Abstract
Persistent hypoxia can cause pulmonary arterial hypertension that may be associated with significant remodeling of the pulmonary arteries, including smooth muscle cell proliferation and hypertrophy. We previously demonstrated that the NADPH oxidase homolog NOX4 mediates human pulmonary artery smooth muscle cell (HPASMC) proliferation by transforming growth factor-β1 (TGF-β1). We now show that hypoxia increases HPASMC proliferation in vitro, accompanied by increased reactive oxygen species generation and NOX4 gene expression, and is inhibited by antioxidants, the flavoenzyme inhibitor diphenyleneiodonium (DPI), and NOX4 gene silencing. HPASMC proliferation and NOX4 expression are also observed when media from hypoxic HPASMC are added to HPASMC grown in normoxic conditions, suggesting autocrine stimulation. TGF-β1 and insulin-like growth factor binding protein-3 (IGFBP-3) are both increased in the media of hypoxic HPASMC, and increased IGFBP-3 gene expression is noted in hypoxic HPASMC. Treatment with anti-TGF-β1 antibody attenuates NOX4 and IGFBP-3 gene expression, accumulation of IGFBP-3 protein in media, and proliferation. Inhibition of IGFBP-3 expression with small interfering RNA (siRNA) decreases NOX4 gene expression and hypoxic proliferation. Conversely, NOX4 silencing does not decrease hypoxic IGFBP-3 gene expression or secreted protein. Smad inhibition does not but the phosphatidylinositol 3-kinase (PI3K) signaling pathway inhibitor LY-294002 does inhibit NOX4 and IGFBP-3 gene expression, IGFBP-3 secretion, and cellular proliferation resulting from hypoxia. Immunoblots from hypoxic HPASMC reveal increased TGF-β1-mediated phosphorylation of the serine/threonine kinase (Akt), consistent with hypoxia-induced activation of PI3K/Akt signaling pathways to promote proliferation. We conclude that hypoxic HPASMC produce TGF-β1 that acts in an autocrine fashion to induce IGFBP-3 through PI3K/Akt. IGFBP-3 increases NOX4 gene expression, resulting in HPASMC proliferation. These observations add to our understanding hypoxic pulmonary vascular remodeling.
vascular remodeling is the hallmark pathological change in pulmonary arterial hypertension (PAH). It collectively refers to intimal, medial, and adventitial thickening due to increases in cell size and number, as well as extracellular matrix accumulation. Vascular remodeling results in luminal narrowing of the pulmonary arteries with subsequent increase in pulmonary arterial resistance. Medial thickening is the result of excessive proliferation and hypertrophy of pulmonary artery smooth cells (PASMC). In almost all forms of PAH, muscularization of normally nonmuscular distal pulmonary arteries occurs (19, 45, 56). Although various mechanisms have been implicated in the pathogenesis of PAH, hypoxia remains the most clinically relevant stimulus of PASMC proliferation and subsequent pulmonary vascular remodeling (45, 56).
Reactive oxygen species (ROS) are important regulators of vascular tone and function (13, 51). In the lung, ROS are implicated in acute hypoxic vasoconstriction (70). Administration of superoxide dismutase significantly attenuates pulmonary vasoconstriction due to hypoxia (38). Moreover, several studies have now shown that agents promoting ROS generation stimulate proliferation of both systemic and PASMC, implicating ROS in the vascular remodeling associated with chronic hypoxia. Again, suppression of endogenous ROS inhibits PASMC proliferation and promotes apoptosis (6, 7, 69). In animal models, ROS have been directly linked to the vascular remodeling associated with chronic hypoxia-induced PAH (25, 39). Furthermore, chronic hypoxia-associated increases in ROS generation may interact with and modulate agonist-mediated pulmonary artery vasoconstrictor responses.
The idea that there is a paradoxical increase in ROS generation during hypoxia, although still controversial, is gaining support. Observations using a variety of experimental techniques, and in many cells and tissue types, support this phenomenon and the related concept that hypoxia-induced ROS may be both a physiological and pathophysiological response to environmental stress (11). Substantiating the feasibility of this apparent paradox is the fact that most oxidases, with the exception of xanthine oxidase, have Km values low enough to support ROS generation at very low intracellular oxygen (O2) concentrations (11, 66).
The sources of ROS in the pulmonary vasculature are not well-defined. However, there is mounting evidence that NADPH oxidases contribute to systemic vascular pathology (55, 60). Homologs to the gp91phox component of the phagocytic NADPH oxidase have been characterized and are collectively referred to as NOX proteins (10, 31). These NOX proteins have been implicated in the pathogenesis of pulmonary vascular remodeling. Mice with the null mutant for gp91phox (now referred to as NOX2) were protected from chronic hypoxia-induced PAH and vascular remodeling (39). In contrast, we observed that smooth muscle cells (SMC) derived from human pulmonary arterial tissue and grown under conditions of normoxia express NOX4 and proliferate by a NOX4-mediated mechanism (57). Mittal and colleagues (46) recently demonstrated that NOX4 is the only NOX homolog increased by hypoxia in murine pulmonary arteries as well as human pulmonary arterial smooth muscle cells (HPASMC). Whether NOX4 contributes to hypoxic pulmonary vascular remodeling remains an important unanswered question.
We now demonstrate that hypoxia induces HPASMC proliferation by a NOX4-mediated mechanism. NOX4 expression is increased by hypoxia due to the autocrine production of transforming growth factor-β1 (TGF-β1) and insulin-like growth factor binding protein-3 (IGFBP-3). In contrast to the mechanisms of TGF-β1 signaling observed in normoxic HPASMC (57), the hypoxic release of TGF-β1 increases IGFBP-3 expression through phosphatidylinositol 3-kinase (PI3K) signaling with subsequent serine/threonine kinase (Akt) phosphorylation. These observations add to our understanding of how hypoxic pulmonary vascular remodeling occurs.
METHODS
Procurement of pulmonary artery tissue.
The University of Utah Institutional Review Board approved collection of human pulmonary arterial tissue from organ donors. Tissue was obtained at the time of thoracic organ procurement, usually within 8 h of the declaration of clinical brain death. At the time of procurement, hearts of donors were beating, and their major organs were adequately perfused to maintain viability required for subsequent organ transplantation. On acquisition, sections of the main pulmonary artery tissue were placed in ice-cold normal saline and transported to the laboratory for isolation of SMC.
Isolation of HPASMC.
HPASMC were obtained by collagenase/elastase (Roche Biochemicals, Indianapolis, IN) digestion of donor main pulmonary artery tissue. Briefly, the adventitia was physically removed, and the endothelial layer was removed by scraping with a blunt edge scalpel. The remaining smooth muscle layer was incubated in collagenase A (1.0 mg/ml) for 24 h at 37°C. This tissue was then minced in a solution of collagenase A (2 mg/ml) and elastase grade II (0.5 mg/ml) and incubated at 37°C in a shaking water bath for 1 h. The tissue fragments were disrupted by pipetting several times and incubated for an additional hour. Undigested tissue was removed by straining through sterile gauze. The cell pellet was washed with sterile PBS, and dispersed cells were plated on gelatin-coated dishes in SMC growth media (Cascade Biologicals, Portland, OR) under standard conditions in a humidified tissue culture maintained at 5% CO2-21% O2. The initial culture is referred to as the “primary culture.” HPASMC were stored at −135°C until used. Cells at passages 3–8 were used in all experiments. Before experiments, cells were grown in a 50:50 mix of SMC growth media and DMEM 10% FCS until 80% confluent. Before exposure to hypoxia or normoxia, the cells were incubated in 1% FCS for 24 h and then placed in DMEM 1% FCS with or without specified inhibitors or blocking antibodies. The PI3K inhibitor LY-294002 (Calbiochem, San Diego, CA) or anti-TGF-β1 antibody (R&D Systems, Minneapolis, MN) was added to HPASMC as described in the figure legends.
Exposure of HPASMC to hypoxia.
HPASMC in DMEM 1% FCS with or without modulating factors or inhibitors were placed inside a humidified Modular Incubator Chamber (Billups-Rothenberg, Del Mar, CA) maintained at 37°C. The chamber was initially flushed for 20 min with a low-oxygen mixture (1% O2-5% CO2, balance nitrogen; Airgas Intermountain, Salt Lake City, UT) flowing at 10 l/min in a closed loop isolated from the ambient atmosphere by water seals applied to both ends of the circuit. The flow was subsequently decreased to 0.5–1.0 l/min. The O2 in the exit limb of gas flow was continuously monitored to confirm desired O2 delivery. The partial pressures of O2 and CO2 and the pH of the growth media were intermittently measured to confirm alterations in O2 tension without significant changes in pH. A gas mixture containing 1% O2 was found to be the most effective in producing the lowest O2 tension in the culture media without causing cell death. Control cells were maintained under identical conditions other than receiving a normoxic gas mixture (21% O2-5% CO2, balance nitrogen).
Preparing conditioned media.
HPASMC were preincubated in low serum media (1% FCS) for 24 h before the addition of inhibitors. The cells were then incubated in 1% or 21% O2 at 37°C. After 72 h, the media were removed, and the volume of the media was noted. The conditioned media were either used immediately or stored at −80°C for use later.
Measurement of H2O2 production by HPASMC.
The assay is based on the detection of H2O2 that reacts with Amplex Red (Molecular Probes, Eugene, OR) in the presence of horseradish peroxidase with a 1:1 stoichiometry producing resorufin. Amplex Red (50 μM) and horseradish peroxidase (5 U/2 ml) were added to cells exposed to hypoxia for indicated periods of time. Fluorescence after H2O2 formation was detected at 37°C in a fluorescence spectrophotometer. The excitation and emission wavelengths were 550 and 585 nm, respectively. Calibration signals were generated using known amounts of H2O2.
Measurement of proliferation by HPASMC.
HPASMC (1 × 105 cells per dish) were plated on gelatin-coated 35-mm dishes in 50:50 SMC growth media-to-DMEM 10% FCS and allowed to attach overnight. Subsequently, cells were incubated for 24 h in 1% FCS. The growth media were then changed to 1% FCS with or without specified inhibitors or blocking antibodies, and cells were incubated at either 1% or 21% O2 at 37°C for 72 h. After 72 h, the dishes were washed twice with PBS, and cells were detached by trypsinization. Cell counts were obtained by hemocytometry. Cell counts were performed in similar fashion to identical dishes of cells immediately before exposure to either 1% or 21% O2 to obtain baseline (0 h) counts. Samples were counted four times and averaged. In selected experiments, proliferation was determined using a previously detailed colorimetric method based on metabolic reduction of the soluble yellow tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to its insoluble purple formazan (5). The absorbance of the MTT formazan reduction product (A540) correlates with cell numbers counted by hemocytometer with an R2 = 0.99 (5).
Real-time quantitative RT-PCR.
Total cellular RNA was isolated from cultured cells using RNeasy protocols (Qiagen, Valencia, CA). First-strand cDNA was reverse-transcribed from 2.0 μg of total RNA using a high capacity cDNA archive kit [Applied Biosystems (ABI), Foster City, CA]. NOX4, IGFBP-3, Smad3, 18S, and polymerase II (Pol2) were quantified using primer pairs supplied by Applied Biosystems on an ABI PRISM 7900HT Sequence Detection System. cDNA (90 ng) was mixed with ABI TaqMan Universal PCR Master Mix and the appropriate ABI TaqMan Gene Expression Assay for the gene of interest. We used the comparative cycle threshold (CT) method (2−ΔΔCT) to calculate relative gene expression under experimental and control conditions normalized to Pol2 or 18S. The results are expressed as fold change over control values (40).
Experiments using RNA interference.
NOX4, Smad3, or IGFBP-3 gene expression was inhibited using RNA interference technology. A SMARTpool consisting of four short or small interfering RNA (siRNA) oligomers for NOX4 (siNOX4), Smad3 (siSmad3), or IGFBP-3 (siIGFBP-3) was obtained from Dharmacon (Lafayette, CO). The Amaxa system was used to transfect 50 nM SMARTpool siRNA targeted to the gene of interest into HPASMC, and cells were allowed to attach overnight. Transfected cells were then incubated with 1% FCS in growth media for 24 h. Fresh growth media with 1% FCS were then applied, and the cells were incubated in 1% or 21% O2. Control cells were transfected under identical conditions with scrambled siRNA oligomers (siSCR) provided by Dharmacon. Preliminary experiments demonstrated that siSCR had no effect on NOX4, Smad3, or IGFBP-3 expression during normoxic or hypoxic conditions. RT-PCR and/or ELISA of the mRNA or protein of interest confirmed successful RNA interference.
Quantification of TGF-β1 and IGFBP-3.
TGF-β1 and IGFBP-3 were measured in the culture media by ELISA according to the manufacturer's instruction (R&D Systems). Data are expressed as fold increase over control.
Assessment of phospho-Akt by Western immunoblotting.
HPASMC (2.5 × 105 cells per dish) were plated on gelatin-coated 100-mm dishes in 50:50 SMC growth media-to-DMEM 10% FCS and allowed to attach overnight. Cells were then incubated for 24 h in 1% FCS. The growth media were changed to fresh 1% FCS with or without anti-TGF-β1 antibodies (50 ng/ml), and cells were incubated at either 1% or 21% O2 at 37°C for 4 or 8 h. Cell lysates were prepared in RIPA buffer supplemented with a full spectrum protease inhibitor cocktail (Roche Biochemicals) and then subjected to SDS-PAGE electrophoresis and subsequent membrane transfer. Human anti-phosphorylated Akt (anti-phospho-Akt)-Ser473 antibodies and anti-Akt antibodies (Cell Signaling Technologies, Danvers, MA) were used for membrane immunostaining. Appropriate dilutions were empirically derived for each antibody.
Statistical analysis.
Data are expressed as means ± SD of four observations unless otherwise indicated. Differences between two groups were compared with the unpaired Student's t-test. Two-tailed tests of significance were used. Differences between multiple groups were compared with one-way analysis of variance. Levels of significance were assumed at P < 0.05.
RESULTS
Hypoxia induces HPASMC proliferation by increasing generation of ROS.
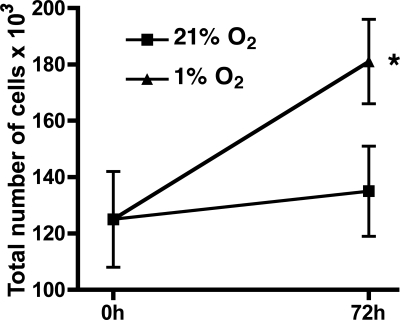
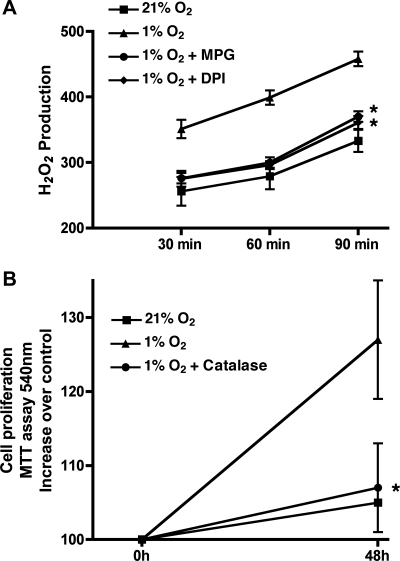
To achieve cellular hypoxia, HPASMC were incubated at 1% O2 for 72 h. Total cell counts were performed at baseline and at 72 h. Hypoxia for 72 h consistently induced HPASMC proliferation (Fig. 1). To investigate the possibility that ROS mediated the observed hypoxia-induced HPASMC proliferation, we determined H2O2 production by HPASMC under control and hypoxic conditions using the Amplex Red fluorescent assay. As demonstrated in Fig. 2A, 48-h exposure to hypoxia substantially increased H2O2 production by HPASMC. The H2O2 accumulation was prevented by the antioxidant N-(2-mercaptopropionyl) glycine (MPG) and by the flavoenzyme inhibitor diphenyleneiodonium (DPI; 2.0 μM). The increased H2O2 production was responsible for the hypoxia-induced HPASMC proliferation as demonstrated by attenuation of proliferation by catalase (Fig. 2B) and MPG (data not shown).
Fig. 1.
Hypoxia induces human pulmonary artery smooth muscle cell (HPASMC) proliferation. Cells were incubated for 72 h in 1% or 21% O2. Baseline and 72-h cell counts were performed. Counts are expressed in total number of cells ×103. Each condition represents the mean of 4 samples ± SD. *P < 0.001, 1% vs. 21% O2 at 72 h.
Fig. 2.
Hypoxic HPASMC proliferation is mediated by reactive oxygen species (ROS). A: hypoxia-induced increased H2O2 production by HPASMC is inhibited by N-(2-mercaptopropionyl) glycine (MPG) and diphenyleneiodonium (DPI). Each condition represents the mean of 3 samples ± SD. *P < 0.001, 1% O2 + MPG or DPI vs. 1% O2 alone. B: scavenging of H2O2 by catalase prevents hypoxia-induced cell HPASMC proliferation. Results are representative of 3 independent experiments. *P < 0.05, 1.0% O2 vs. 1.0% O2 + catalase at 48 h. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
NOX4 is the source of ROS mediating hypoxic cellular proliferation.
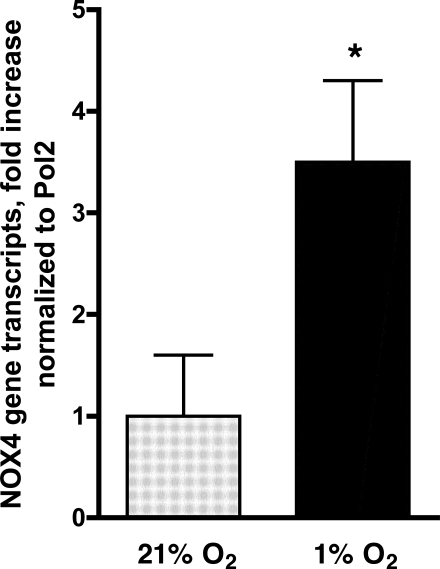
The attenuation of the hypoxia-induced H2O2 generation by DPI suggested a role for an NADPH oxidase. To assess the relevance of an NADPH oxidase, we determined the effect of DPI (2.0 μM) added before hypoxic exposure on HPASMC proliferation. Total cell counts were performed at baseline and at 72 h. DPI inhibited proliferation of cells maintained at 1% O2 for 72 h (Fig. 3A), indicating a central role for an NADPH oxidase. We previously demonstrated that NOX4 is the major NOX homolog forming the NADPH oxidase in HPASMC. To assess the role of NOX4 in hypoxia-induced proliferation, we determined its expression in HPASMC exposed to 1% O2 for 24 h. A 3.5-fold increase in NOX4 mRNA was observed in hypoxic cells compared with cells maintained under normoxic conditions (Fig. 4). To further assess the role of NOX4, we transfected HPASMC with siRNA targeted to NOX4 before hypoxic exposure. At the conclusion of 72 h, cells transfected with NOX4 siRNA were significantly (P < 0.001) fewer in number than control cells (Fig. 3B). These results indicate that the increase in NOX4 expression in hypoxic HPASMC drives cell proliferation.
Fig. 3.
Hypoxic HPASMC proliferation is mediated by NOX4. A: cells were treated with DPI (2.0 μM) before exposure to hypoxia for 72 h. Counts were performed before and after hypoxia (1% O2) and are expressed as total number of cells ×103. Each condition represents the mean of 4 samples ± SD. *P < 0.001, 1% O2 vs. 21% O2 at 72 h. †P < 0.001, 1% O2 vs. 1% O2 + DPI at 72 h. B: HPASMC were transfected with small interfering RNA (siRNA) oligomer for NOX4 (siNOX4) before exposure to hypoxia for 72 h. Counts are expressed in total number of cells ×103. Each condition represents the mean of 4 samples ± SD. *P < 0.001, 1% O2 vs. 21% O2. †P < 0.001, 1% O2 control cells vs. 1% O2 + siNOX4.
Fig. 4.
Hypoxia induces NOX4 gene expression. HPASMC were exposed to 1% O2 for 24 h, and total RNA was isolated. Real-time PCR of reverse-transcribed mRNA was performed using NOX4 primers. Results were normalized to polymerase II (Pol2) and expressed as fold induction over control ± SD. *P < 0.05, 1% vs. 21% O2.
Autocrine factors mediate hypoxic NOX4 expression and resulting cellular proliferation.
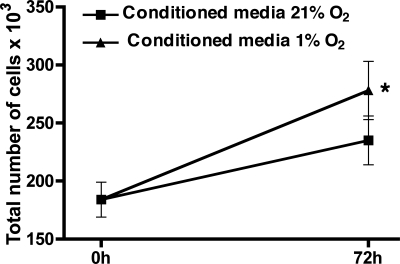
To determine whether hypoxia directly increases NOX4 gene expression in HPASMC or acts through indirect autocrine production of undefined mediators, culture media were removed from cells exposed to 1% O2 for 24 h and added to normoxic cells. Seventy-two hours later, proliferation was assessed. Hypoxia-conditioned media caused HPASMC proliferation, whereas media from normoxic cells did not (Fig. 5). This indicates that hypoxic HPASMC produce soluble mediators that cause proliferation.
Fig. 5.
Culture media from HPASMC exposed to 1% fraction of inspired oxygen (FiO2) for 24 h (“conditioned media”) cause proliferation of normoxic HPASMC. Conditioned media were applied to HPASMC, and cells were exposed to 21% O2 for 72 h. Counts were performed before and after hypoxia and are expressed as total number of cells ×103. Each condition represents the mean of 4 samples ± SD. *P < 0.001, conditioned media from 1% vs. 21% O2 at 72 h.
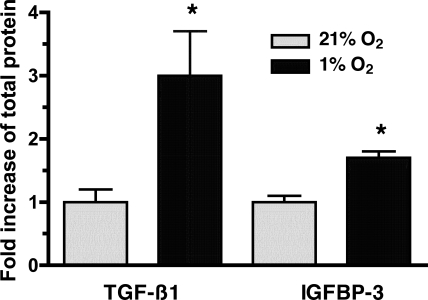
We (57) previously observed that TGF-β1 caused proliferation of HPASMC by a NOX4-mediated mechanism. Hypoxic cells produce both TGF-β1 (53) and IGFBP-3 (64). Affymetrix GeneChip analysis of mRNA obtained from HPASMC indicated that exposure to TGF-β1 induced IGFBP-3 expression 42-fold (unpublished observations). Given this information, the conditioned media were assessed for the presence of TGF-β1 and IGFBP-3 by ELISA. Exposure of HPASMC to 1% O2 for 24 h caused a 3-fold increase in TGF-β1 production (Fig. 6A) and a 1.7-fold increase in IGFBP-3 production (Fig. 6B) compared with HPASMC treated with media obtained from normoxic cells.
Fig. 6.
Hypoxic HPASMC produce transforming growth factor-β1 (TGF-β1) and insulin-like growth factor binding protein-3 (IGFBP-3). HPASMC were exposed to 1% or 21% O2 for 24 h. Cell culture media were assessed for TGF-β1 and IGFBP-3 by ELISA. Values were corrected for changes in volume and protein concentration and compared with media obtained from normoxic cells to determine fold increase. The data represent the means of 3 samples ± SD. *P < 0.05, fold increase in TGF-β1 or IGFBP-3 protein in 1% O2 vs. 21% O2.
TGF-β1 is a proximal mediator in the mechanism of hypoxic HPASMC proliferation.
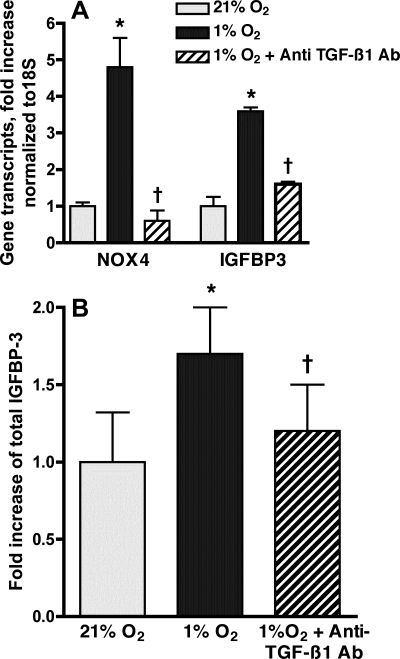
The findings related above suggest that hypoxia may increase NOX4-mediated HPASMC proliferation through autocrine production of TGF-β1 and/or IGFBP-3. The proliferative effects of TGF-β1 may be mediated by the autocrine release of IGFBP-3 (12, 30). Thus we explored whether TGF-β1 serves a proximal role in mediating hypoxia-induced HPASMC proliferation. HPASMC were incubated in 1% or 21% O2 with and without a TGF-β1 blocking antibody (50 ng/ml concentration in media) for 24 h. The anti-TGF-β1 antibody significantly reduced NOX4 and IGFBP-3 gene expression in hypoxic cells (Fig. 7A). Hypoxic release of IGFBP-3 protein into the media was also significantly reduced by anti-TGF-β1 antibody (Fig. 7B). Thus the hypoxia-induced TGF-β1 production subsequently mediates the production of IGFBP-3 by HPASMC.
Fig. 7.
Anti-TGF-β1 antibody (Ab) inhibits hypoxic expression of NOX4 and IGFBP-3. HPASMC were incubated in 1% or 21% FiO2 for 24 h with and without anti-TGF-β1 antibody (50 ng/ml). A: real-time PCR of reverse-transcribed mRNA was performed using IGFBP-3 and NOX4 primers. Values were normalized to 18S and expressed as fold induction over normoxic control. Results are representative of 3 independent experiments. B: IGFBP-3 protein in HPASMC culture media was quantified by ELISA. Results are expressed as fold increase compared with normoxic cells and represent the means of at least 3 samples ± SD. *P < 0.05 1% O2 vs. 21% O2; †P < 0.05, 1% O2 vs. 1% O2 + anti-TGF-β1 antibody.
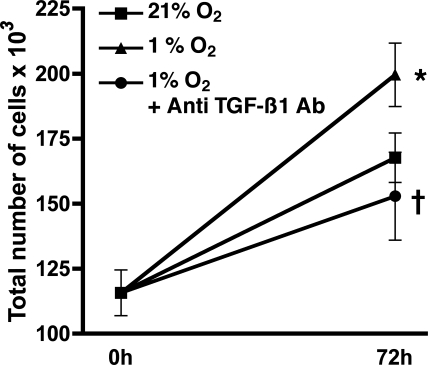
To determine whether TGF-β1 was integral to the mechanism whereby hypoxia results in HPASMC proliferation, cells were incubated in 1% O2 for 72 h in the presence or absence of an anti-TGF-β1 antibody (50 ng/ml). HPASMC proliferation was significantly reduced in presence of the antibody (Fig. 8), indicating that TGF-β1 is a proximal mediator in hypoxia-induced NOX4-mediated cellular proliferation.
Fig. 8.
HPASMC proliferation from hypoxia is inhibited by anti-TGF-β1 antibody. Cells were incubated in 1% or 21% FiO2 for 72 h. Counts were performed before and after hypoxia and are expressed as total number of cells ×103. Each condition represents the mean of 4 samples ± SD. *P < 0.001, 1% O2 vs. 21% O2 at 72 h; †P < 0.001, 1% O2 alone vs. 1% O2 + anti-TGF-β1 antibody at 72 h.
IGFBP-3 mediates hypoxia-induced NOX4 expression.
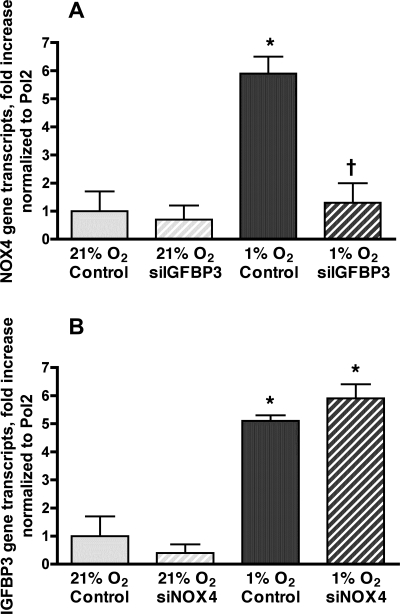
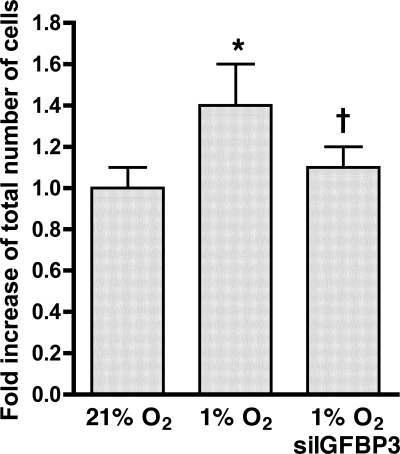
As demonstrated above, blockade of TGF-β1 attenuates hypoxic gene expression of both IGFBP-3 and NOX4. Experiments were performed to define the relationship between the autocrine production of IGFBP-3 and increased NOX4 expression. HPASMC were transfected with siIGFBP-3 or siNOX4 before exposure to 1% O2 for 24 h. Transfection with siRNA targeted to each gene reduced expression of that respective gene to less than 10% of baseline (data not shown). HPASMC transfected with siIGFBP-3 attenuated increased hypoxia-induced NOX4 expression (Fig. 9A). In contrast, transfection of HPASMC with siNOX4 did not alter IGFBP-3 expression due to hypoxia (Fig. 9B). These findings indicate that autocrine production of IGFBP-3 during hypoxia mediates increased NOX4 gene expression. To extend this observation, HPASMC were transfected with siIGFBP-3 and exposed to 1% O2 for 72 h. Baseline and 72 h cell counts were performed. As was observed with NOX4 gene silencing (Fig. 3B), reducing the expression of IGFBP-3 by siRNA inhibited hypoxic HPASMC proliferation (Fig. 10).
Fig. 9.
IGFBP-3 mediates increased NOX4 expression due to hypoxia. HPASMC were transfected with siRNA oligomer for IGFBP-3 (siIGFBP-3) (A) or siNOX4 (B) and incubated in 1% O2 for 24 h. Real-time quantitative RT-PCR (RT-QPCR) of reverse-transcribed mRNA was performed using NOX4 primers (A) or IGFBP-3 primers (B). Values were normalized to Pol2 and expressed as fold induction over normoxic control ± SD. *P < 0.05, 1% O2 vs. 21% O2; †P < 0.05, hypoxic cells transfected with siIGFBP-3 vs. hypoxic control cells.
Fig. 10.
Transfection with siIGFBP-3 prevents hypoxic HPASMC proliferation. Cells were incubated in 1% or 21% O2. Cell counts were performed at 72 h. *P < 0.001, 1% O2 vs. 21% O2; †P < 0.001, hypoxic cells transfected with siIGFBP-3 vs. hypoxic control cells.
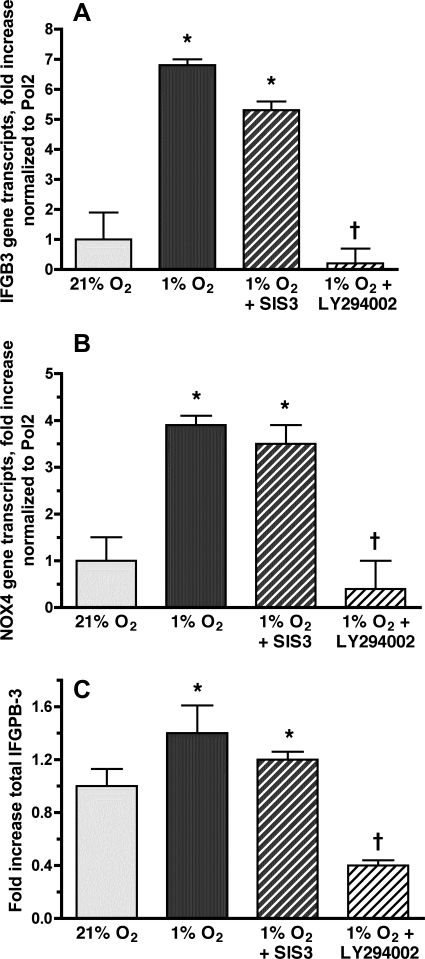
Hypoxic IGFBP-3 and NOX4 expression is mediated by the PI3K/Akt signaling pathway.
TGF-β1 causes NOX4-mediated HPASMC proliferation via the Smad-signaling pathway (57). Since NOX4-mediated hypoxic HPASMC proliferation is also dependent on TGF-β1 (Fig. 8), we hypothesized that Smad signaling would mediate NOX4-mediated hypoxic HPASMC proliferation. Specific inhibitor of Smad3 (SIS3) was added to HPASMC before exposure to 1% O2. After 24 h of hypoxia, SIS3 did not attenuate IGFBP-3 gene expression (Fig. 11A), NOX4 gene expression (Fig. 11B), or IGFBP-3 protein production (Fig. 11C). Likewise, transfection of HPASMC with siSmad3 did not affect the hypoxic expression of either NOX4 or IGFBP-3 (data not shown). TGF-β1 also signals through the PI3K/Akt pathway (24). To determine whether this pathway mediates NOX4 expression in hypoxic HPASMC, cells were treated with the PI3K competitive inhibitor LY-294002 before exposure to 1% O2. LY-294002 caused significant reduction in the hypoxia-induced gene expression of IGFBP-3 (Fig. 11A) and NOX4 (Fig. 11B) and decreased IGFBP-3 protein production (Fig. 11C).
Fig. 11.
Hypoxic induction of IGFBP-3 and NOX4 is mediated by the phosphatidylinositol 3-kinase (PI3K) rather than the Smad signaling pathway. HPASMC were incubated in 1% or 21% O2 for 24 h with or without the PI3K inhibitor LY-294002 (10 nM) or the Smad inhibitor SIS3 (2.2 μM). Total mRNA was isolated from cells and reverse-transcribed, and RT-QPCR was performed using IGFBP-3 (A) or NOX4 (B) primers. Values were normalized to Pol2 and expressed as fold induction over normoxic controls ± SD. *P < 0.05 vs. 21% O2; †P < 0.05, 1% O2 alone vs. 1% O2 + LY-294002. C: cells were exposed to 1% or 21% O2 for 24 h. IGFBP-3 protein in the culture media was quantified by ELISA. Results are expressed as fold increase compared with normoxic controls ± SD. The data represent the means of 3 samples ± SE. *P < 0.05 vs. 21% O2; †P < 0.05, 1% O2 alone vs. 1% O2 + LY-294002.
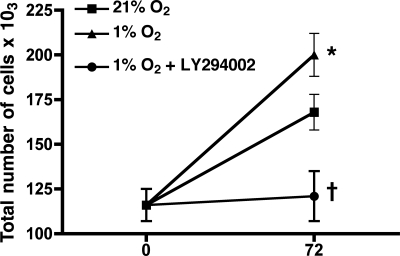
To verify the importance of PI3K in hypoxic HPASMC proliferation, cells were treated with LY-294002 or left untreated before exposure to 1% O2 for 72 h. PI3K inhibition markedly attenuated HPASMC proliferation due to hypoxia (Fig. 12). This is consistent with PI3K signaling mediating increases in IGFBP-3 and, subsequently, NOX4 expression due to autocrine production of TGF-β1 during hypoxia.
Fig. 12.
Hypoxic HPASMC proliferation is attenuated by PI3K inhibition. Cells were incubated in 1% or 21% O2 for 72 h with or without the PI3K inhibitor LY-294002 (10 nM). Cell counts were performed at baseline and at 72 h. Counts are expressed as the number of cells ×103 and represent the average of 4 samples ± SD. *P < 0.001, 1% O2 vs. 21% O2 at 72 h; †P < 0.001 at 72 h, 1% O2 + LY-294002 vs. untreated cells maintained at 1% O2.
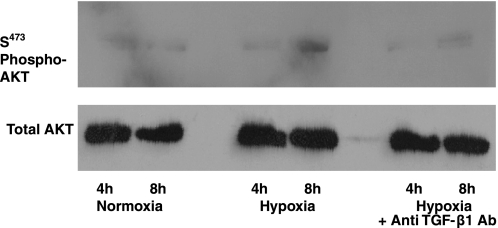
Assessment of the phosphorylation of the PI3K substrate Akt-Ser473 was then performed to confirm activation of PI3K/Akt signaling by hypoxic production of TGF-β1. Anti-TGF-β1 antibodies were added to HPASMC before exposure to 1% O2. Protein immunoblots were probed with antibodies raised against phospho-Akt-Ser473. Exposure to hypoxia for 8 h increased phosphorylation of Akt (Fig. 13). Phosphorylation was attenuated by treatment of cells with the anti-TGF-β antibody, providing additional evidence that TGF-β1 produced by HPASMC during hypoxia activates PI3K/Akt signaling pathways to promote HPASMC proliferation (Fig. 13).
Fig. 13.
Anti-TGF-β1 antibodies inhibit hypoxic Akt phosphorylation (phospho-Akt). HPASMC were exposed to 1% or 21% FiO2 for 4 or 8 h with and without anti-TGF-β antibody (50 ng/ml). Membranes were probed with antibodies raised against phosphorylated Ser473 (S473). To confirm equal protein loading, the blot was stripped and probed for total Akt with anti-Akt antibody.
DISCUSSION
Hypoxia is a clinically important cause of pulmonary vascular remodeling and resulting PAH (45, 56). We (57) previously observed that TGF-β1 induced HPASMC proliferation by a NOX4-mediated mechanism. As hypoxic HPASMC also release TGF-β1 (53), we hypothesized that NOX4 might be relevant to the mechanism of hypoxia-induced HPASMC proliferation. This report confirms the role of NOX4 in hypoxic HPASMC proliferation. Hypoxic induction and autocrine activity of TGF-β1 and IGFBP-3 mediate increases in NOX4 gene expression and subsequent hypoxic cellular proliferation. TGF-β1 stimulates IGFBP-3 production and subsequent NOX4-mediated PASMC proliferation through the PI3K/Akt pathway. A schematic of this scenario is presented in Fig. 14.
Fig. 14.
Working model illustrating the molecular basis for hypoxia-induced HPASMC proliferation. In this model, hypoxia induces TGF-β1 that induces gene expression and synthesis of IGFBP-3 through a mechanism involving PI3K/Akt. IGFBP-3 subsequently induces NOX4. ROS derived from NOX4 mediate HPASMC proliferation.
Originally thought to be relevant only to phagocyte intracellular killing of microbes and byproducts of mitochondrial respiration, ROS are now known to mediate a variety of intracellular processes. Identification of the sources of these ROS has facilitated our understanding of a number of biological processes. The NADPH oxidase responsible for the respiratory burst of phagocytes is a well-characterized source of ROS. It consists of a membrane-associated structure comprised of two proteins known as p22phox and gp91phox. Together, these two proteins form a cytochrome b with electron shuttling capabilities. On activation, several cytosolic proteins (p47phox, p67phox, p40phox, and Rac) move into physical proximity with the membrane-associated cytochrome that catalyzes reduction of molecular oxygen, resulting in a burst of ROS (2). With the description of the first homolog of the gp91phox component of the phagocytic NADPH oxidase (59), it became apparent that the phagocyte is not the sole source of NADPH oxidases. The NOX homologs exist in cells from a variety of tissues, have been localized to numerous subcellular structures and compartments, and are postulated to serve a variety of functions related to cellular signaling, differentiation, and proliferation. In contrast to the NADPH oxidase of phagocytes, the other NOX enzymes produce ROS at lower levels, and some, including NOX4, do not require cytosolic components for activation. NOX4 was originally described as an enzyme highly expressed in the kidney tubular system (21, 54). Given its proximity to the site of erythropoietin-production in the kidney, it was proposed that it might serve as a renal oxygen sensor. NOX4, NOX1, and NOX2 (formerly gp91phox) have all been reported in the systemic vascular smooth muscle (32, 60, 62), and NADPH oxidases of the systemic vascular smooth muscle have been associated with the remodeling of the vascular smooth muscle that occurs in hypertension (63).
In contrast to the systemic circulation, the pulmonary circulation is a low-pressure circuit with far less muscular thickening of the medial layer. The acute response to hypoxia is a reversible contraction of pulmonary artery smooth muscle, which is a protective physiological response that serves to redirect blood to better-ventilated areas of the lung. In contrast, the systemic arterial smooth muscle usually relaxes in response to hypoxia. For these reasons, mechanisms relevant to vascular smooth muscle pathology cannot be assumed to be identical between the two circulations. A thorough evaluation of the identity and function of NOX proteins in both circulatory beds is warranted. We have observed that NOX4 is the NOX that is predominantly expressed in SMC obtained from human pulmonary arteries (57). This is supported by the findings of others (15, 46). As has been verified for NOX4 in other cells, the HPASMC NOX4 is not dependent on cytosolic components for activation and demonstrates low-level constitutive activity (57). With exposure to the appropriate stimulus, NOX4 expression and activity are markedly increased (46, 52, 57, 58, 65), and protein activity appears to diminish rapidly with cessation of transcription (52). Given these attributes, it has been postulated that NOX4 has the potential to function as an inducible “iNOX” (52). Consistent with this theory, increased hypoxic induction of NOX4 gene expression has been observed in the media of murine pulmonary arteries (46), HPASMC (46), and murine neurons (65).
In the present investigation, we observed that hypoxia is a stimulus for increasing expression of NOX4 in HPASMC leading to increased ROS generation and resultant cell proliferation. The critical role of NOX4 in hypoxic HPASMC proliferation was confirmed by the application of the flavoenzyme inhibitor DPI (Fig. 3A) as well as NOX4 siRNA (Fig. 3B). This extends prior findings demonstrating increased expression of NOX4 in hypoxic HPASMC (46) by demonstrating that NOX4 mediates hypoxic HPASMC proliferation. We speculate that during prolonged hypoxia, NOX4 expression and resulting activity are increased. This results in HPASMC proliferation, thickening of the vascular media, and perhaps extension of smooth muscle into more distal regions of the pulmonary circulation, although additional studies are needed to specifically address the role of NOX4 in muscularization of the more distal circulation. Additional studies are also needed to determine the mechanism by which ROS-generated NOX oxidases positively influence proliferative signaling. Previous studies from our laboratory (57) demonstrate that TGF-β1-induced activation of the MAP kinases ERK1/2 in HPASMC is reduced by DPI, suggesting that TGF-β1 facilitates proliferation by upregulating ROS production with transient oxidative inactivation of phosphatases and augmentation of growth signaling cascades. Another potential mechanism, demonstrated in human airway SMC (58), is that NOX4-generated ROS mediate TGF-β-1-induced, redox-dependent phosphorylation of retinoblastoma protein (pRb) and cdc2 kinase, facilitating human airway smooth muscle proliferation.
Members of the TGF-β superfamily are known to modulate PASMC proliferation and have been linked to the development of PAH (19, 27). Bone morphogenetic protein (BMP) type II receptor mutations have been characterized in familial PAH (49), and abnormalities of TGF-β1 signaling have been identified in HPASMC derived from patients with both familial and sporadic idiopathic PAH (48). In addition, alterations in BMP type 1A receptor expression, attributed to increased expression of angiopoietin-1, have been observed in tissue derived from patients with both primary and acquired PAH (17). Mutations in the activin-receptor-like kinase-type 1 receptor increase the risk of PAH development in hereditary hemorrhagic telangiectasia (63a).
TGF-β1 is multifunctional cytokine with numerous activities that are tissue-specific and modulated by the cellular microenvironment (26). For example, TGF-β1 stimulates growth in mesenchymal cells while inhibiting growth in epithelial cells (26). The relevance of TGF-β1 in the mechanism of hypoxic pulmonary hypertension has been established in vivo. Transgenic mice with an inducible dominant-negative mutation in the TGF-β type II receptor demonstrated attenuated right ventricular pressures, right ventricular mass, and pulmonary arterial remodeling and muscularization following exposure to chronic hypoxia (9). We and others (53) have observed increased TGF-β1 production by hypoxic HPASMC. In the present investigation, the functional relevance of TGF-β1 produced by hypoxic HPASMC was demonstrated by the inhibition of NOX4 gene expression in cells pretreated with anti-TGF-β antibody before 24 h of hypoxia in (Fig. 7A). Likewise, anti-TGF-β antibody attenuated HPASMC proliferation following 72 h of hypoxia (Fig. 8). These findings indicate that the mechanism whereby hypoxia causes NOX4-mediated HPASMC proliferation is dependent on autocrine activity of TGF-β1. The mechanism by which TGF-β1 increases during hypoxia is unclear. Hypoxia has been shown to induce expression of the proprotein convertase furin, the proteolytic activator of TGF-β1, due to increases in hypoxia-inducible factor-1 (HIF-1)-mediated furin gene expression (44). This results in increased bioactive TGF-β1 during hypoxia. In addition, TGF-β1 contributes to HIF-1 stabilization by inhibiting expression of prolyl hydroxylase 2 (PHD2) (43). Under normoxic conditions, PHD2 hydroxylates proline residues on the HIF-1 component HIF-1α (3, 18). This targets HIF-1α for ubiquitination by the von Hippel-Lindau tumor suppressor E3 ligase complex and subsequent proteasome-dependent degradation (28, 29). Reductions in PHD2 levels allow persistence of HIF-1α, with resulting increases in HIF-1-mediated gene expression (43). This might result in increased furin expression (44). Thus hypoxia might contribute to progressive increases in TGF-β1 due to proteolytic activation by ever-increasing furin levels. Consistent with this theory, HIF-1α+/− mice exposed to 10% O2 for 3 wk demonstrated attenuation of right ventricular hypertrophy, pulmonary vascular remodeling, and right ventricular pressures compared with HIF-1α+/+ mice (71).
The myriad of activities attributed to TGF-β1 may reflect the different pathways that have been implicated downstream from receptor binding. The classic mechanism whereby TGF-β1 binding to surface receptors produces nuclear effects is the Smad signaling cascade (4, 14). We have observed that HPASMC proliferate in response to exogenous TGF-β1 via Smad2/3 signaling (57). However, PI3K signaling also mediates the effects of TGF-β1 receptor binding (1, 24, 67). PI3K signaling mediates PASMC proliferation in response to platelet-derived growth factor (23) and serotonin (39a). With particular relevance to our studies, PI3K signaling has been demonstrated to mediate hypoxic pulmonary arterial fibroblast proliferation (22). We observed that hypoxic HPASMC increased IGFBP-3 and NOX4 expression by a PI3K-dependent mechanism (Fig. 11). In addition, inhibition of PI3K prevented hypoxic HPASMC proliferation (Fig. 12). PI3K activity leads to phosphorylation of Akt, with resulting cell growth and proliferation (42). Increased phosphorylation of Akt was demonstrated in hypoxic HPASMC (Fig. 13). The application of anti-TGF-β1 antibody to hypoxic cells attenuated Akt phosphorylation. This provides evidence that the autocrine production of TGF-β1 by hypoxic HPASMC is responsible for downstream PI3K-mediated signaling.
The Smad and the PI3K/Akt signaling pathways may not necessarily be mutually exclusive following TGF-β1 stimulation. For example, proliferation of human airway SMC in response to TGF-β1 is mediated by both Smad3 and PI3K signaling (58). However, inhibition of Smad3 signaling with SIS3 did not reduce hypoxic expression of either IGFBP-3 or NOX4 in HPASMC (Fig. 11). Thus, in contrast to the mechanism of TGF-β1 signaling in normoxic HPASMC proliferation (57), we have observed that the PI3K/Akt signaling cascade is activated by TGF-β1 to promote hypoxic HPASMC proliferation.
Our experiments also demonstrate that TGF-β1 contributes to hypoxic HPASMC proliferation by stimulating autocrine secretion of IGFBP-3. IGFBP-3 is a member of a family of proteins that bind to IGFs and modulate their activities (20, 47). IGFBP-3 also has IGF-independent activity in various cell types (20, 47). TGF-β1 causes proliferation of human airway SMC (12) and colon cancer cells (30) by an IGFBP-3-mediated mechanism. Lung tissue from patients with idiopathic pulmonary fibrosis (IPF) has increased levels of IGFBP-3, and fibroblasts from IPF lungs produce IGFBP-3 when stimulated with TGF-β1 (50). IGFBP-3 binds to TGF-β receptor type V (34), but binding does not result in Smad2 or Smad3 phosphorylation (33). Hypoxia increases expression of IGFBP-3 in endothelial cells (35, 36), and IGFBP-3 contributes to vessel preservation, regrowth, and repair in a murine model of oxygen-induced retinopathy (41). In that same model, IGFBP-3 was chemotactic for CD34+ endothelial progenitor cells and enhanced their differentiation into more mature vascular cells, capable of engaging in effective angiogenesis (8). These studies suggest that IGFBP-3 may be integral to vascular homeostasis during hypoxia. We observed an increase in IGFBP-3 expression by hypoxic HPASMC (Fig. 6). Application of anti-TGF-β1 antibodies to cells inhibited both hypoxic IGFBP-3 mRNA and protein expression (Fig. 7), indicating that autocrine production of TGF-β1 mediates IGFBP-3 expression during hypoxia. Inhibition of IGFBP-3 expression by siRNA attenuated hypoxic NOX4 expression (Fig. 9) and cellular proliferation (Fig. 10), whereas siRNA inhibition of NOX4 did not effect IGFBP-3 expression (Fig. 9). This observation indicates that IGFBP-3 is a proximal mediator of hypoxic HPASMC proliferation due to NOX4. Our findings also demonstrate that IGFBP-3 is produced by hypoxic HPASMC in an autocrine manner due to the production of TGF-β1. As described above, PI3K rather than Smad signaling mediates IGFBP-3 expression due to hypoxic TGF-β1 production (Fig. 11).
The findings here reported and those reported by others (15, 46) are somewhat hard to fully reconcile with a recent report in which disruption of the murine NOX2 gene completely abolished chronic hypoxia-induced PAH and vascular remodeling (39). Perhaps attenuated ROS production due to disrupted endothelial, adventitial, and/or phagocytic NOX2 allows unchecked production of endothelial nitric oxide that overwhelms the actions of PASMC NOX4. Absence of ROS generated by NOX2 within the pulmonary arterial vessel wall might also alter NOX4 expression (16). Finally, expression of NOX components may vary among species and in vessels from different sites of the pulmonary circulation.
In summary, our study shows that hypoxia induces HPASMC proliferation by a NOX4-mediated mechanism. The hypoxic expression of NOX4 requires the sequential autocrine production of TGF-β1 and IGFBP-3 as well as PI3K/Akt signaling. Disruption of any of the components of this mechanism might prove useful in attenuation of pulmonary vascular remodeling due to hypoxia.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-67281 (J. Hoidal) and Department of Veterans Affairs salary support for T. Huecksteadt, K. Sanders, T. Kennedy, and J. Hoidal.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 275: 36803–36810, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22: 4082–4090, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertolino P, Deckers M, Lebrin F, ten Dijke P. Transforming growth factor-beta signal transduction in angiogenesis and vascular disorders. Chest 128, Suppl 6: 585S–590S, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Murphy TM, Chitano P, Hoidal JR. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J Biol Chem 274: 20017–20026, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Brennan LA, Steinhorn RH, Wedgweood S, Mata-Greenwood E, Roard EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Brown MR, Miller FJ Jr, Li WB, Ellingson AN, Mozena JD, Chatterjee P, Engelhardt JF, Zwacka RM, Oberley LW, Fang X, Spector AA, Weintraub NL. Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res 85: 524–533, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang KH, Chan-Ling T, McFarland EL, Afzal A, Pan H, Baxter LC, Shaw LC, Caballero S, Sengupta N, Li Calzi S, Sullivan SM, Grant MB. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci USA 104: 10595–10600, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YF, Feng JA, Li P, Xing D, Zhang Y, Serra R, Ambalavanan N, Majid-Hassan E, Opril S. Dominant negative mutation of the TGF-β receptor blocks hypoxia-induced pulmonary vascular remodeling. J Appl Physiol 100: 564–571, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Clanton TL Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol 102: 2379–2388, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Cohen P, Rajah R, Rosenbloom J, Herrick DJ. IGFBP-3 mediates TGF-β1-induced cell growth in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 278: L545–L551, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Demiryurek AT, Wadsworth RM. Superoxide in the pulmonary circulation. Pharmacol Ther 84: 355–365, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Djordjevic T, BelAiba RS, Bonello S, Pfeilschifter J, Hess J, Görlach A. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler Thromb Vasc Biol 25: 519–525, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Djordjevic T, Pogrebniak A, BelAiba RS, Bonello S, Wotzlaw C, Acker H, Hess J, Görlach A. The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free Radic Biol Med 38: 616–630, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, Yuan JX, Deutsch R, Jamieson SW, Thistlethwaite PA. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med 348: 500–509, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 23: 824–854, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97: 8010–8014, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerasimovskaya EV, Tucker DA, Stenmark KR. Activation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J Appl Physiol 98: 722–731, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L354–L363, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, Zhang H, Cui Z, Thannickal VJ. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem 279: 1359–1367, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshikawa Y, Ono S, Suzuki S, Tanikta T, Chida M, Song Noda M C, Tabata T, Voelkel NF, Fujimura S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol 90: 1299–1306, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Huang SS, Huang JS. TGF-beta control of cell proliferation. J Cell Biochem 96: 447–462, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43, Suppl 12: 13S–24S, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Laelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim AV, Heberstreit HF, Mukherji M, Scholfield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Kansra S, Ewton DZ, Wang J, Friedman E. IGFBP-3 mediates TGF beta 1 proliferative response in colon cancer cells. Int J Cancer 87: 373–378, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Lambeth JD NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91phox homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88: 888–894, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Leal SM, Huang SS, Huang JS. Interactions of high affinity insulin-like growth factor-binding proteins with the type V transforming growth factor-beta receptor in mink lung epithelial cells. J Biol Chem 274: 6711–6717, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Leal SM, Liu Q, Huang SS, Huang JS. The type V transforming growth factor beta receptor is the putative insulin-like growth factor-binding protein 3 receptor. J Biol Chem 272: 20572–20576, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Lee WH, Wang GM, Yang XL, Seaman LB, Vannucci SI. Perinatal hypoxia-ischemia decreased neuronal but increased cerebral vascular endothelial IGFBP3 expression. Endocrine 11: 181–188, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Le Jan S, Le Meur N, Cazes A, Philippe J, Le Cunff M, Leger J, Corvol P, Germain S. Characterization of the expression of the hypoxia-induced genes neuritin, TXNIP and IGFBP3 in cancer. FEBS Lett 580: 3395–3400, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 285: L322–L333, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006. [DOI] [PubMed] [Google Scholar]
- 39a.Liu Y, Fanburg BL. Serotonin-induced growth of pulmonary artery smooth muscle requires activation of phosphatidylinositol 3-kinase/serine-threonine protein kinase B/mammalian target of rapamycin/p70 ribosomal S6 kinase 1. Am J Respir Cell Mol Biol 34: 182–191, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Lofqvist C, Chen J, Connor KM, Smith AC, Aderman CM, Liu N, Pintar JE, Ludwig T, Hellstrom A, Smith LE. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci USA 104: 10589–10594, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem 281: 24171–24181, 2006. [DOI] [PubMed] [Google Scholar]
- 44.McMahon S, Grondin F, McDonald PP, Richard DE, Dubois CM. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J Biol Chem 280: 6561–6599, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Meyrick B The pathology of pulmonary artery hypertension. Clin Chest Med 22: 393–404, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Mittal M, Roth M, König P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hänze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol 175: 19–31, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 104: 790–795, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Newman JH, Trembath RC, Morse JA, Grunig E, Loyd JE, Adnot S, Coccolo F, Ventura C, Phillips 3rd JA, Knowles JA, Janssen B, Eickelberg O, Eddahibi S, Herve P, Nichols WC, Elliott G. Genetic basis of pulmonary arterial hypertension: current understanding and future directions. J Am Coll Cardiol 43, Suppl 12: 33S–39S, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Pilewski JM, Liu L, Henry AC, Knauer AV, Feghili-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol 166: 399–407, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhoades RA, Packer CS, Roepke DA, Jin N, Meiss RA. Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can J Physiol Pharmacol 68: 1581–1589, 1990. [DOI] [PubMed] [Google Scholar]
- 52.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheares KK, Jeffery TK, Long L, Yang X, Morrell NW. Differential effects of TGF-β1 and BMP-4 on the hypoxic induction of cyclooxygenase-2 in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 287: L919–L927, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 276: 1417–1423, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-β1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR, Kennedy TP. Nox4 mediates TGF-β1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L1543–L1555, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79–82, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol 22: 21–27, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol 30: 860–866, 2003. [DOI] [PubMed] [Google Scholar]
- 63a.Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I, Simonneau G, Galie N, Loyd JE, Humbert M, Nichols WC, Morrell NW, Berg J, Manes A, McGaughran J, Pauciulo M, Wheeler L. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 345: 325–334, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Tucci M, Nygard K, Tanswell BV, Farber HW, Hill DJ, Han VK. Modulation of insulin-like growth factor (IGF) and IGF binding protein biosynthesis by hypoxia in cultured vascular endothelial cells. J Endocrinol 157: 13–24, 1998. [DOI] [PubMed] [Google Scholar]
- 65.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience 132: 233–238, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Vanderkooi JM, Erecinska M, Silver IA. Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol Cell Physiol 260: C1131–C1150, 1991. [DOI] [PubMed] [Google Scholar]
- 67.Vinals F, Pouyssegur J. Transforming growth factor beta1 (TGF-beta1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-alpha signaling. Mol Cell Biol 21: 7218–7230, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wedgwood S, Dettman RW, Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 281: L1058–L1067, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Wolin MS, Ahmad M, Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol 289: L159–L173, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 103: 691–696, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]