Abstract
Binding of endothelial nitric oxide synthase (eNOS) to the chaperone protein, Hsp90, promotes coupled eNOS synthetic activity. Using resistance level pulmonary arteries (PRA) from 2-day-, 5- to 7-day-, and 12-day-old piglets, we tested the hypothesis that Hsp90-eNOS interactions are developmentally regulated in the early neonatal period. PRA were isolated for coimmunoprecipitation and immunoblot analyses or cannulated for continuous diameter measurements using the pressurized myography technique. NOS inhibition caused less constriction in PRA from 2-day- compared with 5- to 7-day- and 12-day-old piglets. No age-related differences were found in dilation responses to an NO donor or in protein expression of Hsp90, phospho-eNOS (Ser1177), Akt, phospho-Akt, or caveolin-1. Compared with the older animals, PRA from 2-day-old piglets had higher total eNOS expression but displayed less binding of eNOS to Hsp90 and Akt. Hsp90 antagonism with radicicol induced greatest constriction in PRA from 12-day-old piglets. ACh stimulation caused dilation in PRA from 5- to 7-day- and 12-day-old but not 2-day-old animals, despite rapid and equivalent ACh-mediated eNOS phosphorylation (Ser1177) in all three age groups. Hsp90 inhibition abolished ACh-mediated dilation in PRA from the older piglets. ACh failed to stimulate Hsp90-eNOS binding in 2-day-old but induced a significant increase in Hsp90-eNOS coimmunoprecipitation in PRA from the older age groups, which was blocked by Hsp90 antagonism. We conclude that physical interactions between Hsp90 and eNOS mature over the first weeks of life, likely contributing to the postnatal fall in pulmonary vascular resistance and changes in agonist-induced pulmonary vascular responses characteristic of the early neonatal period.
Keywords: Akt, development, nitric oxide
the early postnatal period is one of dramatic changes in the pulmonary circulation. Soon after birth, the neonatal pulmonary circulation undergoes vascular remodeling and a marked drop in pulmonary vascular resistance resulting in an 8- to 10-fold increase in pulmonary blood flow (1, 9, 12, 25, 40). The mechanisms responsible for postnatal changes in pulmonary vascular tone, reactivity, and architecture have long been of interest to pulmonary vascular biologists and neonatologists caring for infants with persistent pulmonary hypertension of the newborn (PPHN). Whereas pulmonary hypertension caused by genetic or environmental factors can occur at any postnatal age, the syndrome and pathobiology of PPHN is unique to the early newborn period. Delineating the mechanisms that underlie the normal postnatal changes in pulmonary vascular tone and reactivity could lead to novel therapies to treat infants who fail to undergo the normal postnatal circulatory transition.
A role for nitric oxide (NO) in normal perinatal circulatory transition is well-established. Prenatal inhibition of nitric oxide synthase (NOS) causes postnatal pulmonary hypertension (1, 11, 19). Differences in pulmonary vascular responses and NOS expression between late fetal and early newborn life have been well-described (3, 26, 36, 38, 42, 47). Juvenile and adult animals demonstrate enhanced endothelium-dependent vasodilation compared with the fetus and newborn (2, 33). Changes in NO production and NOS expression and function have been implicated in these postnatal maturational changes in pulmonary vascular tone and reactivity (2, 10, 23, 24, 31, 33, 36, 38, 47, 49). This manuscript addresses a critical window of time in the first 2 wk of life when little is known about the regulation of NOS activity and the corresponding pulmonary vascular responses.
More recently, the complex regulation of NO production from endothelial NOS (eNOS) has been appreciated, involving a number of posttranslational modifications that are critical to eNOS synthetic activity. We (4) and others (28, 34, 46) have reported that the constitutively expressed chaperone protein, Hsp90, regulates vascular tone and NO production in the lung. The role of Hsp90-eNOS interactions in the early postnatal maturation of pulmonary vascular tone and reactivity remains unclear.
In this study, we tested the hypothesis that Hsp90-eNOS interactions are developmentally regulated in the early neonatal period. We further hypothesized that postnatal alterations in Hsp90 chaperone function has a functional impact on the regulation of pulmonary vascular tone over the first 2 wk of postnatal life. All of our studies in newborn piglets were performed with resistance level pulmonary arteries (PRA) because of their importance in the regulation of pulmonary vascular tone.
MATERIALS AND METHODS
Animals.
Newborn piglets of three different age groups, 2 days (n = 22), 5–7 days (n = 17), and 12 days (n = 15), were preanesthetized with ketamine [30 mg/kg intramuscular (im)] and acepromazine (2 mg/kg im) followed by an intravenous injection of sodium pentobarbital (10 mg/kg) and heparin (1,000 IU/kg iv) and then exsanguinated. Heart and lungs were removed en block and stored in cold, oxygenated physiological bicarbonate solution (PBS) for up to 72 h before study. All experimental protocols were performed in adherence with the National Institutes of Health (NIH) guidelines for the use of experimental animals and approved by the Animal Care and Use Committee of Vanderbilt University Medical Center. The animal resource facility is fully accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC).
Isolation of PRA for measurement of pressurized diameter.
Piglet PRA (80- to 250-μm diameter) were isolated, cannulated, and pressurized for continuous measurement of diameter according to our previously published methods (4, 5, 13, 15, 16, 20). Briefly, PRA measuring <250-μm diameter were dissected in oxygenated Krebs-Henseleit buffer with the following millimolar composition: 118 NaCl, 25 NaHCO3, 4.5 KCl, 1.2 MgCl2·6H2O, 1.2 KH2PO4, 11 dextrose, 2.5 CaCl2·2H2O, and 0.026 EDTA equilibrated with 21% O2, 5% CO2, and balance N2 to maintain pH 7.4. The arteries were transferred to a commercial arteriograph (Living Systems Instrumentation, Burlington, VT) or a custom-made arteriograph chamber (Instrumentation and Model Facility, University of Vermont) where they were cannulated at one end, secured with a single strand of suture, gently flushed free of blood, and cannulated at the distal end. All side branches were tied with a single fiber of 10-0 braided nylon thread to achieve a leak-free preparation. The arteriograph was set on the stage of an inverted microscope (Nikon TMS) with a video camera (Sony XC-73) attached to the viewing tube. Intraluminal pressure was maintained at 15 mmHg by a pressure servo system connected to the proximal cannula. Prewarmed (37°C) buffer was circulated through the vessel chamber at a rate of 30 ml/min. The vessel image was projected on a computer screen, and the vessel diameter was continuously measured by means of a computer-assisted image capture system (SoftEdge and IonWizard 4.4 analysis software; IonOptix, Milton, MA) or on a television monitor with image capture by means of a video scaler (model IV-550; FOR-A, Gainesville, FL).
PRA study protocol.
After a 30-min equilibration period with a stable diameter, vascular smooth muscle and endothelial function were assessed by contraction to KCl (50 mM) or the thromboxane mimetic, U-46619 (0.01 μM), and dilation to ACh (1 μM) or the calcium ionophore, A-23187 (1 μM). Following another 30-min equilibration period, the concentration-dependent effects of the NOS antagonist, NG-nitro-l-arginine methyl ester (l-NAME; 1–100 μM), the NO-independent vasodilator, S-nitroso-N-acetyl penicillamine (SNAP; 1 nM to 10 μM) or the NO-dependent vasodilator, ACh (0.1 nM to 10 μM), were determined. The responses to the Hsp90 antagonist, radicicol (20 nM), on baseline tone and ACh-mediated vasodilation were also assessed in PRA from the three age groups. At the end of each study protocol, PRA were constricted with KCl (120 mM) or U-46619 (0.01 μM) to test for vessel viability. PRA studied on the day of death had similar responses to dilator and constrictor agonists as PRA studied after the lungs were stored for 24–72 h in cold, oxygenated PBS.
Coimmunoprecipitation assays.
Dissected PRA (20–300 μm) were immediately placed in HEPES buffer on ice until quantities of PRA sufficient to perform the coimmunoprecipitation protocols were obtained. Some dissected PRA were immediately snap-frozen in liquid nitrogen, whereas others were incubated in HEPES at 37°C for 20 min in the presence of the Hsp90 antagonist, geldanamycin (1 μM), or vehicle (DMSO). Some of these vessels were subsequently stimulated with ACh (10 μM) or HEPES buffer for an additional 1 min. Supernatants were discarded before the vessels were snap-frozen in liquid nitrogen and stored at −80°C until assayed. Frozen PRA were homogenized on ice in lysis buffer (20 mM Tris·HCl, pH 7.4, 2.5 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 100 mM NaCl, 1.0 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml pepstatin). Protein concentrations of the cell lysates were determined using the Pierce BCA protein assay. To reduce nonspecific binding of proteins, 500 μg of detergent soluble protein were precleared with pansorbin and then incubated for 6 h at 4°C with anti-eNOS monoclonal antibody (1 μg/mg total cell protein; BD Transduction Laboratories, San Diego, CA). Immune complexes were precipitated by overnight incubation at 4°C with protein G-sepharose. The next morning, the beads were washed in lysis buffer and pelleted in a microcentrifuge to remove all unbound protein. The immunoprecipitated samples were heated at 80°C for 15 min in Laemmli loading buffer, and proteins were resolved by SDS-PAGE on an 8% acrylamide gel. Proteins were electroblotted onto polyvinylidene difluoride membranes. The membranes were blocked in 0.1% Tween PBS with 5% nonfat milk and then incubated with the primary antibodies against Hsp90, Akt, or eNOS (1:1,000). Western blots were probed with secondary antibody (goat anti-mouse IgG; 1:2,500) labeled with horseradish peroxidase and visualized using a chemiluminescent substrate (ECL; Amersham Pharmacia Biotech). The bands for each protein were quantified using densitometry.
Western blot analysis.
Frozen samples of PRA dissected from piglets of all three age groups were used for Western blot analysis as previously described (4, 13). All primary antibodies were used in a concentration of 1:1,000. The antibody source was BD Biosciences Pharmingen (San Diego, CA) for anti-eNOS, anti-Hsp90, and anti-phospho-eNOS (Ser1177). The antibody source for anti-Akt, anti-phospho-Akt, and anti-caveolin-1 was Cell Signaling Technology (Danvers, MA). Ten micrograms of PRA protein per lane were loaded for detection of eNOS, Hsp90, and Akt; 1 μg of PRA protein per lane was loaded for detection of caveolin-1; 20 μg of protein were loaded for detection of all phosphorylated proteins.
Statistics.
A linear mixed effects model (29) was used to examine concentration-dependent and age-dependent changes in PRA diameter to l-NAME, SNAP, and ACh. The linear mixed effects model generalizes the commonly used linear regression model by including additional terms such as a random intercept and random slope to control for correlation and thus provide valid inference. As each PRA was exposed to cumulative concentrations of the drug, we included a random intercept and random slope to account for the correlation arising from taking repeated observations on the same PRA (Figs. 1, 2, 6, 7, and 9).
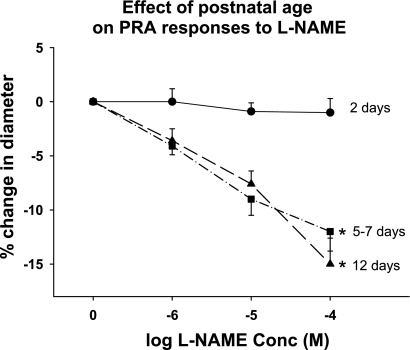
Fig. 1.
Effect of postnatal age on resistance level pulmonary artery (PRA) responses to NG-nitro-l-arginine methyl ester (l-NAME). PRA from piglets aged 2 days (n = 16 PRA from 9 piglets), 5–7 days (n = 7 PRA from 7 piglets), and 12 days (n = 11 PRA from 7 piglets) were exposed to increasing concentrations (Conc) of l-NAME. At each concentration, l-NAME caused a greater constriction in PRA from 5- to 7-day- and 12-day-old piglets than in 2-day-old piglets. *P < 0.001, different from 2-day-old.
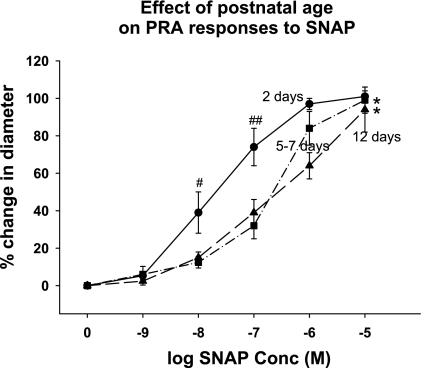
Fig. 2.
Effect of postnatal age on PRA responses to S-nitroso-N-acetyl penicillamine (SNAP). PRA from piglets aged 2 days (n = 8 PRA from 5 piglets), 5–7 days (n = 10 PRA from 10 piglets), and 12 days (n = 8 PRA from 6 piglets) were exposed to increasing concentrations of the NO donor, SNAP. SNAP induced a concentration-dependent dilation in PRA from all 3 age groups. Dilation responses to SNAP were similar in PRA from the 5- to 7- and 12-day-old piglets. Dilation to SNAP was greater in the 2-day-old PRA at intermediate concentrations (10−8 and 10−7 M) of SNAP. *P = 0.001, entire concentration-response curve different from 2-day-old; #P = 0.02, different from 5- to 7-day- and 12-day-old; ##P = 0.001, different from 5- to 7-day- and 12-day-old.
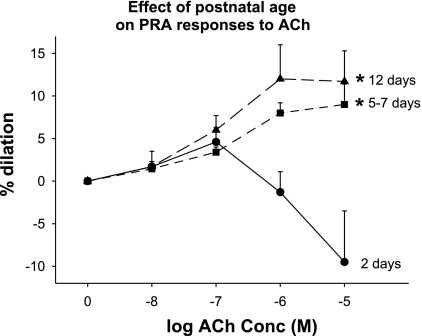
Fig. 6.
Effect of postnatal age on PRA responses to ACh. ACh caused dilation of PRA from 5- to 7-day-old (n = 12 PRA from 12 piglets) and 12-day-old piglets (n = 7 PRA from 7 piglets) but did not induce dilation in PRA from 2-day-old piglets (n = 10 PRA from 5 piglets). *P ≤ 0.002, different from 2-day-old.
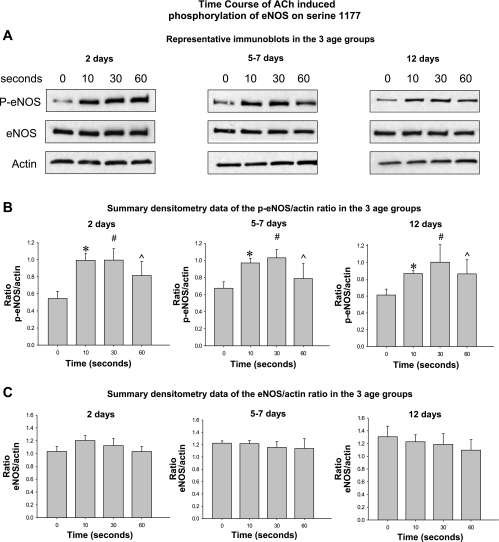
Fig. 7.
Time course of ACh-stimulated phosphorylation of eNOS on Ser1177. In all 3 age groups, ACh (0.1 μM) stimulation for 10, 30, or 60 s increased the amount of eNOS that is phosphorylated on Ser1177 (A and B). No significant differences were detected in the magnitude or time course of eNOS phosphorylation among the 3 age groups. ACh stimulation had no effect on total eNOS at any time point in any of the 3 age groups (C) (n = 5 piglets in each age group). Top: representative Western blots in the 3 age groups. Middle: summary densitometry data showing the ratio of phospho-eNOS relative to actin following stimulation with ACh for 10–60 s in each age group. Bottom: summary densitometry data showing the ratio of total eNOS relative to actin following stimulation with ACh for 10–60 s in each age group. *P = 0.0002, different from time 0; #P = 0.0001, different from time 0; ^P = 0.0129, different from time 0.
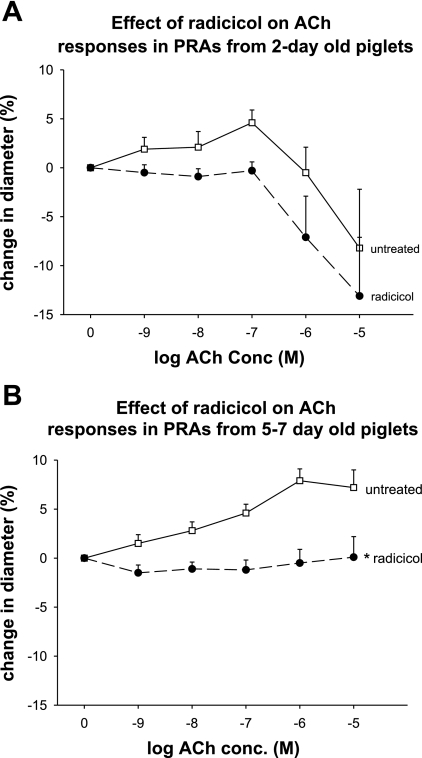
Fig. 9.
A and B: effects of radicicol on ACh responses in PRA from 2-day- and 5- to 7-day-old piglets. Pretreatment of PRA from 2-day-old piglets with radicicol had no significant effect on ACh responses (P = 0.50). In PRA from 5- to 7-day-old piglets, radicicol abolished ACh-mediated dilation. *P < 0.001, different from untreated. n = 8 PRA from 4 2-day-old piglets; n = 7 PRA from 7 5- to 7-day-old piglets.
Nonparametric ANOVA (Kruskal-Wallis or the Wilcoxon signed-rank test) was used to examine the association between protein expression and postnatal age (Figs. 3 and 7). Kruskal-Wallis followed by the Mann-Whitney U test was used to analyze the effect of a single exposure to radicicol on baseline tone (Fig. 5). Coimmunoprecipitation studies were analyzed by one-way ANOVA with Fisher protected least significant differences test (Fig. 4) or by paired t-tests (Fig. 8). Results are reported as P values with P < 0.05 indicating statistical significance.
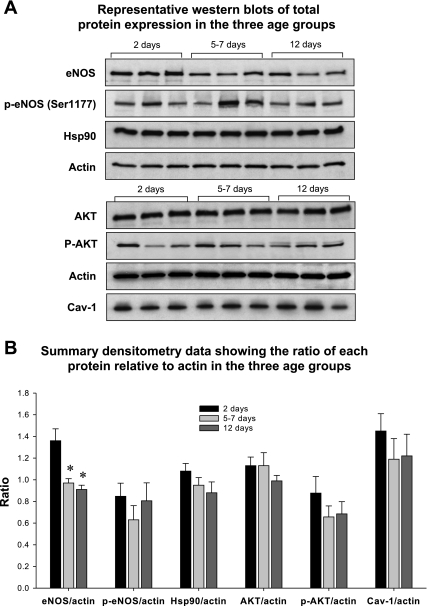
Fig. 3.
Representative Western blots (A) and summary densitometry data (B) of total protein expression of endothelial nitric oxide synthase (eNOS), phospho-eNOS (p-eNOS; Ser1177), heat shock protein 90 (Hsp90), Akt, phospho-Akt, and caveolin-1 (Cav-1) in isolated PRA from the 3 age groups. Total eNOS expression was greater in PRA from 2-day-old piglets compared with the older age groups. No significant differences in total protein expression for any other protein were detected when summary densitometry data of total protein-to-actin ratios were subjected to statistical analysis (*P = 0.002, 2-day-old different from 2 older age groups; n = 9 piglets in each age group).
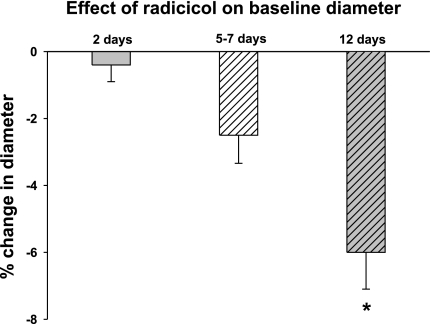
Fig. 5.
Effect of radicicol on basal diameter. Radicicol had no impact on baseline lumen diameter in 2-day-old piglet PRA (n = 9 PRA from 9 piglets) and caused a significantly greater constriction in PRA from the 12-day-old piglets (n = 5 PRA from 5 piglets) compared with the 5- to 7-day-old (n = 12 PRA from 12 piglets) and 2-day-old piglets. *P = 0.01, different from 2-day-old.
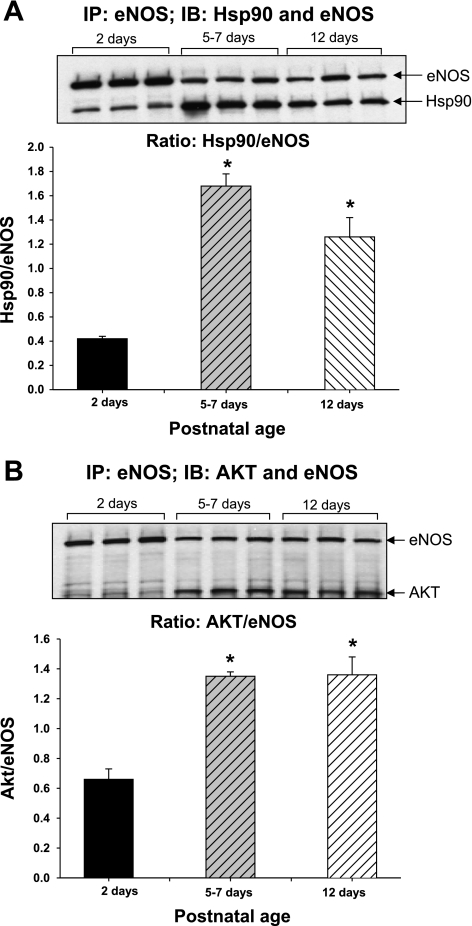
Fig. 4.
Coimmunoprecipitation of eNOS with Hsp90 and Akt. PRA protein homogenates were immunoprecipitated (IP) with anti-eNOS antibody, and the resultant immunoprecipitate was subjected to immunoblot (IB) analysis using antibodies against eNOS, Hsp90, and Akt. A demonstrates that there is less binding of eNOS to Hsp90 in PRA of 2-day-old piglets compared with PRA from piglets aged 5–7 or 12 days. Similarly, less Akt is bound to eNOS in PRA of 2-day-old vs. older piglets (B). *P < 0.05, different from 2-day-old. n = 3 different piglets in each age group.
Fig. 8.
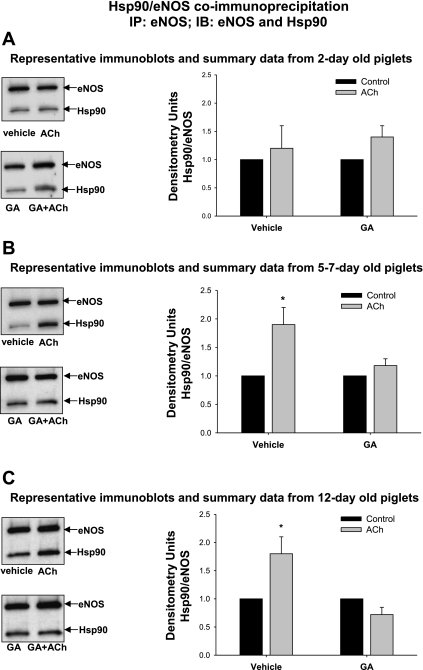
A–C: eNOS-Hsp90 coimmunoprecipitation in PRA from 2-day-, 5- to 7-day-, and 12-day-old piglets. PRA homogenates were immunoprecipitated with anti-eNOS antibody, and the resultant immunoprecipitate was subjected to immunoblot analysis using antibodies against eNOS and Hsp90. ACh (10 μM × 1 min) stimulated eNOS-Hsp90 coimmunoprecipitation in 5- to 7-day- and 12-day-old PRA, whereas ACh fails to enhance the coimmunoprecipitation of Hsp90 and eNOS in 2-day-old PRA. Geldanamycin (GA; 1 μM × 20 min) pretreatment prevents the ACh-stimulated increase in Hsp90-eNOS binding in the older 2 age groups. n = PRA from 10 2-day-old piglets, 8 5-day-old to 7-day-old piglets, and 8 12-day-old piglets; *P < 0.05, different from Vehicle control (DMSO).
RESULTS
To determine the NO-dependent contribution to basal tone at each age group, pressurized PRA were exposed to increasing concentrations (1–100 μM) of the NOS inhibitor, l-NAME. As shown in Fig. 1, PRA from 2-day-old piglets display minimal responses to NOS inhibition. At each l-NAME concentration, PRA from the two older age groups demonstrate a greater constriction compared with the PRA response in 2-day-old animals.
One possible explanation for the failure of l-NAME to cause constriction in PRA from 2-day-old piglets is diminished downstream sensitivity to NO. However, as shown in Fig. 2, SNAP-mediated functional responses are not only intact in PRA from 2-day-old piglets, but also more robust at intermediate concentrations of SNAP.
Protein expressions of eNOS, phospho-eNOS (Ser1177), Hsp90, Akt, phospho-Akt, and caveolin-1 were examined in PRA homogenates from the three age groups. Representative Western blots are shown in Fig. 3A ; Fig. 3B shows the summary densitometry data for each protein relative to actin. No significant age-related differences were noted in the expressions of phospho-eNOS (Ser1177), Hsp90, Akt, phospho-Akt, and caveolin-1. Expression of total eNOS was higher in PRA from 2-day-old piglets than in PRA from the older two age groups.
Despite more total eNOS protein in PRA from 2-day-old piglets (Figs. 3 and 4), coimmunoprecipitation assays demonstrate less binding of eNOS to Hsp90 in PRA from 2-day-old piglets compared with the two older age groups (Fig. 4A). Similarly, there was less Akt bound to eNOS in the 2-day-old vs. older piglets (Fig. 4B).
To demonstrate the functional importance of Hsp90 chaperone function, we examined the effect of the Hsp90 antagonist, radicicol, on basal tone in PRA from the three age groups. As shown in Fig. 5, radicicol induces the greatest constriction in PRA from the 12-day-old piglets.
Agonist-mediated responses in the three age groups are shown in Fig. 6. ACh, a NOS-dependant agonist, causes dilation from basal tone in PRA from 5- to 7-day- and 12-day-old piglets but does not cause dilation in PRA from 2-day-old piglets.
The representative Western blots in Fig. 7A and summary densitometry graphs in Fig. 7B demonstrate a rapid and similar increase in the expression of phospho-eNOS [phospho-eNOS (Ser1177)] following stimulation with ACh (0.1 μM) for 10, 30, or 60 s in all three age groups. The magnitude and time course of phospho-eNOS expression following ACh stimulation did not differ among the three age groups. ACh stimulation had no effect on the expression of total eNOS protein (Fig. 7C).
Figure 8 demonstrates that ACh stimulates the binding of eNOS to Hsp90 with a near doubling of the amount of Hsp90 that coimmunoprecipitates with eNOS in 5- to 7-day- and 12-day-old PRA (Fig. 8, B and C). In contrast, ACh fails to enhance the coimmunoprecipitation of Hsp90 and eNOS in PRA from the 2-day-old piglets (Fig. 8A). Furthermore, the Hsp90 antagonist, geldanamycin, prevents the increase in association between Hsp90 and eNOS stimulated by ACh in the two older age groups.
Lastly, we evaluated the effect of Hsp90 antagonism on vascular responses to ACh. We chose to use radicicol rather than geldanamycin for these functional studies as geldanamycin has been shown to cause redox cycling. Redox cycling may have relatively less impact on physical associations measured by immunoprecipitation. Figure 9A shows that radicicol has no significant effect on ACh responses in 2-day-old piglets. In comparison, radicicol abolishes dilation in PRA from the 5- to 7-day-old animals. This effect is nearly identical to our previously published findings in PRA from 12-day-old piglets (13) where we showed that both radicicol and geldanamycin abolish ACh-mediated dilation.
DISCUSSION
Pulmonary circulatory transition at birth is thought to be regulated, at least in part, by release of NO from eNOS (1, 11, 18, 19, 27). Maturational changes in the regulation of pulmonary vascular tone by NO have been described in a number of species, including rat (10, 26, 36, 49), pig (24), lamb (23, 38), baboon (41), and human (30, 31). Prior publications comparing fetal and postnatal expression of eNOS have demonstrated an increase in NOS expression during gestation that is maximal shortly before or at the time of birth (26, 36) with a subsequent decline postnatally to the low levels observed in adults (36, 47, 49). These studies have been limited by an experimental approach that used whole lung homogenates, cultured endothelial cells, or large conduit pulmonary arteries; the contribution of eNOS from PRA has not been previously addressed.
Novel findings in this study are that physical interactions between Hsp90 and eNOS mature over the first 2 wk of life in PRA. Moreover, we provide evidence that these postnatal changes in Hsp90-eNOS interactions have important functional impact. Specifically, our findings are the first to show that maturation in Hsp90-eNOS interactions underlie, at least in part, the postnatal changes in both basal and agonist-induced pulmonary vascular responses that occur over the first days and weeks of life.
Our data demonstrate that endogenous NO does not play a major role in control of resting tone in 2-day-old piglets but suggest an important role of basal NO release in resting tone of older newborns (Fig. 1). This finding is similar to those of other investigators who have examined the impact of NOS inhibition in lambs (22, 44) and piglets (33) at comparable ages. One possible explanation for the failure of l-NAME to cause constriction in PRA from the 2-day-old animals is diminished downstream sensitivity to NO. However, as shown in Fig. 2, PRA from 2-day-old piglets are capable of robust dilation to the NO donor, SNAP. These findings indicate that downstream sensitivity to NO is not impaired and cannot explain the reduced responses of the younger animals to NOS inhibition. Indeed, at intermediate concentrations, dilation to SNAP was greatest in the 2-day-old PRA. This finding is consistent with that of Berkenbosch et al. (7), who found that an NO donor elicited a more robust dilation in the isolated perfused lungs of 4-day-old compared with 15-day-old piglets. Likewise, sodium nitroprusside induced greater relaxation in large pulmonary artery rings from 2-day- compared with 1-mo-old lambs (37). The mechanisms underlying these findings might be related to increased expression or greater activity of soluble guanylate cyclase (sGC) in newborns compared with more mature animals as reported in carotid and cerebral arteries in the ovine species (48). An increase in pulmonary sGC mRNA, protein levels, and activity in the perinatal period has also been reported in the rat (6, 8). In contrast, Moreno et al. (35) found an age-related increase in dilation to bubbled NO in conduit arteries between 1-day- and 2-wk-old piglets that correlated with increased sGC protein content in the large pulmonary arteries of the older piglets.
We also explored the possibility that age-related changes in NOS expression underlie the functional differences in vascular responses in the three age groups. We found increased, not decreased, total protein expression of eNOS in PRA from the 2-day-old piglets (Figs. 3 and 4) without significant differences in phospho-eNOS (Ser1177), Akt, phospho-Akt, or caveolin-1, a protein when bound to eNOS is thought to inhibit NOS activity (Fig. 3). Prior published studies have compared eNOS expression in fetal, 1-day-old, and juvenile or adult animals, reporting lower eNOS expression in the juvenile and adult animals relative to the fetus and 1-day-old newborn (3, 26, 38). North et al. (36) found eNOS is highest in the late gestation fetus and is similar in 1- and 5-day-old rats. Kawai et al. (26) found no differences in pulmonary eNOS protein or mRNA expression in rats between days 1 and 16 of life, and Chicoine et al. (10) reported similar total eNOS protein in the lungs of 1- and 2-wk-old rats. Sheffield et al. (43) found no significant differences in eNOS expression in postmortem human newborn lung tissue with advancing gestational age or severity of chronic lung disease. Although differences in expression of other NOS isoforms could contribute to maturational changes in vascular responses in the lung, other investigators have reported no differences in iNOS (10) or nNOS (10, 36) expression in whole lung immunoblots over the first 2 wk of life.
Hsp90 is a chaperone protein that is critical to the regulation of eNOS activity (4, 39). Hsp90 acts as a protein scaffold for a multiprotein complex that facilitates phosphorylation by Akt/PKB of eNOS on Ser1177 and activation of the enzyme. Other investigators found that basal amounts of phosphorylated eNOS on Ser1177 (34) and basal Hsp90 protein levels (46) differed between pulmonary artery endothelial cells of fetal and 4-wk-old lambs. However, data on expression of phospho-eNOS and the NOS regulatory proteins, Hsp90 and Akt, in the early postnatal period have, to our knowledge, not been reported previously. Our data indicate that total amounts of Hsp90, Akt, and phospho-Akt are similar in PRA from the three age groups we studied. We did not detect differences in total phosphorylated eNOS (Ser1177) between the three age groups (Fig. 3). Thus the markedly reduced functional response to NOS inhibition in PRA from the 2-day-old piglets compared with the older piglets does not correlate with phospho-eNOS levels and cannot be explained by differences in basal expression of eNOS, Hsp90, or Akt. Others have previously shown a lack of correlation between phospho-eNOS amounts and functional dilation (21, 45). This could occur if eNOS is phosphorylated in an uncoupled state resulting in eNOS-generated superoxide production and in loss of bioavailable NO (39). Factors that impact eNOS uncoupling include limited availability of substrate, arginine (14, 17), or of cofactor, tetrahydrobiopterin (34), or lack of adequate Hsp90-eNOS binding (4, 28, 46). All of these factors could be contributing to the reduced NOS-dependent vascular responses in the 2-day-old piglets.
New findings from this study indicate that interactions between proteins important for regulating eNOS activity change between days 2 and 12 of postnatal life. Our data indicate that at least one explanation for the poor response to the NOS antagonist in the 2-day-old PRA is limited Hsp90-eNOS binding (Fig. 4). Figure 5 showing markedly reduced alterations in basal tone on inhibition of Hsp90 chaperone function with radicicol further supports this concept. Under conditions of limited Hsp90-eNOS binding, phospho-eNOS produces superoxide anion, reflecting uncoupling between electron flow, phosphorylation, and NO production. Moreover, our findings suggest that between days 2 and 12 of life in the piglet, Hsp90-eNOS binding matures so that more eNOS is bound to Hsp90 (Figs. 4A and 5). These findings support a growing body of literature that points to the importance of Hsp90 chaperone function in assuring the correct multiprotein stoichiometry required for coupled production of NO from NOS.
Failure to find a significant difference in the radicicol-mediated constriction from baseline tone in the 2-day-and 5- to 7-day-old animals is not entirely unexpected. We have previously reported in PRA from piglets aged 1–7 days that radicicol did not reduce basal cGMP levels or disrupt preexisting complexes between Hsp90 and eNOS (4). In fact, radicicol augmented Hsp90 binding to eNOS under basal conditions. However, Hsp90 antagonists, such as radicicol, would be expected to modify agonist-induced responses mediated by eNOS or other Hsp90 client proteins. The significant constriction observed in the 12-day-olds may reflect more basal activation of eNOS in pressurized 12-day-old PRA than in pressurized vessels from the younger age groups or may reflect interactions between Hsp90 and a client protein other than eNOS that influences basal tone. In contrast to the functional studies with radicicol, the responses to l-NAME and SNAP primarily reflect inhibition or stimulation, respectively, of the NO-cGMP pathway.
Our data also shed light on the impact of postnatal maturation on agonist-induced eNOS biochemistry and functional responses. As well as regulating basal tone, maturational changes in Hsp90-eNOS interactions could have a significant impact on the ability of the pulmonary vasculature to respond to endogenous or exogenous stimuli that regulate vascular tone via the NO-cGMP signaling pathway. Other investigators have similarly shown that responses to the NOS-dependent agonist, ACh, increase over the first days to months of postnatal life (2, 32, 33, 44, 50). Our data expand on these observations by showing that this postnatal improvement in NOS-dependent dilation is due, at least in part, to a change in the capacity for Hsp90 binding to eNOS in response to agonist stimulation with ACh (Figs. 8 and 9). The ability of ACh to induce enhanced Hsp90-eNOS binding is deficient in 2-day-old piglets (Fig. 8A), and this correlates with the poor ACh-mediated dilation responses in this age group (Fig. 6) despite intact agonist-induced increases in phospho-eNOS (Fig. 7). Thus, similar to our findings in unstimulated PRA and reports by others (21, 45), we show that there is no direct correlation between the amount of agonist-stimulated phospho-eNOS (Ser1177) and Hsp90-eNOS binding or functional dilation.
Agonist-induced increases in phospho-eNOS without functional dilation in the PRA from 2-day-old piglets suggest eNOS uncoupling with production of eNOS-derived superoxide and loss of bioavailable NO. We (4) have previously reported that Hsp90 antagonists, which interfere with Hsp90 binding to eNOS, increase eNOS-derived superoxide production. These studies were performed in cultured endothelial cells, using confocal microscopy and ethidium fluorescence; technical limitations prevent our recapitulating these findings in intact, pressurized PRA from the three age groups. Other limitations of our study include the use of a single agonist, ACh, to stimulate eNOS. It is possible that age-related differences in ACh responses are due, at least in part, to alterations in muscarinic receptor expression or distribution within the pulmonary arterial tree. It is also possible that our findings are unique to ACh and that stimulation with other eNOS agonists, such as bradykinin or A-23187, would yield a different result.
The enhanced agonist-stimulated Hsp90-eNOS association with postnatal maturation in the first 2 wk of life that we report differs from the findings of Mata-Greenwood et al. (34), who failed to detect a developmental increase in Hsp90-eNOS association on stimulation of cultured ovine pulmonary artery endothelial cells with A-23187. Important differences between the study by Mata-Greenwood et al. (34) and our study include the species studied, the developmental ages studied, and the techniques used. Mata-Greenwood et al. (34) looked at Hsp90-eNOS association in cultured endothelial cells from larger vessels from fetal lambs vs. 4-wk-old juvenile sheep, whereas all of our studies were performed in intact vessels from PRA from piglets aged 2–12 days.
Taken together, our findings show that, compared with 5- to 7-day- and 12-day-old piglets, 2-day-old piglets have limited ability to form complex multiprotein interactions between Hsp90, eNOS, and Akt. Our findings also suggest that this limitation in regulatory protein interactions correlates with a reduced role for endogenous NO in the regulation of pulmonary vascular tone in the immediate postnatal period. Moreover, the contribution of NO to pulmonary vascular tone and reactivity increases in the first 2 wk of life in association with enhanced Hsp90-eNOS binding and concomitant with the normal fall in pulmonary vascular resistance, which occurs over the first weeks of life. Thus biophysical interactions between Hsp90 and eNOS, which mature over the first weeks of life, likely contribute to the postnatal fall in pulmonary vascular resistance and changes in pulmonary vascular responses characteristic of the early neonatal period.
GRANTS
The project described was supported by National Heart, Lung, and Blood Institute Grants R01-HL-075511 and R01-HL-068572.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol Heart Circ Physiol 259: H1921–H1927, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Abman SH, Chatfield BA, Rodman DM, Hall SL, McMurtry IF. Maturational changes in endothelium-derived relaxing factor activity of ovine pulmonary arteries in vitro. Am J Physiol Lung Cell Mol Physiol 260: L280–L285, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Arrigoni FI, Hislop AA, Pollock JS, Haworth SG, Mitchell JA. Birth upregulates nitric oxide synthase activity in the porcine lung. Life Sci 70: 1609–1620, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Aschner JL, Foster SL, Kaplowitz M, Zhang Y, Zeng H, Fike CD. Heat shock protein 90 modulates endothelial nitric oxide synthase activity and vascular reactivity in the newborn piglet pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 292: L1515–L1525, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Aschner JL, Smith TK, Kovacs N, Pinheiro JM, Fuloria M. Mechanisms of bradykinin-mediated dilation in newborn piglet pulmonary conducting and resistance vessels. Am J Physiol Lung Cell Mol Physiol 283: L373–L382, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Behrends S, Kempfert J, Mietens A, Koglin M, Scholz H, Middendorff R. Developmental changes of nitric oxide-sensitive guanylyl cyclase expression in pulmonary arteries. Biochem Biophys Res Commun 283: 883–887, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Berkenbosch JW, Baribeau J, Perreault T. Decreased synthesis and vasodilation to nitric oxide in piglets with hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 278: L276–L283, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Bloch KD, Filippov G, Sanchez LS, Nakane M, de la Monte SM. Pulmonary soluble guanylate cyclase, a nitric oxide receptor, is increased during the perinatal period. Am J Physiol Lung Cell Mol Physiol 272: L400–L406, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Cassin S, Dawes GS, Mott JC, Ross BB, Strang LB. The vascular resistance of the foetal and newly ventilated lung of the lamb. J Physiol 171: 61–79, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chicoine LG, Paffett ML, Girton MR, Metropoulus MJ, Joshi MS, Bauer JA, Nelin LD, Resta TC, Walker BR. Maturational changes in the regulation of pulmonary vascular tone by nitric oxide in neonatal rats. Am J Physiol Lung Cell Mol Physiol 293: L1261–L1270, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Cornfield DN, Chatfield BA, McQueston JA, McMurtry IF, Abman SH. Effects of birth-related stimuli on l-arginine-dependent pulmonary vasodilation in ovine fetus. Am J Physiol Heart Circ Physiol 262: H1474–H1481, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Dawes GS, Mott JC, Widdicombe JG, Wyatt DG. Changes in the lungs of the new-born lamb. J Physiol 121: 141–162, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fike CD, Aschner JL, Zhang Y, Kaplowitz MR. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn piglets. Am J Physiol Lung Cell Mol Physiol 286: L1244–L1254, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Fike CD, Kaplowitz MR, Rehorst-Paea LA, Nelin LD. l-Arginine increases nitric oxide production in isolated lungs of chronically hypoxic newborn pigs. J Appl Physiol 88: 1797–1803, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Fike CD, Pfister SL, Kaplowitz MR, Madden JA. Cyclooxygenase contracting factors and altered pulmonary vascular responses in chronically hypoxic newborn pigs. J Appl Physiol 92: 67–74, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Fike CD, Zhang Y, Kaplowitz MR. Thromboxane inhibition reduces an early stage of chronic hypoxia-induced pulmonary hypertension in piglets. J Appl Physiol 99: 670–676, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Fineman JR, Chang R, Soifer SJ. l-Arginine, a precursor of EDRF in vitro, produces pulmonary vasodilation in lambs. Am J Physiol Heart Circ Physiol 261: H1563–H1569, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Fineman JR, Chang R, Soifer SJ. EDRF inhibition augments pulmonary hypertension in intact newborn lambs. Am J Physiol Heart Circ Physiol 262: H1365–H1371, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Fineman JR, Wong J, Morin FC 3rd, Wild LM, Soifer SJ. Chronic nitric oxide inhibition in utero produces persistent pulmonary hypertension in newborn lambs. J Clin Invest 93: 2675–2683, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuloria M, Smith TK, Aschner JL. Role of 5,6-epoxyeicosatrienoic acid in the regulation of newborn piglet pulmonary vascular tone. Am J Physiol Lung Cell Mol Physiol 283: L383–L389, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Go YM, Levonen AL, Moellering D, Ramachandran A, Patel RP, Jo H, Darley-Usmar VM. Endothelial NOS-dependent activation of c-Jun NH2- terminal kinase by oxidized low-density lipoprotein. Am J Physiol Heart Circ Physiol 281: H2705–H2713, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Gordon JB, Tod ML. Effects of Nω-nitro-l-arginine on total and segmental vascular resistances in developing lamb lungs. J Appl Physiol 75: 76–85, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Halbower AC, Tuder RM, Franklin WA, Pollock JS, Forstermann U, Abman SH. Maturation-related changes in endothelial nitric oxide synthase immunolocalization in developing ovine lung. Am J Physiol Lung Cell Mol Physiol 267: L585–L591, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Hislop AA, Springall DR, Buttery LD, Pollock JS, Haworth SG. Abundance of endothelial nitric oxide synthase in newborn intrapulmonary arteries. Arch Dis Child Fetal Neonatal Ed 73: F17–F21, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwamoto HS, Teitel D, Rudolph AM. Effects of birth-related events on blood flow distribution. Pediatr Res 22: 634–640, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Kawai N, Bloch DB, Filippov G, Rabkina D, Suen HC, Losty PD, Janssens SP, Zapol WM, de la Monte S, Bloch KD. Constitutive endothelial nitric oxide synthase gene expression is regulated during lung development. Am J Physiol Lung Cell Mol Physiol 268: L589–L595, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Kinsella JP, McQueston JA, Rosenberg AA, Abman SH. Hemodynamic effects of exogenous nitric oxide in ovine transitional pulmonary circulation. Am J Physiol Heart Circ Physiol 263: H875–H880, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Konduri GG, Bakhutashvili I, Eis A, Pritchard K Jr. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 292: H1812–H1820, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 38: 963–974, 1982. [PubMed] [Google Scholar]
- 30.Levy M, Maurey C, Chailley-Heu B, Martinovic J, Jaubert F, Israel-Biet D. Developmental changes in endothelial vasoactive and angiogenic growth factors in the human perinatal lung. Pediatr Res 57: 248–253, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Lévy M, Maurey C, Dinh-Xuan AT, Vouhé P, Israël-Biet D. Developmental expression of vasoactive and growth factors in human lung. Role in pulmonary vascular resistance adaptation at birth. Pediatr Res 57: 21R–25R, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Lévy M, Souil E, Sabry S, Favatier F, Vaugelade P, Mercier JC, Dall'Ava-Santucci J, Dinh-Xuan AT. Maturational changes of endothelial vasoactive factors and pulmonary vascular tone at birth. Eur Respir J 15: 158–165, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Liu SF, Hislop AA, Haworth SG, Barnes PJ. Developmental changes in endothelium-dependent pulmonary vasodilatation in pigs. Br J Pharmacol 106: 324–330, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mata-Greenwood E, Jenkins C, Farrow KN, Konduri GG, Russell JA, Lakshminrusimha S, Black SM, Steinhorn RH. eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol 290: L232–L241, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno L, Gonzalez-Luis G, Cogolludo A, Lodi F, Lopez-Farre A, Tamargo J, Villamor E, Perez-Vizcaino F. Soluble guanylyl cyclase during postnatal porcine pulmonary maturation. Am J Physiol Lung Cell Mol Physiol 288: L125–L130, 2005. [DOI] [PubMed] [Google Scholar]
- 36.North AJ, Star RA, Brannon TS, Ujiie K, Wells LB, Lowenstein CJ, Snyder SH, Shaul PW. Nitric oxide synthase type I and type III gene expression are developmentally regulated in rat lung. Am J Physiol Lung Cell Mol Physiol 266: L635–L641, 1994. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell DC, Tod ML, Gordon JB. Developmental changes in endothelium-dependent relaxation of pulmonary arteries: role of EDNO and prostanoids. J Appl Physiol 81: 2013–2019, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Parker TA, le Cras TD, Kinsella JP, Abman SH. Developmental changes in endothelial nitric oxide synthase expression and activity in ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 278: L202–L208, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Pritchard KA, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem 276: 17621–17624, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Rudolph AM Fetal and neonatal pulmonary circulation. Annu Rev Physiol 41: 383–395, 1979. [DOI] [PubMed] [Google Scholar]
- 41.Shaul PW, Afshar S, Gibson LL, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC. Developmental changes in nitric oxide synthase isoform expression and nitric oxide production in fetal baboon lung. Am J Physiol Lung Cell Mol Physiol 283: L1192–L1199, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Shaul PW, Farrar MA, Magness RR. Pulmonary endothelial nitric oxide production is developmentally regulated in the fetus and newborn. Am J Physiol Heart Circ Physiol 265: H1056–H1063, 1993. [DOI] [PubMed] [Google Scholar]
- 43.Sheffield M, Mabry S, Thibeault DW, Truog WE. Pulmonary nitric oxide synthases and nitrotyrosine: findings during lung development and in chronic lung disease of prematurity. Pediatrics 118: 1056–1064, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Steinhorn RH, Morin FC 3rd, Gugino SF, Giese EC, Russell JA. Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol Heart Circ Physiol 264: H2162–H2167, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Stepp DW, Ou J, Ackerman AW, Welak S, Klick D, Pritchard KA Jr. Native LDL and minimally oxidized LDL differentially regulate superoxide anion in vascular endothelium in situ. Am J Physiol Heart Circ Physiol 283: H750–H759, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol 293: L1444–L1453, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Wedgwood S, Mitchell CJ, Fineman JR, Black SM. Developmental differences in the shear stress-induced expression of endothelial NO synthase: changing role of AP-1. Am J Physiol Lung Cell Mol Physiol 284: L650–L662, 2003. [DOI] [PubMed] [Google Scholar]
- 48.White CR, Hao X, Pearce WJ. Maturational differences in soluble guanylate cyclase activity in ovine carotid and cerebral arteries. Pediatr Res 47: 369–375, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Xue C, Reynolds PR, Johns RA. Developmental expression of NOS isoforms in fetal rat lung: implications for transitional circulation and pulmonary angiogenesis. Am J Physiol Lung Cell Mol Physiol 270: L88–L100, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Zellers TM, Vanhoutte PM. Endothelium-dependent relaxations of piglet pulmonary arteries augment with maturation. Pediatr Res 30: 176–180, 1991. [DOI] [PubMed] [Google Scholar]