Abstract
We have reported recently that eosinophil-derived basic proteins directly enhance the capsaicin- and electrical stimulation-evoked whole cell responses in rat pulmonary sensory neurons (19). Our present study further elucidates the mechanisms underlying the sensitization of pulmonary afferent nerves induced by these cationic proteins. Our results show that pretreatment with eosinophil major basic protein (MBP; 2 μM, 60 s) significantly enhanced the excitability of isolated rat vagal pulmonary chemosensitive neurons to acid and ATP in the current-clamp mode, but this potentiating effect was absent in the voltage-clamp recordings. The hyperexcitability induced by MBP was not prevented by the blockade of either transient receptor potential vanilloid type-1 receptor (TRPV1) selectively (inhibitor: AMG 9810; 1 μM, 2 min) or all TRPV1–4 channels (inhibitor: ruthenium red; 5 μM, 2 min). In addition, MBP also markedly potentiated the excitability of mouse pulmonary chemosensitive neurons, and no detectable difference was found between those isolated from wild-type and TRPV1 knockout mice. Furthermore, MBP pretreatment affected the decay time and recovery phase of the action potentials evoked by current injections and significantly inhibited both the sustained delayed-rectifier voltage-gated K+ current (IKdr) and the A-type, fast-inactivating K+ current (IKa) in these sensory neurons. In conclusion, our results indicate that the inhibition of IKdr and IKa should, at least in part, account for the hyperexcitability of pulmonary chemosensitive neurons induced by eosinophil-derived cationic proteins, whereas an interaction with TRPV1 channels does not seem to be required for the sensitizing effect of these proteins.
Keywords: eosinophil granule proteins, transient receptor potential vanilloid type-1 receptor, voltage-gated K+ channel, patch clamp
airway infiltration of eosinophils and the release of their granule proteins are the hallmarks of a variety of airway inflammatory diseases including asthma (6, 12). There are four major types of the eosinophil granule-derived, low-molecular-weight and highly cationic proteins: major basic protein (MBP), eosinophil cationic protein, eosinophil peroxidase, and eosinophil-derived neurotoxin; these cationic proteins are believed to play a pathogenic role in airway inflammatory reactions (14). It has been suggested that the cationic charges carried by these proteins are critical in mediating their various bioactive effects in the airways (4, 9). The hypothesis is supported by the following observations: 1) cationic proteins from different origins (including eosinophil granule proteins, cathepsin G, and neutrophil cationic proteins from neutrophil, platelet factor 4 from platelet, and synthetic cationic proteins such as poly-l-lysine) showed similar or comparable effects (8, 9, 16, 28, 40); 2) the effects of cationic proteins could be inhibited by various kinds of negative-charged proteins, such as low-molecular-weight heparin, albumin, dextran sulphate, poly-L-aspartic acid, and poly-L-glutamic acid (4, 9, 10, 16, 18, 19, 27); and 3) the effect of cationic proteins was absent when delivered intravenously instead of intratracheally, presumably because their positive charges were readily neutralized after the delivery into systemic circulation (2, 16, 23, 28).
It is known that most sensory inputs arising from airways and lung structures are conducted in vagus nerves and their branches, and the majority of vagal bronchopulmonary sensory nerves are C-fibers (29). When these C-fiber afferents are activated, sensory inputs encoded in the forms of action potentials conduct impulses to the central nervous system and elicit the pulmonary chemoreflexes (including apnea, bradycardia, and hypotension) and other cardiorespiratory reflex responses, such as bronchoconstriction, mucus hypersecretion, bronchial vasodilation, plasma extravasation, dyspneic sensation, and cough (7, 29, 39). Previous studies from our laboratory have shown that intratracheal instillation of eosinophil-derived or synthetic cationic proteins significantly enhanced the sensitivity of pulmonary C-fibers to both lung inflation and chemical stimuli in anesthetized rats (16, 18, 28). Our recent study has demonstrated that eosinophil-derived cationic proteins can directly potentiate the capsaicin-evoked, transient receptor potential vanilloid type-1 receptor (TRPV1)-mediated whole cell responses and also markedly increase the number of action potentials evoked by electric stimulation in isolated rat vagal pulmonary chemosensitive neurons (19); the later observation suggested a possible involvement of voltage-gated K+ channels (5, 22). The present study was carried out to further elucidate the mechanisms underlying the sensitization of vagal pulmonary sensory nerves induced by these cationic proteins. Four aims were proposed in this study: 1) to determine whether these proteins, such as MBP, upregulate the excitability of pulmonary chemosensitive neurons to chemical stimulants other than capsaicin; 2) to investigate whether an interaction between MBP and TRPV1 plays a critical role in the sensitizing effects of this protein; 3) to investigate the effect of MBP on the action potential firing properties in vagal pulmonary chemosensitive neurons; and 4) to determine whether MBP affects the voltage-gated K+ currents in these neurons.
MATERIALS AND METHODS
The experiments were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Identification of vagal pulmonary sensory neurons.
Cell bodies of vagal sensory nerves arising from airways and lungs reside in nodose and intracranial jugular ganglia. These sensory neurons were identified by retrograde labeling with 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI). Labeling the pulmonary sensory neurons in rats was described previously (26). Briefly, young adult Sprague-Dawley rats (∼150 g) were anesthetized with pentobarbital sodium (40 mg/kg ip) and intubated with a polyethylene catheter (PE 150). DiI was initially sonicated and dissolved in ethanol, diluted in saline (1% ethanol, vol/vol), and then instilled into the lungs (0.2 mg/ml; 0.2 ml × 2) with the animal's head tilted upward at ∼30°. DiI retrograde labeling in mice was performed as follows: mice (age 4–6 wk; weight 15–20 g) were anesthetized with isoflurane (1% in O2) inhalation via a nose cone connected to a vaporizing machine (AM Bickford, Wales Center, NY). A small midline incision was made on the neck skin to expose the trachea. DiI (0.15 mg/ml, 20 μl volume) was then instilled into the lungs via an insulin syringe needle (30-gauge) inserted into the lumen of the trachea; the incision was then closed. All animals recovered undisturbed (7–10 days for rats; 5–7 days for mice) until they were euthanized for the cell culture.
Isolation of nodose and jugular ganglion neurons.
Animals were euthanized with isoflurane inhalation. Nodose and jugular ganglion neurons from rats were isolated as described previously (19). Briefly, nodose and jugular ganglia were extracted, cut into ∼10 pieces, placed in 0.125% type IV collagenase, and incubated at 37°C for 1 h. The ganglion suspension was centrifuged (150 g, 5 min) and supernatant aspirated. The cell pellet was resuspended in 0.05% trypsin for 5 min and centrifuged (150 g, 5 min); the pellet was then resuspended in a modified DMEM/F12 solution [supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μM MEM nonessential amino acids] and gently triturated with a Pasteur pipette. The dispersed cell suspension was centrifuged (500 g, 8 min) through a layer of 15% bovine serum albumin. The pellets were resuspended in the modified DMEM/F12 solution, plated onto poly-l-lysine-coated glass coverslips, and incubated at 37°C in 5% CO2. Neurons from the nodose-jugular complex in mice were isolated following a similar procedure, except that the enzymic digestion was accomplished by incubating the ganglionic tissue in a mixture of collagenase (0.04%) and dispase II (0.04%) for 70 min. Isolated neurons were used within 24–48 h of culture. Although poly-l-lysine used as the substratum is a synthetic cationic protein, it is unlikely to account for any difference in the responses observed in this study because it was present under both baseline and treatment conditions.
Whole cell perforated patch-clamp recording.
Coverslip with neurons was superfused (2 ml/min) continuously by gravity feed (VC-6 perfusion valve controller; Warner Instruments, Hamden, CT) with standard extracellular solution (ECS) containing the following chemicals (in mM): 136 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 0.33 NaH2PO4, 10 glucose, and 10 HEPES; pH was adjusted to 7.4 with NaOH. Whole cell perforated patch clamping (50 μg/ml gramicidin) was performed by using Axopatch 200B/pCLAMP 9.0 (Molecular Devices, Palo Alto, CA). The chemical stimulants were applied by a pressure-driven drug delivery system (ALA-VM8; ALA Scientific Instruments, Westbury, NY) with its tip positioned to ensure that the cell was fully within the stream of the injectate. The resistances of fire-polished glass micropipettes were 2–4 MΩ when filled with the pipette solution consisting of the following (in mM): 92 potassium gluconate, 40 KCl, 8 NaCl, 1 CaCl2, 0.5 MgCl2, 10 EGTA, and 10 HEPES; pH was adjusted to 7.2 with KOH. The series resistance was generally ≤10 MΩ and was not compensated. Data were acquired at 5 kHz and filtered at 2 kHz. The experiments were performed at room temperature (∼22°C).
To record K+ currents, the gigal seal was first established with the use of the standard ECS described above, and sodium-free bath solution was then perfused onto the cell to isolate K+ currents. The bath solution contained (in mM): 140 choline Cl, 3 KCl, 1 MgCl2, 1 CaCl2, 0.1 CdCl2, and 10 HEPES; pH was adjusted to 7.4 with Tris base. The pipette solution contained (in mM): 100 choline Cl, 40 KCl, 1 MgCl2, 10 EGTA, and 10 HEPES; pH was adjusted to 7.2 with Tris base. The current-voltage curves of K+ currents were fitted using a single Boltzmann function: I = A/(1 + exp(−(V − V0.5))/k), where A is the maximum amplitude, V is the test-pulse voltage, V0.5 is the half-activation voltage, and k is the slope factor.
Whole cell patch-clamp recordings were made in pulmonary chemosensitive neurons selected on the basis of the following criteria: 1) labeled with DiI as indicated by fluorescence intensity; 2) smooth surface and spherical shape without visible processes; 3) whole cell capacitance <35 pF; and 4) responding to 1 μM capsaicin. These neurons presumably give rise to pulmonary C-fiber afferents as proposed in our recent studies (26). Although the neurons from rat nodose and jugular ganglia were isolated and studied separately, data from the neurons of these two types of ganglia were pooled for group analysis because no difference between the neurons from the two ganglia was found in this study. Since the nodose and jugular ganglia in mice were not clearly separated anatomically, neurons isolated from the jugular-nodose ganglia complex were studied as one group.
Eosinophil MBP purification.
Eosinophils from patients with marked blood eosinophilia were collected by cytapheresis, and eosinophil granules and granule proteins were purified as previously described (33). Briefly, after cell lysis and granule isolation, granules were solubilized in 0.01 M HCl (pH 2.0) by vigorous suspension with a Pasteur pipette. After centrifugation (13,600 g, 5 min), the supernatant was fractionated on a Sephadex G-50 column equilibrated with 0.025 M sodium acetate (pH 4.3) containing 0.15 M NaCl. Acetate buffer was harvested for vehicle control conditions. Individual protein peaks were pooled, and protein concentrations were determined by absorbance at 280 nm. All eosinophil granule protein preparations were pure as assessed by Coomassie blue staining after SDS-PAGE.
Chemicals.
DiI was purchased from Molecular Probes (Eugene, OR). DMEM/F12 and trypsin were obtained from Invitrogen (Carlsbad, CA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). A stock solution (10 mM) of AMG 9810 or ruthenium red was prepared in dimethyl sulfoxide, and that of others was in ECS. The solutions of chemicals at desired concentrations were prepared daily by dilution with ECS before use. No detectable effect of the vehicles of these chemical agents was found in our preliminary experiments.
Statistical analysis.
Data were analyzed by a one-way ANOVA unless mentioned otherwise. When the ANOVA showed a significant interaction, pair-wise comparisons were made with a post hoc analysis (Fisher's least significant difference). Results were considered significantly different when P < 0.05. Data are means ± SE.
RESULTS
MBP enhanced the action potential firing but not inward currents evoked by acid and ATP in rat vagal pulmonary chemosensitive neurons.
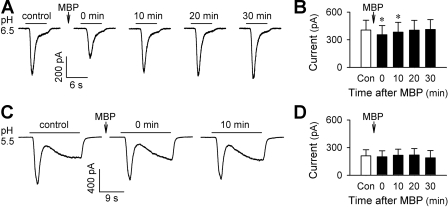
Recent studies from our laboratory as well as others have demonstrated that physiologically/pathophysiologically relevant extracellular acidification can activate the majority of pulmonary sensory neurons (17, 25). In whole cell voltage-clamp recordings, acid elicits either a transient inward current with quick activation and inactivation (reaching the peak during acid application) that is mediated by acid-sensing ion channel (ASIC), or a delayed/sustained inward current (reaching the peak after the termination of acid application) mediated by the activation of TRPV1, or both (17, 31). In the present study, interestingly, MBP displayed distinct effects on the two different types of inward currents evoked by acid (pH 5.5–7.0, 2–18 s). The transient, ASIC-like current was inhibited by the pretreatment with MBP (2 μM, 60 s) (Fig. 1, A and B). The inhibition was modest but significant (P < 0.05; n = 20); it occurred immediately after the pretreatment and lasted less than 20 min (current in pA: 405.4 ± 107.2 at control, 353.9 ± 100.2, 383.1 ± 106.3, and 403.4 ± 107.3 at 0, 10, and 20 min after MBP, respectively). On the other hand, the acid-evoked delayed/sustained, TRPV1-like inward current was not significantly affected by MBP (P > 0.05; n = 14) (Fig. 1, C and D).
Fig. 1.
Effects of major basic protein (MBP) on acid-evoked transient and sustained inward currents in isolated rat vagal pulmonary chemosensitive neurons. A: representative records of pH 6.5-evoked transient currents before and at different time points after pretreatment with MBP (2 μM, 60 s). B: group data showing the inhibiting effect of MBP on the acid (pH 5.5–7.0, 2–18 s)-evoked transient inward current. *Significantly different from the control response (Con) before MBP (P < 0.05, n = 20). C: representative records of pH 5.5-evoked transient and sustained currents before and at different time points after pretreatment with MBP (2 μM, 60 s). D: group data showing that MBP had no significant effect on the acid (pH 5.5–6.5, 6–18 s)-evoked sustained current (P > 0.05, n = 14). The responses at time 0 refer to the capsaicin-induced responses immediately (<1 s) after the termination of MBP pretreatment.
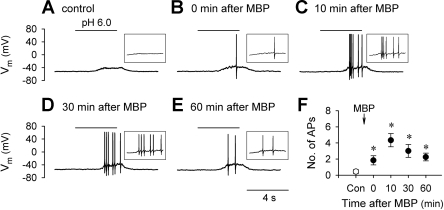
Surprisingly, in current-clamp recordings, MBP pretreatment (2 μM, 60 s) significantly increased the excitability of these sensory neurons in response to acid (Fig. 2). For example, the number of action potentials evoked by acid (pH 5.5–7.0, 2–8 s) was significantly increased almost 10-fold, from 0.4 ± 0.2 at control to 4.3 ± 0.8 at 10 min after MBP (P < 0.05; n = 9) (Fig. 2F). The potentiation of acid-evoked action potentials by MBP pretreatment was observed in all nine neurons tested, including the three neurons in which the acid-evoked inward currents remained unchanged after MBP when tested in voltage-clamp mode (not shown).
Fig. 2.
MBP increases the number of acid-evoked action potentials in rat vagal pulmonary chemosensitive neurons. A–E: representative records in current-clamp mode illustrating the responses to acid (pH 6.0, 4 s) before and at different time points after pretreatment with MBP (2 μM, 60 s). Insets: action potentials triggered by acid shown on a large time scale. F: group data showing that MBP significantly increased the number of action potentials (APs) evoked by acid (pH 5.5–7.0, 2–8 s). Vm, membrane potential. *Significantly different from the control response before MBP (P < 0.05, n = 9).
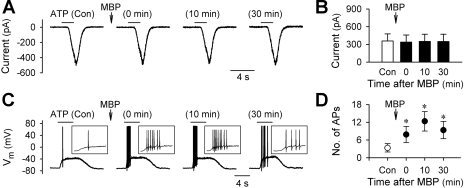
ATP is known to stimulate vagal pulmonary sensory neurons via the activation of P2X receptors (15, 31, 39). In voltage-clamp recordings, pretreatment with MBP (2 μM, 60 s) did not alter the ATP (0.3–1.0 μM, 2–4 s)-evoked whole cell inward current (P > 0.05, n = 8) (Fig. 3, A and B). However, in current-clamp recordings, the number of action potentials evoked by ATP (0.3–1.0 μM, 2–4 s) was significantly increased after MBP; for example, the number of ATP-evoked action potentials was increased from 3.3 ± 1.4 at control to 7.8 ± 2.7, 12.3 ± 2.3, and 9.3 ± 2.9 at 0, 10, and 30 min, respectively, after the MBP pretreatment (P < 0.05, n = 6) (Fig. 3, C and D).
Fig. 3.
Effect of MBP on ATP-evoked whole cell responses in rat vagal pulmonary chemosensitive neurons. A: representative records in voltage-clamp mode illustrating the responses to ATP (0.3 μM, 2 s) before and at different time points after pretreatment with MBP (2 μM, 60 s). B: group data showing that MBP had no significant effect on the ATP (0.3–1.0 μM, 2–4 s)-evoked inward current (P > 0.05, n = 8). C: representative records in current-clamp mode illustrating the responses to ATP (0.3 μM, 4 s) before and at different time points after pretreatment with MBP (2 μM, 60 s). D: group data showing that MBP significantly increased the number of action potentials evoked by ATP (0.3–1.0 μM, 2–4 s). *Significantly different from the control response before MBP (P < 0.05, n = 6).
Effect of TRPV1 antagonist on MBP-evoked hyperexcitability of rat vagal pulmonary chemosensitive neurons.
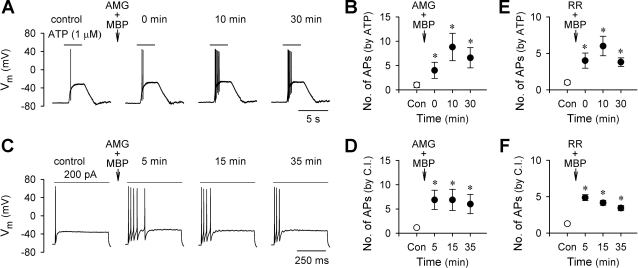
To determine whether TRPV1 plays a critical role in the MBP-induced hyperexcitability of pulmonary chemosensitive neurons, the effect of MBP was studied in the presence of AMG 9810, a selective antagonist of TRPV1 (13, 31). As shown in Fig. 4, pretreatment with AMG 9810 (1 μM, 2 min) did not prevent the MBP (2 μM, 60 s)-induced hyperexcitability of these neurons in response to ATP and current injection. The number of action potentials evoked by ATP (0.3–1.0 μM, 2–3 s) was increased from 1.0 ± 0.5 at control to 8.8 ± 2.8 at 10 min after AMG 9810 and MBP (P < 0.05, n = 5); the number of action potentials evoked by an above-threshold current injection (20–500 pA, 485 ms) was increased from 1.1 ± 0.1 at control to 6.9 ± 2.0 at 5 min after AMG 9810 and MBP (P < 0.05, n = 7) (Fig. 4, C and D).
Fig. 4.
Effects of AMG 9810 and ruthenium red on the MBP-induced hyperexcitability in rat vagal pulmonary chemosensitive neurons. A and C: representative records in current-clamp mode illustrating that AMG 9810 (AMG; 1 μM, 2 min), a selective transient receptor potential vanilloid type-1 receptor (TRPV1) antagonist, did not prevent the MBP (2 μM, 60 s)-induced increase in the number of action potentials evoked by ATP (1 μM, 3 s) and current injection (200 pA, 485 ms), respectively. B and D: group data showing that MBP (2 μM, 60 s) significantly increased the number of action potentials evoked by ATP (0.3–1.0 μM, 2–3 s; n = 5) and current injection (C.I.; 20–500 pA, 485 ms; n = 7), respectively, in the presence of AMG (1 μM, 2 min). E and F: group data showing that MBP (2 μM, 60 s) significantly increased the number of action potentials evoked by ATP (0.3–1.0 μM, 2–3 s; n = 6) and current injection (80–700 pA, 485 ms; n = 8), respectively, in the presence of ruthenium red (RR; 5 μM, 2 min), a nonselective blocker for TRPV1–4 channels. *Significantly different (P < 0.05) from the corresponding control response before MBP.
In a separate group of pulmonary chemosensitive neurons, the effect of ruthenium red, a nonselective but effective inhibitor for TRPV1–4 channels (20), on the sensitizing effect of MBP was investigated. Pretreatment with ruthenium red (5 μM, 2 min) did not block the MBP (2 μM, 60 s)-induced hyperexcitability of these neurons to either ATP (0.3–1.0 μM, 2–3 s; P < 0.05, n = 6) or current injection (80–700 pA, 485 ms; P < 0.05, n = 8) (Fig. 4, E and F). In our preliminary experiments, the effectiveness of the antagonizing effects of AMG 9810 (1 μM) and ruthenium red (5 μM) had been tested. A 2-min pretreatment with either of these two compounds completely abolished the capsaicin-evoked whole cell responses in these neurons (n = 6) (not shown).
Effect of MBP on the excitability of pulmonary chemosensitive neurons from TRPV1+/+ and TRPV1−/− mice.
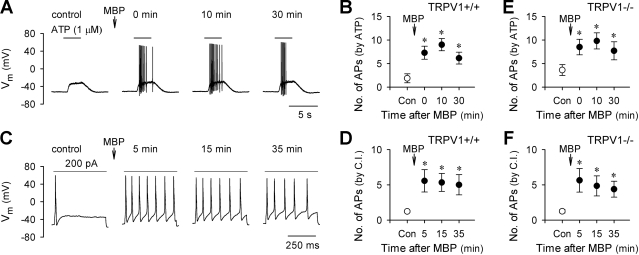
Pretreatment with MBP (2 μM, 60 s) clearly increased the excitability of vagal pulmonary chemosensitive neurons isolated from TRPV1+/+ (C57BL/6) mice (Fig. 5, A–D). Similarly, in the neurons isolated from TRPV1−/− ( TRPV1 knockout) mice, MBP pretreatment (2 μM, 60 s) significantly enhanced their excitabilities in response to ATP (0.3–1.0 μM, 3 s; P < 0.05, n = 6) or current injection (8–400 pA, 485 ms; P < 0.05, n = 8) (Fig. 5, E and F). No obvious difference in the sensitizing effect of MBP was observed between the neurons isolated from TRPV1+/+ and TRPV1−/− mice. In addition, as previously shown in rat neurons, the potentiating effect of MBP lasted more than 30 min in all the neurons tested from these two strains of mice (Fig. 5).
Fig. 5.
MBP-induced hyperexcitability in vagal pulmonary chemosensitive neurons isolated from TRPV1+/+ and TRPV1−/− mice. A and C: representative records in current-clamp mode illustrating the MBP (2 μM, 60 s)-induced hyperexcitability to ATP (1 μM, 3 s) and current injection (200 pA, 485 ms), respectively, in TRPV1+/+ mice. B and D: group data showing that MBP (2 μM, 60 s) significantly increased the number of action potentials evoked by ATP (0.3–1.0 μM, 3 s; n = 7) and current injection (15–300 pA, 485 ms; n = 9), respectively, in TRPV1+/+ mice. E and F: group data showing that MBP (2 μM, 60 s) significantly increased the number of action potentials evoked by ATP (0.3–1.0 μM, 3 s; n = 6) and current injection (8–400 pA, 485 ms; n = 8), respectively, in TRPV−/− mice. *Significantly different (P < 0.05) from the corresponding control response before MBP.
Effect of MBP on the action potential firing properties in rat vagal pulmonary chemosensitive neurons.
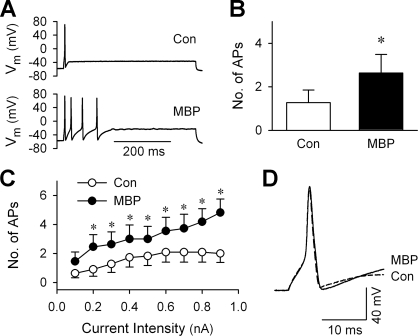
The effect of MBP on the neuron responses to a series of depolarizing current injections (0.1–0.9 nA, increment = 0.1 nA, duration = 485 ms) was tested in current-clamp recording mode. Pretreatment with MBP (2 μM, 60 s) enhanced firing in all 12 neurons tested (Fig. 6). The numbers of action potentials to all but one (0.1 nA) current pulse were significantly increased; for example, the maximum intensity stimulus (0.9 nA) evoked 2.0 ± 0.6 and 4.8 ± 0.9 action potentials before and after MBP, respectively (P < 0.05, n = 12). In consistence with what we reported recently (19), MBP pretreatment did not significantly alter the resting membrane potentials in these sensory neurons (Table 1). When comparing the parameters of the first action potentials evoked by the current injections, MBP did not cause any significant change in the threshold, amplitude, or overshoot but significantly increased the 80% decay time of action potentials. The duration of action potential at 50% maximum amplitude (AP50) and rise time also appeared to be increased after MBP; however, the changes were not statistically significant (Table 1).
Fig. 6.
Effect of MBP on the responses to current injection in rat vagal pulmonary chemosensitive neurons. A: representative records illustrating that pretreatment with MBP (2 μM, 60 s) increased firing in response to a current injection (0.3 nA, 485 ms) from 1 action potential (control trace) to 4 action potentials. B: average increase in the number of action potentials in response to the same current injection after the MBP pretreatment in 12 neurons. C: dependence of firing on the intensity of current injection before and after MBP pretreatment (2 μM, 60 s). *Significantly different (P < 0.05, n = 12) from the corresponding control response before MBP. D: comparison of the membrane potential in a larger scale of the first action potential before (Con) and after MBP as shown in A. Note the change in recovery phase after MBP.
Table 1.
Effect of MBP on characteristics of current injection-evoked action potentials
| Resting Membrane Potential, mV |
Parameters of Action Potential |
||||||
|---|---|---|---|---|---|---|---|
| Threshold, mV | Height, mV | Overshoot, mV | AP50, ms | Rise Time, ms | Time to 80% Decay, ms | ||
| Control | −64.8±1.6 | −22.8±1.6 | 130.5±2.7 | 65.8±2.7 | 3.0±0.5 | 1.1±0.1 | 3.8±0.5 |
| MBP | −64.1±1.9 | −22.7±1.7 | 129.5±3.1 | 65.3±2.6 | 3.3±0.5 | 1.3±0.2 | 4.3±0.7* |
Values are means ± SE. The action potential (AP) parameters were determined for the first AP evoked by the current injection (0.3 nA, 485 ms) as shown in Fig. 6D. AP50, duration of AP at 50% maximum amplitude. The Student's paired t-test was used for statistical comparisons;
Significantly different (P < 0.05) from the corresponding control; n = 12 at each data point. MBP, major basic protein.
Inhibition of voltage-gated K+ currents by MBP in rat vagal pulmonary chemosensitive neurons.
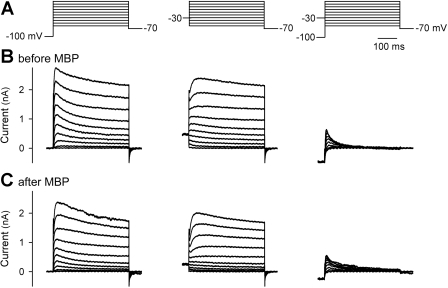
Voltage-gated K+ currents were elicited by a series of 400-ms test pulses ranging from −60 to +40 mV in 10-mV steps recorded as shown in Fig. 7. The command potential protocol was initially undertaken from a holding potential of −100 mV and then repeated in the same neurons from a holding potential of −30 mV. The sustained delayed-rectifier voltage-gated K+ current (IKdr) was measured at the end of depolarizing pulses from a holding potential of −30 mV, and a subtraction of the current waveforms elicited at the two holding potentials was defined as A-type, fast-inactivating K+ current (IKa) (22).
Fig. 7.
Effect of MBP on voltage-gated K+ currents in rat vagal pulmonary chemosensitive neurons. A: depolarizing voltage command protocols used to elicit voltage-gated K+ currents. B and C: representative superimposed current traces before and following (2–15 min after) MBP pretreatment (2 μM, 60 s), respectively. Whole cell K+ currents were elicited by a series of 400-ms test pulses ranging from −60 to +40 mV in 10-mV steps. Left and middle: neurons were held at −100 and −30 mV, respectively, before each pulse. Right: fast inactivating voltage-gated K+ current (IKa) was obtained by subtracting each current in middle from the corresponding current in left.
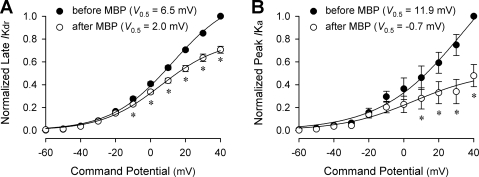
Pretreatment with MBP (2 μM, 60 s) inhibited both IKdr and IKa in vagal pulmonary chemosensitive neurons (Figs. 7 and 8). The amplitudes of IKdr were significantly reduced after pretreatment with MBP in the voltage range of −10 to +40 mV. For example, at +40 mV, the mean IKdr was reduced to 70.6% of the control after MBP pretreatment (P < 0.05, n = 6) (Fig. 8A). Similarly, the amplitudes of IKa were significantly decreased after pretreatment with MBP in the voltage range of +10 to +40 mV; e.g., after MBP pretreatment, the mean IKa at +40 mV was reduced to 47.9% of its control (P < 0.05, n = 6) (Fig. 8B). However, neither the time to reach peak IKdr or IKa amplitude nor the threshold of IKdr or IKa activation (e.g., Fig. 7, B and C), was altered significantly after the MBP pretreatment (P > 0.05, n = 6).
Fig. 8.
Comparison of current-voltage relationships for voltage-gated K+ currents before and after MBP pretreatment in rat vagal pulmonary chemosensitive neurons. A: normalized current-voltage relationships for sustained voltage-gated K+ current (IKdr) before (•) and after (○) MBP (2 μM, 60 s). Currents were measured at the end of 400-ms pulses depolarized from a holding potential of −30 mV as shown in Fig. 7, middle. B: normalized current-voltage relationships for IKa before (•) and after (○) MBP (2 μM, 60 s). Peak currents as shown in Fig. 7, right, were normalized and plotted as a function of voltage. Each set of data are fitted to a single-term Boltzmann function. The half-activation voltages (V0.5) before and after MBP are shown as insets. *Significantly different (P < 0.05) from the corresponding control response before MBP (n = 6 in both A and B).
DISCUSSION
The results of our present study show that, in rat vagal pulmonary chemosensitive neurons, eosinophil MBP significantly enhanced the excitability to acid and ATP in current-clamp mode but not the inward currents evoked by these two chemicals in voltage-clamp recordings. The hyperexcitability induced by MBP was not prevented by the blockade of either TRPV1 alone or all TRPV1–4 channels. In addition, MBP markedly potentiated the excitability of pulmonary chemosensitive neurons isolated from both wild-type and TRPV1 knockout mice. Furthermore, MBP pretreatment markedly affected the decay time and recovery phase of the action potentials evoked by current injections and significantly inhibited the voltage-gated K+ channels, suggesting that the inhibition in IKdr and IKa should, at least in part, account for the hyperexcitability of pulmonary chemosensitive neurons induced by eosinophil-derived cationic proteins.
We have recently reported that eosinophil-derived cationic proteins had a pronounced sensitizing effect on the capsaicin-evoked, TRPV1-mediated whole cell responses in rat vagal pulmonary chemosensitive neurons (19). Although the underlying mechanism was not investigated in that study, the observation that MBP significantly enhanced the capsaicin-evoked both inward current (in voltage clamp) and action potential firing (in current clamp) suggested that the cationic protein could have a direct interaction with TRPV1. Indeed, a recent study by Ahern et al. (1) has demonstrated that polyamines (the organic polycations including spermine, spermidine, and putrescine), by virtue of their cationic charges, can directly activate and sensitize the activity of TRPV1. Their study further suggested that the extracellular acidic residues ASP-646 and Glu-648, which are located near the pore-forming region of TRPV1, play an important role in polyamine regulation. Their hypothesis was supported by the finding that spermine failed to increase the whole-cell current evoked by protons (pH = 4) in TRPV1-expressing oocytes (1). In view of the critical role of positive charges in mediating the effects of eosinophil-derived cationic proteins (4, 9, 10, 16, 19, 27), it is reasonable to propose that these proteins may interact with TRPV1 in a manner similar to polyamines. Such an interaction may offer a plausible explanation as to why MBP enhanced the inward current evoked by capsaicin (19) but did not affect the acid-evoked, TRPV1-mediated inward current (Fig. 1, C and D). However, the mechanism by which MBP exerted a modest and transient inhibition of the acid-evoked, ASIC-mediated transient inward current (Fig. 1, A and B) remains to be elucidated.
Interestingly, our data showed a striking difference between voltage-clamp and current-clamp recordings of the effect of MBP on the acid- or ATP-evoked responses in these neurons; MBP increased only the numbers of action potential firing but not the inward currents evoked by these two chemicals (Figs. 1–3). It has been recently reported that TRPV1 as well as several other TRP channels (e.g., TRPV3 and TRPM8) are voltage sensitive, and physical stimuli such as temperature or the binding of various ligands can shift their voltage-dependent activation curves toward the physiological range of membrane potentials (30, 32, 41). Considering that MBP may act as a potential TRPV1 activator/sensitizer in a manner similar to that of polyamines discussed above, we use antagonists selectively for TRPV1 (AMG 9810) and for all TRPV1–4 (ruthenium red) to evaluate the possibility whether a direct binding of TRPV1 by MBP shifts right the current-voltage curve of the neuron and evokes action potentials when the membrane potential is depolarized by ATP or electrical stimulation. Our results show that blockade of TRPV1 or TRPVs with their antagonists did not prevent the MBP-induced potentiation of action potential firing evoked by ATP or current injection (Fig. 4); our results further demonstrate that the potentiating effect of MBP in TRPV1−/− mice was not different from that in wild-type mice (Fig. 5). These data suggest that a direct interaction between MBP and TRPV1 channels is not involved in the MBP-induced hyperexcitability of pulmonary chemosensitive neurons in response to the stimuli other than the TRPV1 agonists.
In the present study, our results have shown that MBP pretreatment did not significantly alter the resting membrane potentials of pulmonary chemosensitive neurons, nor did it cause any significant change in the threshold, amplitude, or overshoot of the action potentials evoked by current injections; however, the recovery phase and decay time of action potentials were markedly affected by MBP (Fig. 6; Table 1). These data strongly suggested an involvement of the regulation of voltage-gated K+ currents. It is well recognized that voltage-gated K+ channels play important roles in setting the resting potential, repolarizing and hyperpolarizing the cell, and therefore shaping the excitability and firing patterns of cells (22). In view of the complexity and variability of voltage-gated K+ currents (21), we performed a simplified analysis of the effect of MBP on the two kinetically distinct K+ currents, IKdr and IKa. Although the molecular determinants of the channels underlying IKdr are not fully understood, recent studies suggest that this class of K+ currents may comprise the currents from a large diversity of voltage-gated K+ channels, including members from Kv1, Kv2, and Kv3 gene families (3, 21, 24, 35). Previous studies have shown that block of IKdr depolarized the cell membrane, prolonged action potential duration, lowered the threshold for action potential firing, and increased the overall neuronal excitability (5, 11, 24, 34, 37, 38). Surprisingly, the results obtained after MBP treatment in our present study are more complex: no consistent cell membrane depolarization, no significant change in threshold of firing, and only a mild increase in action potential duration (Fig. 6; Table 1). Although we assume that the effect of MBP on K+ currents is mainly charge related, and it may differ from that of other commonly used K+ channel inhibitors/blockers such as tetraethylammonium (34), the mechanism involved in causing the inconsistent changes in the membrane properties seen in our study is not known. The main function of IKa is to dampen developing subthreshold depolarization and lengthen the interspike interval and therefore to slow down the action potential firing rate (22). Recent studies have suggested that IKa is mediated by a variety of voltage-gated K+ channels including that encoded by Kv1.4 and Kv4 family genes (3, 21). Inhibition of IKa has been demonstrated to increase the capability of neurons to fire repetitively (5, 36, 38). In the present study, our results show that both IKdr and IKa were significantly inhibited by MBP (Figs. 7 and 8). Given that the important roles of these two groups of K+ currents play in regulating the excitability of neurons, we propose that the inhibition of IKdr and IKa should, at least in part, account for the MBP-induced hyperexcitability of vagal pulmonary chemosensitive neurons. In addition, an inhibition of voltage-gated K+ channels also provides a reasonable explanation as to why MBP increased only the numbers of acid- or ATP-evoked action potentials in current clamp but not the inward currents evoked by these two chemicals in voltage-clamp recordings when the membrane potential of neurons was held constantly at −70 mV (Figs. 1–3). Although results of this study have demonstrated an inhibitory effect of MBP on these K+ currents, we cannot rule out the possible involvement of other voltage-gated channels (e.g., Ca2+-dependent IK, tetrodotoxin-insensitive INa, etc.) in the sensitizing effect of MBP on these neurons.
Our study has demonstrated that MBP induced hyperexcitability to ATP and current injection in the isolated mice pulmonary sensory neurons (Fig. 5). These results are in general agreement with the findings from rat experiments reported in this and other studies recently reported by our laboratory (16, 19, 28) that showed that eosinophil-derived (e.g., MBP) or synthetic (e.g., poly-l-lysine) cationic proteins significantly enhanced the excitability of pulmonary C-fibers/C-neurons to electrical (current injection), mechanical (lung inflation), and various chemical (e.g., capsaicin, phenyl biguanide, adenosine, ATP) stimulations. It is well documented that bronchopulmonary C-fibers can be stimulated by various endogenous substances (e.g., H+, anandamide, serotonin, etc.), inhaled irritants (e.g., cigarette smoke, SO2, ozone, acid aerosol, etc.), and lung expansion (e.g., increased tidal volume during exercise, sigh, etc.) (29, 39). Under normal conditions, these stimuli may not generate a significant stimulatory effect on bronchopulmonary C-fibers. However, when eosinophils infiltrate into airways under certain pathophysiological conditions (e.g., asthma) and release granule proteins into bronchial tissues, the nonspecific hyperexcitability of the C-fiber endings could be induced by the eosinophil-derived cationic proteins, as demonstrated in the present study. Therefore, the C-fiber afferents could potentially be activated by the above-mentioned stimuli, and the consequent reflexes may contribute to the cardiorespiratory symptoms such as bronchoconstriction, dyspneic sensation, and cough.
In summary, in the light of the recent findings from our laboratory, the present study was aimed to further elucidate the mechanisms underlying the sensitization of vagal pulmonary C-fiber sensory nerves induced by eosinophil-derived cationic proteins. Our results show that eosinophil MBP significantly increased the firing rates of pulmonary chemosensitive neurons in response to acid and ATP challenges. The MBP-induced hyperexcitability to ATP and electrical stimulation did not involve an interaction with TRPV1 channels. More importantly, our data have demonstrated that MBP significantly inhibited both IKdr and IKa in these neurons. Given the important role of the voltage-gated K+ currents in regulating the neuronal excitability, the inhibition of these currents should, at least in part, account for the hyperexcitability of pulmonary C-fibers induced by these cationic proteins.
GRANTS
This study was supported by grants from National Institute of Health HL58686 and HL67379 (L. Lee) and AI09728 (G. Gleich). Q Gu was a Parker B. Francis Fellow in Pulmonary Research.
Acknowledgments
The authors thank Lyo E. Ohnuki for technical assistance and Dr. Brian Delisle for critical reading and helpful comments on this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahern GP, Wang X, Miyares RL. Polyamines are potent ligands for the capsaicin receptor TRPV1. J Biol Chem 281: 8991–8995, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Arseneault D, Maghni K, Sirois P. Selective inflammatory response induced by intratracheal and intravenous administration of poly-l-arginine in guinea pig lungs. Inflammation 23: 287–304, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Baranauskas G Ionic channel function in action potential generation: current perspective. Mol Neurobiol 35: 129–150, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Barker RL, Gundel RH, Gleich GJ, Checkel JL, Loegering DA, Pease LR, Hamann KJ. Acidic polyamino acids inhibit human eosinophil granule major basic protein toxicity. Evidence of a functional role for ProMBP. J Clin Invest 88: 798–805, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birinyi-Strachan LC, Gunning SJ, Lewis RJ, Nicholson GM. Block of voltage-gated potassium channels by Pacific ciguatoxin-1 contributes to increased neuronal excitability in rat sensory neurons. Toxicol Appl Pharmacol 204: 175–186, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola Asthma AM. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161: 1720–1745, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. [DOI] [PubMed] [Google Scholar]
- 8.Coyle AJ, Ackerman SJ, Burch R, Proud D, Irvin CG. Human eosinophil-granule major basic protein and synthetic polycations induce airway hyperresponsiveness in vivo dependent on bradykinin generation. J Clin Invest 95: 1735–1740, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyle AJ, Ackerman SJ, Irvin CG. Cationic proteins induce airway hyperresponsiveness dependent on charge interactions. Am Rev Respir Dis 147: 896–900, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Coyle AJ, Uchida D, Ackerman SJ, Mitzner W, Irvin CG. Role of cationic proteins in the airway hyperresponsiveness due to airway inflammation. Am J Respir Crit Care Med 150: S63–S71, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Crozier RA, Ajit SK, Kaftan EJ, Pausch MH. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J Neurosci 27: 4492–4496, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filley WV, Holley KE, Kephart GM, Gleich GJ. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet 2: 11–16, 1982. [DOI] [PubMed] [Google Scholar]
- 13.Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 313: 474–484, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Gleich GJ Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 105: 651–663, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Gu Q, Kwong K, Lee LY. Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: role of cAMP/PKA signaling pathway. J Neurophysiol 89: 1985–1993, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gu Q, Lee LY. Hypersensitivity of pulmonary C fibre afferents induced by cationic proteins in the rat. J Physiol 537: 887–897, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Q, Lee LY. Characterization of acid-signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 291: L58–L65, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu Q, Lin RL, Vanaman TC, Lee LY. Hypersensitivity of pulmonary chemoreflex induced by poly-l-lysine: role of cationic charge. Respir Physiol Neurobiol 151: 31–43, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Gu Q, Wiggers ME, Gleich GJ, Lee LY. Sensitization of isolated rat vagal pulmonary sensory neurons by eosinophil-derived cationic proteins. Am J Physiol Lung Cell Mol Physiol 294: L544–L552, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci 23: 183–191, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stühmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev 57: 473–508, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Hille B Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001.
- 23.Homma T, Bates JH, Irvin CG. Airway hyperresponsiveness induced by cationic proteins in vivo: site of action. Am J Physiol Lung Cell Mol Physiol 289: L413–L418, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Kayssi A, Amadesi S, Bautista F, Bunnett NW, Vanner S. Mechanisms of protease-activated receptor 2-evoked hyperexcitability of nociceptive neurons innervating the mouse colon. J Physiol 580: 977–991, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 543: 591–600, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong K, Lee LY. PGE2 sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol 93: 1419–1428, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Lee LY, Gu Q. Mechanisms of bronchopulmonary C-fiber hypersensitivity induced by cationic proteins. Pulm Pharmacol Ther 16: 15–22, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Lee LY, Gu Q, Gleich GJ. Effects of human eosinophil granule-derived cationic proteins on C-fiber afferents in the rat lung. J Appl Physiol 91: 1318–1326, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C fibers. Respir Physiol 125: 47–65, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Matta JA, Ahern GP. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol 585: 469–482, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni D, Lee LY. Effect of increasing temperature on TRPV1-mediated responses in isolated rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 294: L563–L571, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: a voltage connection? J Physiol 567: 35–44, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohnuki LE, Wagner LA, Georgelas A, Loegering DA, Checkel JL, Plager DA, Gleich GJ. Differential extraction of eosinophil granule proteins. J Immunol Methods 307: 54–61, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Safronov BV, Bischoff U, Vogel W. Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurones of rat. J Physiol 493: 393–408, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song WJ Genes responsible for native depolarization-activated K+ currents in neurons. Neurosci Res 42: 7–14, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Sonner PM, Filosa JA, Stern JE. Diminished A-type potassium current and altered firing properties in presympathetic PVN neurones in renovascular hypertensive rats. J Physiol 586: 1605–1622, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol 96: 2189–2199, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Tan ZY, Donnelly DF, LaMotte RH. Effects of a chronic compression of the dorsal root ganglion on voltage-gated Na+ and K+ currents in cutaneous afferent neurons. J Neurophysiol 95: 1115–1123, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Taylor-Clark T, Undem BJ. Transduction mechanisms in airway sensory nerves. J Appl Physiol 101: 950–959, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Uchida DA, Ackerman SJ, Coyle AJ, Larsen GL, Weller PF, Freed J, Irvin CG. The effect of human eosinophil granule major basic protein on airway responsiveness in the rat in vivo. A comparison with polycations. Am Rev Respir Dis 147: 982–988, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430: 748–754, 2004. [DOI] [PubMed] [Google Scholar]