Abstract
Phospholamban has been suggested to be a key regulator of cardiac sarcoplasmic reticulum (SR) Ca cycling and contractility and a potential therapeutic target in restoring the depressed Ca cycling in failing hearts. Our understanding of the function of phospholamban stems primarily from studies in genetically altered mouse models. To evaluate the significance of this protein in larger mammalian species, which exhibit Ca cycling properties similar to humans, we overexpressed phospholamban in adult rabbit cardiomyocytes. Adenoviral-mediated gene transfer, at high multiplicities of infection, resulted in an insignificant 1.22-fold overexpression of phospholamban. There were no effects on twitch Ca-transient amplitude or decay under basal or isoproterenol-stimulated conditions. Furthermore, the SR Ca load and Na/Ca exchanger function were not altered. These apparent differences between phospholamban overexpression in rabbit compared with previous findings in the mouse may be due to a significantly higher (1.5-fold) endogenous phospholamban-to-sarco(endo)plasmic reticulum Ca-ATPase (SERCA) 2a ratio and potential functional saturation of SERCA2a by phospholamban in rabbit cardiomyocytes. The findings suggest that important species-dependent differences in phospholamban regulation of SERCA2a occur. In larger mammals, a higher fraction of SERCA2a pumps are regulated by phospholamban, and this may influence therapeutic strategies to enhance cardiac contractility and functional cardiac reserve.
Keywords: calcium, adenoviral, contractility
fine-tuned regulation of cardiac function is mediated by the sympathetic nervous system on a beat-to-beat basis and under increased demand conditions. The signal transduction pathway underlying these effects involves the cAMP axis and key downstream subcellular protein substrates. The major phosphoproteins, regulating Ca cycling and contractility in the heart, are troponin I, the ryanodine receptor, and phospholamban (PLN). PLN regulates the Ca affinity of the sarcoplasmic reticulum (SR) Ca-ATPase. Dephosphorylated PLN is inhibitory; its phosphorylation restores the attenuated SR Ca-ATPase activity, facilitating Ca uptake in the SR and muscle relaxation (21, 28). Consequently, the SR Ca load is augmented, resulting in greater Ca release and increased contractility (6).
The in vivo role of PLN has been elucidated using various genetically altered mouse models (18–20). Collectively, these studies revealed a linear correlation between the expression levels of PLN in the mouse heart and Ca cycling parameters. Thus it has been suggested that PLN may constitute an important therapeutic target in the human failing heart, which exhibits augmented PLN inhibition through increases in the relative levels of PLN vs. sarco(endo)plasmic reticulum Ca-ATPase (SERCA) 2a and dephosphorylation of PLN (7, 14, 23, 27). Indeed, modulation of PLN expression and/or activity appeared to restore function in several models of genetic or experimental heart failure (10, 11, 16, 17, 24).
However, most previous studies on the functional role of PLN in vivo and its therapeutic efficacy have been generated in rodent models, which exhibit significant fundamental differences in cardiac physiology and Ca cycling compared with humans. Specifically, SERCA2a is responsible for nearly all (∼92%) of Ca removal from the cytosol, whereas the Na/Ca exchanger (NCX) contributes only ∼7% during muscle relaxation in mice. In contrast, NCX plays a more prominent role in human hearts where it is responsible for removing 28% of the Ca during each cycle (4), under basal conditions. Therefore, in humans, SERCA2a is initially responsible for a lower percentage of Ca removed compared with mice (and the total rate of SR Ca uptake is also slower in humans), such that the role of the SR is less dominant in the Ca cycling in higher mammalian species. Mouse and rat also have a very brief action potential (lacking a plateau) compared with human or rabbit ventricular myocytes (4). Another difference is the expression of the fast α-myosin heavy chain (MHC) isoform in the mouse, whereas the slow β-MHC isoform is expressed in the human heart. Finally, cardiac reserve, the capacity to increase cardiac output, as well as the capacity to increase heart rate, are minimal in the mouse, whereas these are of paramount importance in humans (21). This cardiac reserve is compromised in heart failure, and it diminishes the heart's ability to regulate contractility (26). Cardiac reserve is connected closely with PLN modulation, insofar as cardiac reserve represents the capacity to increase SR Ca loading (10). Thus the effects of altered PLN levels in humans may be more pronounced or even qualitatively different from those in mice, as suggested by earlier studies (10, 13).
Given the differences in SR Ca cycling between mice and humans, it became important to further characterize the role of PLN in a species exhibiting cardiac Ca cycling properties, electrophysiology, and contractile protein characteristics similar to humans. Therefore, the rabbit was chosen as a model system. Initially, we attempted to elucidate the function of PLN in rabbit hearts through the generation of a model with cardiac-specific PLN overexpression. However, the use of the rabbit β-MHC promoter resulted in high PLN levels in slow-twitch skeletal fibers, and transgenic rabbits exhibited muscular dystrophy characteristics (25). Thus a limited number of viable lines were available for further characterization, and these exhibited relatively low PLN overexpression (∼1.34-fold) levels. Interestingly, these transgenic rabbits indicated no cardiac pathology, and function was normal, precluding further evaluation of PLN functional significance in a model with Ca-cycling characteristics that resemble the human (25).
In the present study, we have extended our examination of the role of PLN overexpression in rabbit cardiomyocytes, using adenoviral-mediated methodology, in an attempt to obtain high levels of PLN overexpression and further evaluate its regulatory effects in rabbit hearts. We show that the highest level of PLN overexpression achieved in isolated rabbit cardiomyocytes was 1.22-fold, and this did not yield any significant alterations in Ca transients. These findings on either acute or chronic overexpression of PLN in the rabbit suggest that only a moderate increase in PLN expression levels can be accommodated in rabbit cardiac SR, and this increase is not sufficient to alter cellular Ca handling under basal or isoproterenol-stimulated conditions.
MATERIALS AND METHODS
Generation of rabbit PLN adenoviruses.
The rabbit PLN cDNA was a gift from Dr. David H. MacLennan (GenBank accession no. M63600). The Ad-Easy XL system was used to generate an adenovirus encoding rabbit PLN wild type (Ad.PLN) in Ad-293 cells. An adenovirus that encoded a green fluorescent protein (Ad.GFP) was generated as a control (Fig. 1A). These viruses were purified using the Adeno-X Virus Mini Purification Kit (Clontech) and titered using the Adeno-X Rapid Titer Kit (Clontech).
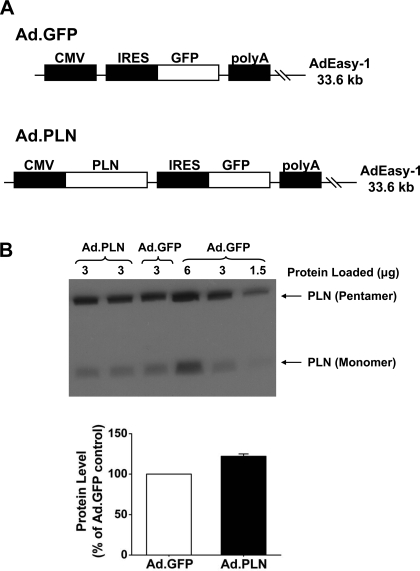
Fig. 1.
A: to create a recombinant adenovirus that expresses wild-type phospholamban (PLN), the cDNA of rabbit PLN was subcloned from the pGEX-6P-3 vector into the pShuttle-IRES-human recombinant green fluorescent protein (GFP)-1 vector and inserted into the AdEasy-1 viral backbone by homologous recombination. An adenovirus expressing GFP was used as a control. B: representative immunoblot of cardiomyocyte lysates infected with GFP (Ad.GFP) or wild-type PLN (Ad.PLN) adenoviruses. The samples were separated by SDS-PAGE and then probed with a PLN monoclonal antibody to show pentameric and monomeric PLN. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Infection with Ad.PLN showed a nonsignificant increase of 1.22-fold in PLN protein levels compared with Ad.GFP infection. Protein levels were normalized to that of Ad.GFP control within the same blot; n = 7 hearts. Values are means ± SE.
Cardiomyocyte Ca-handling measurements.
Left ventricular myocytes were isolated from New Zealand White rabbits to be used for infection by adenoviruses, as previously described (2). Animal research was conducted under approved protocols by institutional animal care and use committees at Loyola University of Chicago (protocol no. LU107290 approval 2/24/2005) and at the University of California, Davis (protocol no. 13259 approval 3/27/2008). Briefly, rabbits were anesthetized by intravenous injection of pentobarbital sodium (50–70 mg/kg). Hearts were excised quickly and placed on a Langendorff perfusion apparatus. Hearts were perfused for 5–7 min with nominally Ca-free Dulbecco's minimum essential medium solution, and then perfusion was switched to the same solution containing 1 mg/ml collagenase. When the heart became flaccid, the ventricles were dispersed and filtered. The cell suspension was washed several times in medium with Ca concentration ([Ca]) = 150 μM. The cardiomyocytes were cultured in modified M199 medium and immediately infected for 2 h with adenovirus (multiplicity of infection = 500 or 1,000) that encoded either green fluorescent protein (Ad.GFP) or wild-type PLN (Ad.PLN).
Ca transients, SR Ca load, and cytosolic Ca removal fluxes were measured at 37°C 24 h after the end of infection. Myocytes were loaded with FURA-PE3, a leakage-resistant version of fura 2 (Teflabs), for 35–40 min with comparable deesterification time. The loading was the minimum needed to produce acceptable signal-to-noise ratios, which minimized dye-dependent Ca buffering. Fluorescence was recorded in three detection channels at 530 nm in response to excitation at 340, 380, and 480 nm delivered in rapid sequential fashion. Signals from 340 and 380 nm were converted to Ca using standard ratiometric methods, after correction for residual (compartmented) fluorescence obtained by lysing each cell at the end of the experiment, as well as cell-free system background. The response to 480 nm was used to verify that GFP was expressed in each selected cell. We did not choose cells where the GFP fluorescence was not discernible from background. For experimental use, cells were superfused with normal Tyrode (NT) solution containing (in mM) 140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 5 HEPES, pH 7.4. Twitches were field stimulated at 1 Hz to steady state. To measure SR Ca content and NCX Ca removal function, stimulation was interrupted, and, 2 s afterward, 10 mM caffeine (in NT) was rapidly applied to release Ca stores. After intracellular Ca concentration ([Ca]i) decline and caffeine washout, steady-state stimulation was resumed. To provide a confirmatory measure of SR Ca loading and to assess Ca removal by the slowest processes (sacrolemmal Ca-ATPase and mitochondrial Ca uptake), the cells were again stimulated at 1 Hz to steady state. Next, electrical stimulation was interrupted, and 0 Na/0 Ca solution (NaCl was replaced by LiCl, and CaCl2 was replaced by 1 mM EGTA) was added (3 s) to the cells, to block NCX. Subsequently, 10 mM caffeine (in 0 Na/0 Ca solution) was restored for 4 s. This resulted in a relatively flat-topped transient (see Fig. 2A). Again, the cells were stimulated to steady state at 1 Hz, 300 nM isoproterenol was applied, and (after ≥3 min) the entire protocol was repeated.
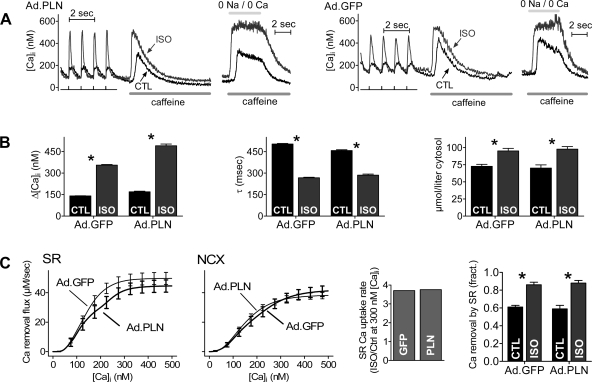
Fig. 2.
Ca transients, sarcoplasmic reticulum (SR) Ca content, and Ca removal system properties were measured in isolated rabbit cardiomyocytes that were infected with adenoviruses encoding GFP (Ad.GFP) as a control or encoding wild-type PLN (Ad.PLN) at a multiplicity of infection of 500 for 24 h. Myocytes were stimulated at a frequency of 1 Hz, and measurements were taken at 37°C. Isoproterenol (ISO, 300 nM) was added to stimulate the cells. A: trace on left, representative Ca transients in GFP-infected myocytes with and without ISO stimulation along with representative caffeine-induced Ca release traces with and without ISO stimulation in 0 Na/0 Ca, which represent relative SR Ca loads (peak amplitude), and representative caffeine-induced release traces with Na [normal Tyrode (NT)]. Trace on right, representative Ca transients in wild-type PLN-infected myocytes with and without ISO stimulation along with representative caffeine-induced Ca release traces with and without ISO stimulation in 0 Na/0 Ca, which represent relative SR Ca loads (peak amplitude), and representative caffeine-induced release traces with Na (NT). B: twitch Ca transient amplitude (nM), twitch Ca transient decay time constant (ms), and SR Ca loading (μmol/l cytosol). C: quantitative analysis of independent SR and Na/Ca exchange (NCX) Ca flux rates vs. intracellular Ca concentration ([Ca]i) in GFP- or PLN-infected myocytes (2 panels on left). Enhancement of sarco(endo)plasmic reticulum Ca-ATPase (SERCA) 2a function by isoproterenol assessed at 300 nM [Ca]i (3rd panel). Relative contributions of SR and NCX Ca flux to twitch [Ca]i decline for wild-type PLN-infected myocytes with and without ISO stimulation (panel on right). Values are means ± SE. GFP: n = 38–41 cells, 11 preparations; PLN: n = 22–26 cells, 7 preparations. Significance: *P < 0.05 vs. control conditions.
We used the combination of steady-state twitch and caffeine-induced Ca transients (± 0 Na/0 Ca solution) to analyze the separate contributions of SERCA2a, NCX, and slow systems (mitochondrial Ca uptake and sarcolemmal Ca-ATPase) to removal of transiently released Ca from the cytosol. The details are discussed extensively in Bassani et al. (1). SR Ca content was calculated from caffeine-induced Ca transient amplitude after converting free [Ca]i values to total cytosolic [Ca], using standard cytosolic buffering constants (15). Because the caffeine-induced Ca transient amplitudes were not different in NT or in 0 Na/0 Ca solution, we combined these data for SR Ca content values. In addition, we compared the steady-state twitch Δ[Ca]i to the Δ[Ca]i in caffeine to provide an indirect estimate of fractional SR Ca release during a twitch.
Quantitative immunoblotting.
For immunoblotting, cultured cardiomyocytes were harvested and lysed for 30 min at 4°C in lysis buffer as described previously (12). Rabbits or mice were anesthetized, and hearts were excised, washed with ice-cold PBS, and then quickly frozen in liquid nitrogen before homogenization. Proteins were then separated by 13% SDS-PAGE and transferred to nitrocellulose membranes. Nonspecific binding sites were blocked for 1 h at room temperature, using 5% dried milk in Tris-buffered saline (pH 7.4) containing 0.1% Tween 20. Membranes were probed overnight at 4°C with a specific primary monoclonal antibody (Affinity Bioreagents, Golden, CO) to PLN or primary monoclonal antibody (Affinity Bioreagents) to SERCA2a. Protein loading was normalized to either endogenous glyceraldehyde-3-phosphate dehydrogenase levels, using a specific primary monoclonal antibody (Abcam, Cambridge, MA), or endogenous calsequestrin levels, using a specific polyclonal antibody (Affinity Bioreagents). A mouse IgG horseradish peroxidase-linked whole antibody (from sheep; GE Healthcare, Buckinghamshire, UK) or a rabbit IgG horseradish peroxidase-linked whole antibody (from donkey; GE Healthcare) was used as a secondary antibody, and incubation time was 1.5 h at room temperature. The ECL Western blotting detection system (GE Healthcare) was used for detection of the signal, and the optical density of the bands was determined by ImageQuant 5.2 software (GE Healthcare).
Statistics.
All results are expressed as means ± SE for n experiments. Comparisons were evaluated by Student's t-test (P < 0.05 was considered statistically significant).
RESULTS AND DISCUSSION
Immunoblotting of infected rabbit cardiomyocytes.
To determine the effect of PLN overexpression in rabbit cardiac cells, isolated cardiomyocytes were infected with GFP (Ad.GFP) as control or wild-type PLN (Ad.PLN) adenoviruses. Quantitative immunoblotting of cell lysates revealed a maximal PLN overexpression level of 1.22-fold (Fig. 1B), which did not reach significance, compared with control virus. Interestingly, this level of overexpression was similar to the level detected in our transgenic rabbits (25). Multiple attempts to increase PLN expression, such as increasing the multiplicity of infection (up to 1,000) or time of infection (up to 72 h), failed to demonstrate higher PLN levels. Thus a multiplicity of infection of 500 and an infection time of 24 h were chosen, since these conditions resulted in the highest attainable levels of PLN expression and preserved cell viability.
Cardiac Ca cycling in infected rabbit cardiomyocytes.
To determine whether the relatively low levels of PLN overexpression in rabbit cells, compared with previous findings in mouse and rat (8, 9, 18), would elicit alterations in SR Ca cycling, Ca transients were measured in infected cardiomyocytes (Fig. 2A). There were no significant differences between the Ad.GFP and Ad.PLN groups under basal conditions for twitch Ca transient amplitude (GFP: 140 ± 9 nM; PLN: 169 ± 24 nM; Fig. 2B). After isoproterenol stimulation, both GFP (355 ± 26 nM)- and PLN (430 ± 46 nM)-infected cells showed significant increases in amplitude compared with basal levels (Fig. 2B). The increases were comparable between the two groups, and they did not differ in unpaired comparisons. Further comparison of the twitch Ca transient decay time constants indicated that these parameters were not significantly different between the GFP (503 ± 27 ms) and the PLN (457 ± 30 ms) cardiomyocytes under basal conditions (Fig. 2B). After isoproterenol simulation, Ca transients decayed significantly faster in both groups compared with basal conditions (GFP: 267 ± 24 ms; PLN: 286 ± 36 ms), but there was no significant difference in unpaired comparisons.
SR Ca load and removal systems in infected rabbit cardiomyocytes.
The lack of effects on Ca transients prompted further studies on the effect of PLN overexpression on SR Ca load. There was no significant difference of SR Ca loading under basal conditions between GFP (73 ± 3 μmol/l cytosol)- and PLN (70 ± 5 μmol/l cytosol)-infected rabbit cardiomyocytes (Fig. 2B). After isoproterenol simulation, both groups showed significantly larger SR Ca loading compared with basal conditions (GFP: 95 ± 4 μmol/l cytosol; PLN: 98 ± 4 μmol/l cytosol), again with no differences between GFP and PLN cardiomyocytes in unpaired comparisons.
To best focus our analysis of Ca removal fluxes from the cytosol (during twitch relaxation) on PLN overexpression and isoproterenol-dependent differences, the percentage of twitch Ca removed via SR uptake or by extrusion on NCX and slower processes (combined) was determined (Fig. 2C). Bar graphs represent the amount removed via SR uptake under control and isoproterenol conditions. For each bar, the combination of NCX and slower processes accounts for the remaining amount (data not shown) that brings the total to 100%. Both GFP (83 ± 2%) and PLN (79 ± 3%) cardiomyocytes had a significantly higher percentage of Ca removed by the SR after isoproterenol stimulation compared with basal conditions (GFP: 61 ± 2%; PLN: 60 ± 3%), but there were no differences between the stimulated groups.
PLN-to-SERCA2a ratio in mouse and rabbit hearts.
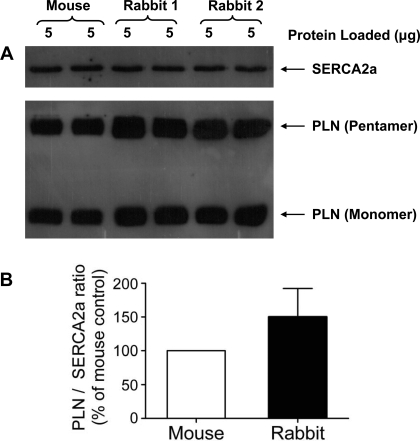
The low levels of “forced” PLN overexpression attainable in rabbit cardiomyocytes and the lack of any effects on cardiomyocyte function prompted us to determine the relative PLN and SERCA2a levels in rabbit and mouse hearts. Quantitative immunoblotting (Fig. 3A) of isolated cardiac homogenates from mice and rabbits showed that the PLN-to-SERCA2a ratio is ∼1.5-fold higher in rabbit vs. mouse hearts (Fig. 3B). This difference in the PLN-to-SERCA2a ratio is significant between the rabbit and mouse. Thus the SERCA2a in normal rabbit hearts is more fully saturated with PLN compared with mouse hearts, and therefore may be more fully inhibited by PLN in the rabbit. This might account for the failure to further slow SR Ca transport rate upon PLN overexpression in rabbit ventricular myocytes. Notably, the relative abundance of PLN pentamers vs. monomers was unaltered between the two species.
Fig. 3.
A: representative immunoblot of mouse and rabbit hearts. The samples were separated by SDS-PAGE and then probed with a SERCA2a monoclonal antibody and a PLN monoclonal antibody to show pentameric PLN and monomeric PLN. Calsequestrin (CSQ) was used as a loading control (not shown). B: rabbit hearts showed a significant 1.5-fold increase in the PLN-to-SERCA2a ratio when compared with mouse hearts. The PLN-to-SERCA2a ratio of the rabbit heart was normalized to the ratio of the mouse heart; n = 3 hearts. Values are means ± SE.
A second factor that could limit the functional effect of PLN overexpression is that the maximum achievable overexpression here was only ∼1.2-fold. It is unclear why this is the case, especially because higher extents of overexpression have been reported in mouse or rat hearts (8, 9, 18) and even in rabbit myocytes after 3 days in culture (22). Conceivably, our results reflect a functional saturation of the SR with PLN, whether due to SERCA2a saturation, overall crowding of the SR membrane (Note that SERCA2a and PLN are already at high concentration in myocytes), or a toxic effect of higher PLN expression levels. Attempts to increase the PLN expression levels by prolongation of the infection time resulted in significant cell death (data not shown; infection was standardized at 2 h in all reported experiments), whereas a higher multiplicity of infection up to 1,000 did not yield any higher PLN levels. There were no increases in cell death at multiplicity of infection up to 1,000 as long as the infection time was maintained at 2 h. Thus either acute overexpression of PLN in isolated cardiomyocytes or chronic overexpression in the heart (25) was associated with a similar low overexpression level in rabbit cardiomyocytes.
Let us consider the present results more quantitatively, while acknowledging the limitations of such with respect to precision. We previously reported that, in mouse heart, ∼40% of the SERCA2a pumps are functionally saturated by PLN (5). The 50% higher PLN-to-SERCA2a ratio we report here in rabbit vs. mouse would suggest that SERCA2a is ∼60% saturated by PLN in rabbit. Considering that the maximal increase in PLN was ∼1.3-fold by adenoviral infection, an increased saturation of rabbit SERCA2a to ∼80% is expected. This would result in a shift of the apparent K1/2 for [Ca]i dependence of SERCA2a function to higher [Ca]i by only 20–40 nM, an effect consistent with our data [K1/2 is increased by 14 nM, not significant (NS) in Fig. 2C]. However, such a small reduction in SERCA2a affinity may not be sufficient to reduce SR Ca content or twitch [Ca]i decline significantly in the presence of prominent NCX function in the rabbit (3).
We hypothesize that, in rabbit myocytes, SERCA2a is nearly saturated with PLN. This would also explain the unaltered isoproterenol responsiveness upon PLN overexpression observed here. In mouse, overexpression of PLN caused a 78% increase in K1/2 of Ca transport via SERCA2a, significantly slowed twitch [Ca]i decline, and enhanced the magnitude of the isoproterenol-induced inotropic and lusitropic effects (18). None of these effects were noted in rabbit cardiomyocytes, and similar limited effects were obtained in transgenic rabbits overexpressing PLN (25). If rabbit myocyte SERCA2a is already nearly fully associated with PLN, then PLN overexpression would neither inhibit the pump further nor allow greater isoproterenol-induced SERCA2a stimulation.
We conclude that, in rabbit ventricular myocytes, unlike mouse and rat myocytes, PLN appears to exert its maximal functional effect on SERCA2a. Moreover, PLN overexpression elicits no additional inhibitory effect on SERCA2a in rabbit nor does it increase the β-adrenergic reserve. It will be interesting to know whether this results from a higher affinity of PLN-SERCA2 complex or simply from the higher baseline expression of PLN vs. SERCA2a in rabbit. In this regard, it will be valuable to know whether human and rabbit ventricular myocytes are similar. This may also be important in understanding altered SERCA2a function in heart failure. For example, if SERCA2a is downregulated in heart failure, such that the PLN-to-SERCA2a ratio is increased (7), the more prominent functional defect may be a reduction in maximal velocity rather than reduced basal Ca affinity (if PLN is already saturating SERCA2a). Thus there are significant species differences in how overexpression of PLN may modulate SERCA2a function in ventricular myocytes. Moreover, interfering with PLN-dependent inhibition of SERCA2a may have stronger functional effects in species like rabbit (and potentially human) where most SERCA2a is basally inhibited by PLN (29).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01 HL-26057, HL-64018, HL-77101, HL-056370, HL-064724, and R37-HL-030077, the Leducq Foundation (E. G. Kranias), and an American Heart Association Post-Doctoral Fellowship (J. R. Waggoner).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol 476: 279–293, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassani JW, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J 68: 1453–1460, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers DM Cardiac Na/Ca exchange function in rabbit, mouse and man: what's the difference? J Mol Cell Cardiol 34: 369–373, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Brittsan AG, Carr AN, Schmidt AG, Kranias EG. Maximal inhibition of SERCA2 Ca(2+) affinity by phospholamban in transgenic hearts overexpressing a non-phosphorylatable form of phospholamban. J Biol Chem 275: 12129–12135, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Brittsan AG, Kranias EG. Phospholamban and cardiac contractile function. J Mol Cell Cardiol 32: 2131–2139, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol 33: 1345–1353, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Dash R, Kadambi V, Schmidt AG, Tepe NM, Biniakiewicz D, Gerst MJ, Canning AM, Abraham WT, Hoit BD, Liggett SB, Lorenz JN, Dorn GW 2nd, Kranias EG. Interactions between phospholamban and beta-adrenergic drive may lead to cardiomyopathy and early mortality. Circulation 103: 889–896, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Davia K, Hajjar RJ, Terracciano CMN, Kent NS, Ranu HK, O'Gara P, Rosenzweig A, Harding SE. Functional alterations in adult rat myocytes after overexpression of phospholamban with use of adenovirus. Physiol Genomics 1: 41–50, 1999. [DOI] [PubMed] [Google Scholar]
- 10.del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation 105: 904–907, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelhardt S, Hein L, Dyachenkow V, Kranias EG, Isenberg G, Lohse MJ. Altered calcium handling is critically involved in the cardiotoxic effects of chronic beta-adrenergic stimulation. Circulation 109: 1154–1160, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Fan GC, Chu G, Mitton B, Song Q, Yuan Q, Kranias EG. Small heat-shock protein Hsp20 phosphorylation inhibits beta-agonist-induced cardiac apoptosis. Circ Res 94: 1474–1482, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, Liggett SB, Dorn 2nd GW, MacLennan DH, Kremastinos DT, Kranias EG. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 111: 869–876, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houser SR, Piacentino IIIV, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol 32: 1595–1607, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Hove-Madsen L, Bers DM. Passive Ca buffering and SR Ca uptake in permeablized rabbit ventricular myocytes. Am J Physiol Cell Physiol 264: C677–C686, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, Date MO, Chrast J, Matsuzaki M, Peterson KL, Chien KR, Ross J Jr. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest 113: 727–736, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janczewski AM, Zahid M, Lemster BH, Frye CS, Gibson G, Higuchi Y, Kranias EG, Feldman AM, McTiernan CF. Phospholamban gene ablation improves calcium transients but not cardiac function in a heart failure model. Cardiovasc Res 62: 468–480, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn 2nd GW, Walsh RA, Kranias EG. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest 97: 533–539, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res 75: 401–409, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Luo W, Wolska BM, Grupp IL, Harrer JM, Haghighi K, Ferguson DG, Slack JP, Grupp G, Doetschman T, Solaro RJ, Kranias EG. Phospholamban gene dosage effects in the mammalian heart. Circ Res 78: 839–847, 1996. [DOI] [PubMed] [Google Scholar]
- 21.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4: 566–577, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Meyer M, Bluhm WF, He H, Post SR, Giordano FJ, Lew WY, Dillmann WH. Phospholamban-to-SERCA2 ratio controls the force-frequency relationship. Am J Physiol Heart Circ Physiol 276: H779–H785, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 92: 778–784, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, Martone ME, Wang Y, Ross J Jr, Kranias EG, Giles WR, Chien KR. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell 99: 313–322, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Pattison JS, Waggoner JR, James J, Martin L, Gulick J, Osinska H, Klevitsky R, Kranias EG, Robbins J. Phospholamban overexpression in transgenic rabbits. Transgenic Res 17: 157–170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pieske B, Beyermann B, Breu V, Loffler BM, Schlotthauer K, Maier LS, Schmidt-Schweda S, Just H, Hasenfuss G. Functional effects of endothelin and regulation of endothelin receptors in isolated human nonfailing and failing myocardium. Circulation 99: 1802–1809, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca(2+)-sensitivity of SERCA2a in failing human myocardium due to reduced serine-16 phospholamban phosphorylation. J Mol Cell Cardiol 31: 479–491, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Simmerman HKB, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev 78: 921–947, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Ziolo MT, Martin JL, Bossuyt J, Bers DM, Pogwizd SM. Adenoviral gene transfer of mutant phospholamban rescues contractile dysfunction in failing rabbit myocytes with relatively preserved SERCA function. Circ Res 96: 815–817, 2005. [DOI] [PubMed] [Google Scholar]