Abstract
NF-κB activation is exaggerated in neonatal organisms after oxidant and inflammatory insults, but the reason for this and the downstream effects are unclear. We hypothesized that specific phosphorylation patterns of IκBα could account for differences in NF-κB activation in hyperoxia-exposed fetal and adult lung fibroblasts. After exposure to hyperoxia (>95% O2), nuclear NF-κB binding increased in fetal, but not adult, lung fibroblasts. Unique to fetal cells, phosphorylation of IκBα on tyrosine 42, rather than serine 32/36 as seen in TNF-α-exposed cells, preceded NF-κB nuclear translocation. In fetal cells stably transfected with an NF-κB-driven luciferase reporter, hyperoxia significantly suppressed reporter activity, in contrast to increased reporter activity after TNF-α incubation. Targeted gene profiling analysis showed that hyperoxia resulted in decreased expression of multiple genes, including proapoptotic factors. Transfection with a dominant-negative IκBα (Y42F), which cannot be phosphorylated on tyrosine 42, resulted in upregulation of multiple proapoptotic genes. In support of this finding, caspase-3 activity and DNA laddering were specifically increased in fetal lung fibroblasts expressing Y42F after exposure to hyperoxia. These data demonstrate a unique pathway of NF-κB activation in fetal lung fibroblasts after exposure to hyperoxia, whereby these cells are protected against apoptosis. Activation of this pathway in fetal cells may prevent the normal pattern of fibroblast apoptosis necessary for normal lung development, resulting in aberrant lung morphology in vivo.
Keywords: IκBα, apoptosis
despite advances in neonatal care and improved survival of very low-birth-weight infants, the incidence of bronchopulmonary dysplasia (BPD) has remained at 25–35% (65). O2 therapy has long been implicated in the pathogenesis of BPD (63). The toxic effects of O2 depend not only on the nature of the exposure, but also on the maturational stage of the organism at the time of exposure. Hyperoxia causes severe lung injury and mortality in adult rodents, but this mortality is less pronounced in similarly exposed neonatal rodents (31). Nonetheless, neonatal animals exposed to hyperoxia develop lesions resembling BPD (5, 26, 33, 43, 66). In vitro studies have demonstrated that hyperoxia results in necrosis, apoptosis, and abnormal differentiation of lung cells (8, 51, 54), which may explain in part the abnormal lung development seen in these studies.
NF-κB has been implicated in the pathogenesis of multiple pulmonary diseases (22). This multisubunit transcription factor activates genes that regulate apoptosis and respond to inflammation and oxidative stress (4, 34, 37). Multiple models have shown increased NF-κB activation in neonates compared with adults after exposure to inflammatory and oxidant stimuli (39, 64, 68). In preterm infants, increased NF-κB activation has been linked to respiratory distress syndrome and an increased risk of developing BPD (10, 18, 20). However, whether this increased activation is a protective response to multiple inflammatory and oxidant stimuli or whether it represents a dysregulated reaction to stress remains unexplored (3, 29, 30, 42). In addition, the downstream effects of activation of this transcription factor on the developing lung are unknown.
In quiescent cells, NF-κB remains sequestered in the cytoplasm bound to its inhibitory protein IκBα (34). With inflammation or oxidant stress, IκBα is phosphorylated, causing dissociation and unmasking of the nuclear localization sequence of NF-κB subunits (37). With an inflammatory stimulus, such as TNF-α, IκBα is phosphorylated on serine residues at positions 32 and 36 and degraded through the proteosomal pathway (37). In addition to this canonical pathway, an atypical pathway of NF-κB activation, where phosphorylation of IκBα occurs on tyrosine 42, has been described (36). This specific phosphorylation occurs after stimulation with pervanadate, nerve growth factor, H2O2, and ischemia-reperfusion (17, 36, 40). The following questions remain to be answered. 1) Does hyperoxia-induced NF-κB activation also rely on this alternative pathway? 2) Are there maturational differences in this pathway? 3) What are the downstream effects of this particular activation pathway?
We used an in vitro fibroblast culture model to provide insights into the effects of hyperoxia on the immature lung. Fibroblasts perform multiple essential functions during lung development. These cells provide vitamin A precursors, elastin, and keratinocyte growth factor (or fibroblast growth factor 7) (9, 21, 27, 44, 49, 52, 53). Additionally, fibroblasts undergo apoptosis during normal lung development (13, 41, 55, 56). Any interruption of these processes could have striking implications for the developing lung, including fibrosis or simplified alveolar structure. In this report, we show that, in lung fibroblasts, hyperoxia-induced NF-κB activation only occurs in fetal cells and via the specific phosphorylation of tyrosine 42 of IκBα. This results in NF-κB nuclear translocation and modulation of apoptotic pathways during oxidative stress.
METHODS
Cell culture and hyperoxic and TNF-α exposure.
RLF-6 fibroblasts (American Type Culture Collection, Manassas, VA), derived from lung tissue of normal, 18-day-gestation Sprague-Dawley rat fetuses, were grown in F12K medium supplemented with 15% fetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin (Mediatech, Herndon, VA) and maintained at 37°C in 5% CO2-95% room air. RLF primary cells (Cell Applications, San Diego, CA), derived from normal adult rat lung tissue, were grown in RLF medium (Cell Applications) and maintained at 37°C in 5% CO2-95% room air. In all experiments, cells were seeded at 30–50% confluence on plastic culture dishes and allowed to adhere overnight before exposure. Hyperoxic exposure was conducted in a humidified chamber (Billups-Rothberg, Del Mar, CA) that was flushed with 95% O2-5% CO2 (hyperoxia) at a flow rate of 20 l/min for 5 min before incubation at 37°C. For experiments in which TNF-α (catalog no. T7539, Sigma, St. Louis, MO) was used, it was diluted in supplemented F12K medium to a concentration of 20 ng/ml and added to cells before collection. For experiments in which H2O2 was used, it was diluted in supplemented F12K medium to a concentration of 1 mM and added to cells before collection.
Preparation of cytosolic and nuclear extractions.
Cells were washed with ice-cold Tris-buffered saline (TBS, pH 7.4) and scraped with 500 μl of TBS + 1 μM protease inhibitor cocktail (catalog no. P8340, Sigma) and 1 μM phosphatase inhibitor cocktail (catalog no. 524625, Calbiochem, Gibbstown, NJ). Nuclear and cytosolic fractions were extracted using a nuclear protein extraction kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions. Protein content was determined by the Bradford method (Bio-Rad Laboratories, Hercules, CA).
Evaluation of nuclear NF-κB binding by EMSA.
A 32P-labeled oligonucleotide with the consensus sequence for NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′; Promega, Madison, WI) was used as a probe to evaluate NF-κB binding ability, as described previously (69). For identification of nonspecific binding of nuclear proteins, competition reactions were performed by addition of 50-fold excess of the non-radio-labeled NF-κB consensus sequence or 50-fold excess of non-radio-labeled mutated NF-κB consensus sequence (5′-AGTTGAGGCGACTTTCCCAGGC-3′; Santa Cruz Biotechnology, Santa Cruz, CA) to the reaction mixtures before electrophoresis. In separate experiments, for identification of the NF-κB subunit proteins in the binding complex, 2.5 μl of p50 or p65 antibody (Calbiochem) were incubated with nuclear proteins for 1 h at 37°C before addition of the radio-labeled probe.
Localization of p65 nuclear translocation in fetal lung fibroblasts.
Immunocytochemical staining of p65, as an index of NF-κB activation, was performed on RLF-6 cells grown on coverslips. Cells were washed with PBS and fixed for 10 min at −20°C with methanol. Cells were permeabilized with saponin, blocked, and washed before incubation with anti-NF-κB p65 antibody (1:50 dilution; Cell Signaling) for 2 h. Cells were washed with PBS and incubated with the secondary antibody Alexa Fluor 594-goat anti-rabbit IgG (1:500 dilution; Molecular Probes) for 1 h. One drop of Vectashield with 4′6-diamidino-2-phenylindole (Vector Laboratories) was added to each coverslip. The coverslips were viewed using an Olympus fluorescent microscope.
Evaluation of IκBα, Bax, p65, caspase-3, and calnexin proteins.
Cytosolic extracts (20 μg) were electrophoresed on a 4–12% polyacrylamide gel (Invitrogen). Proteins were transferred to an Immobilon membrane (Millipore). Membranes were blotted with anti-IκBα (catalog no. sc-847, Santa Cruz Biotechnology), anti-Bax antibody (catalog no. sc-493, Santa Cruz Biotechnology), anti-caspase-3 (Calbiochem), or anti-calnexin (Stressgen). Nuclear extracts (20 μg) were electrophoresed on a 4–12% polyacrylamide gel (Invitrogen). Proteins were transferred to an Immobilon membrane. Membranes were blotted with p65 antibody (Cell Signaling) and anti-acetyl-histone H3 (Upstate Biotechnology).
Phosphatase treatment.
Fetal and adult lung fibroblasts were exposed for the indicated times and solubilized in RIPA buffer. Protein content was determined by the Bradford method, and 20 μg of cell lysate were treated with increasing doses of calf intestinal phosphatase (CIP, 1 U/μl; Roche) or tyrosine-specific phosphatase [protein tyrosine phosphatase (PTP1B), 0.5 mg/ml; Biomol] for 30 min at 37°C. Samples were subjected to SDS-PAGE and immunoblotting with anti-IκBα antibody (see above).
Transfection with vector and wild-type and dominant-negative IκB-α.
To determine the role of phosphorylation of IκBα in hyperoxia-induced NF-κB activation, we transfected fetal cells with an empty vector (V, pcDNA3.1), the vector containing wild-type (WT) IκBα, or dominant-negative mutant IκBα (Y42F), in which phenylalanine is substituted for tyrosine 42. Cells (5 × 104) were plated on 60-mm plates and allowed to achieve 90% confluence. The plasmids (8 μg) were diluted in 500 μl of Opti-MEM (without serum and antibiotics) and then mixed with 500 μl of Opti-MEM containing Lipofectamine 2000 (12 μl; Invitrogen). This mixture was added to 1 ml of DMEM with 10% FBS without antibiotics and placed on fetal cells, which were incubated for 6 h. After incubation, medium was changed to F12K medium supplemented with 15% fetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin. After 24 h of transfection, cells were exposed to hyperoxia (see above). Cells were collected for cytosolic and nuclear extraction as described above. For evaluation of transfection efficiency, the above-described protocol was followed, with cotransfection of 1 μg of phosphorylated enhanced green fluorescent protein with empty vector (8 μg, V, pcDNA3.1), the vector containing WT IκBα (8 μg), or Y42F (8 μg). Cells were subjected to fluorescence-activated cell sorting for green fluorescent protein. For verification of protein expression of the transfected construct Y42F, cytosolic extracts were prepared from transfected cells. Western analysis was performed as described above, and membranes were blotted with anti-IκBα against the COOH terminus (catalog no. sc-371, Santa Cruz Biotechnology) to obviate any loss of antibody affinity due to the mutated residues.
Stable transfection of fetal lung fibroblasts with NF-κB-driven luciferase reporter.
Fetal cells were grown to 90% confluence on a six-well plate and transfected with a luciferase reporter gene driven by four copies of the NF-κB consensus sequence fused to a TATA-like promoter (4 μg of pNFκB-luc; Clontech, Mountain View, CA) and a plasmid containing a puromycin resistance gene (0.5 μg; Invitrogen). The plasmids were diluted in 500 μl of Opti-MEM (without serum and antibiotics) and then mixed with 500 μl of Opti-MEM containing Lipofectamine 2000 (12 μl). This mixture was added to 1 ml of DMEM with 10% FBS without antibiotics and placed on fetal cells, which were incubated for 24 h. After incubation, cells were trypsinized, divided between two 10-cm plates, and mixed with F12K medium supplemented with 15% fetal bovine serum, 100 U/ml penicillin, 100 U/ml streptomycin, and 2.5 μg/ml of puromycin. After 1 wk, single colonies were picked up and passaged 3 times with the puromycin-containing medium. Activation of NF-κB in stably transfected cells was visualized using an in vivo imaging system (Xenogen), as previously described (23). Cells were incubated with 150 μg/ml luciferin for 15 min at room temperature and imaged using the in vivo imaging system. Cells were confirmed to carry the construct by photon emission after stimulation with TNF-α (20 ng/ml).
Evaluation of phosphorylated IκBα via immunoprecipitation.
Fetal and adult lung fibroblasts were exposed for the indicated times, solubilized in RIPA buffer to which 1 μM protease inhibitor cocktail (catalog no. P8340, Sigma, St. Louis, MO) and 1 μM phosphatase inhibitor cocktail (catalog no. 524625, Calbiochem, Gibbstown, NJ) were added, and centrifuged at 14,000 rpm for 15 min, and supernatants were collected as whole cell lysate. Protein concentration of the cell lysate was determined by the Bradford method. Cell lysate was diluted to 1 μg/μl with RIPA buffer. Samples were incubated with agarose beads conjugated to anti-phosphotyrosine antibody, rabbit polyclonal anti-IκBα antibody, or rabbit IgG (catalog nos. sc-7020 AC, sc-847 AC, and sc-2345, respectively, Santa Cruz Biotechnology) for 12 h. Beads were collected via pulse centrifugation (5 s at 14,000 rpm). Supernatant was discarded, and beads were washed twice with ice-cold TBS. Beads were resuspended in 2× SDS loading buffer (50 μl). Immunocomplexes were dissociated from beads by boiling for 5 min. Beads were sedimented by brief centrifugation, and 20 μl of the supernatant were subjected to SDS-PAGE and immunoblotting with anti-phosphotyrosine (catalog no. sc-7040, Santa Cruz Biotechnology), anti-IκBα (catalog no. sc-847, Santa Cruz Biotechnology), or anti-phospho-IκBα tyrosine 42 antibody (ECM Biosciences, Versailles, KY) as described above. To evaluate protein capture by the anti-IκBα (catalog no. sc-847, Santa Cruz Biotechnology) and obviate the cross-reactivity that is observed with use of antibodies raised in the same species, we subjected the supernatant to SDS-PAGE and immunoblotting with mouse monoclonal anti-IκBα (catalog no. sc-1643, Santa Cruz Biotechnology).
Determination of gene expression profile by targeted microarray.
The expression of genes involved in apoptosis was analyzed with Oligo GEArray (SuperArray, Bethesda, MD) according to the manufacturer's protocol. Cells were exposed to room air or hyperoxia (8 h), or they were transfected with Y42F and exposed to room air or hyperoxia (8 h). RNA was isolated using the ArrayGrade total RNA isolation kit (SuperArray). Isolated RNA (3 μg) was labeled and amplified using the TrueLabeling-AMP 2.0 kit (SuperArray). The resulting probes were hybridized to gene-specific cDNA fragments spotted in quadruplicates on the GEArray membranes. The signal of the hybridized spots was measured and analyzed using the GEArray Expression Analysis Suite according to the manufacturer's instruction.
Evaluation of caspase-3 activity and DNA laddering.
Cells were plated on 60-mm plates, and WT IκBα or Y42F was delivered into the cells with Lipofectamine 2000 (see above). Cells were exposed to 24 h of hyperoxia and collected with RIPA buffer without protease inhibitors. Fluorometric caspase-3 substrate (9 μg) was incubated with cell lysate (50 μg) for 90 min at 37°C, and caspase-3 activity was evaluated according to the manufacturer's instructions (Upstate Biotechnology). For evaluation of DNA laddering, genomic DNA was isolated using the BDtract genomic DNA isolation kit (Maxim Biotech, San Francisco, CA). Fragmented DNA was ligated to adaptor DNA fragments and subjected to PCR for amplification of cleaved genomic DNA according to the manufacturer's instructions (PCR kit for DNA ladder assay, Maxim Biotech). Samples were run for 15 min at 100 V on 2% agarose-ethidium bromide gel and visualized using a UV imager.
Statistical analysis.
For comparison between treatment groups, the null hypothesis that no difference existed between treatment means was tested by ANOVA for multiple groups or t-test for two groups (InStat, GraphPad Software). Statistical significance (P < 0.05) between and within groups was determined by Bonferroni's method of multiple comparisons.
RESULTS
Fetal cells activate NF-κB in response to hyperoxia.
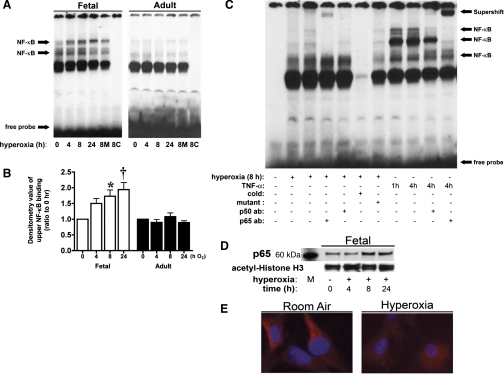
After 8 h of hyperoxia, nuclear extracts from fetal cells demonstrated significantly increased binding to the NF-κB consensus sequence (Fig. 1A). This binding was seen specifically in the upper bands, increased at 8 h, and remained significantly increased through 24 h of hyperoxic exposure (Fig. 1B). In contrast, nuclear extracts from adult cells showed no increased binding in response to hyperoxia (Fig. 1A). These findings indicate a maturational difference in hyperoxia-induced NF-κB activation. To evaluate the involvement of the most abundant NF-κB subunit proteins, p50 and p65, in hyperoxia-induced binding, we performed supershift experiments. Before the binding reaction, anti-p50 or anti-p65 antibody was added to nuclear extracts from fetal cells exposed to 8 h of hyperoxia. Only addition of anti-p65 antibody resulted in a supershift retardation of the binding complex (Fig. 1C). Addition of anti-p50 antibody decreased the density of the band seen with hyperoxic exposure; however, there was no distinct supershift retardation band in fetal cells exposed to hyperoxia (Fig. 1C). This pattern suggests that the p65 subunit participates in regulating gene expression after hyperoxia-induced NF-κB activation. In nuclear extracts from fetal cells exposed to TNF-α (Fig. 1C), EMSA indicated that a much less pronounced increase in nuclear NF-κB consensus sequence binding was induced by hyperoxia than by TNF-α exposure. Also, supershift experiments performed on fetal cells exposed to TNF-α more clearly demonstrated the presence of p65 and p50 in the bands. In support of the EMSA results, Western blot analysis for p65 performed on nuclear extracts isolated from fetal cells demonstrated an increase in nuclear p65 after 8 h of hyperoxia (Fig. 1D). Immunocytochemistry supported nuclear translocation, with decreased cytoplasmic expression of p65 and increased nuclear colocalization after hyperoxia (Fig. 1E).
Fig. 1.
Hyperoxia increases NF-κB consensus sequence binding of nuclear proteins in fetal, but not adult, rat lung fibroblasts. Fetal and adult lung fibroblasts were exposed to room air or hyperoxia (95% O2-5% CO2) for 4, 8, or 24 h. Nuclear extracts were prepared and subjected to EMSA. For each sample, 5 μg of nuclear protein were evaluated to determine NF-κB consensus sequence binding activity. A: representative EMSA. 8M, 50-fold excess of unlabeled mutated NF-κB consensus sequence oligonucleotide added to 8-h sample; 8C, 50-fold excess of unlabeled NF-κB consensus sequence oligonucleotide added to 8-h sample. B: densitometric evaluation of the upper band showing increased binding after hyperoxia. Values are means ± SE of 3 independent experiments for each group. Significantly different from time 0: *P < 0.05; †P < 0.01. C: representative EMSA showing supershift experiments of nuclear extracts from fetal cells exposed to hyperoxia (8 h at 95% O2-5% CO2) or TNF-α (20 ng/ml for 1 or 4 h). Cold, 50-fold excess of unlabeled oligonucleotide added to 8-h sample; mutant, 50-fold excess of mutated oligonucleotide added to 8-h sample; p50 ab, supershift with anti-p50 antibody; p65 ab, supershift with anti-p65 antibody. D: representative Western blot showing p65 in nuclear extracts from fetal lung fibroblasts exposed to hyperoxia, with acetyl-histone H3 as loading control. M, marker. E: immunocytochemical staining of p65 protein in fetal cells exposed to 24 h of hyperoxia. Note cytosolic staining of p65 (red) in cells before exposure to hyperoxia, with decreased p65 cytosolic staining and nuclear colocalization with 4′,6-diamidino-2-phenylindole staining (blue) after hyperoxia.
Hyperoxia induces a slow-migrating IκBα unique to fetal cells.
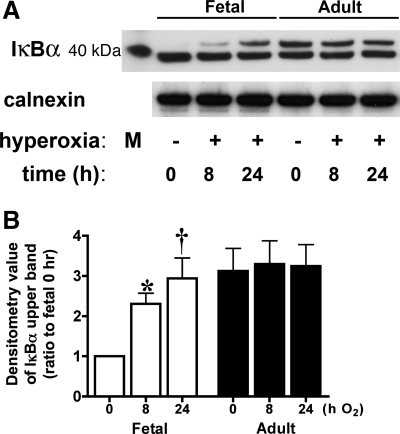
To further define the signaling events upstream of NF-κB nuclear translocation and consensus sequence binding, we evaluated levels of immunoreactive IκBα. Western blot of cytosolic extracts from fetal cells exposed to hyperoxia showed a second ∼40-kDa immunoreactive IκBα band (Fig. 2A) above the major band appearing in control cells. This upper band increased significantly from baseline with hyperoxia and persisted through 24 h of exposure (Fig. 2B). This same band appeared in fetal cells after 4 h of treatment with 1 mM H2O2 (data not shown). In adult cells, two bands were observed in control cells, and neither changed with hyperoxia (Fig. 2A). These data indicate the formation of a variant of IκBα unique to fetal cells exposed to hyperoxia.
Fig. 2.
Hyperoxia results in a slow-migrating IκBα in fetal lung fibroblasts. Fetal and adult lung fibroblasts were exposed to room air or hyperoxia for 8 or 24 h. A: representative Western blot showing IκBα in fetal and adult lung fibroblasts, with 40-kDa marker and calnexin as loading control. M, marker. B: densitometric evaluation of fold increase in the upper band of IκBα after exposure to hyperoxia. Values are means ± SE of 3 independent experiments for each group. Significantly different from time 0: *P < 0.05; †P < 0.001.
Hyperoxia results in specific phosphorylation of IκBα tyrosine residues.
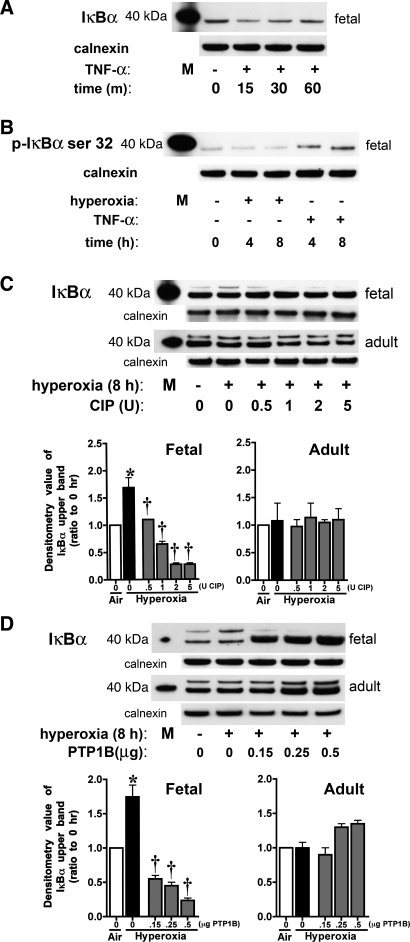
To ensure the specificity of IκBα antibody for the fetal rat lung fibroblast IκBα, we evaluated the IκBα pattern in response to TNF-α. In concert with previously published reports, we observed a decrease in total IκBα by 15 min of TNF-α exposure, followed by reaccumulation and return to baseline by 60 min (Fig. 3A). TNF-α induced an increase in phosphorylation of IκBα at serine 32, which did not change from baseline in cells exposed to hyperoxia (Fig. 3B). To evaluate whether the slow-migrating band seen in hyperoxia represented phosphorylated IκBα, cytosolic extracts from fetal cells exposed to 8 h of hyperoxia were incubated with CIP (Fig. 3C). The upper band was reduced in a dose-dependent manner after incubation with CIP, suggesting that this band was a phosphorylated form of IκBα (Fig. 3C). In contrast, the appearance of the slow-migrating band was not changed by incubation of cytosolic extracts from adult cells exposed to hyperoxia with CIP, suggesting that this band did not represent a phosphorylated form of IκBα (Fig. 3C). Additionally, incubation of the cytosolic extracts from fetal cells exposed to hyperoxia with PTP1B, a phosphotyrosine-specific phosphatase, also resulted in disappearance of the slow-migrating band, suggesting that IκBα is phosphorylated on tyrosine residues after hyperoxia (Fig. 3D). The similar exposure of adult cytosolic extract to PTP1B did not change the appearance of the upper band (Fig. 3D). These results confirm that the upper band appearing in fetal samples after hyperoxia represents IκBα phosphorylated on tyrosine residues, whereas the upper band seen in adult cytosolic extracts is not a phosphorylated form of IκBα. Although the identity of the upper band seen in adult fibroblasts was not elucidated, these experiments prove that it does not represent a phosphorylated form, as seen in fetal cells exposed to hyperoxia.
Fig. 3.
IκBα phosphorylation occurs in fetal cells exposed to hyperoxia. Fetal and adult lung fibroblasts were exposed to TNF-α or hyperoxia. A: representative Western blot showing levels of cytosolic total IκBα in control and TNF-α-treated (20 ng/ml for 15, 30, or 60 min) fetal cells, with 40-kDa marker and calnexin as loading control. M, marker. B: representative Western blot showing cytosolic levels of IκBα phosphorylated on serine 32 (p-IκBα) in control, hyperoxia-exposed (4 or 8 h), and TNF-α-treated (20 ng/ml for 4 or 8 h) fetal cells, with 40-kDa marker and calnexin as loading control. C: representative Western blots showing IκBα pattern after incubation of cytosolic fractions from hyperoxia-exposed fetal and adult cells with 0.5–5 U of calf intestinal phosphatase (CIP), with 40-kDa marker and calnexin as loading control (top), and densitometric evaluation of fold change in the upper band of IκBα after exposure to CIP (bottom). Values are means ± SE of 3 independent experiments for each group. *P < 0.001 vs. time 0. †P < 0.001 vs. 8 h without CIP. D: representative Western blots showing IκBα pattern after incubation of cytosolic fractions from hyperoxia-exposed fetal and adult cells with 0.15–0.5 μg of a tyrosine-specific phosphatase (PTP1B), with 40-kDa marker and calnexin as loading control (top), and densitometric evaluation of fold change in the upper band of IκBα after exposure to PTP1B (bottom). Values are means ± SE of 3 independent experiments for each group. *P < 0.001 vs. time 0. †P < 0.001 vs. 8 h without PTP1B.
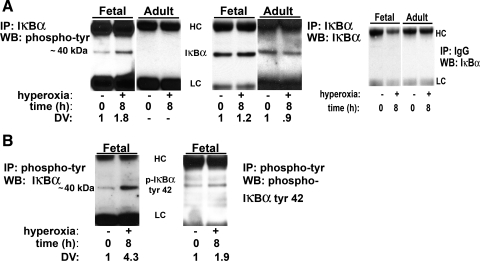
The slow-migrating band results were further evaluated by immunoprecipitation with anti-IκBα antibody and immunoblotting with anti-phosphotyrosine antibody (Fig. 4A ). IκBα phosphorylated on tyrosine residues was found in fetal cells exposed to hyperoxia, whereas no phosphorylation of IκBα on tyrosine residues could be detected on adult cells (Fig. 4A). The reverse experiment, namely, immunoprecipitation with specific phosphotyrosine antibody and immunoblot with anti-IκBα antibody, confirmed these results (Fig. 4B). Specificity of this phosphorylation to tyrosine 42 was determined by immunoprecipitation with IκBα and immunoblot with anti-pIκBα tyrosine 42 (Fig. 4B). Overall, these results demonstrate that phosphorylation of IκBα on tyrosine 42 occurs with hyperoxia-induced NF-κB activation, which is unique to fetal lung cells.
Fig. 4.
Immunoprecipitation (IP) of phosphorylated IκBα on tyrosine residues after hyperoxia occurs only in fetal lung cells. A: immunoprecipitation of IκBα was performed on untreated and hyperoxia-exposed fetal and adult cells, and immunoblot [Western blot (WB)] was performed with anti-phosphotyrosine antibody (left); control immunoprecipitation of IκBα was performed on untreated and hyperoxia-exposed fetal and adult cells, and immunoblot was performed with anti-IκBα antibody (middle; experiments showed adequate pull-down of IκBα); immunoprecipitation with nonspecific IgG was performed on untreated and hyperoxia-exposed fetal and adult cells, and immunoblot was performed with anti-IκBα antibody (right; experiments showed no band with IgG control). B: immunoprecipitation of phosphotyrosine was performed on untreated and hyperoxia-exposed fetal cells, and immunoblot was performed with anti-IκBα antibody or anti-pIκBα tyrosine 42. Hyperoxia, 8 h at 95% O2-5% CO2; DV, densitometric value of immunoprecipitated band. HC, heavy chain IgG; LC, light chain IgG.
Y42F suppresses hyperoxia-induced NF-κB activation.
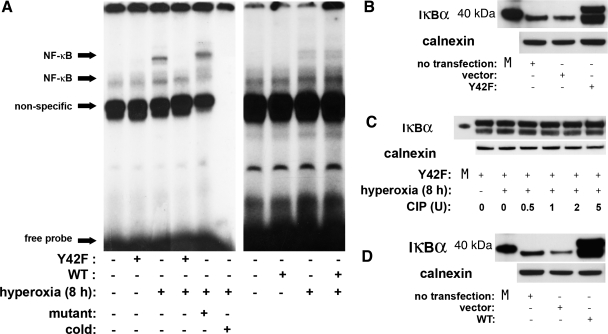
To evaluate the specific contribution of phosphorylation of IκBα at tyrosine 42 on hyperoxia-induced NF-κB activation, we used a mutant form of IκBα. This variant (Y42F), in which phenylalanine is substituted for tyrosine 42, functions as a dominant-negative inhibitor of the atypical pathway (28). Fetal cells were exposed to hyperoxia (8 h) after transfection with a vector containing a mutated variant of IκBα. Transfection efficiency was confirmed to be 50–55% via fluorescein-activated cell sorting analysis (data not shown). Expression of the transfected protein was confirmed by Western blot analysis against IκBα (Fig. 5B). Furthermore, inhibition of tyrosine phosphorylation was shown by incubation of cytosolic extracts from cells exposed to hyperoxia with CIP (Fig. 5C). Phosphatase treatment did not change the appearance of the overexpressed Y42F, suggesting inhibition of IκBα phosphorylation after hyperoxia (Fig. 5C). Nuclear extracts subjected to EMSA showed that Y42F transfection completely prevented hyperoxia-induced NF-κB consensus sequence binding (Fig. 5A), suggesting that phosphorylation of IκBα on tyrosine 42 is essential to hyperoxia-induced NF-κB activation. After transfection of WT IκBα (Fig. 5D), inhibition of nuclear hyperoxia-induced NF-κB consensus sequence binding was not seen (Fig. 5A).
Fig. 5.
Dominant-negative mutant IκBα (Y42F) prevents hyperoxia-induced NF-κB consensus sequence binding of nuclear proteins in fetal lung fibroblasts. A: representative EMSA of 3 independent experiments. Control and transfected [Y42F or wild-type (WT) IκBα] cells were exposed to hyperoxia for 8 h, and nuclear protein binding to NF-κB consensus sequence was evaluated by EMSA. For each sample, 5 μg of nuclear protein were evaluated to determine NF-κB consensus sequence binding activity. Mutant, 50-fold excess of mutated oligonucleotide; cold, 50-fold excess of unlabeled oligonucleotide. B: confirmation of Y42F expression by representative immunoblot for IκB, with 40-kDa marker and calnexin as loading control. M, marker. C: representative Western blots showing IκBα pattern in cells expressing Y42F after incubation of cytosolic fractions from hyperoxia-exposed fetal cells with 0.5–5 U of CIP, with 40-kDa marker and calnexin as loading control. D: confirmation of WT IκBα expression by Western blot, with 40-kDa marker and calnexin as loading control.
Hyperoxia inhibits NF-κB-driven luciferase reporter activity in stably transfected fetal lung fibroblasts.
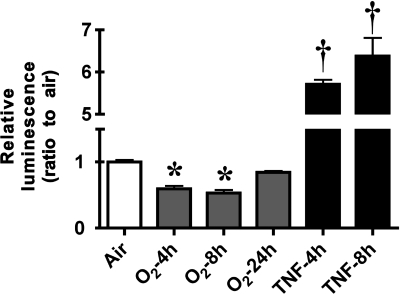
To further define the effect of hyperoxia-induced NF-κB activation on gene transcription, an RLF-6 fetal cell line stably expressing a luciferase reporter gene driven by four copies of the NF- κB consensus sequence fused to a TATA-like promoter (pNFκB-luc) was generated. Cells were exposed to hyperoxia (4, 8, or 24 h) or TNF-α (4 or 8 h). Luciferase activity was determined by measurement of photon emission after incubation with the substrate luciferin. Hyperoxia resulted in a 1.6- and 2-fold reduction in photon emission by 4 and 8 h, respectively. In direct contrast, TNF-α caused a 5.7- and 6.4-fold increase in reporter expression at 4 and 8 h of exposure, respectively (Fig. 6). These data suggest that the NF-κB dimers that bind to the consensus sequence sites in response to hyperoxia suppress expression of downstream genes.
Fig. 6.
Hyperoxia decreases NF-κB-driven luciferase reporter activity. Fetal lung fibroblasts stably transfected with pNFκB-luc were exposed to hyperoxia (4, 8, or 24 h) or incubated with TNF-α (4 or 8 h at 20 ng/ml). Fold induction of photon emission was measured by an in vivo imaging system. Values are means ± SE of 6 independent experiments for each group. Significantly different from time 0: *P < 0.05; †P < 0.001.
Inhibition of hyperoxia-induced NF-κB activation in fetal cells results in upregulation of proapoptotic genes.
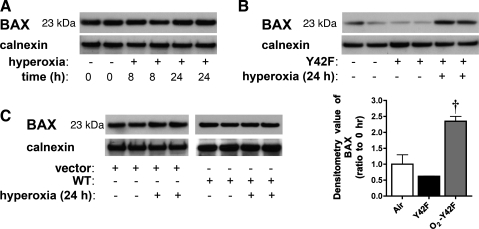
NF-κB is known to regulate apoptosis in response to various stimuli (48). To identify the impact of hyperoxia-induced NF-κB activation on specific genes related to apoptosis, we subjected fetal cells to a targeted gene array analysis. This array included 113 genes related to apoptotic pathways. None of the interrogated genes showed >1.5-fold upregulation after 8 h of hyperoxia. However, multiple genes showed varying degrees of downregulation (Table 1). Among these genes were proapoptotic factors Bcl-2-associated X protein (Bax, 1.8-fold), CASP2 and RIPK1 domain-containing adaptor with death domain (2-fold), and Bcl-10 (1.8-fold). In contrast, cells transfected with Y42F showed a specific upregulation of these proapoptotic genes after hyperoxia [Bax protein (1.9-fold), CASP2 and RIPK1 domain-containing adaptor with death domain (1.9-fold), and Bcl-10 (1.6-fold)]. In support of these findings, Bax protein levels increased when hyperoxia-induced NF-κB activation was prevented with Y42F (Fig. 7). This indicates the specificity of IκBα tyrosine 42 phosphorylation in modulating downstream genes in response to hyperoxia-induced NF-κB activation. Nevertheless, immunoreactive Bax protein was not visibly changed in fetal cells exposed to hyperoxia or in cells transfected with empty vector or WT IκBα and exposed to hyperoxia. This finding is expected, given the length of exposure and the long half-life of Bax protein. Previous reports show that the half-life of Bax exceeds 24 h (6); thus a decrease in gene expression in response to hyperoxia would not be expected to decrease the basal levels, even after exposures of 8–24 h, as in these experiments.
Table 1.
Fold change in genes interrogated by targeted apoptotic pathway array analysis
| Gene | Hyperoxia | Hyperoxia + Y42F Transfection |
|---|---|---|
| CASP2 and RIPK 1 domain-containing adaptor with death domain | 2.2 ↓ | 1.9 ↑ |
| Transformation-related p53 inducible nuclear protein 1 | 2.2 ↓ | 0.8 ↓ |
| Bax | 1.8 ↓ | 1.9 ↑ |
| Bcl-10 | 1.7 ↓ | 1.6 ↑ |
| Baculoviral IAP repeat-containing 3 | 1.6 ↓ | 1.3 ↓ |
| Bcl-2-associated death promoter | 1.6 ↓ | 1.6 ↓ |
Y42F, dominant-negative mutant IκBα; ↓, downregulation; ↑, upregulation.
Fig. 7.
Bax protein levels increase in fetal cells transfected with Y42F and exposed to hyperoxia. A: representative Western blot showing Bax protein levels in fetal cells exposed to hyperoxia (8 or 24 h), with calnexin as loading control. B: representative Western blot showing Bax protein levels in fetal cells exposed to hyperoxia (24 h) after transfection with Y42F, with calnexin as loading control (top) and densitometric evaluation of fold change in Bax protein in fetal cells overexpressing Y42F (bottom). Values are means ± SE of 3 independent experiments for each group. †P < 0.001 vs. Y42F. C: representative Western blot showing Bax protein levels in fetal cells exposed to hyperoxia (24 h) after transfection with empty vector or WT IκBα, with calnexin as loading control. Vector, cells transfected with empty vector. All Western blots are representative of 3 independent experiments.
Preventing hyperoxia-induced NF-κB activation in fetal lung cells results in enhanced apoptosis.
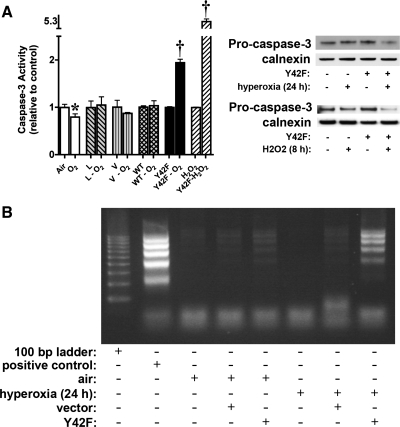
To evaluate whether the change in apoptotic gene expression affected apoptosis, we assessed caspase-3 activity. After hyperoxia, there was a significant decrease in caspase-3 activity in fetal cells, supporting the array data that show modulation of expression of genes regulating apoptosis by NF-κB activation (Fig. 8A). Transfection with vehicle or WT IκBα abrogated the decrease in caspase-3 activity after hyperoxia, which we hypothesize may be due to exposure to the transfection reagent or partial blockade of hyperoxia-induced NF-κB activation by the overexpression of WT IκBα (Fig. 8A). In contrast, cells transfected with Y42F showed a twofold increase in caspase-3 activity. Others have shown that H2O2 activates NF-κB after phosphorylation of IκBα at tyrosine 42 (45, 57, 61). No increase in caspase-3 activity was observed in fetal cells exposed to H2O2 (1 mM, 8 h). However, this oxidant stress resulted in a 5.3-fold increase in caspase-3 activity in fetal cells overexpressing Y42F (Fig. 8A). Western analysis of procaspase-3 on normal fetal cells and cells overexpressing Y42F (Fig. 8A) showed decreased procaspase-3 in cells overexpressing Y42F after exposure to hyperoxia and H2O2, supporting cleavage of this protein and activation of apoptosis. These data support the notion that this pathway is important in protection against apoptosis when fetal cells are exposed to oxidative stress. These results were confirmed by DNA laddering, which was seen only in cells transfected with Y42F after exposure to hyperoxia (Fig. 8B). These findings suggest that the absence of an NF-κB response to hyperoxia results in increased levels of apoptosis.
Fig. 8.
Apoptosis increases in fetal cells transfected with a dominant-negative mutant IκBα and exposed to hyperoxia. Control cells, cells treated with lipofectamine only (L), and cells transfected with empty vector (V), WT IκBα, or Y42F were exposed to room air, H2O2 (8 h), or hyperoxia (24 h). Cell lysate or genomic DNA was collected and subjected to caspase-3 activity assay and Western blot analysis or DNA laddering assay, respectively. A: caspase-3 activity increases in fetal cells transfected with Y42F and exposed to hyperoxia. Left: fold change in caspase-3 activity in cells exposed to hyperoxia compared with respective controls. Air, fetal cells exposed to room air; O2, fetal cells exposed to 24 h of hyperoxia; L, lipofectamine-treated cells exposed to room air; L-O2, lipofectamine-treated cells exposed to 24 h of hyperoxia; V, empty vector-transfected cells exposed to room air; V- O2, empty vector-transfected cells exposed to 24 h of hyperoxia; WT, WT IκBα-transfected cells exposed to room air; WT-O2, WT IκBα-transfected cells exposed to 24 h of hyperoxia; Y42F, Y42F-transfected cells exposed to room air; Y42F-O2, Y42F-transfected cells exposed to 24 h of hyperoxia; H2O2, fetal cells exposed to 1 mM H2O2 for 8 h; Y42F-H2O2, Y42F-transfected cells exposed to 1 mM H2O2. Values are means ± SE of 6 independent experiments for each group. Significantly different from time 0: *P < 0.05; †P < 0.0001. Right: representative Western blot showing levels of procaspase-3 in control fetal cells and cells overexpressing Y42F after exposure to hyperoxia (24 h) or H2O2 (8 h), with calnexin as loading control. B: representative agarose-ethidium bromide gel showing increased DNA laddering in fetal cells transfected with Y42F and exposed to hyperoxia. Positive control, sample DNA provided by manufacturer; air, control cells; hyperoxia, cells exposed to 24 h of hyperoxia; vector, empty vector-transfected cells; Y42F, Y42F-transfected cells.
DISCUSSION
We show here, for the first time, that hyperoxia-induced NF-κB activation occurs via phosphorylation of tyrosine 42 of IκBα. Furthermore, this activation prevents apoptosis in fetal lung cells. In contrast, adult lung fibroblasts show no evidence of hyperoxia-induced NF-κB activation or phosphorylation on tyrosine 42 of IκBα. Thus this mechanism and pattern of NF-κB activation, as well as downstream effects, are unique to hyperoxia and are maturationally regulated.
These data demonstrate that hyperoxia activates NF-κB in fetal lung fibroblasts. However, the literature is somewhat conflicted as to whether hyperoxia results in NF-κB activation. Most studies done on cancer cell lines show no NF-κB response to hyperoxia (1, 25, 47, 50, 67). In A549 alveolar adenocarcinoma cells, nuclear translocation of NF-κB was shown, but this had no effect on the cellular response to hyperoxia (42). In contrast, studies done on multiple primary lung cells and cell lines derived from normal lung tissue show remarkable consistency in their ability to activate NF-κB in response to hyperoxia (19, 30, 32, 58, 60). Cumulatively, these studies suggest that the pathway leading to hyperoxia-induced NF-κB activation may be active only in primary cells and cell lines derived from normal tissue. Furthermore, in the present study, this pathway was active only in fetal cells. Multiple models have shown maturational regulation of NF-κB activation in response to various stimuli, including hyperoxia (2, 39, 64, 68). Our study is consistent with maturational regulation of NF-κB activation. Because of the conflicting reports cited, it has been difficult to understand the role and importance of NF-κB in regulating the cellular response to hyperoxia. Our work sheds light on this complex pathway.
The precise mechanistic effect of tyrosine 42 phosphorylation on the interaction between IκBα and NF-κB dimers remains obscure. Additionally, it is not clear whether phosphorylation of tyrosine 42 precedes degradation of IκBα (40, 46, 57) or, merely, dissociation of NF-κB complexes from this inhibitory protein (7, 36, 45, 59, 61, 71). In our model, we noted phosphorylation of tyrosine 42, but no degradation of IκBα, supporting the hypothesis of dissociation of NF-κB complexes from IκBα.
Our data demonstrate that hyperoxia-induced NF-κB activation suppresses gene expression in a cell line derived from normal lung tissue. Whether this is due to unique subunit conformation, altered DNA binding secondary to subunit oxidation, or some other protein interacting with nuclear NF-κB was not evaluated here. Previous reports demonstrated that the p50 subunit plays a role in modulation of hyperoxia-induced NF-κB activation (68). In this model, the p65 subunit of NF-κB translocates to the nucleus and binds to its consensus sequence after hyperoxia, suggesting that p65 may also be important in modulating gene expression.
Although the link between oxidative stress, NF-κB activation, and tyrosine phosphorylation was first described in 1993 (62), no reports have described this response to hyperoxia. Phosphorylation of IκBα on tyrosine 42 and resultant NF-κB activation modulate a distinct response to hyperoxia in fetal lung fibroblasts. The cellular apoptotic threshold is held in balance by the presence of pro- and antiapoptotic proteins. Classically, NF-κB affects this balance by upregulating expression of proteins that prevent apoptosis (38). Furthermore, there is strong evidence that these pathways are active in mediating hyperoxic injury in the lung. In a study performed on adult rat alveolar epithelial cells, Bax was found to be required for hyperoxia-induced apoptosis (15). Additionally, murine embryonic fibroblasts that do not express Bax and Bak are resistant to hyperoxia-induced cell death (16). In the present study, hyperoxia-induced NF-κB activation prevented apoptosis by downregulating proapoptotic genes, including Bax, and this was reversed by transfection with a dominant-negative IκBα that cannot be phosphorylated on tyrosine 42. Additionally, our results are in agreement with findings of others who demonstrated that blocking NF-κB activation results in upregulation of Bax protein expression (6). Thus hyperoxia-induced NF-κB activation shifts the threshold toward inhibition of apoptosis in fetal lung fibroblasts.
Hyperoxia-induced NF-κB activation may explain in part how this stimulus alters lung development. The lung contains >40 different cell types (11), and it appears that not all cells tolerate hyperoxic exposure in the same way. Although endothelial cells appear very sensitive to oxygen toxicity, type II epithelial cells appear resistant and proliferate in the recovery phase (24). Exposure to hyperoxia appears to prevent the normal differentiation of type II cells to type I cells in the developing lung (70). Lung fibroblasts play an essential role in lung development and alveolarization. As part of normal lung development from the canalicular to the alveolar stage, fibroblasts undergo apoptosis (41), a process that continues through postnatal lung development (13, 55). Any process that interferes with the normal apoptotic process would necessarily adversely affect lung development. Our data show that NF-κB activation prevents apoptosis in hyperoxia-exposed fetal lung fibroblasts. We speculate that altering the normal pattern of fibroblast apoptosis through exposure to hyperoxia during a critical period could contribute to abnormal lung development. Others have shown that hyperoxia causes fibroblast transdifferentiation, alters control of elastin synthesis, and decreases fibroblast growth factor 7 activity (8, 12, 14, 35, 51). Together with our work, these studies suggest that hyperoxic exposure of fibroblasts alters normal lung development. Careful examination in animal models is warranted to confirm this hypothesis.
In summary, phosphorylation of IκBα on tyrosine 42 precedes hyperoxia-induced NF-κB activation in fetal lung fibroblasts. This activation pathway is present in fetal, but not adult, lung fibroblasts. In these cells, NF-κB nuclear translocation results in the downregulation of proapoptotic genes. Functionally, this results in protection against apoptosis following hyperoxia. We speculate that although NF-κB activation may prevent apoptosis in fetal lung fibroblasts exposed to hyperoxia, it may lead to abnormal lung development by disrupting the normal pattern of apoptosis.
GRANTS
This work was funded by National Institute of Child Health and Human Development Pediatric Scientist Development Program Grant K12-HD-00850 (to C. J. Wright) and National Heart, Lung, and Blood Institute Grant RO-1 HL-58752 (to P. A. Dennery).
Acknowledgments
We thank Dr. Yousef Abu-Amer (Washington University, St. Louis, MO) for wild-type and dominant-negative mutant IκBα plasmids and Patrick Fernando for essential help leading to completion of this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allen GL, Menendez IY, Ryan MA, Mazor RL, Wispe JR, Fiedler MA, Wong HR. Hyperoxia synergistically increases TNF-α-induced interleukin-8 gene expression in A549 cells. Am J Physiol Lung Cell Mol Physiol 278: L253–L260, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Alvira CM, Abate A, Yang G, Dennery PA, Rabinovitch M. NF-κB activation in the neonatal mouse lung protects against lipopolysaccharide-induced inflammation. Am J Respir Crit Care Med 175: 805–815, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ankermann T, Reisner A, Wiemann T, Krams M, Kohler H, Krause MF. Topical inhibition of nuclear factor-κB enhances reduction in lung edema by surfactant in a piglet model of airway lavage. Crit Care Med 33: 1384–1391, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell 87: 13–20, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Belik J, Jankov RP, Pan J, Tanswell AK. Chronic O2 exposure enhances vascular and airway smooth muscle contraction in the newborn but not adult rat. J Appl Physiol 94: 2303–2312, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bentires-Alj M, Dejardin E, Viatour P, Van Lint C, Froesch B, Reed JC, Merville MP, Bours V. Inhibition of the NF-κB transcription factor increases Bax expression in cancer cell lines. Oncogene 20: 2805–2813, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Beraud C, Henzel WJ, Baeuerle PA. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-κB activation. Proc Natl Acad Sci USA 96: 429–434, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boros LG, Torday JS, Paul Lee WN, Rehan VK. Oxygen-induced metabolic changes and transdifferentiation in immature fetal rat lung lipofibroblasts. Mol Genet Metab 77: 230–236, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Bostrom H, Willetts K, Pekny M, Leveen P, Lindahl P, Hedstrand H, Pekna M, Hellstrom M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Tornell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85: 863–873, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Bourbia A, Cruz MA, Rozycki HJ. NF-κB in tracheal lavage fluid from intubated premature infants: association with inflammation, oxygen, and outcome. Arch Dis Child Fetal Neonatal Ed 91: F36–F39, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breeze RG, Wheeldon EB. The cells of the pulmonary airways. Am Rev Respir Dis 116: 705–777, 1977. [DOI] [PubMed] [Google Scholar]
- 12.Bruce MC, Bruce EN, Janiga K, Chetty A. Hyperoxic exposure of developing rat lung decreases tropoelastin mRNA levels that rebound postexposure. Am J Physiol Lung Cell Mol Physiol 265: L293–L300, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Bruce MC, Honaker CE, Cross RJ. Lung fibroblasts undergo apoptosis following alveolarization. Am J Respir Cell Mol Biol 20: 228–236, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Bruce MC, Pawlowski R, Tomashefski JF Jr. Changes in lung elastic fiber structure and concentration associated with hyperoxic exposure in the developing rat lung. Am Rev Respir Dis 140: 1067–1074, 1989. [DOI] [PubMed] [Google Scholar]
- 15.Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GR. Reactive oxygen species are required for hyperoxia-induced Bax activation and cell death in alveolar epithelial cells. J Biol Chem 279: 6753–6760, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Budinger GR, Tso M, McClintock DS, Dean DA, Sznajder JI, Chandel NS. Hyperoxia-induced apoptosis does not require mitochondrial reactive oxygen species and is regulated by Bcl-2 proteins. J Biol Chem 277: 15654–15660, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Bui NT, Livolsi A, Peyron JF, Prehn JH. Activation of nuclear factor κB and Bcl-x survival gene expression by nerve growth factor requires tyrosine phosphorylation of IκBα. J Cell Biol 152: 753–764, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao L, Liu C, Cai B, Jia X, Kang L, Speer CP, Sun B. Nuclear factor-κB expression in alveolar macrophages of mechanically ventilated neonates with respiratory distress syndrome. Biol Neonate 86: 116–123, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Cazals V, Nabeyrat E, Corroyer S, de Keyzer Y, Clement A. Role for NF-κB in mediating the effects of hyperoxia on IGF-binding protein 2 promoter activity in lung alveolar epithelial cells. Biochim Biophys Acta 1448: 349–362, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Cheah FC, Winterbourn CC, Darlow BA, Mocatta TJ, Vissers MC. Nuclear factor κB activation in pulmonary leukocytes from infants with hyaline membrane disease: associations with chorioamnionitis and Ureaplasma urealyticum colonization. Pediatr Res 57: 616–623, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Chelly N, Mouhieddine-Gueddiche OB, Barlier-Mur AM, Chailley-Heu B, Bourbon JR. Keratinocyte growth factor enhances maturation of fetal rat lung type II cells. Am J Respir Cell Mol Biol 20: 423–432, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Christman JW, Sadikot RT, Blackwell TS. The role of nuclear factor-κB in pulmonary diseases. Chest 117: 1482–1487, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Contag CH, Spilman SD, Contag PR, Oshiro M, Eames B, Dennery P, Stevenson DK, Benaron DA. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol 66: 523–531, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis 122: 123–143, 1980. [DOI] [PubMed] [Google Scholar]
- 25.D'Angio CT, LoMonaco MB, Johnston CJ, Reed CK, Finkelstein JN. Differential roles for NF-κB in endotoxin and oxygen induction of interleukin-8 in the macrophage. Am J Physiol Lung Cell Mol Physiol 286: L30–L36, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Delemos RA, Coalson JJ, Gerstmann DR, Kuehl TJ, Null DM Jr. Oxygen toxicity in the premature baboon with hyaline membrane disease. Am Rev Respir Dis 136: 677–682, 1987. [DOI] [PubMed] [Google Scholar]
- 27.Dirami G, Massaro GD, Clerch LB, Ryan US, Reczek PR, Massaro D. Lung retinol-storing cells synthesize and secrete retinoic acid, an inducer of alveolus formation. Am J Physiol Lung Cell Mol Physiol 286: L249–L256, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Fan C, Yang J, Engelhardt JF. Temporal pattern of NFκB activation influences apoptotic cell fate in a stimuli-dependent fashion. J Cell Sci 115: 4843–4853, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Franek WR, Horowitz S, Stansberry L, Kazzaz JA, Koo HC, Li Y, Arita Y, Davis JM, Mantell AS, Scott W, Mantell LL. Hyperoxia inhibits oxidant-induced apoptosis in lung epithelial cells. J Biol Chem 276: 569–575, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Franek WR, Morrow DM, Zhu H, Vancurova I, Miskolci V, Darley-Usmar K, Simms HH, Mantell LL. NF-κB protects lung epithelium against hyperoxia-induced nonapoptotic cell death-oncosis. Free Radic Biol Med 37: 1670–1679, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Frank L, Bucher JR, Roberts RJ. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol 45: 699–704, 1978. [DOI] [PubMed] [Google Scholar]
- 32.Guthmann F, Wissel H, Schachtrup C, Tolle A, Rudiger M, Spener F, Rustow B. Inhibition of TNFα in vivo prevents hyperoxia-mediated activation of caspase 3 in type II cells. Respir Res 6: 10, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han RN, Buch S, Tseu I, Young J, Christie NA, Frndova H, Lye SJ, Post M, Tanswell AK. Changes in structure, mechanics, and insulin-like growth factor-related gene expression in the lungs of newborn rats exposed to air or 60% oxygen. Pediatr Res 39: 921–929, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann A, Baltimore D. Circuitry of nuclear factor κB signaling. Immunol Rev 210: 171–186, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Hokuto I, Perl AK, Whitsett JA. FGF signaling is required for pulmonary homeostasis following hyperoxia. Am J Physiol Lung Cell Mol Physiol 286: L580–L587, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF. Tyrosine phosphorylation of IκBα activates NF-κB without proteolytic degradation of IκBα. Cell 86: 787–798, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor κB. Free Radic Biol Med 28: 1317–1327, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Karin M, Lin A. NF-κB at the crossroads of life and death. Nat Immunol 3: 221–227, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Kilpinen S, Henttinen T, Lahdenpohja N, Hulkkonen J, Hurme M. Signals leading to the activation of NF-κB transcription factor are stronger in neonatal than adult T lymphocytes. Scand J Immunol 44: 85–88, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor κB through the phosphorylation of IκBα on tyrosine residues. Cancer Res 54: 1425–1430, 1994. [PubMed] [Google Scholar]
- 41.Kresch MJ, Christian C, Wu F, Hussain N. Ontogeny of apoptosis during lung development. Pediatr Res 43: 426–431, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Zhang W, Mantell LL, Kazzaz JA, Fein AM, Horowitz S. Nuclear factor-κB is activated by hyperoxia but does not protect from cell death. J Biol Chem 272: 20646–20649, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 58: 22–29, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124: 3943–3953, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Livolsi A, Busuttil V, Imbert V, Abraham RT, Peyron JF. Tyrosine phosphorylation-dependent activation of NF-κB. Requirement for p56 LCK and ZAP-70 protein tyrosine kinases. Eur J Biochem 268: 1508–1515, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Mukhopadhyay A, Manna SK, Aggarwal BB. Pervanadate-induced nuclear factor-κB activation requires tyrosine phosphorylation and degradation of IκBα. Comparison with tumor necrosis factor-α. J Biol Chem 275: 8549–8555, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Odoms K, Shanley TP, Wong HR. Short-term modulation of interleukin-1β signaling by hyperoxia: uncoupling of IκB kinase activation and NF-κB-dependent gene expression. Am J Physiol Lung Cell Mol Physiol 286: L554–L562, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Piva R, Belardo G, Santoro MG. NF-κB: a stress-regulated switch for cell survival. Antioxid Redox Signal 8: 478–486, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Post M, Souza P, Liu J, Tseu I, Wang J, Kuliszewski M, Tanswell AK. Keratinocyte growth factor and its receptor are involved in regulating early lung branching. Development 122: 3107–3115, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Rahman I, Mulier B, Gilmour PS, Watchorn T, Donaldson K, Jeffery PK, MacNee W. Oxidant-mediated lung epithelial cell tolerance: the role of intracellular glutathione and nuclear factor-κB. Biochem Pharmacol 62: 787–794, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Rehan V, Torday J. Hyperoxia augments pulmonary lipofibroblast-to-myofibroblast transdifferentiation. Cell Biochem Biophys 38: 239–250, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Roth-Kleiner M, Post M. Similarities and dissimilarities of branching and septation during lung development. Pediatr Pulmonol 40: 113–134, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci USA 86: 802–806, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9: 49–89, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Scavo LM, Ertsey R, Chapin CJ, Allen L, Kitterman JA. Apoptosis in the development of rat and human fetal lungs. Am J Respir Cell Mol Biol 18: 21–31, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Schittny JC, Djonov V, Fine A, Burri PH. Programmed cell death contributes to postnatal lung development. Am J Respir Cell Mol Biol 18: 786–793, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Schoonbroodt S, Ferreira V, Best-Belpomme M, Boelaert JR, Legrand-Poels S, Korner M, Piette J. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of IκBα in NF-κB activation by an oxidative stress. J Immunol 164: 4292–4300, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Shea LM, Beehler C, Schwartz M, Shenkar R, Tuder R, Abraham E. Hyperoxia activates NF-κB and increases TNF-α and IFN-γ gene expression in mouse pulmonary lymphocytes. J Immunol 157: 3902–3908, 1996. [PubMed] [Google Scholar]
- 59.Singh S, Darnay BG, Aggarwal BB. Site-specific tyrosine phosphorylation of IκBα negatively regulates its inducible phosphorylation and degradation. J Biol Chem 271: 31049–31054, 1996. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki Y, Nishio K, Takeshita K, Takeuchi O, Watanabe K, Sato N, Naoki K, Kudo H, Aoki T, Yamaguchi K. Effect of steroid on hyperoxia-induced ICAM-1 expression in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 278: L245–L252, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-κB through tyrosine phosphorylation of IκBα and serine phosphorylation of p65: evidence for the involvement of IκBα kinase and Syk protein-tyrosine kinase. J Biol Chem 278: 24233–24241, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Uckun FM, Schieven GL, Tuel-Ahlgren LM, Dibirdik I, Myers DE, Ledbetter JA, Song CW. Tyrosine phosphorylation is a mandatory proximal step in radiation-induced activation of the protein kinase C signaling pathway in human B-lymphocyte precursors. Proc Natl Acad Sci USA 90: 252–256, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, Susser M, Paneth N, Leviton A. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics 105: 1194–1201, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Vancurova I, Bellani P, Davidson D. Activation of nuclear factor-κB and its suppression by dexamethasone in polymorphonuclear leukocytes: newborn versus adult. Pediatr Res 49: 257–262, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, Everette R, Peters N, Miller N, Muran G, Auten K, Newman N, Rowan G, Grisby C, Arnell K, Miller L, Ball B, McDavid G. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 114: 1305–1311, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110–L117, 1998. [DOI] [PubMed] [Google Scholar]
- 67.Wong HR, Odoms KK, Denenberg AG, Allen GL, Shanley TP. Hyperoxia prolongs tumor necrosis factor-α-mediated activation of NF-κB: role of IκB kinase. Shock 17: 274–279, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Yang G, Abate A, George AG, Weng YH, Dennery PA. Maturational differences in lung NF-κB activation and their role in tolerance to hyperoxia. J Clin Invest 114: 669–678, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang G, Madan A, Dennery PA. Maturational differences in hyperoxic AP-1 activation in rat lung. Am J Physiol Lung Cell Mol Physiol 278: L393–L398, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol 291: L1101–L1111, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Zwacka RM, Zhang Y, Zhou W, Halldorson J, Engelhardt JF. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor κB independently of IκB degradation. Hepatology 28: 1022–1030, 1998. [DOI] [PubMed] [Google Scholar]