulation and hypoestrogenemia in primates produces premenopausal atherosclerosis in the primate model (46,59,60), and provision of hormone contraception has been demonstrated to block this atherosclerotic effect (60,61). Use of a primate model of premenopausal oophorectomy and menopausal hormone therapy demonstrates similar anti-atherosclerotic effects (62).
Adams et al (63) studied nonhuman primate models in cynomolgus macaques to determine the effect of OC on lipoproteins and atherosclerosis. This design compared placebo to two different formulations of OC over 24 months. Despite both OC preparations reducing plasma HDL-C, both had a 50-75% decrease in the extent of atherosclerosis compared to placebo. This study was further stratified by high-risk status defined by total cholesterol/HDL-C ratio >4.5 and found a relatively greater decrease of atherosclerosis by 75-85% in the high-risk group (63).
A second study in cynomolgus macaques was designed to further assess the impact of separate or combined effects of estrogen and progestin in low-dose OC preparations on atherosclerosis progression. This study randomized the monkeys to receive triphasic combined EE/levonorgestrel, triphasic EE alone, levonorgestrel alone, or a placebo. All groups were treated for a 25-month period and continued on a pro-atherogenic diet. Results showed that among the animals treated with EE alone compared with untreated animals, atherosclerosis was reduced by 67% (p<0.05), while the combination EE/levonorgestrel group had a 28% decrease in atherosclerosis, and the levonorgestrel alone group had no effect (64). Further lipid evaluation demonstrated LDL-C particles that were smaller and less esterified in the EE alone or EE/levonorgestrel groups.
Clinical Studies
Current and Immediate Past Contraceptive Hormone Use in Younger and Mid-Life Women
The Nurses’ Health Study, initiated in 1976, published an eight-year self-report prospective study that assessed the risk of myocardial infarction (MI) and OC use in mid-life women (ages 30-55). This study found no increased risk among past users of OC for cardiovascular disease, nonfatal MI or fatal coronary disease when compared to those who had never used OC (65). Additionally there was no association between the duration of use and cardiovascular disease; women who had used OC for more than 10 years had no alteration in risk. Among current OC users, however, there was a 2.5 relative increased risk of adverse cardiovascular events, including cardiovascular death, nonfatal MI and stroke (65). The increase in cardiovascular deaths and nonfatal MI and stroke in current users but not with past use was believed to be associated with the pro-thrombotic effects, and 7 out of 10 of the adverse cardiovascular events occurred in current cigarette smokers (65). Stopping OC was associated with a decline in the risk for adverse cardiovascular events, with a risk ratio (RR) of 0.95 (CI, 0.81-1.11) among past users, suggestive of reversal of the OC pro-thrombotic effects with cessation of use, however other mechanisms such as an anti-atherosclerotic effect could also be contributory.
Other prospective studies consistently show an increased risk of acute MI among women who concomitantly use OC and smoke, and extend the observation to past smokers on OC (65-68). Notably, these studies evaluated OC predominantly with prior generation OC with the relatively higher estrogen doses compared to those currently used. No studies to date have specifically evaluated the newer fourth generation as well as the non-oral contraceptive hormone preparations with regard to current and immediate past use associated adverse cardiovascular events.
Two separate case control studies evaluated the association between OC use and MI, based on the second- and third-generation preparations with differing progestins and reached varying conclusions. Dunn et al (69) performed a community based case-control study of 2,176 women over a 2 years period and found a lower risk ratio (RR) of 1.78 (0.66 to 4.83) for of MI with third generation OC compared to second generation OC use (Table 2). In this study, third generation OC were defined as progestins gestodene or desogestrel combined with EE compared to second generation OC defined as levonorgestrel and noresthisteronone combined with less than 50 mcg of EE. Tanis et al (70) performed a case-control study of 1,173 women over 6 years and concluded that the use of second generation OC, containing levonorgestrel, increased the risk of MI by a RR of 2.3, while third generation, containing desogestrel or gestodene, and other progestins such as cyproterone or norgestimate, did not significantly increase the risk (Table 2). Additionally, this latter study analyzed subjects for the presence of prothrombotic genetic mutations and concluded that there was a non-significant increased risk in subjects with a Factor V Leiden or prothrombin mutation who used third generation OC (RR 1.9, CI, 0.6-5.5).
Table 2.
Observational Clinical Studies of Risk of CVD Events in Women with Current Contraceptive Hormone Use
| Citation | Definitions OC Generations | N | CVD definition | Time of Follow- up (yrs) | Relative Risk (unless stated, compared with non-users) | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Stampfer, et al, 1988(65) | No distinction made with OC generation | Total 62,718 Cases 485 |
All Cardiovascular deaths, nonfatal MI and CVAs |
8 | Past user 0.95 Current user 2.5 |
(0.81-1.11) * (1.3-4.9) * |
| Jick, et al, 1995(44) | 2nd gen: EE < 35μg + LNG 3rd gen: < 35μg + gestodene or desogestrel |
Total 303,470 Cases 15 |
Death related to cardiovascular cause: MI, CVA, PE, cardiac arrest | 3.8 | RR for 3rd generation (desogestrel) users with 2nd generation as baseline 0.4 RR for 3rd generation (gestodene) users 1.4 |
(0.1-2.1) * (0.5-4.5) * |
| WHO, et al, 1996(80) | No distinction made with OC generation. | Total 1309 Cases 368 |
Non fatal first MI |
5 | Current users OR 5.01 in Europe Current users OR 4.78 in developing countries Past users >10yrs 1.61 |
(2.54-9.90) † (2.52-9.07) † (0.62-4.16) |
| Sidney, et al, 1998(81) | No distinction made with OC generation EE with 50μg and < 50μg Progestins with norethindrone and from gonane family |
Total 1264 Cases 271 |
MI | 3.25 | OR for current OC users 0.56 OR for past OC users 0.54 |
(0.21-1.49) † (0.31-0.95) † |
| Dunn, et al, 1999(69) | 2nd gen: EE < 35μg + norethisterone or LNG 3rd gen: EE < 35μg + gestodene or desogestrel |
Total 2176 Cases 448 |
MI | 2 | All combined OC users 1.4 2nd generation users 1.1 3rd generation users 1.96 Adjusted for 3rd vs 2nd users 1.78 |
(0.78-2.52) (0.52-2.30) (0.87-4.39) (0.66-4.83) |
| Dunn, et al, 2001(82) | 2nd gen: EE < 35μg + norethisterone or LNG 3rd gen: EE < 35μg + gestodene or desogestrel |
Total 532 Cases 110 |
Fatal MI | 2 | 2nd generation OC users 2.88 3rd generation OC users 0.83 |
(1.22-6.77) † (0.25-2.81) † |
| Rosenberg et al, 2001(83) | No distinction made with OC generation EE with ≥50, 35-49, <35 μg Progestin with norethindrone, LNG, desogestrel and norgestimate |
Total 6574 Cases 627 |
Non fatal first MI |
14 | OR for current OC use 1.3 Current user OC by type found NS OR around 1 except those with norethindrone (1st generation) RR 2.5 |
(0.8-2.2) (1.1-5.8) |
| Tanis, et al, 2001(70) | 1st gen: lynestrenol 2nd gen: LNG 3rd gen: desogestrel or gestodene, and other progestins, cyproterone or norestimate |
Total 1173 Cases 248 |
MI | 6 | Past 2nd generation users 2.4 Current 2nd generation users 2.7 Past 3rd generation users 1.3 Current 3rd generation users 1.6 |
(1.6-3.6) † (1.6-4.3) (0.8-2.3) (0.9-2.9) |
| Spitzer, et al, 2002(84) | 2nd gen: EE < 35μg + norgestrel or LNG 3rd gen: EE < 35μg + desogestrel or gestodene |
Total 6,464 | MI | Meta- Analysis 7 studies | OR for 3rd generation users 1.13 OR for 2nd generation users 2.18 OR for 3rd compared to 2nd users 0.62 |
(0.66-1.92) (1.62-2.94) (0.38-0.99) |
| Margolis, et al, 2007(2) | 2nd gen: EE < 35μg + norgestrel or LNG 3rd gen: EE < 35μg + desogestrel |
Total 48,321 Cases 214 |
MI | 11 | Past user 1.0* Current user 0.7* |
(0.7-1.4) † (0.4-1.4) |
CVD=cardiovascular disease; CVA = stroke; EE = ethinyl estradiol; LNG = levonorgestrel; MI = myocardial infarction; PE = pulmonary embolism;
age-adjusted RR;
adjusted for cardiac risk factors: age, BMI, smoking status, alcohol intake, physical activity, hypertension, diabetes, menopausal status, see citation for specific adjusted RF.
A recent prospective study from Sweden followed 48,321 women aged 30-49 years old over an average of 11 years. The study, which ended in 2002, was conducted to determine the risk of MI associated with use of OC. During the follow up period, there were 190 non-fatal MI and 24 deaths due to MI. When adjusted for age as well as cardiac risk factors such as hypertension, smoking status and diabetes, the study found no increased risk of MI in both former and current users of OC (Table 2). Additionally, there was no increased risk of MI in women with duration of use of OC, stratified to over 15 years (RR 0.7 CI, 0.4-1.2)(2).
Table 2 summarizes these cardiovascular risk data stratified according to first, second and third generation OC formulations.
Longer-term Prior Contraceptive Hormone Use in Postmenopausal Women
While it is clear that current OC use is associated with an increased risk of MI in women with pre-existing risk factors such as cigarette smoking (66,69,71), insufficient prior data have existed with regard to longer-term past OC use and subsequent cardiovascular disease in the postmenopausal period. There is a relative paucity of data due to: 1) the relatively short population exposure time (OC have only been available for since the 1960s); 2) the decades needed to perform clinical adverse event studies; 3) the additional follow up time needed due to the majority of cardiovascular disease events occurring later in life among older women. Given the animal and human data consistent with anti-atherosclerotic effects of OC, it is reasonable to hypothesize that compared to non-users, women with a history of OC use in their premenopausal years may be relatively protected against atherosclerosis, resulting in a relatively lower cardiovascular disease burden during postmenopause.
Stampfer and coworkers demonstrated a lower RR for adverse coronary disease events of 0.8 (95% confidence intervals, 0.6-1.0) among the past OC users compared to non-prior users in 119,061 women followed for 8 years (65). While these results were statistically significant, there were relatively few adverse coronary disease events in this population with an approximate mean age of 63 years, and this analysis has not been updated. Similar results suggestive of a protective OC effect have been found in smaller studies evaluating adverse cardiac events (72) and coronary angiography (73). A quantitative meta-analysis of 13 studies included in the Stampfer work provided an estimated RR associated with past OC use of 1.01 (95% CI, 0.91 to 1.13), resulting in their conclusion that past OC use had little or no impact on subsequent cardiovascular disease (74).
One study has directly assessed this question using quantitative measures of atherosclerosis. Past OC use and evidence of atherosclerotic coronary artery disease was assessed in 672 postmenopausal women with coronary risk factors and undergoing coronary angiography for suspected ischemia in the Women’s Ischemia Syndrome Evaluation (WISE) study (75). Past OC hormone use was associated with a 2.4 reduced risk of atherosclerotic coronary artery disease measured by quantitative coronary analysis in a core laboratory despite adjustment for age and coronary risk factors (Figure 2). There was no apparent relation between duration of past OC use and the coronary artery disease severity index score, however. Limitations of this observational study included a greater use of menopausal hormone therapy and a higher risk factor burden among the past users of OC, although these factors may have mitigated toward more adverse cardiovascular events and atherosclerosis in this group, respectively.
Figure 2.
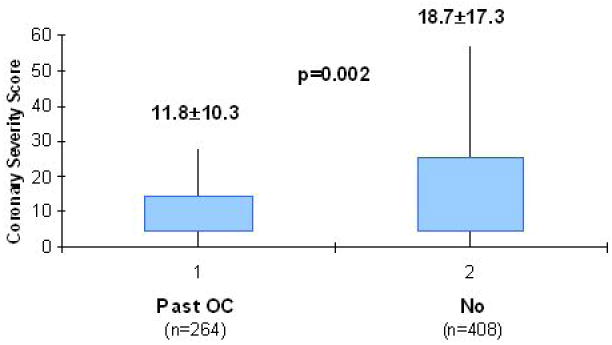
Coronary artery severity score, assessed by quantitative coronary angiography, stratified by reported prior oral contraceptive use. Past OC hormone use was associated with a reduced risk of atherosclerotic coronary artery disease. Reprinted by permission (75).
Current Hormonal Contraceptive Prescribing Guidelines for Women at Elevated Cardiovascular Risk
The American College of Obstetrician and Gynecologists (ACOG) created guidelines for prescribing OC in women with medical conditions, specifically addressing women with cardiovascular risk factors such as hypertension, dyslipidemia, diabetes, smoking, and obesity (76). In addition, ACOG addresses OC use in women older than 35 years. In women with pre-existing hypertension, who are otherwise healthy, OC can be used in well-controlled and monitored women less than 35 years old. If blood pressure remains stable after a few months, then OC may be continued. Current ACOG guidelines recommend pre-treatment fasting lipid profiles in women who are dyslipidemic with monitoring once they have stabilized on an OC. Alternative non-hormonal contraceptive methods, such as an intrauterine device, should be used if the patient has an LDL-C > 160 or multiple cardiac risk factors. OC use in diabetic women, either type I or II, is only appropriate for use in otherwise healthy and less than 35 years. ACOG cautions against prescribing OC in women who smoke and are over the age of 35. Obesity is felt to be an independent risk factor for VTE; therefore, the guideline recommends alternate non-hormonal contraceptive methods. Finally, for women older than 35 years of age, OC with less than 50 mcg EE remain safer than pregnancy in healthy, nonsmoking women, and can be continued until 50-55 years or until menopause. There are no guidelines for transitioning OC to menopausal hormone therapy; however, after the age of 50, measurement of follicle stimulating hormone (FSH) after 6 days off OC to determine menopausal status can provide guidance (77). There are no guidelines about the fourth generation OC to date, thus prudent practice including these as well as the contraceptive transdermal patches is to consider them similar to the other available preparations, and not as safer alternatives. Table 3 summarizes the prescribing guidelines for hormonal contraceptives in women with elevated cardiovascular risk.
Table 3.
Summary of Hormonal Contraceptive Prescribing Guidelines for Women with Elevated Cardiovascular Risk
| Hypertension |
|
| Dyslipidemia |
|
| Diabetes |
|
| Smoking |
|
| Obesity |
|
| Women older than 35 years of age |
|
BMI= body mass index, BP=blood pressure, EE= ethinyl estradiol, IUD= intrauterine device, low density lipoprotein cholesterol, OC=oral contraceptives, VTE=venous thrombo-embolus
Discussion and Recommendations
A variety of basic, animal and human data suggest that contraceptive hormones have anti-atherosclerosis effects, however relatively less is known regarding the impact on thrombosis, vasomotion and arrhythmogenesis, mechanistic pathways which also contribute to cardiovascular risk and benefit. No carefully controlled trials with cardiovascular disease endpoints exist to guide our practice regarding hormonal contraception which is used by over 80% of US women at some point in their lifetime.
Existing observational data with earlier first and second generation, higher dose OC formulations consistently demonstrates small but significantly elevated risks of MI and VTE among current users, particularly smokers, while discontinuation or use of a third generation formulation is associated with a reduction/no elevation in risk. The highest risk of thrombosis appears to occur within the first year of use, appears to be linked with higher estrogen doses, and impacts a select group of women. Newer generation formulations currently in use indicate no increased MI risk for current users, but a persistent increased risk of VTE that is similarly time related.
Measurement of a fasting lipid panel is recommended in women with dyslipidemia prior to use of OC, and alternative non-hormonal contraceptive should be sought if LDL-C is not below 160. Measurement and monitoring of blood pressure is also important to ensure that blood pressure control is not compromised. Women 35 years and older should be assessed for cardiovascular risk including hypertension, smoking, diabetes, nephropathy and other vascular diseases including migraines, prior to OC use. Current WHO and ACOG guidelines for women 35 and older recommend against the use of OC in women with these risk factors (3). OC may be used in the peri-menopausal transition where higher doses of estrogen are needed to suppress ovulation compared to doses needed to treat menopausal symptoms such as hot flashes.
There are no cardiovascular data available for the newest generation contraceptive hormone formulations, including the progestins that lower blood pressure and body weight, as well as the non-oral routes (topical and vaginal). While these newer formulations might be expected to have overall lower risk, specific study is needed. Current guidelines indicate that, as with all medication, contraceptive hormones should be selected and initiated by weighing risks and benefits for the individual patient.
Existing data are mixed with regard to possible protection from early generation OC for atherosclerosis; longer-term cardiovascular follow-up of postmenopausal women with regard to prior OC use, including subgroup information regarding adequacy of ovulatory cycling, the presence of hyperandrogenic conditions, and the presence of prothrombotic genetic disorders, is needed to address this important issue.
Supplementary Material
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, a GCRC grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Denville, New Jersey, the Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, California.
Abbreviations and Acronyms
- ACOG
American College of Obstetrician and Gynecologists
- EE
ethinyl estradiol
- ER
estrogen receptor
- HDL-C
high density lipoprotein cholesterol
- LDL-C
low density lipoprotein cholesterol
- LNG
levonorgestrel
- MI
myocardial infarction
- OC
oral contraceptives
- RR
risk ratio
- VTE
venous thrombo-embolism
Footnotes
Disclosure: There are no relevant conflicts of interest of any of the authors to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chandra A, M G, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of U.S. Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital Health Stat. 2005;23 [PubMed] [Google Scholar]
- 2.Margolis KL, Adami HO, Luo J, Ye W, Weiderpass E. A prospective study of oral contraceptive use and risk of myocardial infarction among Swedish women. Fertil Steril. 2007;88:310–6. doi: 10.1016/j.fertnstert.2006.11.206. [DOI] [PubMed] [Google Scholar]
- 3.Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262–70. doi: 10.1056/NEJMcp0708481. [DOI] [PubMed] [Google Scholar]
- 4.DeStefano F, Merritt RK, Anda RF, Casper ML, Eaker ED. Trends in nonfatal coronary heart disease in the United States, 1980 through 1989. Arch Intern Med. 1993;153:2489–94. [PubMed] [Google Scholar]
- 5.Bairey Merz CN, Johnson BD, Sharaf BL, et al. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2003;41:413–9. doi: 10.1016/s0735-1097(02)02763-8. [DOI] [PubMed] [Google Scholar]
- 6.Solomon CG, Hu FB, Dunaif A, et al. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87:2013–7. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- 7.Snell-Bergeon JK, Dabelea D, Ogden LG, et al. Reproductive history and hormonal birth control use are associated with coronary calcium progression in women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2008;93:2142–8. doi: 10.1210/jc.2007-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991;265:1861–7. [PubMed] [Google Scholar]
- 9.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 10.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–65. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 11.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 12.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 13.Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356:2639–41. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J Am Coll Cardiol. 2006;47:1741–53. doi: 10.1016/j.jacc.2005.10.076. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–6. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitazawa T, Hamada E, Kitazawa K, Gaznabi AK. Non-genomic mechanism of 17 beta-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J Physiol. 1997;499(Pt 2):497–511. doi: 10.1113/jphysiol.1997.sp021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stubblefield P, Carr-Ellis S, Kapp N. In: Berek & Novak’s Gynecology. 14. Berek JS, editor. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 18.Sitruk-Ware R. Pharmacological profile of progestins. Maturitas. 2004;47:277–83. doi: 10.1016/j.maturitas.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Krattenmacher R. Drospirenone: pharmacology and pharmacokinetics of a unique progestogen. Contraception. 2000;62:29–38. doi: 10.1016/s0010-7824(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 20.Cerel-Suhl SL, Yeager BF. Update on oral contraceptive pills. Am Fam Physician. 1999;60:2073–84. [PubMed] [Google Scholar]
- 21.Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception. 2006;73:229–34. doi: 10.1016/j.contraception.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Petitti DB. Clinical practice. Combination estrogen-progestin oral contraceptives. N Engl J Med. 2003;349:1443–50. doi: 10.1056/NEJMcp030751. [DOI] [PubMed] [Google Scholar]
- 23.Oddsson K, Leifels-Fischer B, de Melo NR, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. 2005;71:176–82. doi: 10.1016/j.contraception.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Sibai BM, Odlind V, Meador ML, Shangold GA, Fisher AC, Creasy GW. A comparative and pooled analysis of the safety and tolerability of the contraceptive patch (Ortho Evra/Evra) Fertil Steril. 2002;77:S19–26. doi: 10.1016/s0015-0282(01)03264-2. [DOI] [PubMed] [Google Scholar]
- 25.Baird DT, Glasier AF. Hormonal contraception. N Engl J Med. 1993;328:1543–9. doi: 10.1056/NEJM199305273282108. [DOI] [PubMed] [Google Scholar]
- 26.Jones DR, Schmidt RJ, Pickard RT, Foxworthy PS, Eacho PI. Estrogen receptor-mediated repression of human hepatic lipase gene transcription. J Lipid Res. 2002;43:383–91. [PubMed] [Google Scholar]
- 27.Sitruk-Ware RL, Menard J, Rad M, et al. Comparison of the impact of vaginal and oral administration of combined hormonal contraceptives on hepatic proteins sensitive to estrogen. Contraception. 2007;75:430–7. doi: 10.1016/j.contraception.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Endrikat J, Klipping C, Cronin M, et al. An open label, comparative study of the effects of a dose-reduced oral contraceptive containing 20 microg ethinyl estradiol and 100 microg levonorgestrel on hemostatic, lipids, and carbohydrate metabolism variables. Contraception. 2002;65:215–21. doi: 10.1016/s0010-7824(01)00316-x. [DOI] [PubMed] [Google Scholar]
- 29.Skouby SO, Endrikat J, Dusterberg B, et al. A 1-year randomized study to evaluate the effects of a dose reduction in oral contraceptives on lipids and carbohydrate metabolism: 20 microg ethinyl estradiol combined with 100 microg levonorgestrel. Contraception. 2005;71:111–7. doi: 10.1016/j.contraception.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Barkfeldt J, Virkkunen A, Dieben T. The effects of two progestogen-only pills containing either desogestrel (75 microg/day) or levonorgestrel (30 microg/day) on lipid metabolism. Contraception. 2001;64:295–9. doi: 10.1016/s0010-7824(01)00269-4. [DOI] [PubMed] [Google Scholar]
- 31.Chasan-Taber L, Willett WC, Manson JE, et al. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation. 1996;94:483–9. doi: 10.1161/01.cir.94.3.483. [DOI] [PubMed] [Google Scholar]
- 32.Cardoso F, Polonia J, Santos A, Silva-Carvalho J, Ferreira-de-Almeida J. Low-dose oral contraceptives and 24-hour ambulatory blood pressure. Int J Gynaecol Obstet. 1997;59:237–43. doi: 10.1016/s0020-7292(97)00239-7. [DOI] [PubMed] [Google Scholar]
- 33.Narkiewicz K, Graniero GR, D’Este D, Mattarei M, Zonzin P, Palatini P. Ambulatory blood pressure in mild hypertensive women taking oral contraceptives. A case-control study. Am J Hypertens. 1995;8:249–53. doi: 10.1016/0895-7061(95)96212-3. [DOI] [PubMed] [Google Scholar]
- 34.Suthipongse W, Taneepanichskul S. An open-label randomized comparative study of oral contraceptives between medications containing 3 mg drospirenone/30 microg ethinylestradiol and 150 microg levonogestrel/30 microg ethinylestradiol in Thai women. Contraception. 2004;69:23–6. doi: 10.1016/j.contraception.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Oelkers W, Foidart JM, Dombrovicz N, Welter A, Heithecker R. Effects of a new oral contraceptive containing an antimineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism. J Clin Endocrinol Metab. 1995;80:1816–21. doi: 10.1210/jcem.80.6.7775629. [DOI] [PubMed] [Google Scholar]
- 36.Chasan-Taber L, Willett WC, Stampfer MJ, et al. A prospective study of oral contraceptives and NIDDM among U.S. women. Diabetes Care. 1997;20:330–5. doi: 10.2337/diacare.20.3.330. [DOI] [PubMed] [Google Scholar]
- 37.Kim C, Siscovick DS, Sidney S, Lewis CE, Kiefe CI, Koepsell TD. Oral contraceptive use and association with glucose, insulin, and diabetes in young adult women: the CARDIA Study. Coronary Artery Risk Development in Young Adults. Diabetes Care. 2002;25:1027–32. doi: 10.2337/diacare.25.6.1027. [DOI] [PubMed] [Google Scholar]
- 38.Gol M, Akan P, Dogan E, Karas C, Saygili U, Posaci C. Effects of estrogen, raloxifene, and hormone replacement therapy on serum C-reactive protein and homocysteine levels. Maturitas. 2006;53:252–9. doi: 10.1016/j.maturitas.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Hu P, Greendale GA, Palla SL, et al. The effects of hormone therapy on the markers of inflammation and endothelial function and plasma matrix metalloproteinase-9 level in postmenopausal women: the postmenopausal estrogen progestin intervention (PEPI) trial. Atherosclerosis. 2006;185:347–52. doi: 10.1016/j.atherosclerosis.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Cauci S, Di Santolo M, Culhane JF, Stel G, Gonano F, Guaschino S. Effects of third-generation oral contraceptives on high-sensitivity C-reactive protein and homocysteine in young women. Obstet Gynecol. 2008;111:857–64. doi: 10.1097/AOG.0b013e31816a2476. [DOI] [PubMed] [Google Scholar]
- 41.Prior JC, Vigna YM, Schechter MT, Burgess AE. Spinal bone loss and ovulatory disturbances. N Engl J Med. 1990;323:1221–7. doi: 10.1056/NEJM199011013231801. [DOI] [PubMed] [Google Scholar]
- 42.Clarkson TB, Kaplan JR, Shively CA, Klein KP. Benefits of exogenous oestrogen in inhibiting stress-related coronary artery atherosclerosis. Br J Obstet Gynaecol. 1996;103(Suppl 13):73–8. discussion 78-9. [PubMed] [Google Scholar]
- 43.Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP., Jr Venous thromboembolic disease in users of low-estrogen combined estrogen-progestin oral contraceptives. Contraception. 2004;70:3–10. doi: 10.1016/j.contraception.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Jick H, Jick SS, Gurewich V, Myers MW, Vasilakis C. Risk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestagen components. Lancet. 1995;346:1589–93. doi: 10.1016/s0140-6736(95)91928-7. [DOI] [PubMed] [Google Scholar]
- 45.Lafitte C, Even C, Henry-Lebras F, de Toffol B, Autret A. Migraine and angina pectoris by coronary artery spasm. Headache. 1996;36:332–4. doi: 10.1046/j.1526-4610.1996.3605332.x. [DOI] [PubMed] [Google Scholar]
- 46.Williams JK, Shively CA, Clarkson TB. Determinants of coronary artery reactivity in premenopausal female cynomolgus monkeys with diet-induced atherosclerosis. Circulation. 1994;90:983–7. doi: 10.1161/01.cir.90.2.983. [DOI] [PubMed] [Google Scholar]
- 47.Reis SE, Gloth ST, Blumenthal RS, et al. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994;89:52–60. doi: 10.1161/01.cir.89.1.52. [DOI] [PubMed] [Google Scholar]
- 48.Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81:1680–7. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
- 49.Gilligan DM, Quyyumi AA, Cannon RO., 3rd Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89:2545–51. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- 50.Herrington DM, Werbel BL, Riley WA, Pusser BE, Morgan TM. Individual and combined effects of estrogen/progestin therapy and lovastatin on lipids and flow-mediated vasodilation in postmenopausal women with coronary artery disease. J Am Coll Cardiol. 1999;33:2030–7. doi: 10.1016/s0735-1097(99)00128-x. [DOI] [PubMed] [Google Scholar]
- 51.Hermsmeyer RK, Mishra RG, Pavcnik D, et al. Prevention of coronary hyperreactivity in preatherogenic menopausal rhesus monkeys by transdermal progesterone. Arterioscler Thromb Vasc Biol. 2004;24:955–61. doi: 10.1161/01.ATV.0000126372.14332.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mishra RG, Hermsmeyer RK, Miyagawa K, et al. Medroxyprogesterone acetate and dihydrotestosterone induce coronary hyperreactivity in intact male rhesus monkeys. J Clin Endocrinol Metab. 2005;90:3706–14. doi: 10.1210/jc.2004-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–4. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- 54.Boyle MB, MacLusky NJ, Naftolin F, Kaczmarek LK. Hormonal regulation of K+-channel messenger RNA in rat myometrium during oestrus cycle and in pregnancy. Nature. 1987;330:373–5. doi: 10.1038/330373a0. [DOI] [PubMed] [Google Scholar]
- 55.Seth R, Moss AJ, McNitt S, et al. Long QT syndrome and pregnancy. J Am Coll Cardiol. 2007;49:1092–8. doi: 10.1016/j.jacc.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 56.Miller D, Waters DD, Warnica W, Szlachcic J, Kreeft J, Theroux P. Is variant angina the coronary manifestation of a generalized vasospastic disorder? N Engl J Med. 1981;304:763–6. doi: 10.1056/NEJM198103263041306. [DOI] [PubMed] [Google Scholar]
- 57.Cheng J. Evidences of the gender-related differences in cardiac repolarization and the underlying mechanisms in different animal species and human. Fundam Clin Pharmacol. 2006;20:1–8. doi: 10.1111/j.1472-8206.2005.00384.x. [DOI] [PubMed] [Google Scholar]
- 58.Kurokawa J, Tamagawa M, Harada N, et al. Acute effects of oestrogen on the guinea pig and human IKr channels and drug-induced prolongation of cardiac repolarization. J Physiol. 2008;586:2961–73. doi: 10.1113/jphysiol.2007.150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002;99:381–8. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- 60.Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosom Med. 1996;58:598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Kaplan JR, Adams MR, Anthony MS, Morgan TM, Manuck SB, Clarkson TB. Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arterioscler Thromb Vasc Biol. 1995;15:2094–100. doi: 10.1161/01.atv.15.12.2094. [DOI] [PubMed] [Google Scholar]
- 62.Wagner JD, Clarkson TB, St Clair RW, Schwenke DC, Shively CA, Adams MR. Estrogen and progesterone replacement therapy reduces low density lipoprotein accumulation in the coronary arteries of surgically postmenopausal cynomolgus monkeys. J Clin Invest. 1991;88:1995–2002. doi: 10.1172/JCI115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adams MR, Clarkson TB, Shively CA, Parks JS, Kaplan JR. Oral contraceptives, lipoproteins, and atherosclerosis. Am J Obstet Gynecol. 1990;163:1388–93. doi: 10.1016/0002-9378(90)91353-e. [DOI] [PubMed] [Google Scholar]
- 64.Adams MR, Anthony MS, Manning JM, Golden DL, Parks JS. Low-dose contraceptive estrogen-progestin and coronary artery atherosclerosis of monkeys. Obstet Gynecol. 2000;96:250–5. doi: 10.1016/s0029-7844(00)00891-7. [DOI] [PubMed] [Google Scholar]
- 65.Stampfer MJ, Willett WC, Colditz GA, Speizer FE, Hennekens CH. A prospective study of past use of oral contraceptive agents and risk of cardiovascular diseases. N Engl J Med. 1988;319:1313–7. doi: 10.1056/NEJM198811173192004. [DOI] [PubMed] [Google Scholar]
- 66.Croft P, Hannaford PC. Risk factors for acute myocardial infarction in women: evidence from the Royal College of General Practitioners’ oral contraception study. BMJ. 1989;298:165–8. doi: 10.1136/bmj.298.6667.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jensen G, Nyboe J, Appleyard M, Schnohr P. Risk factors for acute myocardial infarction in Copenhagen, II: Smoking, alcohol intake, physical activity, obesity, oral contraception, diabetes, lipids, and blood pressure. Eur Heart J. 1991;12:298–308. doi: 10.1093/oxfordjournals.eurheartj.a059894. [DOI] [PubMed] [Google Scholar]
- 68.Salonen JT. Oral contraceptives, smoking and risk of myocardial infarction in young women. A longitudinal population study in eastern Finland. Acta Med Scand. 1982;212:141–4. doi: 10.1111/j.0954-6820.1982.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 69.Dunn N, Thorogood M, Faragher B, et al. Oral contraceptives and myocardial infarction: results of the MICA case-control study. BMJ. 1999;318:1579–83. doi: 10.1136/bmj.318.7198.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanis BC, V M, Kemmeren JM, Cats VM, Helmerhorst FM, Algra A, Van Der Graaf Y, Rosendaal FR. Oral Contraceptives and the Risk of Myocardial Infarction. N Engl J Med. 2001;345:1787–93. doi: 10.1056/NEJMoa003216. [DOI] [PubMed] [Google Scholar]
- 71.Lidegaard O. Smoking and use of oral contraceptives: impact on thrombotic diseases. Am J Obstet Gynecol. 1999;180:S357–63. doi: 10.1016/s0002-9378(99)70696-4. [DOI] [PubMed] [Google Scholar]
- 72.Victory R, DS C, Diamond M, McNeeley SG, Vista-Deck D, Hendrix S. Adverse cardiovascular disease outcomes are reduced in women with a history of oral contraceptive use: results from the Women’s Health Initiative database. Fertil Steril. 2004;82(Suppl 2):S52–53. [Google Scholar]
- 73.Engel HJ, Engel E, Lichtlen PR. Coronary atherosclerosis and myocardial infarction in young women--role of oral contraceptives. Eur Heart J. 1983;4:1–6. doi: 10.1093/oxfordjournals.eurheartj.a061365. [DOI] [PubMed] [Google Scholar]
- 74.Stampfer MJ, Willett WC, Colditz GA, Speizer FE, Hennekens CH. Past use of oral contraceptives and cardiovascular disease: a meta-analysis in the context of the Nurses’ Health Study. Am J Obstet Gynecol. 1990;163:285–91. doi: 10.1016/0002-9378(90)90569-s. [DOI] [PubMed] [Google Scholar]
- 75.Merz CN, Johnson BD, Berga S, Braunstein G, Reis SE, Bittner V. Past oral contraceptive use and angiographic coronary artery disease in postmenopausal women: data from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Fertil Steril. 2006;85:1425–31. doi: 10.1016/j.fertnstert.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 76.ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453–72. doi: 10.1097/00006250-200606000-00055. [DOI] [PubMed] [Google Scholar]
- 77.Van Winter JT, Bernard ME. Oral contraceptive use during the perimenopausal years. Am Fam Physician. 1998;58:1373–7. 1381–2. [PubMed] [Google Scholar]
- 78.Godsland IF, Crook D, Simpson R, et al. The effects of different formulations of oral contraceptive agents on lipid and carbohydrate metabolism. N Engl J Med. 1990;323:1375–81. doi: 10.1056/NEJM199011153232003. [DOI] [PubMed] [Google Scholar]
- 79.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001;87:937–41. A3. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 80.Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349:1202–9. [PubMed] [Google Scholar]
- 81.Sidney S, Siscovick DS, Petitti DB, et al. Myocardial infarction and use of low-dose oral contraceptives: a pooled analysis of 2 US studies. Circulation. 1998;98:1058–63. doi: 10.1161/01.cir.98.11.1058. [DOI] [PubMed] [Google Scholar]
- 82.Dunn NR, Arscott A, Thorogood M. The relationship between use of oral contraceptives and myocardial infarction in young women with fatal outcome, compared to those who survive: results from the MICA case-control study. Contraception. 2001;63:65–9. doi: 10.1016/s0010-7824(01)00172-x. [DOI] [PubMed] [Google Scholar]
- 83.Rosenberg L, Palmer JR, Rao RS, Shapiro S. Low-dose oral contraceptive use and the risk of myocardial infarction. Arch Intern Med. 2001;161:1065–70. doi: 10.1001/archinte.161.8.1065. [DOI] [PubMed] [Google Scholar]
- 84.Spitzer WO, Faith JM, MacRae KD. Myocardial infarction and third generation oral contraceptives: aggregation of recent studies. Hum Reprod. 2002;17:2307–14. doi: 10.1093/humrep/17.9.2307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


