Abstract
Intestinal ischemia after trauma-hemorrhagic shock (T/HS) results in gut barrier dysfunction and the production/release of biologically active and tissue injurious factors in the mesenteric lymph, which, in turn, causes acute lung injury and a systemic inflammatory state. Since T/HS-induced lung injury is associated with pulmonary endothelial and epithelial cell programmed cell death (PCD) and was abrogated by mesenteric lymph duct ligation, we sought to investigate the cellular pathways involved. Compared with trauma-sham shock (T/SS) rats, a significant increase in caspase-3 and M30 expression was detected in the pulmonary epithelial cells undergoing PCD, whereas apoptosis-inducing factor (AIF), but not caspase-3, was detected in endothelial cells undergoing PCD. This AIF-mediated pulmonary endothelial PCD response was validated in an in situ femoral vein assay where endothelial cells were found to express AIF but not caspase-3. To complement these studies, human umbilical vein endothelial cell (HUVEC), human lung microvascular endothelial cell (HLMEC), and human alveolar type II epithelial cell (A549) lines were used as in vitro models. T/HS lymph induced the nuclear translocation of AIF in HUVEC and HLMEC, and caspase inhibition in these cells did not afford any cytoprotection. For proof of principle, AIF silencing in HUVEC reversed the cytotoxic effects of T/HS on cell viability and DNA fragmentation. In A549 cells, T/HS lymph activated caspase-3-mediated apoptosis, which was partially abrogated by N-benzyloxycarbonyl-Val-Ala-Asp (zVAD). Additionally, T/HS lymph did not cause the nuclear translocation of AIF in A549 cells. Collectively, T/HS-induced pulmonary endothelial PCD occurs via an AIF-dependent caspase-independent pathway, whereas epithelial cells undergo apoptosis by a caspase-dependent pathway.
Keywords: acute lung injury, apoptosis-inducing factor, caspase-3
it has been recognized for over 25 yr that the development of a dysregulated inflammatory response as well as acute lung injury (ALI) and the development of the multiple organ dysfunction syndrome (MODS) complicates the recovery of patients with severe trauma (16). Although this relationship between trauma-hemorrhagic shock (T/HS) and pulmonary dysfunction has been recognized for many years and studied extensively in patients and animal models, the pathogenesis of T/HS-induced pulmonary microvascular and alveolar epithelial damage is not well-understood. Nevertheless, it is clear that hemorrhagic shock and major trauma are associated with intestinal ischemia, loss of gut barrier function, and the intestine becoming a proinflammatory organ. Most recently, we have shown experimentally that T/HS-induced lung injury can be prevented by ligating the main mesenteric lymph duct exiting the intestine (17, 35) and that the injection of T/HS mesenteric lymph into naïve animals recreates the lung injury observed with actual T/HS (48). Additionally, our earlier in vitro studies have demonstrated that mesenteric lymph from T/HS, but not trauma-sham shock lymph (T/SS) rats, causes endothelial cell apoptosis and activation as well as primes neutrophils (2, 13, 18). Thus, based on our in vivo and in vitro findings, we have proposed that early post-T/HS-induced lung injury is related to gut-derived factors carried in the mesenteric lymph (19). Since T/HS-induced lung injury is associated with pulmonary endothelial and epithelial cell death and was abrogated by mesenteric lymph duct ligation (35), the goal of the current study was to investigate the cellular mechanisms associated with pulmonary endothelial and alveolar cell death.
In recent years, the mode of programmed cell death (PCD) depends on the stimulus as well as the cell type studied (9). There are multiple types of PCD including apoptosis, caspase-independent cell death, oncosis, and necrosis (32, 51). Multiple types of PCD have been documented to occur in ALI models (39, 43, 52). Thus, in this research, both in vivo and in vitro studies investigated whether common or different types of PCD contributed to T/HS-induced pulmonary alveolar epithelial and endothelial cell injury. The rationale for studying the mechanisms by which alveolar epithelial cells undergo cell death was based on earlier studies demonstrating that impairment of the alveolar-capillary barrier plays a role in the pathogenesis of ALI and acute respiratory distress syndrome (ARDS) (5, 54) and that epithelial cell apoptosis represents an important mechanism in the development of ALI (44). This “epithelial cell hypothesis” has been mainly tested in models of direct ALI. However, in indirect ALI models such as hemorrhagic shock and sepsis, it is recognized that endothelial activation and injury is a common denominator in the pathophysiology of ALI (22, 41). In our T/HS model, the role of caspase-independent PCD was primarily evaluated by investigating the role of apoptosis-inducing factor (AIF), a mitochondrial apoptotic factor that triggers caspase-independent cell death (50). AIF has been demonstrated to play a role in renal cell death after ischemia (26), neuronal cell death after focal cerebral ischemia (33), as well as ischemia-reperfusion (I/R)-mediated cardiomyocyte apoptosis (30). Our findings demonstrated that different PCD pathways account for T/HS-induced pulmonary endothelial and epithelial cell death, specifically with endothelial cell death occurring primarily via an AIF-dependent, caspase-independent process, whereas epithelial cell apoptosis involves, at least in part, a caspase-dependent PCD.
MATERIALS AND METHODS
Animals.
Adult, male, specific pathogen-free Sprague-Dawley rats (Charles River Laboratories, Portage, MI) weighing 350–450 g were used after a minimum 7-day acclimation period. The animals were housed under barrier conditions and kept at 25°C with a 12:12-h light-dark cycle. Rats were allowed free access to water and chow (Teklad 22/5 Rodent Diet W-8640; Harlan Teklad, Madison, WI). All animals were maintained in accordance with the recommendations of the “Guide for the Care and Use of Laboratory Animals,” and the New Jersey Medical School Animal Care and Use Committee approved all experiments.
T/HS model.
Rats were subjected to a nonlethal model of T/HS as previously described (17). Briefly, male rats were anesthetized with sodium pentobarbital (50 mg/kg) injected intraperitoneally, following which they underwent a 5-cm laparotomy (trauma), and their mean arterial blood pressure was reduced to 30 mmHg and maintained between 30 and 40 mmHg level for 90 min by reinfusing or withdrawing blood as needed. The rats were resuscitated at the end of the shock period by reinfusing the shed blood. The T/SS rats were anesthetized and underwent a 5-cm laparotomy, and their femoral arteries and jugular vein were cannulated, but no blood was withdrawn or infused. Three hours after the end of the 90-min T/HS or T/SS period, the rats were killed, and the lungs were removed and then processed for in situ apoptosis detection, immunohistochemistry, and immunofluorescence studies. Three hours after the end of the 90-min T/HS or T/SS period (i.e., 4.5 h after the induction of T/HS), the rats were killed, the lungs were removed, and then the lung samples were processed for in situ apoptosis detection.
Collection of mesenteric lymph.
Mesenteric lymph used in this study was collected from additional groups of male rats subjected to T/HS or T/SS as previously described (17). The collected lymph was centrifuged at 400 g for 15 min to remove cellular debris, diluted 1:1 with PBS, aliquoted, and flash-frozen at −80°C. Lymph samples collected during the first 3 h after resuscitation were used in this study. In all of the in vitro experiments, T/HS and T/SS lymph was tested at a 5 or 10% concentration. These concentrations were chosen because lung injury is present by 3 h after the end of the T/HS period, and the total volume of lymph produced during the shock period plus the 3-h postshock resuscitation period averaged 1.8 ml for a 300-g rat. Since the blood volume of the rat is ∼6% of its body weight, the rat's blood volume would be ∼18 ml. Thus the volume of lymph produced during this period would be ∼10% of the blood volume. Hence, testing 5–10% lymph concentrations appeared to be physiologically relevant.
In situ femoral vein model.
To further test which PCD pathways are involved in T/HS lymph-induced endothelial cell death, T/HS or T/SS lymph or medium was injected into the right and left femoral veins of naïve rats that had been ligated proximally and distally as previously described (35). Briefly, after the veins were ligated, they were cannulated with a PE-10 catheter through a hole made close to the distal ligature and then washed free of blood with PBS. After washing was completed, T/HS lymph (10% vol/vol) was placed in one femoral vein, and T/SS lymph or medium was placed in the other vein. After a 3-h exposure period, the veins were removed and fixed overnight, following which they were processed for immunohistochemistry and immunofluorescence studies as described below.
Immunohistochemistry.
Paraffin blocks containing lung tissue or femoral vein specimens were cut in 6-μm thick sections and stained for TdT-mediated dUTP nick end labeling (TUNEL)-positive cells using the VasoTACS In Situ Apoptosis Detection Kit (Trevigen, Gaithersburg, MD) and caspase-3-positive cells using the SignalStain Cleaved Caspase-3 (Asp175) IHC Detection Kit (Cell Signaling Technology, Beverly, MA) according to the manufacturer's instructions. Lung sections were also stained for the M30, a caspase-cleaved cytokeratin 18 neoepitope used as a specific apoptotic epithelial cell marker (31a). Slides were incubated with M30 CytoDeath Biotin (DiaPharma, Columbus, OH) at 1:50 and counterstained with the VectaStain ABC Kit (Vector Laboratories, Burlingame, CA) as previously described (42). When viewed under a standard light microscope, the number of TUNEL- and caspase-3-positive cells per 100 high-power fields (hpf) and the number of M30-positive cells per 40 hpf were counted in a blinded fashion for each animal.
Immunofluorescence staining for AIF.
For AIF staining, frozen lung tissue and femoral vein specimens were cut in 4-μm sections, mounted onto slides at room temperature, fixed in cold acetone for 10 min, and then washed in PBS. All incubations were done at room temperature. After blocking in 10% normal blocking serum for 20 min, the slides were incubated with goat anti-AIF polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at a concentration of 5.0 μg/ml in 1.5% normal blocking serum. The slides were washed in PBS extensively and then incubated with Alexa 488-conjugated secondary antibody (Molecular Probes, Eugene, OR) for 1 h at a concentration of 1.5 μg/ml in 3% normal blocking serum. For double labeling for AIF and von Willebrand factor, an endothelial cell-specific marker, the slides were incubated with mouse monoclonal antibody against AIF (Santa Cruz Biotechnology) and rabbit polyclonal antibody against von Willebrand factor (Millipore, Billerica, MA) for 1 h at concentrations of 5.0 μg/ml in 1.5% normal blocking serum. The slides were washed extensively in PBS and incubated with the secondary antibodies conjugated to FITC for AIF detection and to rhodamine for von Willebrand factor (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at 5 μg/ml in PBS with 3% normal blocking serum. After several washes in PBS, the fluorescence signals for AIF and von Willebrand factor were visualized by Axiovert 200 and analyzed using AxioVision 4.6 software (Carl Zeiss, Thornwood, NY). The number of AIF-positive cells per 30 hpf were counted in a blinded fashion for each animal.
Cell culture and reagents.
Human umbilical vein endothelial cells (HUVEC) and human lung microvascular endothelial cells (HLMEC) were purchased from Lonza (Walkersville, MD). Both HUVEC and HLMEC were grown in endothelial growth medium (Lonza) containing 2% FBS. HUVEC were only used from passages 3 to 5, and HLMEC were only used from passages 5 to 7. A549 and CCL-95.1 cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA). A549 cells were cultured in Ham's F-12 nutrient mixture with 10% FBS, and CCL-95.1 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA).
Preparation of whole cell extracts and Western blotting analysis.
For preparation of whole cell extracts (WCEs), both floating and adherent cells were collected and homogenized in lysis buffer [1% Nonidet P-40, 150 mM NaCl, 50 mM Tris·HCl, pH 8.0, 0.5 mM EDTA, 1 mM DTT, 0.5 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 1 mM NaVO3, and 1 μg/ml each for aprotinin, leupeptin, and pepstatin]. After 30-min incubation in ice, the lysates were centrifuged, and the supernatants were collected. Protein concentrations were determined by the Coomassie brilliant blue reaction (Bio-Rad, Hercules, CA). Proteins in WCEs were resolved on 4–12% Bis-Tris gradient SDS-PAGE (Invitrogen), transferred to a nitrocellulose membrane (Amersham, Arlington Heights, IL), and blocked with 5% milk in TBS-T (50 mM Tris·HCl, pH 7.5, 140 mM NaCl, 0.1% Tween). The primary antibodies used in our studies were the following: goat anti-AIF (1:1,000; Santa Cruz Biotechnology), rabbit anti-cleaved caspase-3 (1:1,000; Cell Signaling Technology), and rabbit anti-β-actin (1:1,000; Santa Cruz Biotechnology). Following an overnight incubation with the primary antibody at 4°C, the membrane was washed four times in TBS-T, subsequently incubated for 1 h at room temperature with horseradish peroxidase-conjugated anti-rabbit or anti-goat secondary antibodies (1:2,000; Santa Cruz Biotechnology), and developed with enhanced chemiluminescence (Amersham). Transfer efficiency was assessed by staining with Ponceau S (Bio-Rad). Western blots were analyzed by densitometry using an AlphaImager 3400 imaging system and AlphaEase FC software (Alpha Innotech, San Leandro, CA).
AIF translocation and M30 immunofluorescence studies.
For both AIF localization and M30 expression, HUVEC, A549, or HLMEC were seeded onto coverslips and fixed in methanol. Cells were incubated for 1 h at 37°C with either goat anti-AIF antibody (1:100; Santa Cruz Biotechnology) as previously described (55) or mouse anti-M30 antibody (1:50; DiaPharma) as previously described (42). The cells were washed extensively in TNT (100 mM Tris·HCl, pH 7.5, 150 mM NaCl, 0.05% Tween) and subsequently incubated for 1 h at 37°C with Alexa 488-conjugated secondary antibody for AIF detection (1:200; Molecular Probes) or FITC-conjugated secondary antibody for M30 detection (1:100; Santa Cruz Biotechnology). Nuclear morphology was detected by counterstaining the nuclei with propidium iodide (PI; 1:100). Cells were mounted, and green, red, and yellow fluorescence was visualized by Axiovert 200 and analyzed using AxioVision 4.6 software (Carl Zeiss).
AIF knockdown by siRNA.
HUVEC were transiently transfected in a Nucleofector system (Amaxa, Köln, Germany) according to the manufacturer's protocol with 3 μg of SMARTpool small interfering RNA (siRNA) against human AIF (Dharmacon, Lafayette, CO). Additionally, mock transfections of HUVEC without any siRNA were done. After 72 h at 37°C, WCEs were prepared, and AIF protein expression was examined by Western blotting as described above. In parallel, cells were treated with either medium, 5% T/HS, or 5% T/SS for 3 h, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and DNA fragmentation assays were performed as described below.
Cell viability MTT assay.
HLMEC and A549 cells were seeded at 2 × 104 cells per well in a 96-well plate (Corning Glassware, Corning, NY) for 24 h before the addition of medium, 1 μM staurosporine (STS; Sigma-Aldrich, St. Louis, MO), 5% T/HS or T/SS lymph for 3 h. STS was used as an apoptotic inducer as well as positive control for caspase-3 and AIF expression. For caspase inhibition studies, 100 μM zVAD-FMK (Alexis, San Diego, CA) or DMSO, the vehicle control, was added to cells for 1 h before the addition of medium, STS, T/HS, or T/SS lymph. After the 3-h incubation, cell viability was measured using the MTT-based cell cytotoxicity assay kit (Sigma-Aldrich) as described earlier (35). The medium condition was used as the baseline and expressed as 100% viability.
DNA fragmentation assay.
To determine the extent of apoptosis, DNA fragmentation was assessed using the nucleosome ELISA (Cell Death Detection ELISA; Roche, Indianapolis, IN) according to the manufacturer's instructions. Briefly, genomic DNA was isolated from cells, and free nucleosomes were detected by anti-histone biotin and anti-DNA horseradish peroxidase immunoreagents. After 2 h, a stop solution was added, and the number of free nucleosomes was determined spectrophotometrically on a Dynatech MR 500 at a wavelength of 450/595 nm.
Statistical analysis.
All data were analyzed by analysis of variance followed by Tukey-Kramer multiple comparisons test and are expressed as the means ± SD. Statistical significance was considered to be reached when P ≤ 0.05.
RESULTS
Different PCD effectors are associated with T/HS-induced pulmonary alveolar epithelial and endothelial cell death.
The overall goal of our in vivo studies is to characterize the pathways of endothelial and alveolar epithelial cell death after trauma-hemorrhage as well as the role of mesenteric lymph in this process. The T/HS model (laparotomy plus 90 min of hemorrhagic shock at 30–40 mmHg) represents a global I/R injury. The addition of a laparotomy is important because tissue injury is a component of clinical traumatic hemorrhage and the combined insult of hemorrhage plus tissue injury results in an inflammatory result that more closely reflects trauma patients all of whom have soft tissue injury and require instrumentation with vascular cannulation. (15). As shown in Fig. 1A and validated by our earlier study (35), rats subjected to T/HS, but not T/SS, had increased evidence of pulmonary PCD as determined by TUNEL assay. Significant increases in TUNEL-positive endothelial and nonendothelial cells were found in the lungs of the T/HS group (Fig. 1B). To further investigate the apoptotic mechanisms involved in T/HS-induced lung injury, we chose to determine whether caspase-3 activation was associated with pulmonary PCD. Cleaved caspase-3 activation was measured in the lungs of rats subjected to T/HS and T/SS by immunohistochemistry. In the alveolus, which consists of mainly epithelial and endothelial cells, caspase-3 appears to be activated primarily in alveolar epithelial cells by T/HS (Fig. 2A). A 4.4-fold significant increase of cleaved caspase-3-positive lung nonendothelial cells was found in rats undergoing T/HS compared with T/SS (Fig. 2B). Neither T/HS nor T/SS had any effect on caspase-3 expression in endothelial cells (0.0 ± 0.0). To provide more evidence those alveolar epithelial cells underwent caspase-3-dependent apoptosis, our lung sections were stained for the M30 neoepitope. M30 is a cytokeratin 18 that is cleaved by caspases 3, 6, 7, and 9 and acts as a specific apoptotic epithelial cell marker (31a). In Fig. 2, C and D, M30-positive lung epithelial cells were markedly increased after T/HS but not T/SS (P < 0.0001). Our results suggest that T/HS induces alveolar epithelial cell apoptosis via a caspase-3-dependent pathway.
Fig. 1.
Trauma-hemorrhagic shock (T/HS)-induced pulmonary cell death. A: TdT-mediated dUTP nick end labeling (TUNEL) staining of lung tissue from rat subjected to T/HS. Arrows indicate endothelial (Endo) and nonendothelial apoptotic cells that stained blue. Magnification, ×100, and inset, ×40. B: number of TUNEL-positive endothelial and nonendothelial cells per 100 high-power fields (hpf) in rats subjected to trauma-sham shock (T/SS) or T/HS. Data are shown as mean values ± SD (n = 4 per group). *P < 0.01 vs. T/SS.
Fig. 2.
T/HS induces alveolar epithelial cell apoptosis via caspase-3-dependent pathway. A: caspase-3 immunohistochemical staining of lung tissue from rat subjected to T/HS. Arrows indicate cleaved caspase-3-positive alveolar epithelial cells. Magnification, ×100. B: number of cleaved caspase-3-positive epithelial cells per 100 hpf. Data are shown as mean values ± SD (n = 4–6 per group). *P < 0.01 vs. T/SS. C: M30 immunohistochemical staining of lung tissue from rats subjected to T/SS or T/HS. Magnification, ×40. D: number of M30-positive alveolar epithelial cells per 40 hpf. Data are shown as mean values ± SD (n = 4–6 per group). *P < 0.001 vs. T/SS.
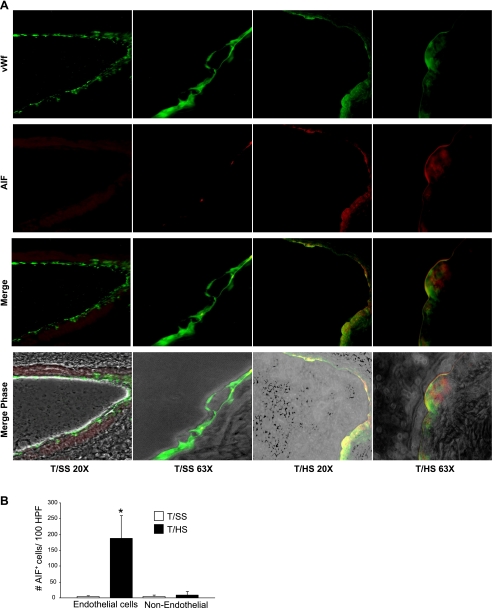
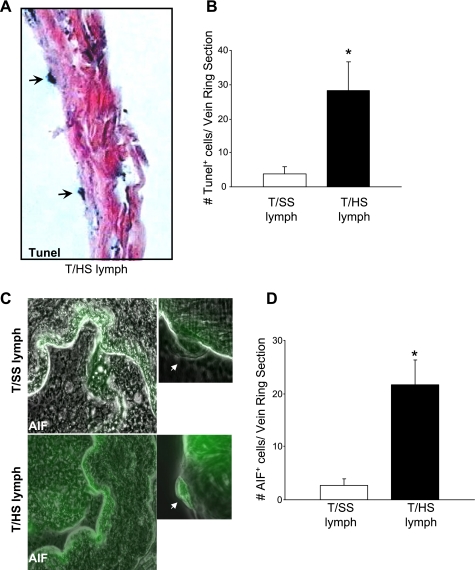
Since T/HS-induced pulmonary endothelial cell PCD does not involve caspase-3 activation, and our earlier electron microscopy studies of T/HS rat lungs (35) documented morphological features characteristic of apoptosis-like PCD such as less compact nuclear condensation and cell shrinkage (32), we investigated whether AIF, a caspase-independent mitochondrial factor mediates T/HS-induced endothelial cell PCD. Using lung sections from rats subjected to T/HS or T/SS, double immunofluorescent staining for AIF (red) and von Willebrand factor (green), an endothelial-specific marker was performed (Fig. 3A). Colocalization of AIF and von Willebrand factor (yellow) was only evident after T/HS but not T/SS. In Fig. 3B, T/HS increased AIF expression by 35-fold in endothelial cells (P < 0.01) and by 2.2-fold in nonendothelial cells (P < 0.01) relative to T/SS. To further validate that AIF is associated with T/HS-induced pulmonary endothelial cell PCD, we tested whether T/HS lymph directly activated AIF expression in an in vivo femoral vein model. Our earlier study (35) demonstrated that that T/HS lymph caused femoral vein endothelial cell PCD. After the femoral veins of normal rats were infused with T/HS or T/SS mesenteric lymph for 3 h, vein ring sections were processed for TUNEL, AIF fluorescence, and caspase-3 immunohistochemistry. A pronounced increase in the number of TUNEL-positive (Fig. 4, A and B) and AIF-positive (Fig. 4, C and D) endothelial cells per vein ring section was observed with T/HS lymph compared with T/SS lymph (P < 0.01). Caspase-3 activation was not detected in any of the femoral vein endothelial cells exposed to T/HS or T/SS lymph (0.0 ± 0.0). These findings were consistent with our pulmonary studies and suggested that an AIF-dependent, caspase-independent PCD pathway was associated with T/HS lymph-induced endothelial cell death.
Fig. 3.
T/HS induces apoptosis-inducing factor (AIF) expression in pulmonary endothelial cells. A: AIF and von Willebrand factor (vWf) immunofluorescent staining in lung tissue of rats subjected to T/SS or T/HS. Magnifications shown are ×20 and ×63. B: number of AIF-positive endothelial and nonendothelial cells per 100 hpf from rats subjected to T/HS or T/SS. Data are shown as mean values ± SD. *P < 0.01 vs. T/SS (n = 4 per group).
Fig. 4.
AIF activation is associated with T/HS lymph-induced rat femoral endothelial cell death. A: TUNEL staining of normal rat femoral vein incubated with 10% T/HS mesenteric lymph for 3 h. Arrows depict TUNEL-positive endothelial cells. Magnification, ×40. B: number of TUNEL-positive endothelial per vein ring section incubated with 10% T/SS or T/HS lymph. Data are shown as mean values ± SD (n = 4). *P < 0.01 vs. T/SS lymph. C: AIF immunofluorescent staining in normal rat femoral veins incubated with 10% T/SS or T/HS lymph for 3 h. Magnification, ×40. Inset: magnification image of an endothelial cell (×100). D: number of AIF-positive endothelial cells per vein ring section incubated with 10% T/SS or T/HS lymph. Data are shown as mean values ± SD (n = 4 per group). *P < 0.01 vs. T/SS lymph.
T/HS lymph causes endothelial cell death via a noncaspase, AIF-mediated PCD pathway.
To complement our in vivo studies, HUVEC and HLMEC were used as an in vitro model system to further study the response of endothelial cells to T/HS lymph. Our earlier studies have documented that T/HS lymph markedly reduces HUVEC and rat pulmonary microvascular endothelial cells cell viability after a 3-h exposure (18). Since endothelial cells from different vascular beds may respond differently to the same or similar stimuli, and our in vivo studies focused on the cytotoxic effects of T/HS during lung injury, we repeated our viability assays with low passage number HLMEC. Both T/HS lymph and STS, a positive inducer of caspase-3 and AIF expression (27), markedly reduced the viability of HLMEC by P < 0.001 vs. T/SS, albeit at different levels (Fig. 5). Whereas the addition of N-benzyloxycarbonyl-Val-Ala-Asp (zVAD) did not afford any protection in HLMEC exposed to T/HS lymph (P > 0.05 vs. T/HS), a significant increase of HLMEC viability was seen in cells exposed to STS in the presence of zVAD (P < 0.001). Thus our viability study using HLMEC are consistent with our earlier observations with HUVEC, thereby suggesting that T/HS lymph induces endothelial cell apoptosis via a caspase-3-independent mechanism.
Fig. 5.
Inhibition of caspases by N-benzyloxycarbonyl-Val-Ala-Asp (zVAD) does not protect against the cytotoxic effects of T/HS lymph in human lung microvascular endothelial cells (HLMEC). HLMEC were pretreated with zVAD (100 μM) or DMSO vehicle control for 1 h and then exposed to medium, staurosporine (STS; 1 μM), and 5% T/HS and 5% T/SS lymph for 3 h. Cell death was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay. In the bar graph, the data are expressed as mean values ± SD. *P < 0.001 vs. STS plus zVAD (n = 5 per condition).
Having shown that the induction of AIF was associated with T/HS-induced pulmonary endothelial apoptosis, we examined whether T/HS lymph induced the translocation of AIF from the mitochondria to the nucleus in endothelial cells. Colocalization studies with AIF and the nuclear marker PI were performed in both HUVEC and HLMEC exposed to T/HS or T/SS lymph for 3 h. As shown in Figs. 6A and 7A, AIF expression was excluded from the nucleus and appeared diffuse in the cytoplasm and perinuclear regions in HUVEC and HLMEC exposed to medium or T/SS lymph. In contrast, when HUVEC and HLMEC were exposed to T/HS lymph or STS, AIF was redistributed into the nucleus (green-red merge). Incubation of HUVEC or HLMEC with T/HS lymph caused a significant increase in the number of AIF-positive cells (P < 0.001; Fig. 6B and Fig. 7B) relative to incubation with T/SS lymph or medium. T/HS lymph, but not T/SS lymph, induced AIF expression in HUVEC as early as 1 h of exposure and persisted for 3 h (data not shown). These studies demonstrate an association between the induction of AIF and PCD as a consequence of T/HS.
Fig. 6.
T/HS lymph induces the nuclear translocation of AIF in human umbilical vein endothelial cells (HUVEC). A: HUVEC were exposed to medium (Med), STS (200 nM), and 5% T/HS and 5% T/SS lymph for 3 h, immunostained for AIF (green), and examined by immunofluorescent microscopy. The nuclei were stained with propidium iodide (PI; red). In HUVEC exposed to medium or T/SS lymph, AIF expression was excluded from the nucleus and appeared diffuse in the cytoplasm and perinuclear regions, whereas in HUVEC exposed to T/HS or STS, AIF was redistributed into the nucleus (green-red merge). Magnification, ×63. B: the mean values ± SD shown in the bar graphs for the number of AIF-positive stained cells per 100 cells (*P < 0.001 vs. T/SS and medium) were from 4 independent experiments.
Fig. 7.
T/HS lymph induces the nuclear translocation of AIF in HLMEC. A: HLMEC were exposed to medium, STS (200 nM), and 5% T/HS and 5% T/SS lymph for 3 h, immunostained for AIF (green), and examined by immunofluorescent microscopy. The nuclei were stained with PI (red). In HLMEC exposed to medium or T/SS lymph, AIF expression was excluded from the nucleus and appeared diffuse in the cytoplasm and perinuclear regions, whereas in HLMEC exposed to T/HS or STS, AIF was redistributed into the nucleus (green-red merge). Magnification, ×63. B: the mean values ± SD shown in the bar graphs for the number of AIF-positive stained cells per 100 cells (*P < 0.001 vs. T/SS or medium) were from 4 independent experiments.
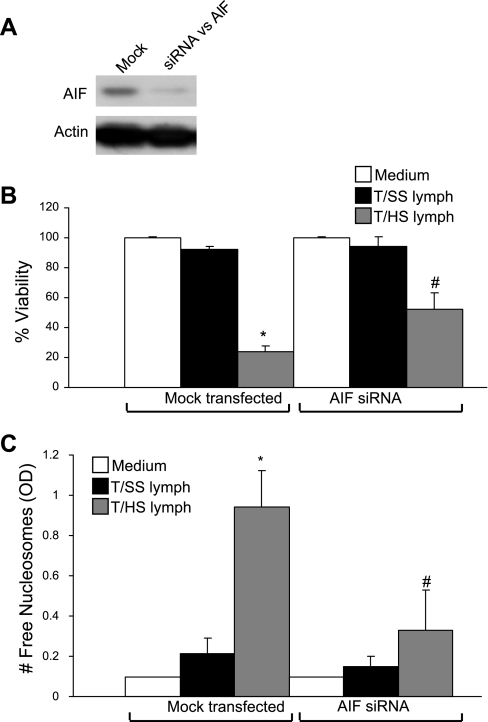
For proof of principle, we tested whether loss of AIF expression by RNA interference blocked T/HS lymph-induced HUVEC cell death. Endogenous AIF protein levels were markedly reduced in HUVEC transfected with AIF siRNA compared with mock-transfected HUVEC (Fig. 8A). In both MTT (Fig. 8B) and nucleosomal release ELISA (Fig. 8C) assays, HUVEC transfected with AIF siRNA reduced the inhibitory effects of T/HS lymph on viability by 28% (P < 0.001 vs. mock-transfected cells) and resulted in a 3-fold decrease in the release of free nucleosomes (P < 0.001 vs. mock-transfected cells). Similar protective effects by AIF siRNA were observed in the MTT viability assay when the duration of exposure to T/HS lymph was increased from 3 to 24 h (data not shown), thereby indicating that this early protective effect by siRNA AIF persisted. These studies suggest a causal relationship between the induction of AIF and T/HS-induced endothelial cell PCD.
Fig. 8.
Knockdown of AIF expression inhibits T/HS-induced endothelial cell apoptosis. A: whole cell extracts (WCEs) were prepared from HUVEC that were mock transfected or transfected with small interfering RNA (siRNA) against AIF for 72 h. AIF and actin protein levels were determined by Western blotting. B and C: after 48–72 h of transfection, HUVEC were exposed to medium and 5% T/HS and 5% T/SS lymph for 3 h, and cell viability and DNA fragmentation were assessed by MTT cell viability assay (B) and nucleosomal release ELISA (C). The optical density (OD) correlates with the number of free nucleosomes. In the bar graphs, the data are expressed as mean values ± SD for percent cell viability (B) and the number of free nucleosomes (C). *P < 0.001 vs. mock-transfected HUVEC incubated with medium or T/SS lymph, and #P < 0.001 vs. mock-transfected HUVEC incubated with T/HS lymph (n = 6–18 per condition).
Since silencing of AIF did not fully protect HUVEC from the cytotoxic effects of T/HS lymph, we determined whether T/HS-induced HUVEC PCD was associated with the induction of EndoG, another mitochondrial factor implicated in caspase-independent PCD. After a 3-h exposure with T/HS lymph, EndoG protein levels were significantly induced in HUVEC compared with T/SS lymph (Fig. 9, A and B).
Fig. 9.
Endonuclease G (EndoG) is activated in HUVEC exposed to T/HS lymph. A: WCEs from HUVEC were analyzed for EndoG and actin expression by Western blotting. B: densitometry was performed to quantify EndoG and actin expression. In the bar graph, the data are expressed as mean values ± SD for EndoG/actin. *P < 0.001 vs. T/SS lymph (n = 6–10 per condition).
T/HS lymph causes alveolar epithelial cell death via a caspase-3-dependent, AIF-independent apoptotic pathway.
Using A549 cells as a model for alveolar type II epithelial cells, we examined whether T/HS induced A549 cell death via a caspase-3-dependent mechanism. A significant increase in the expression of M30-positive apoptotic cells was observed in A549 cells exposed to T/HS lymph compared with T/SS lymph (Fig. 10, A and B). Consistent with these findings, T/HS lymph reduced A549 cell viability to 75%, whereas T/SS lymph had a stimulatory effect on A549 cell viability (Fig. 10C). The addition of zVAD protected A549 cells from T/HS lymph with cell viability increasing from 75 to 90% (P < 0.05). Similarly, a reduction of cell viability by STS was inhibited by the presence of zVAD. Albeit modest, this cytoprotection is consistent with studies showing that STS induces cell death by both caspase-dependent and caspase-independent pathways (8). The protective effect of zVAD on T/HS lymph-induced A549 cell viability was not short-lived and persisted after an 18-h incubation period (from 63 ± 14 to 81 ± 11%; P < 0.05). zVAD also protected a second epithelial cell line (the type I epithelial CCL-95.1) after exposure to T/HS lymph. The viability of CCL-95.1 cells incubated with T/HS lymph in the presence of zVAD was increased after a 3-h (63 ± 12 to 82 ± 13%; P < 0.05) or 18-h (40 ± 15 to 59 ± 15%; P < 0.05) incubation period.
Fig. 10.
Inhibition of caspases by zVAD protects against T/HS lymph-induced A549 cell apoptosis. A: A549 cells were exposed to 5% T/HS and 5% T/SS lymph for 3 h, immunostained with M30 (green), and examined by immunofluorescence. The nuclei were stained with PI (red). B: the mean values ± SD shown in the bar graph for the number of M30 apoptotic A549 cells counted per 100 cells. *P < 0.001 vs. T/SS lymph. C: A549 cells were pretreated with zVAD (100 μM) or DMSO vehicle control for 1 h and then exposed to medium, STS (1 μM), and 5% T/HS and 5% T/SS lymph for 3 h. Cell death was assessed by MTT cell viability assay. In the bar graph, the data are expressed as mean values ± SD for percent cell viability. *P < 0.01 STS vs. STS, and **P < 0.01 T/HS vs. T/HS and zVAD (n = 6–8 per condition).
Having shown that caspases are involved in T/HS lymph-induced pulmonary epithelial cell death but not endothelial cell death, we measured caspase-3 protein levels in A549 and HUVEC. As shown in Fig. 11A, pronounced expression of both 19- and 17-kDa cleaved caspase-3 proteins was detected in A549 cells exposed to T/HS lymph compared with T/SS lymph samples. In HUVEC, three of the four T/HS lymph samples induced caspase-3 cleavage but at significantly lower levels than observed in A549 cells (Fig. 11B).
Fig. 11.
T/HS lymph induces caspase-3 activation in A549 cells. WCEs prepared from A549 (A) and HUVEC (B) exposed to medium and 5% T/SS lymph for 3 h were examined for cleaved caspase-3 protein levels by Western blotting. STS was used as a positive control for apoptosis in HUVEC (200 nM) and A549 (1 μM) for 3 h. Data are representative of 3–4 independent lymph or medium samples per condition. The numbers represent individual samples per condition.
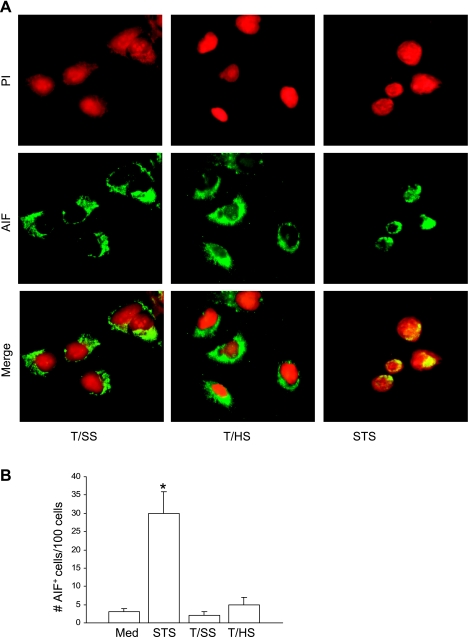
Since caspase inhibition by zVAD did not completely protect A549 cells from T/HS lymph-induced apoptosis, we determined whether T/HS lymph induced AIF nuclear translocation in A549 cells exposed to T/HS lymph for 3 h. As shown in Fig. 12A, AIF expression was excluded from the nucleus in A549 cells exposed to medium, T/SS, or T/HS lymph, whereas in A549 cells exposed to STS, AIF was redistributed into the nucleus (green-red merge). A significant increase in the number of AIF-positive cells was only seen in A549 cells exposed to STS but not to T/HS lymph or T/SS lymph (Fig. 12B). This finding indicates that AIF does not play a significant role in T/HS-induced pulmonary epithelial cell apoptosis.
Fig. 12.
T/HS lymph does not induce nuclear translocation of AIF in A549 cells. A: A549 cells were exposed to STS (1 μM) and 5% T/HS and 5% T/SS lymph for 3 h, immunostained for AIF (green), and examined by immunofluorescence. The nuclei were stained with PI (red). In A549 cells exposed to T/SS or T/HS, AIF expression was excluded from the nucleus, whereas in A549 cells exposed to STS, AIF was redistributed into the nucleus (green-red merge). Original magnification, ×63. B: the mean values ± SD shown in the bar graphs for the number of AIF-positive stained cells per 100 cells (*P < 0.001 vs. all groups) were from 3 independent experiments.
DISCUSSION
It is well-accepted that host-derived endogenous factors play a critical role in the induction and perpetuation of the systemic inflammatory response as well as in the development of ARDS and MODS (16). However, neither the source nor the exact identities of these critical proinflammatory organ and cell injury-inducing factors are completely known. Our previous work showing that T/HS-induced lung injury, neutrophil activation, as well as endothelial cell activation and injury are due to gut-derived factors carried in the mesenteric lymph and not the portal blood (19) has begun to address one of these issues; it has identified the ischemic gut as a source of factors leading to early post-T/HS lung injury as well as the induction of a systemic inflammatory state. That is, T/HS mesenteric lymph appears to be a biologically relevant fluid that is directly involved in transducing the hypotensive effects of trauma-hemorrhage into a proinflammatory and tissue-injurious event. Although the exact factors responsible for T/HS mesenteric lymph cytotoxic effects are yet to be identified, modified albumin species and lipid factors have been implicated (28, 47). Furthermore, previous work demonstrates that neither bacteria nor their products are responsible for the cytotoxic effects of T/HS lymph (1) and that this effect is not cytokine-mediated (12). Nonetheless, the ability to prevent T/HS-induced lung injury and pulmonary endothelial and epithelial PCD by lymph duct ligation (35) combined with the ability to collect and test the effects of T/HS lymph in controlled in vitro studies provides a unique opportunity to investigate the potential mechanisms and cellular pathways involved in the development of T/HS-induced pulmonary PCD.
The combination of our in vivo studies and the use of T/HS lymph as a tool in in situ and in vitro studies indicate that T/HS-induced pulmonary endothelial cell PCD and epithelial cell PCD occur via different death pathways. Although both animal and clinical studies have documented that ALI and ARDS are associated with increased pulmonary cellular apoptosis (14, 35, 36), the mechanisms by which this occurs is incompletely understood as are the pathways involved. Furthermore, these studies have largely focused on the pulmonary epithelial cell population (36, 42, 43). Yet, in cases of indirect ALI, where lung damage is secondary to a systemic insult, such as after trauma-hemorrhage or nonpulmonary sepsis, the injury-inducing factors reach the lung via the systemic circulation, with the endothelium being the first pulmonary cell population exposed to these factors. This is in contrast to direct causes of ALI, such as aspiration or pneumonia, where the pulmonary epithelial cell populations are initially exposed to the inciting agents. Thus we believed it was important to investigate both the endothelial cell as well as the pulmonary epithelial cell PCD response to T/HS. Additionally, since the factors involved in the pulmonary injurious response change over time, and the factors initiating the injury response may not be the same as those potentiating or perpetuating injury, we chose to study an early endpoint of T/HS-induced lung injury to allow us to distinguish primary injurious factors from secondary ones. More importantly, if these primary injurious factors (i.e., the initial response) are identified, they can be targeted and modulated such that the later injurious responses can be abrogated or mitigated.
Based on this strategy, we have found that the in vivo pulmonary endothelial PCD response differs from the epithelial response in that the endothelial cell death pathway appears to involve a noncaspase-mediated pathway in contrast to the epithelial cells where cell death is associated with caspase activation. Although there are a number of in vivo studies investigating the effects of hemorrhagic shock on lung PCD (3, 4, 14, 24, 25, 29, 35, 42, 43, 49), most have been descriptive studies (3, 4, 14, 24, 25, 29, 49), and none have investigated both the endothelial and epithelial cell populations. The one set of mechanistic studies by Perl et al. (42, 43) examined pulmonary epithelial cell apoptosis in a combined hemorrhagic shock-abdominal sepsis model. They found that the apoptotic pulmonary epithelial cells were TUNEL- and caspase-3-positive and that the epithelial apoptotic response occurred via a caspase-dependent mechanism that involved activation of the extrinsic Fas receptor (42, 43). These animal studies were consistent with studies in patients with ALI where pulmonary epithelial apoptosis appears to occur, in part, via a Fas-mediated process (36). Thus our in vivo pulmonary epithelial cell apoptosis results showing that these cells are TUNEL-, caspase-3-, and M30-positive but AIF-negative support the above studies. Although there are no studies besides our own focusing on pulmonary endothelial cell PCD after T/HS (35), studies by Mura et al. (39), utilizing a localized model of gut ischemia-induced lung, where the superior mesenteric artery is occluded, found that lung injury was associated with endothelial cell oncosis and the endothelial cells were TUNEL-positive but caspase-3-negative. Thus our results are consistent with and validate the study of Mura et al. (39). Furthermore, in our in situ femoral vein model, endothelial cells undergoing T/HS-induced PCD were found to be AIF-positive and caspase-3-negative. This finding further substantiates the notion that T/HS lymph is able to directly induce endothelial cell PCD via a caspase-independent and AIF-mediated apoptotic pathway.
Having shown that two different types of PCD participate in endothelial and epithelial T/HS-induced lung injury and that T/HS lymph appears to mediate this response (35), we studied the mechanisms underlying T/HS lymph-induced endothelial cell (HUVEC, HLMEC) and alveolar type epithelial (A549) cell death. These in vitro endothelial cell studies validated our in vivo studies to the extent that T/HS lymph increased AIF protein levels and induced the translocation of AIF from the cytoplasm to the nucleus, and siRNA-mediated AIF knockdown significantly abrogated the cytotoxic effects of T/HS lymph on HUVEC. However, since AIF knockdown was not fully cytoprotective, it appears that other factors besides AIF mediate the cytotoxic effects of T/HS lymph. EndoG, another mitochondrial protein that has been shown to mediate caspase-independent PCD (11), was also markedly increased in HUVEC exposed to T/HS lymph. EndoG has been reported to play a deleterious role in different models of I/R injury (6, 31). The observation that both AIF and EndoG induction is associated with T/HS lymph-induced HUVEC PCD is similar to a recent study showing that wood smoke extract-induced lung endothelial cell PCD is both caspase-independent and is associated with AIF and EndoG translocation from the cytoplasm to the nucleus (34). Although the signaling pathways by which T/HS lymph induced an AIF response were not studied, reactive oxygen species, elevated intracellular calcium levels, as well as poly(ADP-ribose) polymerase (PARP) have been implicated in AIF release from the mitochondria in the absence of caspase activation (11). However, based on earlier pharmacological studies, we have documented that neither inhibition of PARP nor intracellular calcium reduces T/HS lymph-induced HUVEC cell death (40). However, since this study did show that the antioxidants vitamin E and nordihydroguairetic acid were both protective, it seems likely that T/HS lymph-induced HUVEC cytotoxicity involves reactive oxygen species (40). These in vitro results with T/HS lymph differ from other studies investigating the mechanisms of endothelial cell PCD where different stimuli were used to induce an apoptotic response. For example, cytokines such as TNF (20) and endotoxin (7) induce endothelial cell apoptosis via a caspase-dependent process. Thus, in interpreting the role of various cell death pathways, it is also important to consider the specific stimulus used to induce PCD. This notion is highlighted by a recent study showing that whereas caspase-3 was increased in the livers of rats subjected to hemorrhagic shock, caspase inhibition did not prevent liver injury, although it did prevent the caspase-3 induction (38). Yet, in a model of endotoxic shock, where liver injury was TNF-dependent, caspase-3 inhibition did prevent liver injury (23). Likewise, it is well-recognized that endothelium from different vascular beds may respond different to the same or similar stimuli (45). In this context, hemorrhagic shock-induced mesenteric vasculature hyperpermeability and apoptosis has recently been shown to be a caspase-mediated event (10). Thus the pulmonary endothelial cell response observed to T/HS in the current study should not be extrapolated to other vascular beds or organs and may not occur in conditions associated with high TNF or endotoxin levels.
The in vivo T/HS-induced lung epithelial cell response was also complimented by our in vitro T/HS lymph pulmonary epithelial cell studies. Specifically, caspase-3-positive and AIF-negative epithelial cells were detected in lungs from rats subjected to T/HS compared with T/SS. Our in vitro studies showed that T/HS lymph activated caspase-3 and M30 in A549 cells and that the cytotoxic effects of T/HS lymph in A549 cells were partially rescued by the pan-caspase inhibitor zVAD. Additionally, our finding that T/HS lymph did not cause the translocation of AIF to the nucleus in A549 cells validates our in vivo data. Although A549 cells are widely used in studying mechanisms of alveolar epithelial injury, one must recognize that A549 cells are derived from a lung adenocarcinoma and that these cells may respond differently to stress and proapoptotic factors than primary alveolar epithelial cells (21). Nevertheless, this caspase-dependent cell death pathway is consistent with human studies showing that bronchoalveolar fluid from patients with ARDS induces epithelial cell death via a Fas-dependent, caspase-mediated pathway (36) as well as animal studies by Perl et al. (42, 43).
Like endothelial cells, neither the exact factors in T/HS lymph nor the signaling pathways involved in inducing epithelial cell death are known. Additionally, the explanation for why T/HS as well as T/HS lymph induce pulmonary epithelial cell apoptosis primarily via a caspase-dependent pathway, whereas these same insults lead to endothelial cell apoptosis primarily via a caspase-independent pathway, is unknown. In fact, one limitation of using T/HS lymph as a reagent in performing mechanistic studies is the fact that this is a biological fluid containing numerous factors, and the exact factors responsible for inducing epithelial or endothelial cell apoptosis are not known. Thus, in contrast to other agents generally used to study apoptotic mechanisms in vitro, such as endotoxin, cytokines, or oxidants, T/HS lymph is a complex biological fluid. Although studying a complex biological fluid, such as T/HS lymph, has potential limitations, it also has advantages. For example, since T/HS lymph is generated in vivo, it has the advantage that observations on its effects are more likely to be biologically and physiologically relevant than studies using single agents. Nonetheless, as the biologically active cytotoxic factors present in T/HS lymph are identified, it will be important to study them. Another potential limitation of focusing on lymph is that factors released primarily into plasma that could induce PCD of epithelial and endothelial cells would not be considered. We believe that this possibility is unlikely based on our in vivo studies showing that lymph duct ligation prevented pulmonary injury (46) and our earlier in vitro study showing that postshock portal vein plasma had minimal cytotoxic activity in endothelial cells (53).
In summary, the observations made with T/HS lymph provide unique information on the effects of actual T/HS on pulmonary endothelial and epithelial cells that could not be obtained by studying single isolated factors.
GRANTS
This research was supported by National Institute of General Medical Sciences Grants GM-059841 (E. A. Deitch) and T32 GM-069330 (F. J. Caputo) and the Thomas P. Infusino Endowment (D. Barlos).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams CA Jr, Xu DZ, Lu Q, Deitch EA. Factors larger than 100 kd in post-hemorrhagic shock mesenteric lymph are toxic for endothelial cells. Surgery 129: 351–363, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Adams JM, Hauser CJ, Adams CA Jr, Xu DZ, Livingston DH, Deitch EA. Entry of gut lymph into the circulation primes rat neutrophil respiratory burst in hemorrhagic shock. Crit Care Med 29: 2194–2198, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Alam HB, Austin B, Koustova E, Rhee P. Resuscitation-induced pulmonary apoptosis and intracellular adhesion molecule-1 expression in rats are attenuated by the use of Ketone Ringer's solution. J Am Coll Surg 193: 255–263, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Ayuste EC, Chen H, Koustova E, Rhee P, Ahuja N, Chen Z, Valeri CR, Spaniolas K, Mehrani T, Alam HB. Hepatic and pulmonary apoptosis after hemorrhagic shock in swine can be reduced through modifications of conventional Ringer's solution. J Trauma 60: 52–63, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 3: 35–56, 1982. [PubMed] [Google Scholar]
- 6.Bahi N, Zhang J, Llovera M, Ballester M, Comella JX, Sanchis D. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J Biol Chem 281: 22943–22952, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bannerman DD, Eiting KT, Winn RK, Harlan JM. FLICE-like inhibitory protein (FLIP) protects against apoptosis and suppresses NF-kappaB activation induced by bacterial lipopolysaccharide. Am J Pathol 165: 1423–1431, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belmokhtar CA, Hillion J, Ségal-Bendirdjian E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 20: 3354–3362, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Candé C, Vahsen N, Garrido C, Kroemer G. Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ 11: 591–595, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Childs EW, Tharakan B, Hunter FA, Tinsley JH, Cao X. Apoptotic signaling induces hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol 292: H3179–H3189, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 23: 2785–2796, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Davidson MT, Deitch EA, Lu Q, Osband A, Feketeova E, Németh ZH, Haskó G, Xu DZ. A study of the biologic activity of trauma-hemorrhagic shock mesenteric lymph over time and the relative role of cytokines. Surgery 136: 32–41, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Dayal SD, Haskó G, Lu Q, Xu DZ, Caruso JM, Sambol JT, Deitch EA. Trauma/hemorrhagic shock mesenteric lymph upregulates adhesion molecule expression and IL-6 production in human umbilical vein endothelial cells. Shock 17: 491–495, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Deb S, Sun L, Martin B, Talens E, Burris D, Kaufmann C, Rich N, Rhee P. Lactated ringer's solution and hetastarch but not plasma resuscitation after rat hemorrhagic shock is associated with immediate lung apoptosis by the up-regulation of the Bax protein. J Trauma 49: 47–53, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Deitch EA Animal models of sepsis and shock: a review and lessons learned. Shock 9: 1–11, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Deitch EA Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 216: 117–134, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deitch EA, Adams C, Lu Q, Xu DZ. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery 129: 39–47, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Deitch EA, Adams CA, Lu Q, Xu DZ. Mesenteric lymph from rats subjected to trauma-hemorrhagic shock are injurious to rat pulmonary microvascular endothelial cells as well as human umbilical vein endothelial cells. Shock 16: 290–293, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Deitch EA, Xu D, Kaiser VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci 11: 520–528, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Denecker G, Vercammen D, Declercq W, Vandenabeele P. Apoptotic and necrotic cell death induced by death domain receptors. Cell Mol Life Sci 58: 356–370, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiser T, Atabai K, Jarreau PH, Ware LB, Pugin J, Matthay MA. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1β-dependent mechanism. Am J Respir Crit Care Med 163: 1384–1388, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Hooper WC, Mensah GA, Haworth SG, Black SM, Garcia JG, Langleben D. Vascular endothelium summary statement V: pulmonary hypertension and acute lung injury: public health implications. Vascul Pharmacol 46: 327–329, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNFα-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol 160: 3480–3486, 1998. [PubMed] [Google Scholar]
- 24.Jaskille A, Alam HB, Rhee P, Hanes W, Kirkpatrick JR, Koustova E. d-lactate increases pulmonary apoptosis by restricting phosphorylation of bad and eNOS in a rat model of hemorrhagic shock. J Trauma 57: 262–269, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Jernigan TW, Croce MA, Fabian TC. Apoptosis and necrosis in the development of acute lung injury after hemorrhagic shock. Am Surg 70: 1094–1098, 2004. [PubMed] [Google Scholar]
- 26.Jones EA, Shoskes DA. The effect of mycophenolate mofetil and polyphenolic bioflavonoids on renal ischemia reperfusion injury and repair. J Urol 163: 999–1004, 2000. [PubMed] [Google Scholar]
- 27.Joseph B, Marchetti P, Formstecher P, Kroemer G, Lewensohn R, Zhivotovsky B. Mitochondrial dysfunction is an essential step for killing of non-small cell lung carcinomas resistant to conventional treatment. Oncogene 21: 65–77, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser VL, Sifri ZC, Dikdan GS, Berezina T, Zaets S, Lu Q, Xu DZ, Deitch EA. Trauma-hemorrhagic shock mesenteric lymph from rat contains a modified form of albumin that is implicated in endothelial cell toxicity. Shock 23: 417–425, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Kiang JG, Lu X, Tabaku LS, Bentley TB, Atkins JL, Tsokos GC. Resuscitation with lactated Ringer solution limits the expression of molecular events associated with lung injury after hemorrhage. J Appl Physiol 98: 550–556, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Kim GT, Chun YS, Park JW, Kim MS. Role of apoptosis-inducing factor in myocardial cell death by ischemia-reperfusion. Biochem Biophys Res Commun 309: 619–612, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Lee BI, Lee DJ, Cho KJ, Kim GW. Early nuclear translocation of endonuclease G and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Neurosci Lett 386: 23–27, 2005. [DOI] [PubMed] [Google Scholar]
- 31a.Leers MP, Kölgen W, Björklund V, Bergman T, Tribbick G, Persson B, Björklund P, Ramaekers FC, Björklund B, Nap M, Jörnvall H, Schutte B. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol 187: 567–572, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2: 589–598, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Nemoto M, Xu Z, Yu SW, Shimoji M, Andrabi SA, Haince JF, Poirier GG, Dawson TM, Dawson VL, Koehler RC. Influence of duration of focal cerebral ischemia and neuronal nitric oxide synthase on translocation of apoptosis-inducing factor to the nucleus. Neuroscience 144: 56–65, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu PL, Chen YL, Chen YH, Lin SJ, Kou YR. Wood smoke extract induces oxidative stress-mediated caspase-independent apoptosis in human lung endothelial cells: role of AIF and EndoG. Am J Physiol Lung Cell Mol Physiol 289: L739–L749, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Lu Q, Xu DZ, Davidson MT, Haskó G, Deitch EA. Hemorrhagic shock induces endothelial cell apoptosis, which is mediated by factors contained in mesenteric lymph. Crit Care Med 32: 2464–2470, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Martin TR, Hagimoto N, Nakamura M, Matute-Bello G. Apoptosis and epithelial injury in the lungs. Proc Am Thorac Soc 2: 214–220, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauriz JL, González P, Jorquera F, Olcoz JL, González-Gallego J. Caspase inhibition does not protect against liver damage in hemorrhagic shock. Shock 19: 33–37, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Mura M, Andrade CF, Han B, Seth R, Zhang Y, Bai XH, Waddell TK, Hwang D, Keshavjee S, Liu M. Intestinal ischemia-reperfusion-induced acute lung injury and oncotic cell death in multiple organs. Shock 28: 227–238, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Osband AJ, Deitch EA, Lu Q, Zaets S, Dayal S, Lukose B, Xu DZ. The role of oxidant-mediated pathways in the cytotoxicity of endothelial cells exposed to mesenteric lymph from rats subjected to trauma-hemorrhagic shock. Shock 20: 269–273, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Pelosi P, D'Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, Barbas CS, Chiaranda M, Gattinoni L. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl 42: 48s–56s, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol 167: 1545–1559, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perl M, Chung CS, Perl U, Lomas-Neira J, de Paepe M, Cioffi WG, Ayala A. Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury. Am J Respir Crit Care Med 176: 591–601, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perl M, Lomas-Neira J, Chung CS, Ayala A. Epithelial cell apoptosis and neutrophil recruitment in acute lung injury- a unifying hypothesis? What have we learned from small interfering RNAs. Mol Med 14: 465–475, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg RD, Aird WC. Vascular-bed–specific hemostasis and hypercoagulable states. N Engl J Med 340: 1555–1564, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Sambol JT, Xu DZ, Adams CA, Magnotti LJ, Deitch EA. Mesenteric lymph duct ligation provides long term protection against hemorrhagic shock-induced lung injury. Shock 14: 416–420, 2000. [PubMed] [Google Scholar]
- 47.Sarin EL, Moore EE, Moore JB, Masuno T, Moore JL, Banerjee A, Silliman CC. Systemic neutrophil priming by lipid mediators in post-shock mesenteric lymph exists across species. J Trauma 57: 950–954, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Senthil M, Watkins A, Barlos D, Xu DZ, Lu Q, Abungu B, Caputo F, Feinman R, Deitch EA. Intravenous injection of trauma-hemorrhagic shock mesenteric lymph causes lung injury that is dependent upon activation of the inducible nitric oxide synthase pathway. Ann Surg 246: 822–830, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Shires GT, Browder LK, Steljes TP, Williams SJ, Browder TD, Barber AE. The effect of shock resuscitation fluids on apoptosis. Am J Surg 189: 85–91, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 294: L632–L641, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Tang PS, Tsang ME, Lodyga M, Bai XH, Miller A, Han B, Liu M. Lipopolysaccharide accelerates caspase-independent but cathepsin B-dependent death of human lung epithelial cells. J Cell Physiol 209: 457–467, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Upperman JS, Deitch EA, Guo W, Lu Q, Xu D. Post-hemorrhagic shock mesenteric lymph is cytotoxic to endothelial cells and activates neutrophils. Shock 10: 407–414, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress sundrome. Am J Respir Crit Care Med 163: 1376–1383, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297: 259–263, 2002. [DOI] [PubMed] [Google Scholar]