Abstract
The purpose of the present study was to determine for the first time the qualitative and quantitative impact of varying degrees of interatrial shunting on right heart dynamics and systemic perfusion in subjects with chronic pulmonary hypertension (CPH). Eight dogs underwent 3 mo of progressive pulmonary artery banding, following which right atrial and ventricular end-systolic and end-diastolic pressure-volume relations were calculated using conductance catheters. An 8-mm shunt prosthesis was inserted between the superior vena cava and left atrium, yielding a controlled model of atrial septostomy. Data were obtained 1) preshunt or “CPH”; 2) “Low-Flow” shunt; and 3) “High-Flow” shunt (occluding superior vena cava forcing all flow through the shunt). With progressive shunting, right ventricular pressure fell from 72 ± 19 mmHg (CPH) to 54 ± 17 mmHg (Low-Flow) and 47 ± 17 mmHg (High-Flow) (P < 0.001). Cardiac output increased from 1.5 ± 0.3 l/min at CPH to 1.8 ± 0.4 l/min at Low-Flow (286 ± 105 ml/min, 15% of cardiac output; P < 0.001), but returned to 1.6 ± 0.3 l/min at High-Flow (466 ± 172 ml/min, 29% of cardiac output; P = 0.008 vs. Low-Flow, P = 0.21 vs. CPH). There was a modest rise in systemic oxygen delivery from 252 ± 46 ml/min at CPH to 276 ± 50 ml/min at Low-Flow (P = 0.07), but substantial fall to 222 ± 50 ml/min at High-Flow (P = 0.005 vs. CPH, P < 0.001 vs. Low-Flow). With progressive shunting, bichamber contractility did not change (P = 0.98), but the slope of the right atrial end-diastolic pressure volume relation decreased (P < 0.04), consistent with improved compliance. This study demonstrated that Low-Flow interatrial shunting consistently improved right atrial mechanics and systemic perfusion in subjects with CPH, while High-Flow exceeded an “ideal shunt fraction”.
Keywords: right heart failure, atrial septostomy right ventricular overload
atrial septostomy has been employed as a high-risk therapeutic option for patients with chronic pulmonary hypertension (CPH) refractory to maximum medical therapy (7, 16–18, 21, 22, 25); however, results are often unpredictable, due to a lack of understanding of the qualitative and quantitative impact of varying degrees of acute and chronic atrial shunting on cardiac output and peripheral perfusion. A fine balance likely exists between increased left heart throughput and systemic arterial desaturation following atrial septostomy, such that a defect that is too small will not increase cardiac output sufficiently to impact oxygen delivery, while a defect that is too large will pass a critical level of desaturation, such that systemic oxygen delivery falls. The purpose of the present experimental study was to determine the mechanisms by which interatrial shunting improves systemic perfusion and determine whether there is a shunt fraction beyond which hemodynamic consequences become detrimental.
METHODS
Initial Surgical Preparation
Eight adult mongrel dogs of either sex (20–25 kg) were anesthetized using a standard protocol, as previously described by our laboratory (8, 9, 20). A median sternotomy was performed, leaving the pericardium intact, and a 5-Fr pressure catheter (Access Technologies, Skokie, IL) was introduced into the right ventricular (RV) free wall through a purse string suture. An inflatable Silastic band (16-mm diameter, Access Technologies, Skokie, IL) was secured around the distal main pulmonary artery (PA). The PA band and RV pressure (RVP) catheter were tunneled through the left and right lateral chest walls, respectively, and connected to small reservoirs covered with silicone membranes that allowed injection of saline and pressure monitoring. Reservoirs were buried in a subcutaneous pocket, and the sternum was closed.
Creation of Chronic RVP Overload
Approximately 1 wk after the initial operation, RVP overload was initiated in a stepwise manner with progressive inflation of the PA band. Inflation of the band was performed weekly (0.3–0.5 ml), increasing RVP by 10–20 mmHg at each interval. Weekly inflations were performed until clinical signs of right heart failure developed (ascites, dyspnea, peripheral edema) an average of 111 ± 17 days after the initial operation.
Controlled Surgical Model of Atrial Septostomy
Following the creation of severe CPH, animals underwent a second open-chest, closed-pericardium experimental study. Initially, two micromanometer-tipped pressure catheters (Millar Instruments, Houston, TX) were zeroed in a 37°C water bath for 30 min. Repeat sternotomy was performed to access the heart, and a 7-Fr pressure catheter (Millar MPC-500) was advanced through the left mammary artery to the aortic arch to record central arterial pressure. A 7-Fr Millar pressure catheter was inserted through the left atrial (LA) appendage to measure LA pressure (LAP), ultrasonic flow probes (10–12-mm perivascular probes with a T206 Flowmeter, Transonic Systems, Ithaca, NY) were placed around the superior (SVC) and inferior vena cava (IVC) to measure right atrial (RA) inflow, and a tourniquet was positioned around the IVC to allow transient caval occlusion (preload alteration during data collection). Mini-pericardiotomies (1 cm) were performed over the anterior RV free wall and RA appendage to introduce 6-Fr (Millar SPR-843) and 5-Fr (Millar SPR-766) combined pressure-volume (PV) conductance catheters through purse string sutures. The RV catheter was inserted just below the pulmonary valve and positioned toward the RV apex to measure RVP and chamber conductance using dual-field technology with 12 electrodes 4 mm apart. The RA catheter was positioned along the long axis of the RA so that its tip rested at the RA-IVC junction to measure RA pressure (RAP) and chamber conductance using dual-field technology with 10 electrodes 4 mm apart. The RA and RV PV catheters were connected to two signal conditioner processors (Sigma 5DF, CD Leycom, Zoetermeer, The Netherlands).
A 2-cm right-sided pericardiotomy was performed just above the Waterston's groove, and 100 U/kg of heparin were administered. Through purse string sutures in the SVC (at its junction with the RA) and the LA wall (at its junction with the right superior pulmonary vein), a custom-made composite metallic shunt with 8-mm tubing was inserted to allow diversion of RA inflow to the LA. To measure continuous flow through the shunt, a custom-made ultrasonic pump-tubing flow probe was calibrated specifically for our needs (Transonics Systems, Ithaca, NY).
Data Acquisition
Data were obtained during three different hemodynamic states: 1) SVC open and shunt clamped (“CPH”); 2) SVC open and shunt open (“Low-Flow” shunting); and 3) SVC occluded (by a tourniquet) and shunt open, effectively forcing all SVC flow through the shunt (“High-Flow” shunting). During each data-acquisition run, surface ECG, LAP, RAP, RVP, aortic pressure, SVC flow, IVC flow, RA and RV conductance, and shunt flow were acquired at 200 Hz and processed using custom-designed computer software. The RAP and RVP signals were differentiated with respect to time to calculate instantaneous RA change in pressure over time (dP/dt) and RV dP/dt. To minimize the effects of intrathoracic pressure variation, the respirator was temporarily interrupted at end expiration during data collection for 10–15 s. After steady-state data were obtained (3–5 beats), slow, progressive vena caval occlusion was performed to generate RA and RV PV loops over a wide physiological range of filling pressures. Arterial blood gases were drawn from the aortic root at CPH, Low-Flow, and High-Flow.
At the conclusion of the experiment, the animals were euthanized using pentothal sodium (1 g iv), followed by potassium chloride (80 meq iv), and proper positioning of the catheters was confirmed. All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health. The study was approved by the Washington University School of Medicine Animal Studies Committee and conducted according to Washington University policy.
Data Analysis
RA elastance (contractility).
RA contractile performance was assessed using the “atrial end-systolic” PV relationship (RA-ESPVR; chamber elastance), as previously described (8, 20, 1, 13). Atrial end-systolic pressure (RAPA-ES) and volume (RA-VolA-ES) points were determined for each cardiac cycle during preload reduction, and, by least squares linear regression, a straight line was fitted to the following equation:
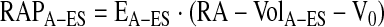 |
where EA-ES (mmHg/ml) is the slope (RA chamber elastance) and V0 (ml) is the volume-axis intercept.
RA stiffness.
Static RA stiffness was defined as the slope of the “atrial end-diastolic” P-V relation (8, 20). Atrial end-diastolic pressure (RAPA-ED) and volume (RA-VolA-ED) points were determined at the time of maximum RA volume (corresponding to tricuspid valve opening and the RAP V-wave) for each cardiac cycle during preload reduction. By least squares linear regression, a straight line was fitted to the following equation:
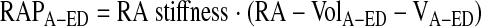 |
where RA stiffness (ml/mmHg) and VA-ED (ml) are the slope and RA diastolic equilibrium volume, respectively.
RV elastance and stiffness.
Similar mathematical methods were used to calculate RV elastance and stiffness as the slope of the RV ESPVR and end-diastolic PV relationship (EDPVR) as follows:
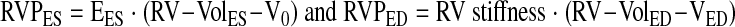 |
where RVPES is end-systolic RVP; EES is end-systolic slope; RV-VolES is end-systolic RV volume; RVPED is end-diastolic RVP; RV-VolED is end-diastolic RV volume; and VED is diastolic volume-axis intercept, and where end-systole was defined as the time of maximum RVP-to-RV volume ratio and end-diastole as the time of maximum RV volume.
RA reservoir and conduit function.
Reservoir and conduit function of the RA were calculated by integrating RA inflow (combined SVC and IVC flow) during RV systole (reservoir) and RV diastole (conduit), as previously described (8, 9). Integrated RA inflow volume during the reservoir and conduit phases was divided by total RA inflow volume (stroke volume) to yield reservoir function and conduit function as a percentage of total RA inflow.
Statistical Analysis
All data are reported as means ± SD. Hemodynamic data obtained during steady-state recording are reported as the average of three consecutive beats. Data recorded from the three different hemodynamic experimental phases (CPH, Low-Flow, High-Flow) were compared using repeated-measures ANOVA. All statistical analyses were performed using SigmaStat 2.03 (SPSS, Chicago, IL).
RESULTS
Figure 1 illustrates typical changes from one animal recorded during the three experimental hemodynamic states. Table 1 summarizes steady-state data with chronic PA banding (CPH) and with Low-Flow and High-Flow shunting. With Low-Flow shunting, shunt flow averaged 286 ± 105 ml/min (15% of cardiac output), RVP fell by 25% from 72 ± 19 to 54 ± 17 mmHg (P < 0.001), cardiac output increased from 1.5 ± 0.3 to 1.8 ± 0.4 l/min (P < 0.001), and there was no change in heart rate (P = 0.24). With High-Flow shunting, shunt volume increased to 466 ± 172 ml/min (29% of cardiac output), RVP fell to 47 ± 17 mmHg (P = 0.03 vs. Low-Flow, P < 0.001 vs. CPH), and cardiac output returned to 1.6 ± 0.3 l/min (P = 0.008 vs. Low-Flow, P = 0.21 vs. CPH) with no change in heart rate (P = 0.32 vs. Low-Flow, P = 0.78 vs. CPH). In addition, while RAP fell by 57% with progressive shunting (P < 0.001), LAP rose by 30% (P = 0.003). There was no significant change in maximum RV volume (P = 0.15) or RV stroke volume (total RA inflow, P = 0.51) with shunting. With progressive shunting, left ventricular (LV) stroke volume initially increased from 15 ± 6 ml/beat at CPH to 17 ± 5 ml/beat at Low-Flow, but returned to 16 ± 6 ml/beat with High-Flow shunting. While mean aortic pressure did not change with shunting (P = 0.58), systemic vascular resistance fell with Low-Flow (P = 0.04) due to the significant rise in cardiac output.
Fig. 1.
A: our instrumentation for data acquisition included a right atrial (RA) and right ventricular (RV) conductance catheter, a left atrial pressure (LAP) catheter, and two ultrasonic flow probes around the superior (SVC) and inferior vena cava. B: intraoperative image illustrating the following: 1, flow probe around the SVC; 2, flow probe around the inferior vena cava; 3, RA conductance catheter; 4, RV conductance catheter; and 5, shunt prosthesis with flow probe and its connection between the SVC and left atrial wall at the site of the right pulmonary vein inflow.
Table 1.
Hemodynamic data recorded with chronic pulmonary arterial banding (CPH), after opening the shunt (Low-Flow), and after forcing all SVC flow through the shunt by occluding the SVC (High-Flow)
| CPH | Low-Flow | High-Flow | |
|---|---|---|---|
| Shunt flow, ml/min | 0 | 286±105* | 466±172*† |
| Cardiac output, l/min | 1.5±0.3 | 1.8±0.4* | 1.6±0.3† |
| Shunt fraction, %cardiac output | 0 | 15±4* | 29±9*† |
| Heart rate, beats/min | 109±15 | 106±14 | 103±11 |
| LV stroke volume, ml/beat | 15±6 | 17±5* | 16±6 |
| RV stroke volume, ml/beat | 15±6 | 14±7 | 13±5 |
| Mean aortic pressure, mmHg | 68±22 | 61±32 | 65±36 |
| Maximum RV pressure, mmHg | 72±19 | 54±17* | 47±15* |
| Mean RA pressure, mmHg | 8.8±1.5 | 5.8±1.8* | 3.8±1.3*† |
| Mean LA pressure, mmHg | 8.3±1.5 | 10.6±2.6* | 10.8±2.5* |
| Maximum RV volume, ml | 27±6 | 27±5 | 26±5 |
| Maximum RA volume, ml | 25±5 | 23±8 | 22±8 |
| Maximum RV dP/dt, mmHg/s | 661±296 | 598±371 | 479±196*† |
| Maximum RA dP/dt, mmHg/s | 81±27 | 77±27 | 58±23*† |
| Systemic vascular resistance, dyn·s·cm−5 | 3,101±1,076 | 2,370±1,075* | 3,010±1,577 |
Values are means ± SD; n = 8. SVC, superior vena cava; CPH, chronic pulmonary hypertension; LV, left ventricular; RV, right ventricular; RA, right atrial; LA, left atrial; dP/dt, change in pressure over time.
P < 0.05 vs. CPH;
P < 0.05 vs. Low-Flow (repeated-measures ANOVA).
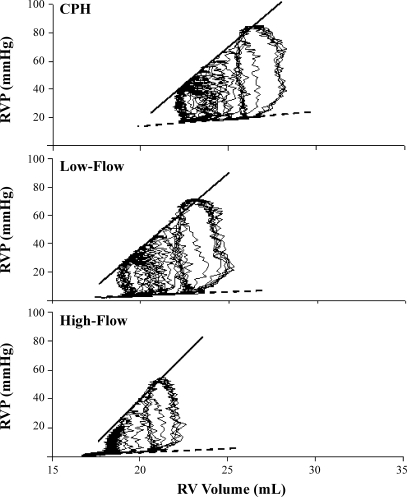
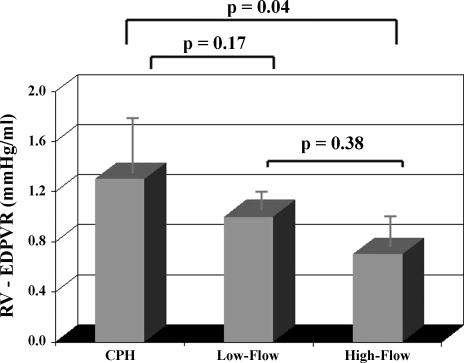
Typical RV PV loops from the same animal depicted in Fig. 1 are illustrated in Fig. 2. For all animals, RV elastance (contractility) did not significantly change from CPH to Low-Flow or subsequent High-Flow shunting (P = 0.12) (Table 2), but there was a decline in RV diastolic stiffness at High-Flow (P = 0.04 vs. CPH) (Fig. 3).
Fig. 2.
Typical RV pressure (RVP)-volume loops from the same animal depicted in Fig. 1. In this animal, RV elastance (contractility) remained unchanged, as evidenced by no change in the slope of the RV-end-systolic pressure-volume relationship (ESPVR; solid lines). In contrast, the RV-end-diastolic pressure-volume relationship (EDPVR; dotted lines) slightly declined with progressive shunting, consistent with increased RV compliance. CPH, chronic pulmonary hypertension.
Table 2.
RV elastance (contractility) and chamber stiffness as recorded with chronic pulmonary arterial banding (CPH), after opening the shunt (Low-Flow), and after forcing all SVC flow through the shunt by occluding the SVC (High-Flow)
| CPH | Low-Flow | High-Flow | |
|---|---|---|---|
| RV elastance, mmHg/ml | 12±7 | 10±2 | 11±3 |
| RV V0, ml | 21±4 | 19±4 | 21±4 |
| Correlation coefficient (r2) | 0.92±0.07 | 0.94±0.07 | 0.95±0.06 |
| RV stiffness, mmHg/ml | 1.3±0.5 | 1.0±0.2 | 0.9±0.3* |
| RV VED, ml | 23±4 | 20±6 | 18±4* |
| Correlation coefficient (r2) | 0.94±0.02 | 0.94±0.05 | 0.92±0.01 |
Values are mean ± SD; n = 8. V0, volume-axis intercept; VED, end-diastolic volume.
P < 0.05 vs. CPH (repeated-measures ANOVA).
Fig. 3.
Effects of progressive interatrial shunting in subjects with chronic RVP overload on RV diastolic stiffness (compliance) for the entire series (n = 8). Data obtained from each animal were recorded: 1) preshunt (CPH); 2) after opening the shunt (Low-Flow); and 3) after forcing all SVC flow through the shunt (High-Flow). Data represent means ± SD (repeated-measures ANOVA).
Typical RA PV loops during CPH and with progressive shunting at Low-Flow and High-Flow from the same animal depicted in Figs. 1 and 2 are illustrated in Fig. 4. Shunting did not impact RA elastance compared with CPH (P = 0.99) (Table 3), but there was a significant decline in the slope of the RA EDPVR with Low-Flow shunting (P < 0.02 vs. CPH), consistent with improved RA compliance (Fig. 5). Furthermore, the RA was decompressed as evidenced by a significant change in RA V0 (P = 0.004 vs. CPH). Both parameters of RA compliance remained at this level after creation of the High-Flow state (P = 0.03 vs. CPH, P = 0.89 vs. Low-Flow).
Fig. 4.
Typical sequence of RAP-volume loops over a wide range of preloads taken from the same animal depicted in Figs. 1 and 2. In this animal, RA elastance (contractility) was unchanged (solid lines), but the slope of the RA-EDPVR (dotted lines) substantially declined after opening the shunt (Low-Flow), consistent with improved RA compliance.
Table 3.
RA elastance (contractility) and chamber stiffness as recorded with chronic pulmonary arterial banding (CPH), after opening the shunt (Low-Flow), and after forcing all SVC flow through the shunt by occluding the SVC (High-Flow)
| CPH | Low-Flow | High-Flow | |
|---|---|---|---|
| RA elastance, mmHg/ml | 1.3±0.4 | 1.2±0.6 | 1.2±0.6 |
| RA V0, ml | 18±3 | 11±7* | 12±5* |
| Correlation coefficient (r2) | 0.92±0.06 | 0.89±0.05 | 0.96±0.02 |
| RA stiffness, mmHg/ml | 1.2±0.5 | 0.7±0.1* | 0.7±0.4* |
| RA VED, ml | 21±4 | 11±6* | 9±7* |
| Correlation coefficient (r2) | 0.87±0.06 | 0.92±0.05 | 0.91±0.09 |
Values are mean ± SD; n = 8.
P < 0.05 vs. CPH (repeated-measures ANOVA).
Fig. 5.
Effects of progressive interatrial shunting in subjects with chronic RVP overload on RA diastolic stiffness (compliance) for the entire series (n = 8). Data obtained from each animal were recorded: 1) preshunt (CPH); 2) after opening the shunt (Low-Flow); and 3) after forcing all SVC flow through the shunt (High-Flow). Data represent means ± SD (repeated-measures ANOVA).
Figure 6 summarizes changes in RA reservoir and conduit function. At CPH, reservoir function accounted for 82 ± 21% of RA inflow (12 ± 6 ml) and conduit function for 18 ± 4% (3 ± 2 ml). With subsequent shunting, there was a decline in reservoir function to 61 ± 21% of RA inflow (8 ± 4 ml) at Low-Flow (P = 0.002 vs. CPH), and 68 ± 18% of RA inflow (8 ± 4 ml) at High-Flow (P = 0.02 vs. CPH, P = 0.27 vs. Low-Flow).
Fig. 6.
RA reservoir-to-conduit ratio with chronic pulmonary arterial banding alone (CPH), and after employing the controlled surgical model of atrial septostomy at Low-Flow and High-Flow (forcing all SVC flow through the shunt).
Arterial blood-gas samples from the three different hemodynamic states are summarized in Table 4. There was a modest rise in systemic oxygen delivery from 252 ± 46 ml/min at CPH to 276 ± 50 ml/min at Low-Flow (P = 0.09), which significantly fell to 222 ± 50 ml/min at High-Flow (P = 0.005 vs. CPH, P < 0.001 vs. Low-Flow).
Table 4.
Data from arterial blood gases drawn from the aortic root with chronic pulmonary arterial banding (CPH), after opening the shunt (Low-Flow), and after forcing all SVC flow through the shunt by occluding the SVC (High-Flow)
| CPH | Low-Flow | High-Flow | |
|---|---|---|---|
| O2 saturation, % | 94±2 | 90±2* | 87±3*† |
| O2 content, ml/dl | 16.6±1.9 | 14.8±1.5* | 13.8±2.1* |
| Systemic O2 transport, ml/min | 252±46 | 276±50 | 222±50*† |
| Systemic O2 transport index, ml·min−1·m−2 | 298±46 | 325±49 | 262±55*† |
Values are mean ± SD; n = 8.
P < 0.05 vs. CPH;
P < 0.05 vs. Low-Flow (repeated-measures ANOVA).
DISCUSSION
Patients with CPH and a patent foramen ovale have been observed to live longer than those without such a defect (24). However, a patent foramen ovale is likely beneficial only if sufficient right-to-left shunting occurs to increase cardiac output (16). In an attempt to mimic the hemodynamic consequence of a patent foramen ovale, atrial septostomy was employed for selected patients with recurrent syncope or significant right heart failure refractory to medical treatment (3, 6, 10, 26). Reported beneficial effects include prolonged survival with deferral of transplantation and improved quality of life, symptoms, and hemodynamic function (16, 18, 21, 23, 28). However, due to severe RA dilatation and resultant loss of anatomical landmarks, atrial septostomy in critically ill end-stage patients with CPH is technically challenging, and procedure-related morbidity and mortality remain an issue. Atrial septostomy has been associated with a risk of intraprocedural and postprocedural mortality up to 30% in several series (22, 18, 16, 21, 28), most commonly, secondary to progressive hypoxia, right heart failure, and ventricular arrhythmias.
Two previous experimental studies have addressed atrial shunting in pulmonary hypertension (2, 15). Austen and colleagues in 1964 (2) demonstrated improved exercise capacity and an estimated increase in cardiac output with right-to-left shunting in dogs, but their experimental model could not quantify or control the degree of shunting that occurred. Kawaguchi and co-investigators (15) reported improved systemic oxygen uptake and survival in CPH rats with an interatrial shunt, but again, the degree of shunting could not be defined. In the present study, Low-Flow shunting (15% of cardiac output) improved RA diastolic compliance by 42% and caused a shift of the RA reservoir-to-conduit ratio toward physiological conditions. It has been suggested that, with chronic RVP overload, enhanced RA distensibility (improved diastolic compliance) is key to maintain filling of the stiffened ventricle (8).
The positive impact of enhanced atrial compliance and maintained reservoir to conduit properties on global cardiac performance has previously been reported for the LA (4, 11, 12, 14). In an elegant computer model, Suga (27) also suggested that increased atrial distensibility would improve cardiac performance and concluded that a “flexible atrium” (increased receptacle capabilities) could substantially impact the heart's output. Interestingly, in the present report, despite a significant 25% decline in maximum RVP, RV systolic and diastolic properties were not acutely affected by interatrial shunting, potentially due to the pronounced morphological ventricular changes consequent to chronic RVP overload (8).
In the present investigation, with Low-Flow shunting (15% of cardiac output), LV preload increased (elevated LAP), augmenting LV stroke volume and cardiac output. In the healthy LV in our model of CPH, this can be attributed to the Frank-Starling mechanism. According to the Frank-Starling mechanism, as the heart is stretched in response to increased preload, it augments its contraction force at the expense of increased myocardial oxygen consumption. Thus the finding that, at the High-Flow state, cardiac output dropped back to baseline may be explained by the critical decline of arterial oxygen content that presumably limited LV myocardial oxygen delivery. The rise in cardiac output by 20% not only compensated for the decline in arterial oxygen saturation due to right-to-left shunting, but was associated with a trend toward improved systemic O2 delivery (P = 0.07). In contrast, with High-Flow shunting (29% of cardiac output), the beneficial effects of Low-Flow shunting were abated, evidenced by no further augmentation of LV stroke volume and a decline in cardiac output and systemic oxygen transport. None of the assessed parameters of right heart mechanics or systemic perfusion, with the exception of RV diastolic compliance, improved with High-Flow shunting compared with Low-Flow shunting, suggesting that a shunt fraction of 29% exceeds the ideal.
Potential Limitations
The purpose of the present investigation was to delineate the impact of interatrial shunting in subjects with chronic RVP overload on RA and RV mechanics and systemic perfusion. Previous studies using chemical toxicants to injure the pulmonary vasculature elegantly simulated the etiology of clinical CPH, since it is the peripheral pulmonary vascular tree that is predominantly affected in patients, but these studies are limited by their ability to stimulate only a modest elevation in PA pressure (5, 29). For the present investigation, a method for creating chronic RVP overload was selected to model the hemodynamic consequences of severe CPH on the right heart (8, 9). Although the present study included a chronic surgical instrumentation to mimic CPH, it was designed to compare acute hemodynamic changes following interatrial shunting. Thus the analytic methods employed were not dependent on absolute volume measurements and were consistent with previous studies involving the RA (20). The authors considered the use of an adjustable device to create an atrial septal defect, which would have been more physiological. However, the major shortcoming of such a device is the difficulty of directly and accurately measuring the real-time interatrial shunt flow. Although conductance catheters have been reliably used in open chest animal models under general anesthesia before (19), the limitations of such a model should be mentioned. Potential drawbacks include that physiological responses to hemodynamic changes, such as the carotid chemoreceptor-mediated response or the vagal response, may be blunted.
In summary, Low-Flow interatrial shunting (15% of cardiac output) consistently improved RA compliance by unloading the RA and made the RA work more economically, as evidenced in a maintained RA elastance (contractility) and a shift of RA reservoir to conduit ratio toward physiological baseline conditions. Increased LV preload augmented LV stroke volume, culminating in a rise is cardiac output. The augmentation of cardiac output compensated for a decline in oxygen content due to right-to-left shunting and was associated with a trend toward improved systemic O2 delivery. In contrast, with High-Flow shunting (29% of cardiac output), the beneficial effects were reversed and, in part, impaired beyond baseline, indicating that High-Flow shunting exceeded the “ideal shunt fraction”. By conducting this experimental study, it was our hope to contribute to a better understanding of the distinct physiological consequences of atrial septostomy at different shunt sizes, so that it may become a more predictable and thus more widely applicable therapeutic approach in end-stage patients with CPH. Present data suggest that atrial septostomy should be performed, yielding an initial shunt fraction of 15–20% of cardiac output.
GRANTS
This study was supported by grant HL-92088 from the National Heart, Lung, and Blood Institute and by a Foundation Research Grant from the Thoracic Surgery Foundation for Research and Education. A. Zierer was supported by a DFG-Research-Fellowship of the German Research Foundation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alexander J, Sunagawa K, Chang N, Sagawa K. Instantaneous pressure-volume relation of the ejecting canine left atrium. Circ Res 61: 209–219, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Austen WG, Morrow AG, Berry WB. Experimental studies of the surgical treatment of primary pulmonary hypertension. J Thorac Cardiovasc Surg 48: 448–455, 1964. [PubMed] [Google Scholar]
- 3.Barst RJ Role of atrial septostomy in the treatment of pulmonary vascular disease. Thorax 55: 95–96, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman AW, Kovacs SJ. Left atrial conduit volume is generated by deviation from the constant-volume state of the left heart: a combined MRI-echocardiographic study. Am J Physiol Heart Circ Physiol 286: H2416–H2424, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Chen EP, Craig DM, Bittner HB, Davis RD, Van Trigt P. Pharmacological strategies for improving diastolic dysfunction in the setting of chronic pulmonary hypertension. Circulation 97: 1606–1612, 1998. [DOI] [PubMed] [Google Scholar]
- 6.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115: 343–349, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Doyle RL, McCrory D, Channick RN, Simonneau G, Conte J. Surgical treatments/interventions for pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 126: 63S–71S, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Gaynor SL, Maniar HS, Bloch JB, Steendijk P, Moon MR. Right atrial and ventricular adaptation to chronic right ventricular pressure overload. Circulation 112: 212–218, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Gaynor SL, Maniar HS, Prasad SM, Steendijk P, Moon MR. Reservoir and conduit function of right atrium: impact on right ventricular filling and cardiac output. Am J Physiol Heart Circ Physiol 288: H2140–H2145, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Haworth SG Pulmonary hypertension in the young. Heart 88: 658–664, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoit BD, Gabel M. Influence of left ventricular dysfunction on the role of atrial contraction: an echocardiographic-hemodynamic study in dogs. J Am Coll Cardiol 36: 1713–1719, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Hoit BD, Shao Y, Gabel M. Left atrial systolic and diastolic function accompanying chronic rapid pacing-induced atrial failure. Am J Physiol Heart Circ Physiol 275: H183–H189, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Hoit BD, Shao Y, Gabel M, Walsh RA. In vivo assessment of left atrial contractile performance in normal and pathological conditions using a time-varying elastance model. Circulation 89: 1829–1838, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Hoit BD, Shao Y, Tsai LM, Walsh RA. Altered left atrial compliance after atrial appendectomy. Influence on left atrial and ventricular filling. Circ Res 72: 167–175, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi AT, Kawashima Y, Ishibashi-Ueda H, Yanase M, Murakami T, Yagihara T, Kunieda T. Right-to-left interatrial shunt in rats with progressive pulmonary hypertension. J Thorac Cardiovasc Surg 106: 1072–1080, 1993. [PubMed] [Google Scholar]
- 16.Kerstein D, Levy PS, Hsu DT, Hordof AJ, Gersony WM, Barst RJ. Blade balloon atrial septostomy in patients with severe primary pulmonary hypertension. Circulation 91: 2028–2035, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Klepetko W, Mayer E, Sandoval J, Trulock EP, Vachiery JL, Dartevelle P, Pepke-Zaba J, Jamieson SW, Lang I, Corris P. Interventional and surgical modalities of treatment for pulmonary arterial hypertension. J Am Coll Cardiol 43: 73S–80S, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kurzyna M, Dabrowski M, Bielecki D, Fijalkowska A, Pruszczyk P, Opolski G, Burakowski J, Florczyk M, Tomkowski WZ, Wawrzynska L, Szturmowicz M, Torbicki A. Atrial septostomy in treatment of end-stage right heart failure in patients with pulmonary hypertension. Chest 131: 977–983, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Lips DJ, van der Nagel T, Steendijk P, Palmen M, Janssen BJ, van Dantzig JM, de Windt LJ, Doevendans PA. Left ventricular pressure-volume measurements in mice: comparison of closed-chest vs. open-chest approach. Basic Res Cardiol 99: 351–359, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Maniar HS, Prasad SM, Gaynor SL, Chu CM, Steendijk P, Moon MR. Impact of pericardial restraint on right atrial mechanics during acute right ventricular pressure load. Am J Physiol Heart Circ Physiol 284: H350–H357, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Reichenberger F, Pepke-Zaba J, McNeil K, Parameshwar J, Shapiro LM. Atrial septostomy in the treatment of severe pulmonary arterial hypertension. Thorax 58: 797–800, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rich S, Dodin E, McLaughlin VV. Usefulness of atrial septostomy as a treatment for primary pulmonary hypertension and guidelines for its application. Am J Cardiol 80: 369–371, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Rothman A, Sklansky MS, Lucas VW, Kashani IA, Shaughnessy RD, Channick RN, Auger WR, Fedullo PF, Smith CM, Kriett JM, Jamieson SW. Atrial septostomy as a bridge to lung transplantation in patients with severe pulmonary hypertension. Am J Cardiol 84: 682–686, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Rozkovec A, Montanes P, Oakley CM. Factors that influence the outcome of primary pulmonary hypertension. Br Heart J 55: 449–458, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandoval J, Rothman A, Pulido T. Atrial septostomy for pulmonary hypertension. Clin Chest Med 22: 547–560, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43: 5S–12S, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Suga H Importance of atrial compliance in cardiac performance. Circ Res 35: 39–43, 1974. [DOI] [PubMed] [Google Scholar]
- 28.Thanopoulos BD, Georgakopoulos D, Tsaousis GS, Simeunovic S. Percutaneous balloon dilatation of the atrial septum: immediate and midterm results. Heart 76: 502–506, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zakrzewicz A, Hecker M, Marsh LM, Kwapiszewska G, Nejman B, Long L, Seeger W, Schermuly RT, Morrell NW, Morty RE, Eickelberg O. Receptor for activated C-kinase 1, a novel interaction partner of type II bone morphogenetic protein receptor, regulates smooth muscle cell proliferation in pulmonary arterial hypertension. Circulation 115: 2957–2968, 2007. [DOI] [PubMed] [Google Scholar]