Abstract
Chronic hypoxia during the course of pregnancy is a common insult to the fetus. However, its long-term effect on the pulmonary vasculature in adulthood has not been described. In this study, the vasorelaxation responses of conduit pulmonary arteries in adult female sheep that were chronically hypoxic as fetuses and raised postnatally at sea level were investigated. Vessel tension studies revealed that endothelium-dependent relaxation responses were attenuated in pulmonary arteries from adult sheep that experienced prenatal hypoxia. Endothelial nitric oxide synthase (eNOS) protein expression was unchanged, but eNOS activity was significantly decreased in pulmonary arteries from prenatally hypoxic sheep. Protein expression of eNOS partners, caveolin-1, calmodulin, and heat shock protein 90 (Hsp90) did not change following prenatal hypoxia. However, the association between eNOS and caveolin-1, its inhibitory binding partner, was significantly increased, whereas association between eNOS and its stimulatory partners calmodulin and Hsp90 was greatly decreased. Furthermore, phosphorylation of Ser1177 in eNOS decreased, whereas phosphorylation of Thr495 increased, in the prenatally hypoxic pulmonary arteries, events that are related to eNOS activity. These data demonstrate that prenatal hypoxia results in persistent abnormalities in endothelium-dependent relaxation responses of pulmonary arteries in adult sheep due to decreased eNOS activity resulting from altered posttranslational regulation.
Keywords: endothelial nitric oxide synthase, caveolin-1, calmodulin, heat shock protein 90
a considerable body of epidemiological data support the idea that the development of diseases in later life, such as type 2 diabetes, hypertension, and cardiovascular disease, is linked to an abnormal intrauterine environment that results in growth retardation (3, 10, 28). Chronic in utero hypoxia during gestation is a common insult to the fetus and results in fetal intrauterine growth restriction, which is independent of malnutrition (2, 23, 33, 43, 51, 54). Among all the stresses to which the fetus is subjected, perhaps the most important and clinically relevant is hypoxia. Living at high altitude, smoking cigarettes, or exposure to carbon monoxide air pollution during pregnancy can result in fetal hypoxia. In addition, many clinical conditions in pregnant women, such as anemia, hemoglobinopathy, cord compression, placental insufficiency, and heart, lung, or kidney diseases can cause chronic hypoxia in the fetuses. Evidence from studies in various experimental animal models indicates that undernutrition during pregnancy (5, 13, 32, 36, 46) or fetal exposure to elevated glucocorticoid levels (42, 45) results in systemic vascular dysfunction in adult offspring. Several studies have demonstrated that fetal hypoxia results in impaired endothelium-dependent relaxation responses in systemic vessels. A study by Payne et al. (47) showed that endothelium-dependent nitric oxide (NO)-mediated vascular relaxation was inhibited in intrauterine growth-restricted offspring of pregnant rats with reduced uteroplacental perfusion. Williams et al. (53) observed that maternal hypoxia during gestation also resulted in impaired NO-mediated endothelial function in adult offspring, an effect mediated by reduced NO bioavailability. However, the long-term effects of prenatal hypoxia on endothelium-dependent NO-mediated vascular relaxation in adult pulmonary arteries are largely unknown.
NO is generated in endothelial cells from conversion of l-arginine to l-citrulline by the enzymatic action of endothelial nitric oxide synthase (eNOS). The regulation of eNOS activity is complex. A range of transcriptional and posttranscriptional mechanisms regulates the protein expression levels of eNOS. The eNOS enzyme reversibly associates with a diverse family of protein partners that regulate eNOS subcellular localization, catalytic function, and biological activity. eNOS enzyme activity and subcellular localization are intimately controlled by posttranslational modifications including phosphorylation, S-nitrosylation, and acylation (8). In the pulmonary artery isolated from hypoxia-induced pulmonary hypertensive rats, decreased eNOS activity resulted from abnormal interaction between eNOS and its regulatory proteins (44). However, the effect of fetal hypoxia on endothelial eNOS-NO signaling pathway in adult pulmonary arteries is unknown.
Studies have suggested that the pulmonary circulation in females is more sensitive to late effects of perinatal hypoxia. Resting pulmonary arterial pressure and/or pulmonary vascular resistance in adult rats were slightly but significantly elevated by perinatal hypoxia when pooled male and female rats were studied (27, 34) but not in experiments on male rats only (30). Hampl et al. (31) also found that perinatal hypoxia caused long-term alterations of pulmonary circulation and right heart that were more pronounced in female than in male rats.
Therefore, the objectives of this study were to test the hypothesis that long-term prenatal hypoxia affects endothelium-dependent relaxation responses in pulmonary arteries of adult female sheep. Our results demonstrate that prenatal hypoxia results in persistent impairment in endothelium-dependent relaxation responses of pulmonary arteries in adult sheep. This is due to decreased activity of eNOS resulting from abnormal protein-protein interactions between eNOS and its partner proteins and an altered phosphorylation status of eNOS.
MATERIALS AND METHODS
Animals.
Pregnant ewes carrying single or twin fetuses were obtained from Nebeker Ranch, Lancaster, CA [altitude, ∼300 m; arterial partial pressure of O2 (PaO2), 102 ± 2 Torr]. To induce chronic hypoxia in the fetuses, some pregnant sheep were transported to Barcroft Laboratory, White Mountain Research Station, Bishop, CA (altitude, 3,801 m; PaO2, 60 ± 2 Torr) at 30 days of gestation (term being 150 days) and kept there for ∼110 days. The ewes were brought down to sea level immediately before delivery, and the newborn lambs were raised at sea level for 14 mo. Control pregnant ewes were kept at sea level until delivery, and the newborn lambs were raised at sea level for 14 mo.
We used 25 adult female sheep in this study. Sheep were anesthetized with thiamylal (10 mg/kg iv) and then euthanized with T-61 (euthanasia solution; Hoechst-Roussel, Somerville, NJ). All procedures and protocols used in the present study were approved by the Animal Research Committees of Los Angeles Biomedical Research Institute and Loma Linda University (37).
Pulmonary artery preparation and vessel tension study.
The lungs were immediately removed, and the fourth- and fifth-generation pulmonary arteries were dissected free of parenchyma and cut into rings (length, 5 mm) in ice-cold modified Krebs-Ringer bicarbonate buffer (in mM: 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, and 11.1 glucose). The outside diameters of pulmonary arteries of control and prenatally hypoxic sheep were 3.53 ± 0.25 (n = 8) and 3.97 ± 0.27 mm (n = 9), respectively (P > 0.05).
Vessel rings were suspended in organ chambers filled with 10 ml of modified Krebs-Ringer bicarbonate solution maintained at 37 ± 0.5°C and aerated with 95% O2-5% CO2 (pH 7.4). Each ring was suspended via two stirrups that were passed through the lumen: one stirrup was anchored to the bottom of the organ chamber, and the other one was connected to a strain gauge (model FT03C; Grass Instrument, Quincy, MA) for the measurement of isometric force (18).
At the beginning of the experiment, each vessel ring was stretched to its optimal resting tension. This was achieved by stepwise stretching, in 0.1-g increments, until the contractile response to 100 mM KCl reached a plateau. The optimal resting tension was 0.8 ± 0.06 (n = 8) and 0.68 ± 0.07 g (n = 9) for the control and prenatally hypoxic arteries, respectively (P > 0.05).
Vessels were allowed to equilibrate for 1 h after they were brought to their optimal resting tension. Relaxation responses were determined in vessel rings preconstricted with 6 × 10−9 M endothelin (ET)-1. A-23187 (an endothelium-dependent but receptor-independent vasodilator)- or DETA-NONOate (a NO donor)-induced responses were determined at least 30 min after the administration of nitro-l-arginine (10−4 M, an inhibitor of NOS). In all experiments, indomethacin (10−5 M) was present to prevent the possible interference of vasoactive prostanoids (16).
eNOS activity assay.
eNOS activity was measured using a kit from Cayman Chemical (Ann Arbor, MI) according to the manufacturer's instructions. Isolated pulmonary arteries were homogenized in 5 volumes of ice-cold homogenization buffer containing 25 mM Tris·HCl (pH 7.4), 1 mM EDTA, and 1 mM EGTA. The homogenates were sonicated on ice and centrifuged at 10,000 g for 15 min at 4°C. The supernatants were assayed for eNOS activity by measuring the biochemical conversion of [14C]arginine to [14C]citrulline. Aliquots (10 μl) of supernatant were added to a ice-cold reaction buffer (volume, 40 μl) containing 31.25 mM Tris·HCl (pH 7.4), 3.75 μM tetrahydrobiopterin, 1.25 μM flavin adenine dinucleotide, 1.25 μM flavin adenine mononucleotide, 1.25 mM reduced nicotinamide adenine dinucleotide phosphate (NADPH), 0.75 mM CaCl2, and 0.05 μCi [14C]arginine monohydrochloride. Calmodulin was presented in the reaction samples with a final concentration of 0.1 μM. The reaction samples were incubated at room temperature for 60 min. Reactions were terminated by adding 400 μl of stop buffer containing 50 mM HEPES (pH 5.5) and 5 mM EDTA to the reaction samples. The equilibrated resin provided was thoroughly resuspended, and 100 μl of the equilibrated resin were added to each reaction sample. The spin cups were placed into cup holders, and the reaction samples were transferred to spin cups. The spin cups and holders were centrifuged in a microcentrifuge at full speed for 30 s, and then spin cups were removed from cup holders and the eluates were transferred to scintillation vials. Scintillation fluid was added to each vial, and the radioactivity was quantitated in a liquid scintillation counter. Assays were performed in triplicate with total counts and background count controls. The percent citrulline formed in the reaction in relation to total possible counts was determined as follows: %conversion = [(reaction cpm − background cpm)/total cpm] × 100. eNOS activity for each sample is expressed as percent conversion per microgram of protein. Protein concentration in supernatant was measured using Bradford's procedure, with bovine serum albumin as a standard.
Immunoblotting analysis and immunoprecipitation.
Isolated pulmonary arteries were flash frozen in liquid nitrogen and homogenized in 4 volumes of ice-cold lysis buffer containing 50 mM β-glycerophosphate (pH 7.4), 150 mM NaCl, 1.5 mM EGTA, 1 mM EDTA, 1% Triton X-100, 100 mM NaF, 2 mM Na3VO4, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 2 μg/ml pepstatin A. After centrifugation at 13,200 g for 15 min at 4°C, the supernatant was used for Western blot analysis to measure eNOS, caveolin-1, calmodulin, and Hsp90 protein expressions. Protein concentration of the supernatant was measured using Bradford's method, with bovine serum albumin as the standard. Aliquots of the supernatant, each containing 60 μg of protein, were subjected to SDS-PAGE and electrically transferred to nitrocellulose membrane, and nonspecific binding sites were blocked with Tris-buffered saline (TBS) containing 5% (wt/vol) nonfat dry powdered milk for 1 h at room temperature. After two brief washes with TBS containing 0.1% Tween 20 (TBST), the blots were then incubated in 1:3,000-diluted anti-eNOS monoclonal antibody (Sigma-Aldrich, St. Louis, MO), 1:5,000-diluted anti-caveolin-1 polyclonal antibody (Abcam), 1:3,000-diluted anti-calmodulin polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), 1:1,000-diluted anti-Hsp90 monoclonal antibody (Abcam), and 1:10,000-diluted anti-actin monoclonal antibody (EMD Chemicals, La Jolla, CA) overnight at 4°C. After three washes in TBST, the blots were incubated in 1:3,000 (for eNOS)-, 1:6,000 (for caveolin-1)-, 1:5,000 (for calmodulin)-, 1:1,000 (for Hsp90)-, and 1:20,000 (for actin)-diluted horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies for 1 h at room temperature. After three more washes in TBST, the blots were exposed to X-ray film using SuperSignal West Pico chemiluminescent substrate (Pierce Biotechnology, Rockford, IL) and developed. The relative density of the blot was determined with UN-SCAN-IT software (Silk Scientific, Orem, UT), and the blot densities were normalized to that of actin. In experiments to examine the association of caveolin-1, calmodulin, and Hsp90 with eNOS or to detect the phospho-Ser1177 and phospho-Thr495 eNOS protein expression, isolated pulmonary arteries from control and prenatally hypoxic sheep were first incubated in the Krebs-Ringer bicarbonate buffer (37°C, 95% O2-5% CO2, pH 7.4) containing 10−5 M indomethacin for 30 min. ET-1 (6 × 10−9 M) and acetylcholine (10−4 M) were then added sequentially with a 30-min interval. At the end of the incubation, vessels were flash frozen in liquid nitrogen and homogenized as described above. The supernatant was immunoprecipitated with anti-eNOS polyclonal antibody (Sigma-Aldrich) using Dynabeads protein G (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Briefly, aliquots of the supernatant, each containing 1,500 μg of protein, were precleared by incubating with 50 μl of beads for 1 h at 4°C to minimize nonspecific binding. Fresh beads (50 μl) were washed with citrate phosphate buffer (pH 5.0) and then coated with 10 μg of anti-eNOS polyclonal antibody (Sigma-Aldrich). The bound antibody was cross-linked to the beads using 20 mM dimethyl pimelimidate in 0.2 M triethanolamine (pH 8.2). The precleared supernatant was then added to the cross-linked beads and incubated overnight at 4°C. The protein-Ig-Dynabeads protein G complex was washed with PBS containing 0.1% Tween 20. Finally, protein was eluted with 0.1 M citrate (pH 2.0) and determined by immunoblotting using antibodies against eNOS, caveolin-1, calmodulin, Hsp90, phospho-Ser1177 eNOS (Cell Signaling Technology), and phospho-Thr495 eNOS (Cell Signaling Technology).
Reagents.
The following reagents were used (unless otherwise specified, all were obtained from Sigma-Aldrich): A-23187, acetylcholine, nitro-l-arginine, indomethacin, ET-1 (American Peptide, Sunnyvale, CA), DETA-NONOate (Cayman Chemical), NADPH, and [14C]arginine monohydrochloride (>300 mCi/mmol, 50 μCi/ml; Amersham Biosciences).
Indomethacin (10−5 M) was prepared in equal molar Na2CO3, and DETA-NONOate (10−4 M) was prepared freshly in 0.01 M NaOH. These concentrations of Na2CO3 and NaOH did not significantly affect the pH of the solution in the organ chamber. A-23187 was dissolved in DMSO, and the maximal bath concentration of DMSO attained during any experiment was <0.1%, which had no independent effect on vessel tension. NADPH (10 mM) was freshly prepared in 10 mM Tris·HCl (pH 7.4). The other reagents were prepared using distilled water.
Data analyses.
Contractions are expressed in grams. Relaxations are expressed as percentages of contraction of vessels to endothelin-1. Data are means ± SE. Student's t-test for unpaired observations was used to compare the mean values of two groups. Comparison of mean values of more than two groups was made using one-way ANOVA with the Student-Newman-Keuls test for post hoc testing of multiple comparisons. Statistical significance was accepted when the P value (two tailed) was <0.05. In all experiments, n represents the number of animals.
RESULTS
Vessel tension studies.
Pulmonary arteries of normoxic and prenatally hypoxic sheep were preconstricted with 6 × 10−9 M ET-1 to comparable tension levels before testing the effect of A-23187, acetylcholine, and DETA-NONOate (3.16 ± 0.59 vs. 3.48 ± 1.20 g, n = 8, P > 0.05). To exclude the involvement of cyclooxygenase products, we administered indomethacin (10−5 M) at the beginning of the experiments. For some experiments, nitro-l-arginine (10−4 M, an inhibitor of NOS) was added to the organ chamber. These inhibitors had no effect on the resting tension of pulmonary arteries (data not shown, n = 8, P > 0.05). nitro-l-arginine (10−4 M) also had no significant effect on ET-1-induced constrictor responses of arteries from control and prenatally hypoxic sheep (3.2 ± 0.96 vs. 4.2 ± 1.05 g, n = 8, P > 0.05).
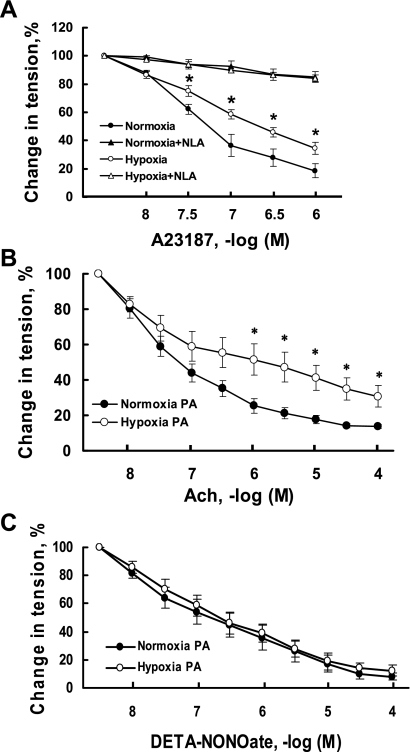
After vessel tension was stable, vasorelaxation responses to A-23187, a calcium ionophore, were examined in the presence and absence of nitro-l-arginine (10−4 M). The calcium ionophore A-23187 is a receptor-independent activator of the endothelium, and maximal response to this drug is a reasonable estimate of maximum endothelium-dependent vasodilator capacity. In the presence of nitro-l-arginine, there were no differences in the relaxation responses to A-23187 between arteries from prenatally hypoxic sheep and controls. However, in the absence of nitro-l-arginine, the relaxation responses to A-23187 were significantly depressed in pulmonary arteries from prenatally hypoxic sheep compared with controls (Fig. 1A).
Fig. 1.
Effect of prenatal hypoxia on relaxation responses of intrapulmonary arteries. A: effect of prenatal hypoxia on relaxation responses of intrapulmonary arteries to A-23187 in the presence (triangles) or absence (circles) of nitro-l-arginine (NLA). NLA (10−4 M) was administrated before normoxic control (filled symbols) or prenatally hypoxic vessels (open symbols) were preconstricted to a similar tension with ET-1 (6 × 10−9 M). Relaxation is expressed as a percentage of vessel tension before the addition of A-23187. Values are means ± SE; n = 6 for prenatally hypoxic and n = 7 for control group. B: relaxation responses of intrapulmonary arteries to acetylcholine (ACh). Pulmonary vessels of control (normoxia PA) or prenatally hypoxic groups (hypoxia PA) were preconstricted to a similar tension with ET-1 (6 × 10−9 M). Relaxation is expressed as a percentage of vessel tension before the addition of ACh. Values are means ± SE; n = 6 for each group. C: relaxation responses of intrapulmonary arteries to DETA-NONOate, an exogenous NO donor. NLA (10−4 M) was administrated before normoxic control or prenatally hypoxic vessels were preconstricted to a similar tension with ET-1 (6 × 10−9 M). Relaxation is expressed as a percentage of vessel tension before the addition of DETA-NONOate. Values are means ± SE; n = 6 for each group. *P < 0.05, significantly different from pulmonary arteries from normoxic control.
Acetylcholine is a potent vasodilator that can induce NO-mediated endothelium-dependent relaxation responses in pulmonary vessels. Acetylcholine elicited a smaller relaxation of pulmonary arteries obtained from prenatally hypoxic sheep than from control sheep (Fig. 1B). The relaxation responses of pulmonary arteries to DETA-NONOate, an exogenous NO donor, were determined at least 30 min after the administration of nitro-l-arginine (10−4 M, an inhibitor of NOS), to exclude the involvement of endogenous NO. There were no significant differences between the relaxation responses to exogenous NO of pulmonary arteries from control and prenatally hypoxic sheep (Fig. 1C).
eNOS activity and protein expression.
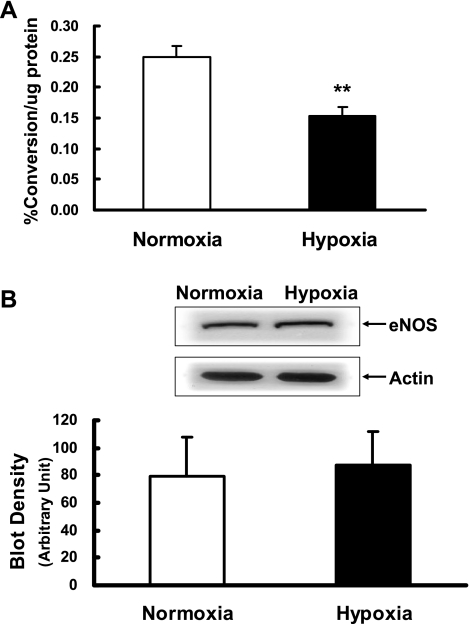
eNOS activity assay measuring the biochemical conversion of [14C]arginine to [14C]citrulline showed that eNOS activity was greatly decreased in pulmonary arteries from prenatally hypoxic sheep compared with control sheep (Fig. 2A). Immunoblot analysis showed that there was no significant difference between the protein expression levels of eNOS in pulmonary arteries from control and prenatally hypoxic sheep (Fig. 2B).
Fig. 2.
Endothelial nitric oxide synthase (eNOS) activity and protein expression. A: eNOS activity in intrapulmonary arteries isolated from control (normoxia) and prenatal hypoxia groups. Values are means ± SE; n = 7 for each group. **P < 0.01, significantly different from intrapulmonary arteries from normoxic control. B: eNOS protein expression in intrapulmonary arteries isolated from control (normoxia) and prenatal hypoxia groups. Representative Western blots for eNOS and actin are shown at top; blot density obtained from densitometric scanning of eNOS normalized to actin is shown at bottom. Values are means ± SE; n = 7 for each group.
Interaction of eNOS with caveolin-1, calmodulin, and Hsp90 proteins.
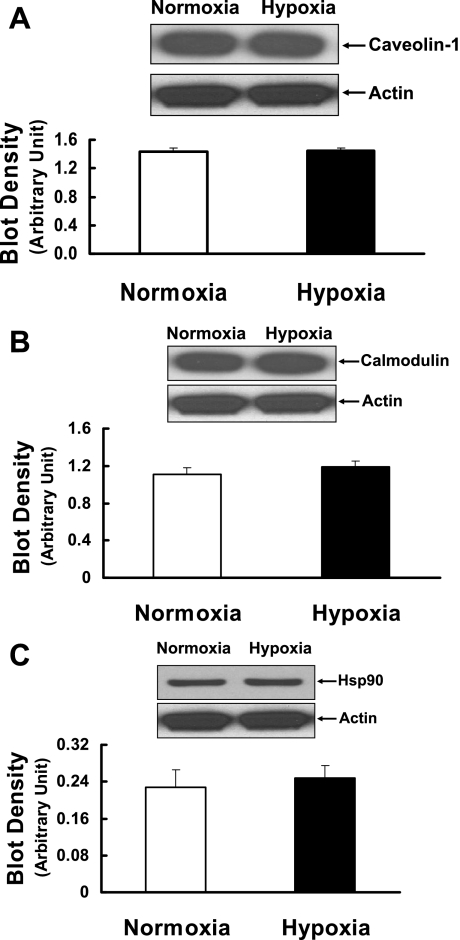
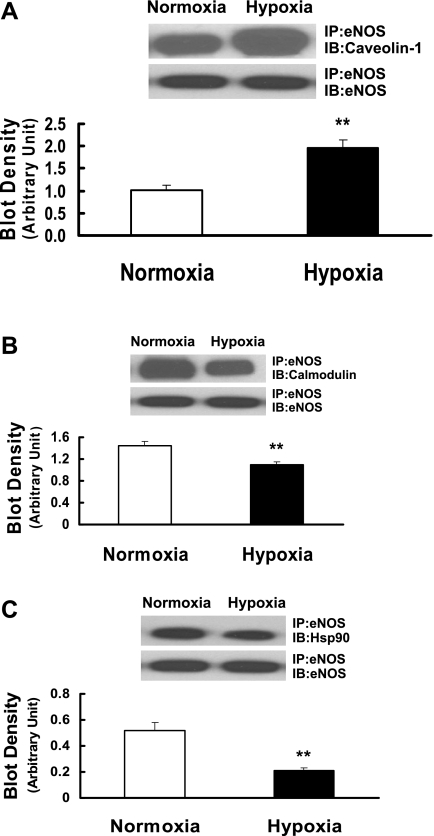
To elucidate further the mechanisms responsible for the impairment of eNOS activity induced by prenatal hypoxia, we examined eNOS regulatory binding partners such as caveolin-1, calmodulin, and Hsp90 and their interactions with eNOS. As shown in Fig. 3, A–C, protein expression levels of caveolin-1, calmodulin, and Hsp90 in pulmonary arteries of prenatally hypoxic sheep were not significantly different from those of control sheep. To determine the interaction of eNOS with caveolin-1, calmodulin, and Hsp90, eNOS immune complex was isolated using anti-eNOS antibody, separated by SDS-PAGE, and then probed with antibodies against caveolin-1, calmodulin, and Hsp90. In pulmonary arteries of prenatally hypoxic sheep, tighter coupling between eNOS and caveolin-1 was observed compared with normoxic sheep (Fig. 4A). In contrast, the association of calmodulin with eNOS was significantly reduced in pulmonary arteries of prenatally hypoxic sheep compared with control sheep (Fig. 4B). In addition, in pulmonary arteries of prenatally hypoxic sheep, less Hsp90 protein was detected in eNOS immune complex than in normoxic sheep (Fig. 4C).
Fig. 3.
Caveolin-1 (A), calmodulin (B), and Hsp90 (C) protein expression in intrapulmonary arteries isolated from control (normoxia) and prenatal hypoxia groups. Representative Western blots for caveolin-1, calmodulin, Hsp90, and actin are shown at top; blot density obtained from densitometric scanning of caveolin-1, calmodulin, and Hsp90 normalized to actin is shown at bottom. Values are means ± SE; n = 5 for each group.
Fig. 4.
Interaction between eNOS and caveolin-1 (A), calmodulin (B), and Hsp90 (C) in pulmonary arteries isolated from control (normoxia) and prenatal hypoxia groups. Tissue lysates were immunoprecipitated (IP) with anti-eNOS antibody and immunoblotted (IB) with antibodies recognizing caveolin-1, calmodulin, and Hsp90. To ensure equal loading in each lane, blots were reprobed with anti-eNOS antibody. The extent of caveolin-1, calmodulin, and Hsp90 associated with eNOS was quantified by scanning densitometry and normalized to eNOS. Values are means ± SE; n = 5 for each group. **P < 0.01, significantly different from intrapulmonary arteries from normoxic control.
eNOS phosphorylation.
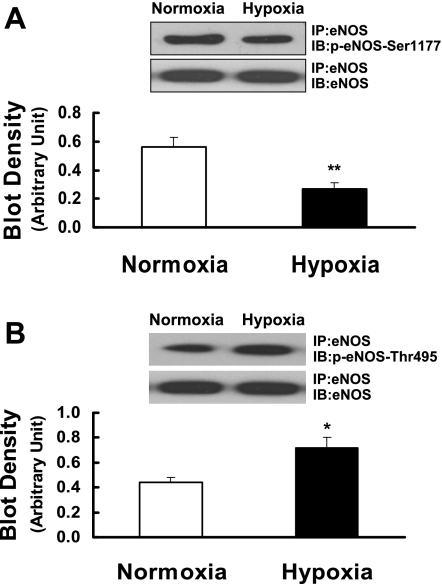
To determine the phosphorylation status of eNOS, eNOS was immunoprecipitated with anti-eNOS antibody and then probed with antibodies against Ser1177- and Thr495-phosphorylated eNOS. In pulmonary arteries of prenatally hypoxic sheep, the phosphorylation of Ser1177 of eNOS was significantly decreased compared with normoxic sheep (Fig. 5A). In contrast, the phosphorylation of Thr495 of eNOS was markedly increased compared with normoxic sheep (Fig. 5B).
Fig. 5.
Ser1177 (A) and Thr495 (B) phosphorylation of eNOS in pulmonary arteries isolated from control (normoxia) and prenatal hypoxia groups. Tissue lysates were immunoprecipitated with anti-eNOS antibody and immunoblotted with antibodies recognizing Ser1177- or Thr495-phosphorylated eNOS. To ensure equal loading in each lane, blots were reprobed with anti-eNOS antibody. The level of Ser1177 or Thr495 phosphorylation of eNOS was quantified by scanning densitometry and normalized to eNOS. Values are means ± SE; n = 5 for each group. *P < 0.05; **P < 0.01, significantly different from intrapulmonary arteries from normoxic control.
DISCUSSION
In this study, we examined the long-term effects of prenatal hypoxia on endothelium-dependent relaxation responses in conduit pulmonary arteries of adult female sheep. We previously reported that chronic high-altitude hypoxia in the ovine fetus results in abnormal relaxation responses in fetal conduit pulmonary arteries (19). The current study determined the long-lasting effects of prenatal hypoxia on postnatal pulmonary vascular function by examining endothelium-dependent relaxation responses in pulmonary arteries from adult sheep. Although there have been reports about effects of perinatal hypoxia on pulmonary artery vasoreactivity of adult animals (38, 48), this is the first study that demonstrates endothelial dysfunction in pulmonary arteries of adult animal following chronic prenatal hypoxia.
Both endothelial and smooth muscle cell diversity exist between proximal conduit and peripheral resistance pulmonary vessels (49, 50). The segmental differential responses to vasoactive substances may have important implications for the regulation of resistance in this low-tone vascular bed (35). Various studies including ours demonstrate that the reactivities of conduit pulmonary vessels are actively regulated and may play a substantial role in the regulation of pulmonary circulation (9, 11, 15–19, 41).
In the present study, to determine the long-term effect of prenatal hypoxia on relaxation responses in pulmonary arteries, we determined vasorelaxation responses to A-23187 in the presence or absence of nitro-l-arginine. A-23187 directly enhances calcium entry into endothelial cells and stimulates eNOS to release NO. As a calcium ionophore, A-23187 is receptor independent, and therefore this agent allows us to determine the function of eNOS even in the presence of abnormalities in receptor function (29), and we can elicit maximal activation of endothelium-dependent calcium-mediated vasorelaxation responses (52). Relaxation responses to A-23187 were significantly attenuated in pulmonary arteries from prenatally hypoxic sheep compared with controls. In the presence of nitro-l-arginine, an inhibitor of NOS, relaxation responses to A-23187 of pulmonary arteries were virtually eliminated. These results suggest that attenuated endothelium-dependent relaxation responses in pulmonary arteries of prenatally hypoxic sheep are due to altered eNOS function. Such a notion is further supported by the findings that relaxation to acetylcholine, which causes pulmonary arteries to relax through endothelium-derived nitric oxide (1), was also attenuated in the prenatal hypoxic arteries.
We found that DETA-NONOate, an exogenous NO donor, after the administration of nitro-l-arginine (an inhibitor of NOS), caused similar relaxation of pulmonary arteries from long-term prenatally hypoxic sheep and normoxic controls. These results suggest that attenuated endothelium-dependent vasodilatation of pulmonary vessels by long-term prenatal hypoxia at high altitude results from altered function of eNOS.
We determined the activity of eNOS to elucidate the underlying mechanisms by which prenatal hypoxia affects pulmonary artery endothelium-dependent vasodilation. eNOS activity assay showed that eNOS activity was significantly decreased in pulmonary arteries of prenatally hypoxic sheep compared with control sheep. These results demonstrate that long-term prenatal hypoxia attenuates endothelium-dependent relaxation responses of pulmonary arteries in adult female sheep by altering eNOS activity. However, Western blot analyses showed that there were no significant differences between the protein expression levels of eNOS in pulmonary arteries from normoxic and prenatally hypoxic sheep. These results suggest that the decreased eNOS activity in pulmonary arteries from prenatally hypoxic sheep is not due to a decreased eNOS protein expression but may result from the eNOS inactivity at posttranslational level.
To elucidate the posttranslational mechanisms underlying the regulation of eNOS activity by long-term prenatal hypoxia, we investigated the interactions of eNOS and its regulatory partner proteins such as caveolin-1, calmodulin, and Hsp90. Caveolin-1 inhibits eNOS that is present in caveolae via a direct interaction between a specific amino acid sequence within caveolin-1, called the scaffolding domain, and a motif in the oxygenase domain of eNOS that is rich in aromatic amino acid residues (20). In addition, an interaction between caveolin-1 and the reductase domain of eNOS also has been demonstrated (22). The binding of caveolin-1 to both the oxygenase and reductase domain of eNOS compromises its ability to bind calmodulin (20, 22). Intracellular calcium level is a critical determinant of eNOS activity, because maximal catalytic function of eNOS requires calmodulin binding to an ∼50-amino acid domain to facilitate transfer of electrons between the enzyme's reductase and oxygenase domains (6). Binding of calmodulin disrupts the inhibitory interaction between caveolin-1 and eNOS (40). Another protein that has been demonstrated to interact with eNOS is the 90-kDa Hsp90 (21). Binding of Hsp90 to eNOS in response to histamine, VEGF, or shear stress increases eNOS activity by facilitating the calmodulin-induced displacement of caveolin from eNOS (25). This increase in eNOS activity is inhibited by the Hsp90 inhibitor geldanamycin (21). After various stimulations, the increase in intracellular calcium induces the binding of calmodulin to eNOS, resulting in disruption of eNOS-caveolin-1 interaction, and eNOS translocates from caveolae into cytosol. On binding with calmodulin, eNOS generates NO, which is enhanced by interaction with Hsp90 (24, 25).
The major finding of this study is that in the pulmonary arteries from prenatally hypoxic sheep, coupling of caveolin-1 to eNOS is increased while caveolin-1 protein expression remains unchanged, compared with normoxic controls. Moreover, without changing the protein expression levels of calmodulin or Hsp90, interactions of eNOS with either calmodulin or Hsp90 decreased in the prenatally hypoxic pulmonary arteries. Therefore, tighter coupling between eNOS and caveolin-1, dissociation of calmodulin and Hsp90 from eNOS may account for the impairment of eNOS activity observed in the prenatally hypoxic pulmonary arteries.
Phosphorylation and dephosphorylation are posttranslational modification mechanisms underlying the regulation of eNOS activity. There are numerous potential phosphorylation sites, but most is known about the functional consequences of phosphorylation of a serine residue (human eNOS sequence, Ser1177; bovine eNOS sequence, Ser1179) in the reductase domain and a threonine residue (human eNOS sequence, Thr495; bovine eNOS sequence, Thr497) within the calmodulin-binding domain. Phosphorylation at Ser1177 is stimulatory, whereas phosphorylation at Thr495 is inhibitory (4). The activation of eNOS catalytic function by Ser1177 phosphorylation is due to the inhibition of calmodulin dissociation from eNOS and also enhancement of the internal rate of eNOS electron transfer (7, 14, 39). On the other hand, phosphorylation at Thr495 attenuates the binding of calmodulin to eNOS (12, 26). Thus we next investigated the phosphorylation of Ser1177 and Thr495 of eNOS in prenatally hypoxic pulmonary arteries and revealed that eNOS Ser1177 phosphorylation was attenuated, whereas eNOS Thr495 phosphorylation was increased, compared with normoxic control sheep. These results suggest that the process of phosphorylation and dephosphorylation are impaired in the pulmonary arteries from prenatally hypoxic sheep.
Basal release of endothelium-derived NO modulates various vasoconstrictor responses, including those of ET-1. In this study, we did not specifically address this issue. Alteration in basally released NO and differential relaxation responses of resistance vs. conduit arteries in this animal model need to be investigated in the future.
In summary, our results demonstrate that prenatal hypoxia results in persistent abnormalities in endothelium-dependent relaxation responses of conduit pulmonary arteries in adult female sheep and that this is due to decreased activity of eNOS. The impairment of eNOS activity may be explained by altered interaction of eNOS with its regulatory partner proteins such as caveolin-1, calmodulin, and Hsp90. Alterations in phosphorylation and dephosphorylation of key serine and threonine residues in eNOS also may be involved in the impairment of eNOS activity in the prenatally hypoxic pulmonary arteries in adult sheep.
GRANTS
This study was supported in part by the National Institutes of Health (NIH) Grants HL 059435 and HL 075187 (to J. U. Raj) and NIH Grant P01 HD 31226 (to L. D. Longo).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adnot S, Raffestin B, Eddahibi S. NO in the lung. Respir Physiol 101: 109–120, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol 285: H983–H990, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ Fetal origins of coronary heart disease. Br Med J 311: 171–174, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem 278: 14841–14849, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res 54: 83–90, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chen PF, Wu KK. Characterization of the roles of the 594–645 region in human endothelial nitric-oxide synthase in regulating calmodulin binding and electron transfer. J Biol Chem 275: 13155–13163, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res 75: 247–260, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards JC, Ignarro LJ, Hyman AL, Kadowitz PJ. Relaxation of intrapulmonary artery and vein by nitrogen oxide-containing vasodilators and cyclic GMP. J Pharmacol Exp Ther 228: 33–42, 1984. [PubMed] [Google Scholar]
- 10.Edwards LJ, Coulter CL, Symonds ME, McMillen IC. Prenatal undernutrition, glucocorticoids and the programming of adult hypertension. Clin Exp Pharmacol Physiol 28: 938–941, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Feletou M, Girard V, Canet E. Different involvement of nitric oxide in endothelium-dependent relaxation of porcine pulmonary artery and vein: influence of hypoxia. J Cardiovasc Pharmacol 25: 665–673, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Franco Mdo C, Arruda RM, Dantas AP, Kawamoto EM, Fortes ZB, Scavone C, Carvalho MH, Tostes RC, Nigro D. Intrauterine undernutrition: expression and activity of the endothelial nitric oxide synthase in male and female adult offspring. Cardiovasc Res 56: 145–153, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, Figeys D, Harrison DG, Berk BC, Aebersold R, Corson MA. Identification of flow-dependent endothelial nitric oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem 274: 30101–30108, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Zhou H, Raj JU. Heterogeneity in role of endothelium-derived NO in pulmonary arteries and veins of full-term fetal lambs. Am J Physiol Heart Circ Physiol 268: H1586–H1592, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Zhou H, Ibe BO, Raj JU. Prostaglandins E2 and I2 cause greater relaxations in pulmonary veins than in arteries of newborn lambs. J Appl Physiol 81: 2534–2539, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Tolsa JF, Raj JU. Heterogeneity in endothelium-derived nitric oxide-mediated relaxation of different sized pulmonary arteries of newborn lambs. Pediatr Res 44: 723–729, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Dhanakoti S, Trevino EM, Sander FC, Portugal AM, Raj JU. Effect of oxygen on cyclic GMP-dependent protein kinase-mediated relaxation in ovine fetal pulmonary arteries and veins. Am J Physiol Lung Cell Mol Physiol 285: L611–L618, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Portugal AD, Negash S, Zhou W, Longo LD, Usha Raj J. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol 292: L678–L684, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem 272: 25437–25440, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392: 821–824, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh S, Gachhui R, Crooks C, Wu C, Lisanti MP, Stuehr DJ. Interaction between caveolin-1 and the reductase domain of endothelial nitric-oxide synthase. Consequences for catalysis. J Biol Chem 273: 22267–22271, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res 49: 490–494, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 280: F193–F206, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric oxide synthase, hsp90 and caveolin-1 complex in vitro: evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem 275: 22268–22272, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Greif DM, Kou R, Michel T. Site-specific dephosphorylation of endothelial nitric oxide synthase by protein phosphatase 2A: evidence for crosstalk between phosphorylation sites. Biochemistry 41: 15845–15853, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Hakim TS, Mortola JP. Pulmonary vascular resistance in adult rats exposed to hypoxia in the neonatal period. Can J Physiol Pharmacol 68: 419–424, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35: 595–601, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Hammond LC, Bonnet C, Kemp PJ, Yates MS, Bowmer CJ. Chronic hypoxia up-regulates expression of adenosine A1 receptors in DDT1-MF2 cells. Biochem Pharmacol 67: 421–426, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Hampl V, Herget J. Perinatal hypoxia increases hypoxic pulmonary vasoconstriction in adult rats recovering from chronic exposure to hypoxia. Am Rev Respir Dis 142: 619–624, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Hampl V, Bibova J, Ostadalova I, Povysilova V, Herget J. Gender differences in the long-term effects of perinatal hypoxia on pulmonary circulation in rats. Am J Physiol Lung Cell Mol Physiol 285: L386–L392, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Holemans K, Gerber R, Meurrens K, De Clerck F, Poston L, Van Assche FA. Maternal food restriction in the second half of pregnancy affects vascular function but not blood pressure of rat female offspring. Br J Nutr 81: 73–79, 1999. [PubMed] [Google Scholar]
- 33.Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health 87: 1003–1007, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keith IM, Tjen-A-Looi S, Kraiczi H, Ekman R. Three-week neonatal hypoxia reduces blood CGRP and causes persistent pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 279: H1571–H1578, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Kemp BK, Smolich JJ, Cocks TM. Evidence for specific regional patterns of responses to different vasoconstrictors and vasodilators in sheep isolated pulmonary arteries and veins. Br J Pharmacol 121: 441–450, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 86: 217–222, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Long W, Zhang L, Longo LD. Fetal and adult cerebral artery KATP and KCa channel responses to long-term hypoxia. J Appl Physiol 92: 1692–1701, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Marino M, Bény JL, Peyter AC, Bychkov R, Diaceri G, Tolsa JF. Perinatal hypoxia triggers alterations in K+ channels of adult pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 293: L1171–L1182, 2007. [DOI] [PubMed] [Google Scholar]
- 39.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem 275: 6123–6128, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem 272: 25907–25912, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Miike T, Shirahase H, Kanda M, Kunishiro K, Kurahashi K. Regional heterogeneity of substance P-induced endothelium-dependent contraction, relaxation, and -independent contraction in rabbit pulmonary arteries. Life Sci 83: 810–814, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Molnar J, Howe DC, Nijland MJ, Nathanielsz PW. Prenatal dexamethasone leads to both endothelial dysfunction and vasodilatory compensation in sheep. J Physiol 547: 61–66, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore LG Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol 4: 141–156, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem 277: 44085–44092, 2002. [DOI] [PubMed] [Google Scholar]
- 45.O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab 287: E863–E870, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol 530: 141–152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne JA, Alexander BT, Khalil RA. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension 42: 768–774, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Peyter AC, Muehlethaler V, Liaudet L, Marino M, Di Bernardo S, Diaceri G, Tolsa JF. Muscarinic receptor M1 and phosphodiesterase 1 are key determinants in pulmonary vascular dysfunction following perinatal hypoxia in mice. Am J Physiol Lung Cell Mol Physiol 295: L201–L213, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Stenmark KR, Frid MG. Smooth muscle cell heterogeneity: role of specific smooth muscle cell subpopulations in pulmonary vascular disease. Chest 114: 82–90, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Stevens T, Phan S, Frid MG, Alvarez D, Herzog E, Stenmark KR. Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc 5: 783–791, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unger C, Weiser JK, McCullough RE, Keefer S, Moore LG. Altitude, low birth weight, and infant mortality in Colorado. JAMA 259: 3427–3432, 1988. [PubMed] [Google Scholar]
- 52.Weksler BB, Ley CW, Jaffe EA. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A23187. J Clin Invest 62: 923–930, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams SJ, Hemmings DG, Mitchell JM, McMillen IC, Davidge ST. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol 565: 125–135, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zamudio S, Droma T, Norkyel KY, Acharya G, Zamudio JA, Niermeyer SN, Moore LG. Protection from intrauterine growth retardation in Tibetans at high altitude. Am J Phys Anthropol 91: 215–224, 1993. [DOI] [PubMed] [Google Scholar]