Abstract
Excessive neutrophil elastase (NE) activity and altered vascular endothelial growth factor (VEGF) signaling have independently been implicated in the development and progression of pulmonary emphysema. In the present study, we investigated the potential link between NE and VEGF. We noted that VEGF165 is a substrate for NE. Digestion of purified VEGF165 with NE generated a partially degraded disulfide-linked fragment of VEGF. Mass spectrometric analysis revealed that NE likely cleaves VEGF165 at both the NH2 and COOH termini to produce VEGF fragment chains ∼5 kDa reduced in size. NE treatment of VEGF-laden endothelial cell cultures and smooth muscle cells endogenously expressing VEGF generated VEGF fragments similar to those observed with purified VEGF165. NE-generated VEGF fragment showed significantly reduced binding to VEGF receptor 2 and heparin yet retained the ability to bind to VEGF receptor 1. Interestingly, VEGF fragment showed altered signaling in pulmonary artery endothelial cells compared with intact VEGF165. Specifically, treatment with VEGF fragment did not activate extracellular-regulated kinases 1 and 2 (ERK1/2), yet resulted in enhanced activation of protein kinase B (Akt). Treatment of monocyte/macrophage RAW 264.7 cells with VEGF fragment, on the other hand, led to both Akt and ERK1/2 activation, increased VEGFR1 expression, and stimulated chemotaxis. These findings suggest that the tissue response to NE-mediated injury might involve the generation of diffusible VEGF fragments that stimulate inflammatory cell recruitment and activation via VEGF receptor 1.
Keywords: lung injury, proteases, extracellular matrix, vascular endothelial growth factor receptors, heparin binding
pulmonary emphysema is defined by airspace enlargement as a result of alveolar septal cell death. Seminal findings, first reported in the 1960s, identified a strong link between emphysema and a deficiency in α1-antiprotease, leading to the elastase-antielastase imbalance hypothesis implicating neutrophil-released elastase as an important component to emphysema progression (36, 37, 79). However, it has become clear that the pathogenesis of emphysema is extremely complex and involves a wide range of biochemical and biomechanical components. Recent information suggests a convergence, to a certain degree, that identifies the inability of the lung to respond sufficiently to various forms of injury as a hallmark of disease (6, 68). In particular, excessive damage and lack of repair of the lung extracellular matrix (ECM) appear to play a central role in the ultimate loss of pulmonary function. In this light, we have focused on how injury to the pulmonary ECM alters its ability to control the access and response of cells to growth factors (7, 11, 12, 18, 65). In the present study, we report an interesting connection between neutrophil elastase (NE) and vascular endothelial growth factor (VEGF) with implications for lung injury and repair.
NE is a potent protease capable of degrading key components of connective tissue, including elastin (36, 37, 42, 70). Under conditions of tissue injury, elastase and an array of peptides, proteins, and other enzymes are released in the ECM by polymorphonuclear neutrophils. According to the protease-antiprotease hypothesis, for normal repair to occur, the subsequent activity of elastase is balanced by an antielastase screen of endogenous inhibitors, the predominant one being α1-antiprotease. However, under circumstances of overwhelming stimuli, oxidative inactivation, or genetic deficiency, the natural balance is disrupted, and a situation develops for elastase to evade local inhibitors, contributing to tissue destruction and the development of emphysema. NE is certainly not acting alone, since it has become clear that a large number of enzymes participate in lung injury. In particular, the large family of matrix metalloproteinases (MMPs) has been implicated in many aspects of lung pathology. Indeed, animals deficient in macrophage elastase (MMP12) are less susceptible to cigarette smoke-induced emphysema, and activation of MMP9 and MMP12 by interleukin-13 overexpression induces airspace enlargement (32, 82). NE has also been directly implicated in this process, since mice deficient in this protease show ∼40% as much airspace enlargement as wild-type counterparts in response to cigarette smoke (67). Thus there is considerable need to better understand the consequences of NE injury within the pulmonary system.
VEGF is a critical factor involved in the development, growth, and survival of blood vessels (20, 66). Although the major tyrosine kinase receptors VEGFR1 (Flt-1) and VEGFR2 (KDR, flk-1) are expressed on vascular endothelial cells, there is also considerable evidence of wide expression of VEGFR1 within pulmonary epithelial cells, monocytes, and macrophages (23, 24). VEGF stimulates endothelial cell proliferation, migration, survival, and vascular permeability, with most of these activities being attributed to signaling through VEGFR2 and its tyrosine kinase activity. VEGFR1, on the other hand, is a kinase-impaired receptor whose role in mediating VEGF activity remains to be fully delineated (61). Targeted deletion of VEGFR1 resulted in early embryonic lethality because of excessive and unregulated blood vessel growth, suggesting that this receptor mediates a negative growth/survival signal in endothelial cells (25, 26, 43). Although the role of VEGFR1 signaling in endothelial cells remains controversial, recent data indicate that the nature of VEGFR1 signaling may vary depending on the cell type where it is expressed. For instance, VEGFR1 signaling in nonendothelial cells such as monocytes and trophoblasts stimulates cell migration and proliferation (3, 5, 14, 19).
The lung is among the organs with the highest levels of VEGF (48). Indeed, angiogenesis is clearly critical to pulmonary development (78), and even partial loss of endothelial function can drastically impair alveolarization (17). Recent evidence also suggests that VEGF contributes a protective activity against injury and oxidative stress. In this regard, a series of studies have implicated VEGF in experimental and human emphysema. Pharmacological inhibition of VEGFR2 activity and lung-targeted inactivation of the VEGF gene result in an emphysematic phenotype in rats and mice, respectively (41, 73). In human patients with emphysema, the expression of VEGF is reduced, and apoptosis is evident when compared with normal patients (40). In a separate study, patients with emphysema showed decreased levels of VEGF protein in sputum (39). Moreover, VEGF blockage induced the expression of oxidative stress markers and apoptosis in rats, leading to emphysema that was prevented by a superoxide dismutase mimetic, suggesting a link between VEGF and oxidative stress (76).
As a further link between VEGF and emphysema, a recent report shows that cigarette smoke exposure reduced VEGF and VEGFR2 levels in rats and that human patients with chronic obstructive pulmonary disease showed reduced VEGFR2 expression (49). Interestingly, lung-targeted overexpression of VEGF induced interleukin-13 and an asthma-like phenotype with inflammation associated with an increased number of infiltrating leukocytes, edema, and parenchymal and vascular remodeling (44). Overexpression of placental growth factor (PlGF), a VEGF-like protein that binds VEGFR1 but not VEGFR2, caused emphysema with enlarged airspaces, increased septal cell apoptosis, and reduction in the number of endothelial cells (75). This range of observations clearly implicates VEGF as a factor that is under strict control within the healthy lung, since both decreased and increased levels show the potential to contribute to disease. An interesting link between these new observations implicating VEGF as a component of emphysema and the classic elastase-antielastase hypothesis is the fact that VEGF is normally stored within the ECM where it binds to heparan sulfate proteoglycans (HSPGs) and fibronectin (59, 64, 77). Thus elastase injury to the lung ECM is likely to have an impact on the storage, release, and activity of VEGF. Although the above studies have contributed significant information regarding the consequences of too much or too little VEGF in the lung, there is little information on how VEGF interactions with the ECM might be involved in the normal and pathological injury response in the lung.
In the present study, we investigated the possible link between NE-mediated injury and the VEGF pathway. We evaluated the sensitivity of VEGF165 to NE digestion and noted that NE partially degrades VEGF165 to generate a VEGF fragment that shows significantly reduced VEGFR2 and heparin binding activity, yet retains VEGFR1 binding. Interestingly, the NE-generated VEGF fragment showed altered signaling in pulmonary artery endothelial cells and in RAW 264.7 macrophage/monocyte cells compared with intact VEGF165. These findings suggest that the release of a VEGF fragment with altered activity from ECM may be an additional consequence of NE injury of pulmonary tissue.
MATERIALS AND METHODS
Materials
Human recombinant VEGF165, recombinant human tumor necrosis factor-α (TNF-α), platelet-derived growth factor (PDGF)-AA, Quantikine human and rat VEGF immunoassay enzyme-linked immunosorbent assays (ELISAs), recombinant human VEGFR2/Fc chimera and VEGFR1/Fc chimera were purchased from R&D systems (Minneapolis, MN). Fibroblast growth factor 2 (FGF2) was from Chiron (Sunnyvale, CA). 125I-Bolton Hunter reagent was obtained from PerkinElmer (Boston, MA). 125I-labeled VEGF165, -FGF2, -TNF-α, and -PDGF were prepared using a modified Bolton-Hunter procedure as previously described (29, 55). Heparin was from Neoparin (San Leonardo, CA). BSA was obtained from American Bioanalytical (Natick, MA). RPMI-1640 low glucose media was purchased from Lonza Walkersville (Walkersville, MD). DMEM, PBS, penicillin/streptomycin, l-glutamine, and HEPES buffer were obtained from Invitrogen (Carlsbad, CA). CASE Kit for protein kinase B (Akt) S473 was purchased from SuperArray (Frederick, MD). Calf serum (CS) and FBS were purchased from Hyclone (Logan, Utah). Primary antibodies for phosphor-extracellular-regulated kinase 1/2 (ERK1/2), total ERK1/2, Akt, and phospho-Akt were obtained from Cell Signaling Technology (Danvers, MA). Anti-VEGF polyclonal antibodies raised to full-length VEGF (Upstate no. 06–565) and to a synthetic peptide encompassing an internal region conserved among all VEGF isoforms (Millipore no. 07-1376) were obtained from Millipore (Billerica, MA). Human neutrophil MMP9 and VEGFR kinase inhibitor III were purchased from Calbiochem (San Diego, CA). NE (human) and Porcine Pancreatic Elastase (PPE) were obtained from Elastin Products (Owensville, MO). 4-Aminophenylmercuric acetate was purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture
Bovine aortic endothelial cells (BAEC passage 5–15) were maintained in low-glucose DMEM, supplemented with 10% CS, 5 mM l-glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate. Bovine pulmonary artery endothelial cells (bPAEC passage 5–10) were purchased from Cambrex Bio Science Walkersville and maintained in low-glucose DMEM, supplemented with 10% FBS, 5 mM l-glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate. Mouse macrophage/monocyte RAW 264.7 cells were propagated in RPMI-1640 with 5 mM l-glutamine, 10% heat-inactivated FBS, 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate (63). FBS was inactivated by heating for 30 min in a 56°C water bath, mixing every 10 min. Neonatal rat aortic smooth muscle cells (SMCs) were isolated from Sprague-Dawley rats, ages 1–3 days as described (22), and maintained in low-glucose DMEM, with 10% FBS, 5 mM l-glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate and 1% nonessential amino acids. Neonatal rat lung fibroblasts were isolated and maintained in low-glucose DMEM, with 5% FBS, 5 mM l-glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate and 1% nonessential amino acids (27).
VEGF Fragment Generation
125I-VEGF165 was treated with varying NE or PPE concentrations in 44 mM sodium bicarbonate, pH 7.4, for various times at 37°C. Mock-treated 125I-VEGF165 was incubated in 44 mM sodium bicarbonate, pH 7.4 at 37°C. The reaction was stopped by adding 1 μM di-isopropyl fluorophosphate (DFP), and samples were subjected to 15% SDS-PAGE under reducing and nonreducing conditions, followed by gel fixation and phosphor screen visualization of the bands. For large VEGF fragment (VEGFf) preparations, the samples (NE and mock treated) were dialyzed (10-kDa molecular weight cut off, Slide-A-Lyzer; Pierce, Rockford, IL) against PBS at 4°C to remove DFP. The concentration of 125I-VEGF165 and 125I-VEGFf in the samples was determined by trichloracetic acid precipitation. A silver stain kit (Owl Scientific, Portsmouth, NH) was also used for visualization of the protein bands. Further purification of VEGFf was performed using heparin-Sepharose CL6B beads (GE Healthcare Bio-Science, Uppsala, Sweden). VEGFf at various NaCl concentrations in PBS was incubated with heparin-Sepharose beads (1:1 slurry) for 1 h at 4°C while rotating. Heparin-bound VEGF was separated from the unbound VEGFf by centrifugation at 1,000 g for 3 min. Supernatants were analyzed by SDS-PAGE and phosphor screen visualization.
Mass Spectrometry
To evaluate the physical alterations of VEGF caused by NE digestion, we used carrier-free, purified recombinant VEGF165. Disulfide bonds in VEGF and VEGF treated with NE were reduced with dithiotheritol (10 mM, 100°C, 15 min), and all cysteine residues were blocked by treatment with iodoacetamide in the dark (100 mM, 57°C, 30 min). Full-length and elastase-treated VEGF were subjected to 12% SDS-PAGE followed by Coommassie blue staining. The Coommassie-stained VEGF bands were excised, cut further to 1-mm cubes, and subjected to in-gel trypsin digestion as previously described (46). Extracted peptides were dried to completion by vacuum centrifugation.
Liquid chromatography-mass spectrometry/mass spectrometry data were obtained using a LTQ Orbitrap (Thermofisher, San Jose, CA) mass spectrometer. Dried peptides were resuspended in 10 μl of 5% acetonitrile/3% acetic acid, and 4 μl were loaded on a pulled fused silica microcapillary column (125 μm ID, 12-cm bed) packed with C18 reverse-phase resin (Magic C18AQ, 5 μm particles; 200 A° pore size; Michrom Bioresources, Auburn, CA). Peptides were resolved using an Agilent 1100 series binary pump across a 30-min linear gradient of 8–25% acetonitrile in 0.2% formic acid at a 250 μl/min flow rate. In each data collection cycle, one full mass spectrometry (MS) scan (375:1,600 mass-to-charge ratio) was acquired in the Orbitrap (6 × 104 resolution setting; automatic gain control target of 106) followed by 10 data-dependent MS/MS scans in the LTQ (AGC target 5,000; threshold 3,000) using the 10 most abundant ions for collision-induced dissociation for fragmentation. The method dynamically excluded previously selected ions for 30 s, singly charged ions, and unassigned charged states.
Raw files obtained from the data collection were converted into mzXML format using the ReAdW program (http:/sashimi.sourceforge.net/software_glossolalia.html). Monoisotopic precursor ion and charge state information for each acquired MS/MS spectra were extracted by in-house software. SEQUEST search algorithm was used to search the MS/MS spectra against the HUMAN.NCI database. The search parameters for posttranslational modifications included a static modification of 57.02146 Da on cysteine (carboxyamidomethylation) and dynamic modification of 15.99491 Da for methionine (oxidation) residues.
VEGF165 and VEGF165 Fragment Binding Assays
Binding to VEGFR1/R2.
Binding assays were performed with VEGFR chimeras by incubating a range of 125I-VEGF165 and 125I-VEGFf concentrations (0.05, 0.1, 0.25, 0.5, and 1 nM) with Fc-VEGFR1 or VEGFR2 (0.1 nM) in binding buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, and 1 mg/ml BSA) for 2 h at 4°C. The bound complexes were pulled down with magnetic protein-A beads (New England Biolabs, Beverly, MA). The beads were washed three times with binding buffer, and 125I-VEGF165/125I-VEGFf associated with the beads was measured using a Coba Auto-Gamma 5005 counter (Packard Instruments, Meridian, CT).
Binding to heparin.
Ninety-six-well plates that have been functionalized by plasma polymerization to contain a controlled layer of amines (generously supplied by Plasso Technology, Sheffield, UK) were used. Heparin (1 μg/ml in PBS) was complexed to the surface of the plate overnight at room temperature (RT). The plates were washed and then incubated with 125I-VEGF165 and 125I-VEGFf in binding buffer (0.15 M NaCl, 25 mM HEPES, pH 7.5) for 2 h at 4°C. Unbound VEGF/VEGFf was washed, and heparin-bound VEGF/VEGFf was extracted with 1 M NaCl, 25 mM HEPES (pH 7.5), and 0.5% Triton X-100. Radioactivity was counted using a Coba Auto-Gamma 5005 counter.
VEGF binding and release from endothelial cells.
BAECs were plated at 5 × 104 cells/well density in a 24-well plate and grown to subconfluence. Cells were incubated with binding buffer [25 mM HEPES, pH 7.5 in DMEM (without bicarbonate) containing 0.1% BSA] for 10 min at 4°C to inhibit endocytosis and binding site turnover. 125I-VEGF and VEGFf were added to the cells and incubated for 2.5 h at 4°C. Unbound VEGF/VEGFf was washed away, and cells were incubated with 44 mM NaHCO3, pH 7.4, with and without PPE (0.5 μg/ml) for various times at RT. The quantity of 125I-VEGF165/125I-VEGFf in the PPE digest was determined by counting the radioactivity in a gamma counter. The state of the 125I-VEGF165 was evaluated by subjecting samples to 15% SDS-PAGE followed by phosphor image analysis. PPE was used in these experiments to avoid the complications of heparan sulfate-mediated inhibition of NE (71). The VEGFf generated by PPE treatment of purified VEGF165 was indistinguishable to that generated by NE.
VEGFf Release from Smooth Muscle and Fibroblast Cell Cultures
Rat aortic SMCs and rat lung fibroblast cells were kept in culture for 4 and 3 wk, respectively. At the onset of the experiment, cultures were rinsed with 44 mM NaHCO3, pH 7.4. Cells were then incubated with 44 mM NaHCO3 with and without PPE or NE (5 μg/ml) for the indicated time (15 or 30 min) at 37°C. The NaHCO3 solutions (referred to as elastase digests) were collected, and elastase was inactivated with 1 μM DFP. Samples were centrifuged at 800 g for 10 min at 4°C, and the supernatants were concentrated using Amicon ultra centrifugal filter devices (10,000 MWCO; Millipore). The presence of VEGF and VEGFf was evaluated using VEGF ELISA. Samples were also subjected to 15% SDS-PAGE and analyzed by immunoblot to visualize VEGF and VEGFf released.
Cell Signaling
ERK1/2 and Akt activation were evaluated in response to VEGF and VEGFf. bPAEC and RAW 264.7 cells were plated at 105 cells/ml in six-well dishes. After 24 h, the cells were serum starved overnight in 0.5% serum to quiesce the cells. The cells were incubated in medium (DMEM, 25 mM HEPES, 1 mg/ml BSA) for 90 min followed by treatment with VEGF and VEGFf at 37°C for 10 min. After the incubation, the medium was removed, and the cells were extracted in lysis buffer (150 mM NaCl, 10 mM Tris, pH 7.5, 1 mM EDTA, 1 mM EGTA, 0.5% Nonidet P-40, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 0.2 mM sodium vanadate). Lysates were centrifuged, and the supernatant was subjected to 12% SDS-PAGE and analyzed by immunoblot to measure phosphorylated (p) ERK1/2, total ERK1/2, pAkt, and total Akt. pAkt and total Akt were also measured using a cell-based CASE Kit. RAW and bPAEC cells were seeded in 96-well tissue culture-treated plates at 15,000 cells/well. Later (24 h), cells were serum starved overnight in serum-free media. VEGF or VEGFf were added directly to the cultured media (0.45 nM), and cells were incubated at 37°C for 10 min. After the incubation, the levels of pAkt and total Akt were measured according to the SuperArray Kit protocol.
Real-Time PCR
RAW 264.7 cells were grown to ∼70% confluence, serum starved overnight in 0.5% FBS, and treated with 0.45 nM VEGF/VEGFf for 30 h. Total RNA was extracted from the cells in 4 M guanidinium thiocyanate as described previously (80). Genomic DNA was removed by incubation with RNase-free DNase I (M0303S; New England BioLabs) in the presence of RNase inhibitor. The RNA was annealed with oligo(dT) and random hexamer primers, and first-strand synthesis was carried out with MuLV reverse transcriptase. Negative controls were performed without reverse transcriptase. Real-time PCR was performed on ABI 7300 using ABI TaqMan gene expression assays as follows: VEGFR1 Mm00438980 and the Eukaryotic 18S rRNA Endogenous Control, 4308329. The cycling parameters were 50°C for 10 min, 95°C for 2 min, 45 cycles of 95°C for 15 s, and 60°C for 1 min. Results were calculated using the ΔΔCT method using 18S rRNA as the endogenous control.
Cell Migration
Wells in the upper chamber of a 96-well multiscreen-MIC plate (8 μm pore size; Millipore) were coated with 60.5 μg/ml type I collagen overnight at RT. The following day, RAW 264.7 cells (5,000 cells/well) were seeded in serum-free media without phenol red dye. The wells in the lower plate filled with serum-free media with or without chemoattractant and the upper plate containing cells were placed on top of the feeder plate and incubated for 4 h at 37°C. After the migration period, the upper plate containing cells was separated from the feeder plate; media were discarded, and the migrated cells on the outer side of the membrane were detached by incubating in TrypZean solution (Sigma-Aldrich) for 30 min at 37°C. Detachment solution containing migrated cells was transferred to an ultraviolet transparent 96-well plate, and the cells were stained and detected using the CyQUANT NF cell proliferation kit (Invitrogen). Fluorescence was read using excitation at 485 nm and emission at 530 nm using a plate reader (Tecan Infinite M200, San Jose, CA).
Statistical Analysis
Statistical significance of data included in this paper was evaluated using ANOVA followed by the Newman-Keul's multiple-comparison t-test. Differences were considered significant when P values were <0.05. Data are presented as the means of replicate samples ± SE or ± SD as indicated in the legends to Figs. 1–10.
Fig. 1.
125I-labeled vascular endothelial growth factor (VEGF) degradation by neutrophil elastase (NE). A: 0.82 nM 125I-VEGF was incubated with and without 20 μg/ml NE in 44 mM sodium bicarbonate buffer, pH 7.4 for the indicated time at 37°C. The reaction was stopped by adding 1 μM di-isopropyl fluorophosphate (DFP), and the samples were subjected to 15% SDS-PAGE under reducing conditions, followed by PhosphorImager analysis. B: 125I-VEGF (0.82 nM) was incubated with the indicated concentration of NE for 30 min at 37°C and applied to SDS-PAGE as in A. C: 15% SDS-PAGE of NE-digested (20 μg/ml; 30 min at 37°C) 125I-VEGF was conducted under reducing and nonreducing conditions. 125I-protein bands were visualized by PhosphorImager analysis. bME, β-mercaptoethanol.
Fig. 10.
Schematic representation of the proposed elastase-mediated VEGF cleavage and release of VEGFf. In this representation, VEGF is bound within the extracellular matrix (ECM). Upon stress or injury, neutrophils are recruited to the site of tissue damage and secrete elastase. Elastase cleaves VEGF, altering its ECM binding (reduced heparin binding), and releases it to act on surrounding cells. The released VEGFf would potentially bind to VEGFR1 on endothelial cells, leading to Akt activation. VEGFf having no affinity for VEGFR2 will not activate its downstream events, yet might alter the distribution of intact VEGF on VEGFR1 vs. VEGFR2 (data not shown). VEGFf might also act on macrophage/monocytic cells through VEGFR1, leading to ERK1/2 and Akt activation. Activation of VEGFR1 by VEGFf could mediate macrophage recruitment to the site of injury, where macrophages would be able to participate in tissue repair/remodeling. This schematic representation is simplified and is not intended to exclude the possibility that other proteases (e.g., MMPs) may also influence VEGF release during tissue injury and inflammation.
RESULTS
Elastase Partially Degrades VEGF
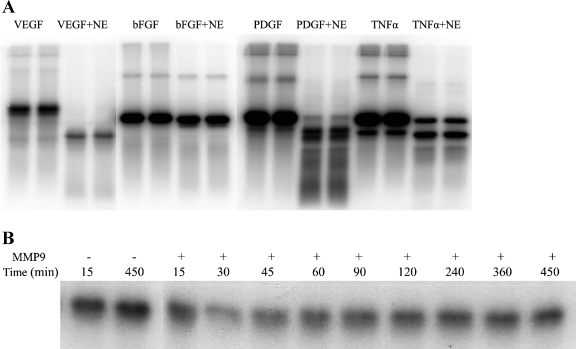
Uncontrolled elastase activity is a hallmark of lung pathology. Recent studies have also revealed that disruption of VEGF-mediated endothelial cell survival can disrupt lung function and can lead to the development of an emphysematic phenotype. VEGF is normally stored in ECM, where it binds to HSPGs and fibronectin, and is essential for endothelial cell survival and repair in the peripheral lung. To explore the possible connection between elastase-mediated lung injury and VEGF, we assessed the sensitivity of VEGF165 to NE cleavage. 125I-VEGF165 was incubated with a range of NE concentrations for 30 min. We observed that VEGF165 was partially degraded by NE and that, after 30 min of incubation, most of the VEGF165 was cleaved to a lower-molecular-mass fragment (Fig. 1A). NE concentrations as low as 10 μg/ml led to significant VEGF digestion and production of a smaller VEGFf (Fig. 1B). Interestingly, the major VEGFf generated appeared to be significantly resistant to further digestion with NE since increasing NE concentrations up to 50 μg/ml did not show further degradation.
Active VEGF is a disulfide-linked dimer; hence, we assessed whether the NE-generated VEGFf remains disulfide-linked by comparing the migration of 125I-VEGF and 125I-VEGFf in SDS-PAGE under reducing and nonreducing conditions. The major band of intact VEGF migrated with apparent molecular mass of ∼44 kDa under nonreducing conditions, corresponding to a disulfide-linked VEGF dimer. Under reducing conditions, the major VEGF band showed a relative molecular mass of ∼22 kDa. NE-digested VEGF showed a major band at ∼17 kDa under reducing conditions. However, under nonreducing conditions, VEGFf showed a major band of ∼34 kDa, indicating that the majority of VEGFf is also a disulfide-linked dimer (Fig. 1C).
To analyze the physical alterations in VEGF when treated with NE, we digested purified recombinant VEGF165 with NE, fully reduced and blocked all cysteine residues, and visualized the products using SDS-PAGE and silver staining. In the first lane in Fig. 2A, there is a band corresponding to intact mock-treated VEGF at ∼22 kDa. Fully reduced and disulfide-blocked VEGFf formed after NE treatment produced a distribution of bands with the major species migrating to ∼17 kDa. There was a clear distinction between VEGFf and NE (NE migrates to ∼29 kDa).
Fig. 2.
VEGF fragment characterization and mass spectrometry. A and B: carrier-free VEGF165 (100 μg/ml) was incubated with and without 20 μg/ml NE in 44 mM sodium bicarbonate buffer, pH 7.4 for 30 min at 37°C. The reaction was stopped by adding 1 μM DFP, and the samples were reduced and cysteine blocked by treatment with dithiotheritol and iodoacetamide. Samples were resolved on 12% SDS-PAGE under reducing conditions, and protein bands were visualized either by silver staining (A) or Coomassie blue staining for mass spectrometry analysis (B). VEGF bands in Coomassie blue-stained gels were excised and subjected to in-gel trypsin digestion, and then analyzed by mass spectrometry. Sequences recovered from mock-treated VEGF and NE-treated VEGF are underlined in bold.
VEGF and VEGFf were also analyzed by mass spectrometry. Liquid chromatography-MS/MS was performed, and the data were searched using SEQUEST set to a mass tolerance of 1.1 D. Both VEGF and VEGFf bands produced peptides that were identified as human VEGF165. Figure 2B shows mock-treated VEGF sequence coverage retrieved by mass spectrometry compared with sequences retrieved from VEGFf. Comparison of the two indicates that VEGF165 is likely cleaved by NE on the NH2 terminus and COOH terminus to generate a major fragment that is ∼5 kDa smaller in size than intact VEGF165. There are likely also some cleavage sites within the protein that produce the smaller proteins observed when the protein is fully reduced (Fig. 2A).
VEGFf is Released from VEGF-Laden Endothelial Cell Cultures
Because we were able to observe VEGFf formation in vitro, we expanded our studies to tissue culture, where we used BAECs, which are known to bind and incorporate a considerable amount of VEGF in their ECM (29). 125I-VEGF165 was incubated with BAECs until binding reached equilibrium. Unbound VEGF was removed, and the VEGF that remained bound to the ECM and VEGFR was released by incubation in buffer in the presence and absence of PPE. We observed a rapid release of bound 125I-VEGF as early as 5 min after elastase treatment was initiated (Fig. 3A) with the total quantity of VEGF released being greater in the elastase condition compared with control. Interestingly, when we visualized the state of the released VEGF using SDS-PAGE and autoradiography, we noted the appearance of VEGFf along with intact VEGF in the elastase-treated cultures (Fig. 3B). Thus elastase was able to generate VEGFf by digesting VEGF-impregnated cell cultures.
Fig. 3.
125I-VEGF165 binding and release from endothelial cells. A: confluent cultures of bovine aortic endothelial cells were incubated with 0.23 nM 125I-VEGF165 at 4°C for 2 h, and unbound 125I-VEGF165 was removed by washing the cells three times in binding buffer. The 125I-VEGF165-bound cells were incubated in 44 mM sodium bicarbonate buffer, pH 7.4, ±0.5 μg/ml porcine pancreatic elastase (PPE) for the indicated time. Released 125I-VEGF165 was counted using a γ-counter. •, 125I-VEGF released with PPE; ○, 125I-VEGF released in bicarbonate buffer. Each data point represents the mean of triplicate determinations ± SD. Statistical analysis revealed a significant difference between PPE and buffer-treated cells (p < 0.01). The experiment was repeated more than three times with similar results. B: 44 mM sodium bicarbonate buffer, pH 7.4, containing released 125I-VEGF165 was collected from the cells from three separate wells after the indicated incubation period and was subjected to 15% SDS-PAGE and PhosphorImager analysis.
To determine if elastase can release endogenously synthesized VEGF from cells, we treated lung fibroblast cultures with PPE (5 μg/ml, 15 min) or buffer alone and used a quantitative ELISA to measure VEGF and noted increased levels of VEGF released in the PPE-treated cells (3.2 ± 0.2 pg/ml in PPE digests compared with 0.0 pg/ml in control digests). Although the effect of PPE on lung fibroblasts was reproducible, the low levels of VEGF released prevented further analysis of the fragmented state of the elastase-released VEGF. Thus, to investigate the state of elastase-released endogenous VEGF, we used primary SMCs that produce high levels of VEGF when maintained in culture for prolonged periods. SMCs were cultured for 4 wk to allow for the accumulation of VEGF within the ECM and then subjected to PPE treatment (5 μg/ml; 30 min) and VEGF ELISA. PPE treatment resulted in release of VEGF (61.2 ± 14.0 pg/ml in the PPE digests compared with 6.4 ± 1.6 pg/ml in the control digests).
These samples and similar ones from NE-treated cells were subjected to SDS-PAGE and Western blot analysis with two separate VEGF antibodies. Control and elastase digests showed a number of anti-VEGF immunoreactive bands in the molecular mass range consistent with the presence of the various VEGF isoforms. However, lower-molecular-mass bands were observed in the PPE and NE digests that were not present in the untreated samples. There were also some interesting differences in the bands recognized by the two VEGF antibodies. There were VEGFf bands similar to those observed with human recombinant VEGF165 in the blots analyzed with the polyclonal antibody raised to full-length VEGF (Fig. 4, A and C), whereas there were also smaller-fragment bands observed with the antibody raised to the internal region of VEGF (Fig. 4, B and D). Although the exact epitopes recognized by these antibodies are not known, it is possible that the various bands represent fragments of particular VEGF isoforms that are selectively recognized by the two antibodies used. Importantly, these data indicate that endogenous VEGFf appear to be generated when VEGF-rich cell cultures are subjected to elastase treatment.
Fig. 4.
Endogenous VEGF and VEGF fragments are released from smooth muscle cells (SMCs). SMCs were maintained in culture for 4 wk and then subjected to treatment with 44 mM NaHCO3 with or without 5 μg/ml PPE (A and B) or 5 μg/ml NE (C and D) for 30 min at 37°C. Elastase digests were collected, and 1 μM was DFP added. Digests were centrifuged (800 g, 10 min at 4°C) and concentrated (10,000 MWCO centrifugal devices). PPE (A and B) and NE (C and D) digest samples were subjected to 15% SDS-PAGE and analyzed by immunoblot with anti-VEGF polyclonal antibody raised to full-length VEGF165 (no. 06–565; A and C) or anti-VEGF polyclonal antibody raised to the internal region of VEGF (no. 07-1376; B and D).
Specificity of VEGFf Formation
To evaluate the specificity of elastase generation of VEGFf, we subjected other growth factors (FGF2, PDGF) and cytokines (TNF-α) to NE digestion (Fig. 5A). We observed that PDGF was also cleaved to generate a smaller fragment. This is an interesting finding, given that PDGF is a structural homolog of VEGF, characterized by highly conserved cysteine residues making a cysteine knot motif (53). Interestingly, TNF-α did not appear to be cleaved to a smaller elastase-resistant fragment; instead, the band intensity of intact TNF-α was reduced compared with the control, indicating that TNF-α is more fully digested by NE. FGF2 seems to be considerably resistant to NE cleavage, with NE-treated FGF2 being of similar intensity as intact FGF2.
Fig. 5.
Evaluating the specificity of VEGF degradation. A: 50 ng/ml of 125I-VEGF165, 125I-fibroblast growth factor 2 (FGF2), 125I-platelet-derived growth factor (PDGF), and 125I-tumor necrosis factor (TNF)-α were incubated with 20 μg/ml NE in 44 mM sodium bicarbonate buffer, pH 7.4, at 37°C for 30 min. The reaction was stopped by boiling in reducing SDS-PAGE sample buffer, and samples were subjected to 15% SDS-PAGE, followed by gel fixation and PhosphorImager analysis. B: 125I-VEGF165 was treated with 10 μg/ml activated matrix metalloproteinase (MMP) 9 [activation by 4-aminophenylmercuric acetate (APMA) (72)] for the indicated times at 37°C, and the reaction was terminated by boiling in reducing SDS-PAGE sample buffer. Samples were subjected to 15% SDS-PAGE, followed by gel fixation and PhosphorImager analysis.
To test whether other ECM proteases cleave VEGF to produce partially digested fragments, we treated VEGF with MMP9, since MMP9 has also been implicated in pulmonary matrix destruction and the development of emphysema. Even with prolonged incubations of 125I-VEGF165 with MMP9, little VEGF degradation was detected (Fig. 5B). Under these same conditions, these MMP9 samples (10, 2.5, and 1 μg/ml) effectively solubilized 1 mg/ml of its known target protein Gelatin type B (data not shown). Hence, the cleavage of VEGF165 by NE does not appear to reflect a general property of all extracellular proteases.
VEGFf Shows Altered Heparin and Receptor Binding
To investigate the activity of the elastase-generated VEGFf, we prepared large batches of VEGFf and mock-treated VEGF for comparison. PhosphorImager analysis revealed >98% reduction in the full-length VEGF by NE (Fig. 6A). Assuming that elastase modulates VEGF upon lung injury, which would lead to altered ability of VEGF to bind to HSPG sites within the ECM, we performed experiments to assess VEGFf binding to heparin. Ninety-six-well plates containing an amine-coated surface were complexed with heparin, and VEGF/VEGFf binding was examined. The results indicate that VEGFf showed reduced ability to bind to heparin compared with intact VEGF (Fig. 6B). This is consistent with mass spectrometry data that indicate that VEGF is cleaved on the COOH terminus, which contains the major heparin-binding domain. Knowing that VEGFf has a much lower affinity for heparin binding than intact VEGF, we used this characteristic to further purify VEGFf from the intact VEGF in the master preparation. To do so, we incubated VEGFf with heparin-Sepharose beads with increasing salt concentrations. Proteins bound to heparin-Sepharose beads were separated from the unbound fractions by centrifugation, and the unbound solution was examined by SDS-PAGE and PhosphorImager analysis. At very low salt concentration, such as 0.15 M, most of the intact VEGF and VEGFf remained bound to heparin; thus, we see a very light band representing VEGFf that had not bound to the heparin-Sepharose beads. When the salt concentration was increased to 0.25 M, intact VEGF still remained bound to heparin, whereas the majority of the VEGFf was dissociated from heparin and released in the supernatant. Only at the highest salt concentrations tested (0.5 M) did we begin to observe intact VEGF dissociation from heparin. Therefore, we used heparin-Sepharose with 0.25 M NaCl to purify VEGFf from the small amount of intact VEGF present in the NE-treated VEGF preparations.
Fig. 6.
VEGF fragment (VEGFf) binding to heparin. A: a batch of VEGFf was prepared by incubating 125I-VEGF165 (33.3 μg/ml) with NE (20 μg/ml) in 44 mM sodium bicarbonate buffer, pH 7.4 for 30 min at 37°C. The reaction was stopped by adding 1 μM DFP, and the samples were dialyzed exhaustively (10-kDa MWCO) against PBS at 4°C. A fraction was analyzed by 15% SDS-PAGE followed by gel fixation and PhosphorImager visualization. B: 96-well plates were coated with 1 μg/ml heparin by overnight incubation in PBS. Plates were incubated with 125I-VEGF and 125I-VEGFf (0.11, 0.23, 0.68, and 1.14 nM) in binding buffer (0.15 M NaCl, 25 mM HEPES, pH 7.5) for 2 h at 4°C, and bound VEGF/VEGFf was extracted with 1 M NaCl, 25 mM HEPES (pH 7.5), and 0.5% Triton X-100. Samples were counted in a gamma counter. •, 125I-VEGF bound; ○, 125I-VEGFf bound. Each data point is the mean of quadruplicate determinations ± SD. The binding of intact VEGF and VEGFf to heparin was significantly different (P < 0.01). Similar results were observed in three separate experiments. C: 125I-VEGFf with various NaCl concentrations in PBS (0.15, 0.24, 0.3, and 0.5 M) was incubated with heparin-Sepharose beads (1:1 slurry) for 1 h at 4°C while rotating. Heparin-bound VEGF was separated from the unbound VEGFf by centrifugation at 1,000 g for 3 min. Supernatants were collected and analyzed by 15% SDS-PAGE and phosphor screen visualization.
We proceeded to determine whether VEGFf retained the ability to bind to its major tyrosine kinase receptors VEGFR1 and VEGFR2 using VEGFR-Fc chimeras. Intact VEGF was pulled down with VEGFR1-Fc and VEGFR2-Fc chimeras, indicating its binding affinity for both receptors (Fig. 7A). However, VEGFf was pulled down complexed with VEGFR1-Fc only, and very little VEGFf was pulled down complexed to the VEGFR2-Fc chimera (Fig. 7B). The results indicate that VEGFf has retained the ability to bind to VEGFR1, whereas it has lost the ability to bind VEGFR2.
Fig. 7.
Binding of VEGF and VEGFf to VEGF receptor (VEGFR) 1 and VEGFR2. 125I-VEGF165 (A) and 125I-VEGFf (B) were incubated with Fc-VEGFR1 or -R2 (0.1 nM) for 2 h; complexes were pulled down with magnetic protein-A beads, and the quantity of 125I-VEGF/VEGFf was counted in the gamma counter. filled bars. VEGF/VEGFf associated with the protein A beads with no receptors present; open bars, VEGF/VEGFf bound to R2; gray bars, VEGF/VEGFf bound to R1. Each data point represents the mean of triplicate determinations ± SD. Similar results were observed in four separate experiments. A: binding of VEGF to R1 and R2 was significantly different than when no receptors were present (P < 0.05). B: binding of VEGFf to R1 was significantly different than when no receptors were present (P < 0.05). There was no statistically significant binding of VEGFf to R2 compared with that observed with no receptor [P = not significant (NS)].
VEGF Activity is Altered by Elastase Cleavage
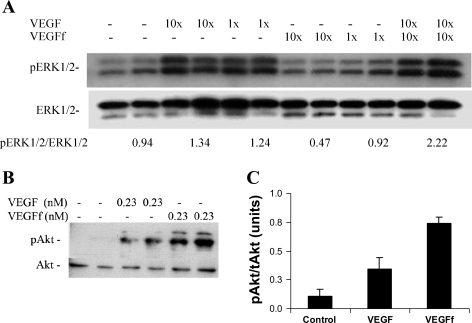
We tested VEGFf activity in endothelial cells by looking at the activation of ERK1/2, a major downstream target of VEGFR2 signaling (35). VEGFf compared with mock-treated VEGF did not show significant stimulation of ERK1/2. Interestingly, when VEGFf was added along with VEGF, there was an increase in ERK1/2 activation compared with that observed with VEGF alone (∼2-fold, Fig. 8A). These data suggest that VEGFf might potentiate VEGF activity, potentially by binding to VEGFR1 and preventing intact VEGF binding, thus increasing the fraction of VEGF available to bind VEGFR2. We also investigated Akt activation upon VEGF and VEGFf stimulation and noted that Akt was activated by both intact and cleaved VEGF (Fig. 8B) with the response to VEGFf being slightly greater than that observed with intact VEGF. Costimulation of endothelial cells with VEGF and VEGFf did not show increased Akt activation above that observed with VEGFf alone (data not shown). The Akt activation was confirmed using a cell-based ELISA as well where VEGFf showed twofold greater Akt activation than intact VEGF (Fig. 8C). These findings suggest that VEGFf binding to VEGFR1 can mediate Akt activation in these cells.
Fig. 8.
VEGFf activities on endothelial cells. A: serum-starved bovine pulmonary artery endothelial cells (bPAEC) were treated with VEGF or VEGFf (10× = 0.23 nM or 1× = 0.023 nM) or both for 10 min. Cells were extracted from duplicate wells and analyzed by immunoblot for activated extracellular signal-regulated kinase (ERK) 1/2 [phosphorylated (p) ERK1/2] and total ERK1/2. pERK1/2/ERK1/2 indicates the average density ratio of the activated pERK and total ERK bands for each condition. B: serum-starved bPAEC were treated with VEGF and VEGFf (0.23 nM each) for 10 min at 37°C. The cells from duplicate wells were extracted and analyzed by immunoblot for phosphorylated protein kinase B (Akt) and total Akt levels. C: bPAEC were treated with VEGF and VEGFf (0.45 nM each), and the CASE Akt enzyme-linked immunosorbent assay (ELISA) was used to determine Akt activation. Each data point is the mean of triplicate determinations ± SE. ANOVA followed by multiple-comparison t-tests reveal significant differences between all treatment groups (P < 0.01).
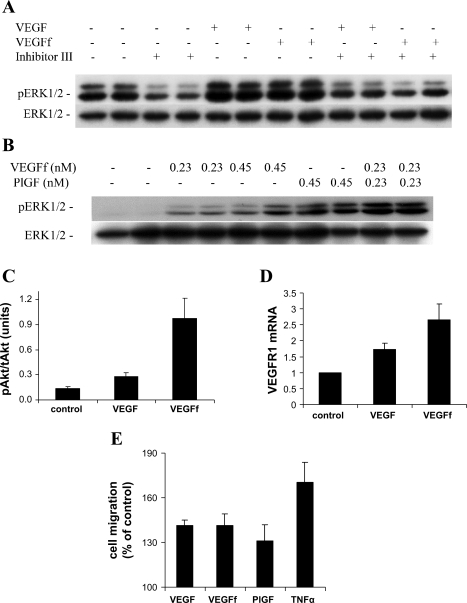
To further explore the biological effects mediated by VEGFf, the mouse macrophage/monocyte cell line RAW 264.7 was used. This cell line expresses only VEGFR1 and has no detectable VEGFR2 expression. RT-PCR analysis confirmed the expression of VEGFR1 mRNA in these cells, whereas VEGFR2 mRNA was undetectable, even after 45 cycles (data not shown). When we treated RAW 264.7 cells with VEGF and VEGFf, we saw that both were similarly capable of activating ERK1/2 (Fig. 9A). This is in contrast to the response we observed with endothelial cells, where VEGFf alone had no effect (Fig. 8A). To evaluate whether the ERK1/2 activation was mediated through VEGFR1, we preincubated cells with a known VEGFR kinase inhibitor (VEGFR kinase inhibitor III), before stimulation with VEGF or VEGFf. The VEGF and VEGFf effects on ERK1/2 activation were completely abolished in the presence of the VEGFR kinase inhibitor (Fig. 9A). To ensure that the effect of VEGFf is not complicated by the presence of DFP-inactivated NE in the VEGFf preparation, we treated cells with NE that had been inactivated with DFP and observed no ERK1/2 activation (data not shown). It is known that PlGF has strong homology to VEGF and binds and mediates its effects through VEGFR1 (14). Thus, we tested the effects of PlGF on RAW 264.7 cells and observed ERK1/2 activation, similar to that observed with VEGF/VEGFf (Fig. 9B).
Fig. 9.
VEGFf activities on RAW 264.7 cells. A: RAW 264.7 cells were treated for 1.5 h with VEGFR kinase inhibitor III (10 μM) or left untreated and then incubated with VEGF and VEGFf (0.23 nM each) for 10 min. Cells from duplicate wells were extracted and analyzed by immunoblot for pERK1/2 and total ERK1/2. B: RAW 264.7 cells were treated with VEGFf (0.23 and 0.45 nM) and placental growth factor (PlGF, 0.45 and 0.23 nM) for 10 min; cells from duplicate wells were extracted and analyzed for pERK1/2 and total ERK1/2. C: RAW 264.7 cells were treated with VEGF and VEGFf (0.45 nM each), and Akt activation was measured using the CASE Akt ELISA (n = 6; each point is average ± SE): ANOVA (P < 0.01); VEGF and VEGFf vs. control (P < 0.01); VEGF vs. VEGFf (P < 0.01). D: RAW 264.7 cells were treated with 0.45 nM VEGF or VEGFf for 30 h; mRNA was isolated, and real-time PCR was performed using ABI TaqMan gene expression assays: VEGFR1 and the eukaryotic 18S rRNA endogenous control (n = 9; each point is average ± SE): ANOVA (P < 0.01); VEGF and VEGFf vs. control (P < 0.001); VEGF vs. VEGFf (P < 0.05). E: RAW 264.7 cells (5,000 cells/well) were seeded on the upper membrane of Transwell cell migration chambers. VEGF, VEGFf, PlGF, and TNF-α (0.45, 0.45, 0.45, and 1 nM, respectively) were added to the lower chamber. The cells were incubated for 4 h at 37°C, and migrated cells were detected using phosphorescence (n = 4; each point is average ± SE): ANOVA (P < 0.05) all growth factors vs. control (P < 0.05). Similar results were obtained in four separate experiments.
We also evaluated Akt activation in RAW 264.7 cells in response to VEGFf treatment. Similar to our observations with endothelial cells, both VEGF and VEGFf activated Akt, and VEGFf appeared to be more effective (Fig. 9C). VEGF is known to stimulate the expression of VEGFR1 (4); thus, we tested the effects of VEGF and VEGFf on VEGFR1 mRNA levels. RAW 264.7 cells were treated with 0.45 nM VEGF or VEGFf for 30 h, and total RNA was isolated and analyzed by real-time PCR. Figure 9D shows that both VEGF and VEGFf treatment increase VEGFR1 mRNA levels and that VEGFf was more potent in doing so.
Because it had been reported that VEGF and PlGF are monocyte chemoattractants (14), we assessed whether VEGFf has any effect on monocyte migration. To do so, we added VEGF, VEGFf, PlGF, or TNF-α to the lower chamber of a migration plate and measured RAW 264.7 cell migration after 4 h. VEGF, VEGFf, and PlGF stimulated RAW cell migration to a similar extent, and TNF-α was somewhat more effective at the concentration tested. The addition of serum to the lower chamber enhanced RAW migration as well, as previously reported (57) (data not shown).
DISCUSSION
VEGF is a critical factor for normal vascular development and angiogenesis, with even single allele deletions being embryonic lethal between days 11 and 12 (13). In addition to its well-known role as an endothelial cell survival factor and mitogen, recent data have suggested important roles for VEGF in other cell types. For example, VEGF has been demonstrated to stimulate monocyte chemotaxis (5, 14), enhance colony formation of granulocyte macrophage progenitors (9), and increase B cell production (31). VEGF has also been suggested to stimulate surfactant production by alveolar type II cells (15) and induce distal airway epithelial cell proliferation (8) in explant and whole lung studies; however, studies with isolated alveolar type II cells suggest that these effects are indirect and likely reflect paracrine interactions involving additional lung cell types (30, 62). Thus the function of VEGF within adult tissues and organs is likely to involve multiple cell types and be controlled at several levels. Consequently, it is not surprising that numerous studies have implicated VEGF and VEGF family members as active participants in lung pathology, with both loss of VEGF function and excessive activity leading to dysfunction.
In the present study, we investigated the potential link between NE, a well-known mediator of lung injury, and VEGF. We observed that VEGF is subject to partial cleavage by NE, leading to the generation of a VEGFf with altered activity. Whereas intact VEGF165 binds VEGFR1 and -2 and heparin, the NE-generated VEGFf shows a selective loss of binding to VEGFR2 and heparin while retaining VEGFR1 binding. Interestingly, the altered receptor binding translated to a loss of signaling potential in endothelial cells, as noted by reduced ERK1/2 activation, yet no loss of activity in RAW 264.7 monocyte/macrophage cells. Moreover, the NE-generated VEGFf showed enhanced ability to stimulate Akt phosphorylation. Although we do not yet know if the NE-generated VEGFf plays important roles in mediating the pulmonary response to injury, our findings suggest that the role of extracellular proteolytic processing of VEGF should be considered as this process is more fully studied.
The generation of bioactive VEGFf by NE was not reflective of digestion by all extracellular proteases, since MMP9 did not lead to VEGFf production. In addition, NE digestion did not appear to lead to the production of partially cleaved fragments with all growth factors/cytokines tested. Indeed, FGF2, which has been shown to be released from ECM storage sites by elastase injury (12), appeared to be significantly resistant to NE cleavage, whereas TNF-α and PDGF-AA showed somewhat distinct digestion profiles. Interestingly, NE digestion of PDGF-AA, a structural analog of VEGF, appeared to lead to the generation of a fragment similar to that observed with VEGF. It will be important to consider the potential functions of these processed forms of growth factors in mediating the pulmonary response to proteolytic injury.
VEGF165 is secreted by a variety of cell types in the lung, but a significant fraction remains bound to the ECM (59). ECM-bound VEGF has been shown to be released by plasmin cleavage, which generates a bioactive VEGFf (33). Recent findings have also shown that VEGF can be modulated by other proteases as well (2, 45). Here we show that VEGF bioavailability and activity might also be controlled by processing through the action of NE. Hence, at sites of tissue injury or inflammation, NE released by activated neutrophils will cause ECM degradation, and potentially the processing of VEGF to a form that will selectively activate cells via VEGFR1 (Fig. 10). Our finding that VEGF is processed to a new form by NE is interesting in light of a number of recent findings that have identified cryptic protein activities generated by proteolytic attack on the ECM (16). In particular, a large number of endogenous angiogenesis inhibitors have been demonstrated to be derived by the proteolytic processing of other ECM proteins such as type XVIII collagen, type IV collagen, perlecan, and fibulins 1 and 5 (16, 38, 50, 54, 56, 81). Moreover, even nonprotein components of the ECM, such as hyaluronan and heparan sulfate, have been demonstrated to function alternatively as inhibitors or stimulators of angiogenesis depending on the size of the polysaccharide chain, indicating a mechanism of control related to ECM degradation (60, 69). Thus the proteolytic processing of ECM-resident VEGF might also have important implications for angiogenic control in response to tissue injury and damage.
Analysis of NE-produced VEGFf by MS indicates that NE cleaves VEGF at its NH2 and COOH terminus, with some suggestion that there might also be cleavage sites within the internal region of the protein chain as well (Fig. 2). Internal cleavage would predict the generation of fragments of lower molecular mass than the major fragment we observe under reducing SDS-PAGE; however, it is important to note that the highly disulfide-linked cystine knot structure might allow intrachain disulfide bonds to resist standard reduction conditions (51, 52). In any case, the cleavage within the canonical heparin-binding domain in the COOH terminus of VEGF (21) is consistent with the loss of heparin affinity of the VEGFf compared with the intact VEGF165. The decreased affinity for heparin binding would potentially make NE-generated VEGFf more diffusible such that the ECM-released fragments could stimulate cells (e.g., macrophages) at sites distant to the primary site of NE action. Thus the powerful proteolytic activity of NE might not only participate in enhancing migration of neutrophils by removing connective tissue, but it might also generate active VEGFf that could contribute to further inflammatory cell recruitment and activation.
VEGFf has lost the ability to bind VEGFR2, the main mediator of VEGF activities, whereas it has retained the ability to bind VEGFR1 (Fig. 7). The function of VEGFR1 has been an active topic of debate, with studies indicating that VEGFR1 functions mainly as a “decoy” receptor, preventing VEGF from binding to VEGFR2 on the vascular endothelium (58). However, the expression of VEGFR1 on nonendothelial cell types such as pulmonary epithelial cells and monocyte/macrophages suggests additional roles for this receptor. VEGF is a strong activator of ERK1/2 via VEGFR2 (35) in endothelial cells, and, consistent with this finding, VEGFf did not cause ERK1/2 activation in the pulmonary endothelial cells when added alone. Interestingly, when added together with intact VEGF165, VEGFf appeared to potentiate ERK1/2 activation (Fig. 8A). This finding suggests that VEGFf might shield intact VEGF from binding to VEGFR1, making it more available to mediate its effects through VEGFR2 on endothelial cells. A similar function has been suggested for the VEGFR1 ligand PlGF (47). However, when we investigated Akt activation, a major factor implicated in VEGF-mediated cell survival (28, 74), we were surprised to find that VEGFf was a more potent activator of Akt compared with the intact VEGF (Fig. 8). These findings suggest that VEGFf can signal via VEGFR1 to activate Akt in endothelial cells. Although VEGFR1 certainly shows impaired kinase activity compared with VEGFR2, site-directed mutagenesis has identified several phosphorylated residues in VEGFR1 capable of interacting with SH2-domain proteins such as phosphatidylinositol 3-kinase (34).
The observation that VEGF and PlGF are potent activators of monocyte/macrophage migration (1, 5) led us to investigate the effects of the NE-generated VEGFf in the mouse macrophage/monocyte cell line RAW 264.7. RAW 264.7 cells express VEGFR1 with no detectable VEGFR2, and treatment of these cells with VEGFf led to Akt and ERK1/2 activation, increased VEGFR1 expression, and stimulation of chemotaxis. The ability of VEGFf and intact VEGF165 to activate ERK1/2 in RAW 264.7 cells, in a VEGFR2-independent manner, suggests that distinct signaling pathways are present in these cells that are not in endothelial cells. Thus it is possible that VEGFf can activate a range of activities in nonendothelial cells within elastase-damaged lungs via cell type-specific VEGFR1 signaling.
In the present study, we report that VEGF165 is a substrate of NE and that the partial digestion of VEGF produces a fragment with altered activity and potentially altered function compared with intact VEGF. Although it is not clear what role NE-generated VEGFf play in mediating the tissue response to injury in the lung, it is intriguing to speculate that the functional response to VEGFf might relate to the ratio of VEGFf to intact VEGF. Whereas NE-digestion of ECM might release both fragmented and intact VEGF through the action of NE directly on VEGF and indirectly on VEGF-binding sites [e.g., HSPG (10, 12)]; it is possible that, under certain conditions, VEGFf can function to enhance the activity of VEGF on endothelial cells, and further function by diffusing to distant sites to recruit macrophages to the site of injury (Fig. 10). However, chronic or excessive elastolysis might lead to complete conversion of VEGF to VEGFf, with the concomitant loss of critical endothelial activities and excessive inflammatory cell activation. A more complete understanding of the role of VEGF within the lung will need to consider the possible function of proteolytic fragments of VEGF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-56200 and HL-088572 and by a departmental grant from the Massachusetts Lions Eye Research Fund.
Acknowledgments
We thank Alfred G. Tamayo for providing helpful advice and protocols for the use of RAW 264.7 cells. We are very grateful to Dr. Steven Gygi and the Harvard Medical School laboratory for assistance with the mass spectrometric analysis of VEGF and VEGFf. We thank Celeste Rich for providing primary neonatal rat lung fibroblasts and aortic smooth muscle cells and for valuable assistance in conducting real-time PCR analysis of VEGFR1 expression.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res 62: 2749–2752, 2002. [PubMed] [Google Scholar]
- 2.Ai S, Cheng X, Inoue A, Nakamura K, Okumura K, Iguchi A, Murohara T, Kuzuya M. Angiogenic activity of bFGF and VEGF suppressed by proteolytic cleavage by neutrophil elastase. Biochem Biophys Res Commun 364: 395–401, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Athanassiades A, Lala PK. Role of placenta growth factor (PIGF) in human extravillous trophoblast proliferation, migration and invasiveness. Placenta 19: 465–473, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Barleon B, Siemeister G, Martiny-Baron G, Weindel K, Herzog C, Marme D. Vascular endothelial growth factor up-regulates its receptor fms-like tyrosine kinase 1 (FLT-1) and a soluble variant of FLT-1 in human vascular endothelial cells. Cancer Res 57: 5421–5425, 1997. [PubMed] [Google Scholar]
- 5.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87: 3336–3343, 1996. [PubMed] [Google Scholar]
- 6.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 22: 672–688, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Black LD, Brewer KK, Morris SM, Schreiber BM, Toselli P, Nugent MA, Suki B, Stone PJ. Effects of elastase on the mechanical and failure properties of engineered elastin-rich matrices. J Appl Physiol 98: 1434–1441, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Brown KR, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol 281: L1001–L1010, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Broxmeyer HE, Cooper S, Li ZH, Lu L, Song HY, Kwon BS, Warren RE, Donner DB. Myeloid progenitor cell regulatory effects of vascular endothelial cell growth factor. Int J Hematol 62: 203–215, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Buczek-Thomas JA, Chu CL, Rich CB, Stone PJ, Foster JA, Nugent MA. Heparan sulfate depletion within pulmonary fibroblasts: implications for elastogenesis and repair. J Cell Physiol 192: 294–303, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Buczek-Thomas JA, Lucey EC, Stone PJ, Chu CL, Rich CB, Carreras I, Goldstein RH, Foster JA, Nugent MA. Elastase mediates the release of growth factors from lung in vivo. Am J Respir Cell Mol Biol 31: 344–350, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Buczek-Thomas JA, Nugent MA. Elastase-mediated release of heparan sulfate proteoglycans from pulmonary fibroblast cultures. A mechanism for basic fibroblast growth factor (bFGF) release and attenuation of bfgf binding following elastase-induced injury. J Biol Chem 274: 25167–25172, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 271: 17629–17634, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8: 702–710, 2002. [DOI] [PubMed] [Google Scholar]
- 16.D'Amore PA, Ng YS. Tales of the cryptic: unveiling more angiogenesis inhibitors. Trends Mol Med 8: 313–315, 2002. [DOI] [PubMed] [Google Scholar]
- 17.DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, Davies PF, Savani RC. Loss of PECAM-1 function impairs alveolarization. J Biol Chem 281: 8724–8731, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Dicamillo SJ, Yang S, Panchenko MV, Toselli PA, Naggar EF, Rich CB, Stone PJ, Nugent MA, Panchenko MP. Neutrophil elastase-initiated EGFR/MEK/ERK signaling counteracts stabilizing effect of autocrine TGF-beta on tropoelastin mRNA in lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 291: L232–L243, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol 174: 215–222, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Dvorak HF Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20: 4368–4380, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Fairbrother WJ, Champe MA, Christinger HW, Keyt BA, Starovasnik MA. Solution structure of the heparin-binding domain of vascular endothelial growth factor. Structure 6: 637–648, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Faris B, Tan OT, Toselli P, Franzblau C. Long-term neonatal rat aortic smooth muscle cell cultures: a model for the tunica media of a blood vessel. Matrix 12: 185–188, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25: 581–611, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Fong GH, Klingensmith J, Wood CR, Rossant J, Breitman ML. Regulation of flt-1 expression during mouse embryogenesis suggests a role in the establishment of vascular endothelium. Dev Dyn 207: 1–10, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66–70, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Foster JA, Rich CB, Miller MF. Pulmonary fibroblasts: an in vitro model of emphysema. Regulation of elastin gene expression. J Biol Chem 265: 15544–15549, 1990. [PubMed] [Google Scholar]
- 28.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273: 30336–30343, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Goerges AL, Nugent MA. Regulation of vascular endothelial growth factor binding and activity by extracellular pH. J Biol Chem 278: 19518–19525, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Grubor B, Meyerholz DK, Lazic T, DeMacedo MM, Derscheid RJ, Hostetter JM, Gallup JM, DeMartini JC, Ackermann MR. Regulation of surfactant protein and defensin mRNA expression in cultured ovine type II pneumocytes by all-trans retinoic acid and VEGF. Int J Exp Pathol 87: 393–403, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med 193: 1005–1014, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277: 2002–2004, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 267: 26031–26037, 1992. [PubMed] [Google Scholar]
- 34.Igarashi K, Isohara T, Kato T, Shigeta K, Yamano T, Uno I. Tyrosine 1213 of Flt-1 is a major binding site of Nck and SHP-2. Biochem Biophys Res Commun 246: 95–99, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Ilan N, Mahooti S, Madri JA. Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J Cell Sci 111: 3621–3631, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Janoff A Elastase in tissue injury. Annu Rev Med 36: 207–216, 1985. [DOI] [PubMed] [Google Scholar]
- 37.Janoff A Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis 132: 417–433, 1985. [DOI] [PubMed] [Google Scholar]
- 38.John H, Radtke K, Standker L, Forssmann WG. Identification and characterization of novel endogenous proteolytic forms of the human angiogenesis inhibitors restin and endostatin. Biochim Biophys Acta 1747: 161–170, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Kanazawa H, Asai K, Hirata K, Yoshikawa J. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med 114: 354–358, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 163: 737–744, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106: 1311–1319, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol 451: 1–10, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood 99: 2397–2407, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, Elias JA. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 10: 1095–1103, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol 169: 681–691, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Gerber SA, Rudner AD, Beausoleil SA, Haas W, Villen J, Elias JE, Gygi SP. Large-scale phosphorylation analysis of alpha-factor-arrested Saccharomyces cerevisiae. J Proteome Res 6: 1190–1197, 2007. [DOI] [PubMed] [Google Scholar]
- 47.MacGabhann F, Popel AS. Model of competitive binding of vascular endothelial growth factor and placental growth factor to VEGF receptors on endothelial cells. Am J Physiol Heart Circ Physiol 286: H153–H164, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci USA 95: 15809–15814, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marwick JA, Stevenson CS, Giddings J, Macnee W, Butler K, Rahman I, Kirkham PA. Cigarette smoke disrupts the VEGF165-VEGFR2 receptor signaling complex in rat lungs and patients with COPD: morphological impact of VEGFR2 inhibition. Am J Physiol Lung Cell Mol Physiol 290: L897–L908, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Mongiat M, Sweeney SM, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem 278: 4238–4249, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Muller YA, Christinger HW, Keyt BA, de Vos AM. The crystal structure of vascular endothelial growth factor (VEGF) refined to 1.93 A resolution: multiple copy flexibility and receptor binding. Structure 5: 1325–1338, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Muller YA, Heiring C, Misselwitz R, Welfle K, Welfle H. The cystine knot promotes folding and not thermodynamic stability in vascular endothelial growth factor. J Biol Chem 277: 43410–43416, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Muller YA, Li B, Christinger HW, Wells JA, Cunningham BC, de Vos AM. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci USA 94: 7192–7197, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res 74: 85–89, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nugent MA, Edelman ER. Kinetics of basic fibroblast growth factor binding to its receptor and heparan sulfate proteoglycan: a mechanism for cooperativity. Biochemistry 31: 8876–8883, 1992. [DOI] [PubMed] [Google Scholar]
- 56.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res 65: 3967–3979, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Paavola CD, Hemmerich S, Grunberger D, Polsky I, Bloom A, Freedman R, Mulkins M, Bhakta S, McCarley D, Wiesent L, Wong B, Jarnagin K, Handel TM. Monomeric monocyte chemoattractant protein-1 (MCP-1) binds and activates the MCP-1 receptor CCR2B. J Biol Chem 273: 33157–33165, 1998. [DOI] [PubMed] [Google Scholar]
- 58.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269: 25646–25654, 1994. [PubMed] [Google Scholar]
- 59.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 4: 1317–1326, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Presta M, Leali D, Stabile H, Ronca R, Camozzi M, Coco L, Moroni E, Liekens S, Rusnati M. Heparin derivatives as angiogenesis inhibitors. Curr Pharm Des 9: 553–566, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Rahimi N VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front Biosci 11: 818–829, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raoul W, Chailley-Heu B, Barlier-Mur AM, Delacourt C, Maitre B, Bourbon JR. Effects of vascular endothelial growth factor on isolated fetal alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 286: L1293–L1301, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Raschke WC, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell 15: 261–267, 1978. [DOI] [PubMed] [Google Scholar]
- 64.Rhodes JM, Simons M. The extracellular matrix and blood vessel formation: not just a scaffold. J Cell Mol Med 11: 176–205, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rich CB, Nugent MA, Stone P, Foster JA. Elastase release of basic fibroblast growth factor in pulmonary fibroblast cultures results in down-regulation of elastin gene transcription. A role for basic fibroblast growth factor in regulating lung repair. J Biol Chem 271: 23043–23048, 1996. [DOI] [PubMed] [Google Scholar]
- 66.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci 114: 853–865, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol 163: 2329–2335, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am J Respir Cell Mol Biol 32: 367–372, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol 26: 58–68, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Snider GL, Ciccolella DE, Morris SM, Stone PJ, Lucey EC. Putative role of neutrophil elastase in the pathogenesis of emphysema. Ann NY Acad Sci 624: 45–59, 1991. [DOI] [PubMed] [Google Scholar]
- 71.Spencer JL, Stone PJ, Nugent MA. New insights into the inhibition of human neutrophil elastase by heparin. Biochemistry 45: 9104–9120, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Stricklin GP, Jeffrey JJ, Roswit WT, Eisen AZ. Human skin fibroblast procollagenase: mechanisms of activation by organomercurials and trypsin. Biochemistry 22: 61–68, 1983. [DOI] [PubMed] [Google Scholar]
- 73.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 97: 1559–1566, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Thakker GD, Hajjar DP, Muller WA, Rosengart TK. The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem 274: 10002–10007, 1999. [DOI] [PubMed] [Google Scholar]
- 75.Tsao PN, Su YN, Li H, Huang PH, Chien CT, Lai YL, Lee CN, Chen CA, Cheng WF, Wei SC, Yu CJ, Hsieh FJ, Hsu SM. Overexpression of placenta growth factor contributes to the pathogenesis of pulmonary emphysema. Am J Respir Crit Care Med 169: 505–511, 2004. [DOI] [PubMed] [Google Scholar]
- 76.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 29: 88–97, 2003. [DOI] [PubMed] [Google Scholar]
- 77.Vlodavsky I, Fuks Z, Ishai-Michaeli R, Bashkin P, Levi E, Korner G, Bar-Shavit R, Klagsbrun M. Extracellular matrix-resident basic fibroblast growth factor: implication for the control of angiogenesis. J Cell Biochem 45: 167–176, 1991. [DOI] [PubMed] [Google Scholar]
- 78.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev 92: 55–81, 2000. [DOI] [PubMed] [Google Scholar]
- 79.Weiss SJ Tissue destruction by neutrophils. N Engl J Med 320: 365–376, 1989. [DOI] [PubMed] [Google Scholar]
- 80.Wolfe BL, Rich CB, Goud HD, Terpstra AJ, Bashir M, Rosenbloom J, Sonenshein GE, Foster JA. Insulin-like growth factor-I regulates transcription of the elastin gene. J Biol Chem 268: 12418–12426, 1993. [PubMed] [Google Scholar]
- 81.Xie L, Palmsten K, MacDonald B, Kieran MW, Potenta S, Vong S, Kalluri R. Basement membrane derived fibulin-1 and fibulin-5 function as angiogenesis inhibitors and suppress tumor growth. Exp Biol Med (Maywood) 233: 155–162, 2008. [DOI] [PubMed] [Google Scholar]
- 82.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ Jr, Chapman HA Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest 106: 1081–1093, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]