Abstract
Normal pregnancy is associated with reduced blood pressure (BP) and decreased pressor response to vasoconstrictors, even though the renin-angiotensin system is upregulated. Angiotensin II (ANG II) activates both angiotensin type 1 receptors (AT1Rs) and angiotensin type 2 receptors (AT2Rs). Although the role of the AT1R in vascular contraction is well documented, the role of the AT2R in vascular relaxation, particularly during pregnancy, is less clear. It was hypothesized that the decreased BP and vasoconstriction during pregnancy was, at least in part, due to changes in AT2R amount, distribution, and/or postreceptor mechanisms of vascular relaxation. To test this hypothesis, systolic BP was measured in virgin and pregnant (day 19) Sprague-Dawley rats. Isometric contraction/relaxation was measured in isolated aortic rings, and nitric oxide (NO) production was measured using 4-amino-5-methylamino-2′,7′-difluorescein fluorescence. AT1R and AT2R mRNA expression and protein amount were measured in tissue homogenates using real-time RT-PCR and Western blots, and their local distribution was visualized in cryosections using immunohistochemistry and immunofluorescence. BP was lower in pregnant than virgin rats. Phenylephrine (Phe) caused concentration-dependent contraction that was reduced in the aorta of pregnant compared with virgin rats. Treatment with the AT2R antagonist PD-123319 caused greater enhancement of Phe contraction, and the AT2R agonist CGP-42112A caused greater relaxation of Phe contraction in the aorta of pregnant than virgin rats. ANG II plus the AT1R blocker losartan induced greater NO production in the aorta of pregnant than virgin rats. RT-PCR revealed increased mRNA expression of vascular endothelial NO synthase (eNOS), little change in AT1Rs, and increased AT2Rs in pregnant compared with virgin rats. Western blots revealed an increased protein amount of activated phospho-eNOS, little change in AT1Rs, and increased AT2Rs in pregnant compared with virgin rats. Immunohistochemistry and immunofluorescence analysis in aortic sections of virgin rats revealed abundant AT1R staining in tunica media that largely colocalized with actin in vascular smooth muscle and less AT2Rs mainly in the tunica intima and endothelium. In pregnant rats, AT1R staining in the smooth muscle layer and adventitia was reduced, and endothelial AT2R staining was enhanced. These data suggest an enhanced AT2R-mediated vascular relaxation pathway involving increased expression/activity of endothelial AT2Rs and increased postreceptor activated phospho-eNOS, which may contribute to the decreased BP during pregnancy.
Keywords: endothelium, nitric oxide, nitric oxide synthase, vascular smooth muscle, blood pressure
during normal pregnancy, significant cardiovascular changes occur to meet the metabolic needs of the mother and fetus. Pregnancy-associated changes in hemodynamics include increased cardiac output and reduced blood pressure (BP) and vascular resistance (29). Upregulation of the renin-angiotensin system is associated with several forms of hypertension (58, 69). Although the renin-angiotensin system is upregulated during normal pregnancy, a reduction in BP and a decreased pressor response to ANG II are observed (2, 25, 29); the vascular mechanisms involved are unclear.
ANG II activates not only the angiotensin type 1 receptor (AT1R) to produce vasoconstriction (31, 52, 66, 67) but also the angiotensin type 2 receptor (AT2R) to induce the release of vasodilator substances (24, 32, 39, 62). Although the role of the AT1R in vascular contraction and hypertension is well characterized (30, 54, 65), the role of the AT2R in vascular relaxation and BP regulation, particularly during pregnancy, is not clearly understood. A few studies (11, 63, 64) have suggested a potential role for ANG II receptor subtypes in BP control in pregnant rats and mice; however, BP regulation is a complex process that involves changes in renal, neural, hormonal, and vascular control mechanisms (6, 28, 43, 63). Also, it has been previously shown that the vascular responsiveness to vasoconstrictors is diminished during pregnancy (16, 29, 42, 43), although the role of the AT2R in mediating the pregnancy-associated reduction in vasoconstriction is unclear.
The AT1R is widely expressed in blood vessels and the kidney and mediates most of the effects of ANG II (30, 54, 65). The AT2R has been identified, cloned, and sequenced (7, 9). However, knowledge about the function of the AT2R is still evolving. While both the AT1R and AT2R are expressed in renal tissues and vessels, they contribute differently to renal cellular growth, tubular functions, and hemodynamics (62, 65). In many instances, the AT2R mediates responses that counteract the effects of the AT1R (30, 49).
Studies have shown that the amount of vascular AT2Rs is greater in female than male spontaneously hypertensive rats, and evidence has suggested a role for estrogen in the gender differences in AT2R expression (60). Also, the AT2R is expressed heavily in fetal and placental tissues compared with adult tissues (7, 44, 68). These observations led us to hypothesize that the decreased BP and vascular responsiveness to vasoconstrictors during pregnancy could be due to increased expression of the vascular AT2R.
Upregulation of the AT2R system could be manifested not only as an increase in the amount of AT2Rs but also as an increase in the signaling mechanisms downstream from receptor activation. Endothelium-dependent pathways of vascular relaxation are enhanced during pregnancy (17, 29, 43). Also, the expression of inducible nitric oxide (NO) synthase (NOS) is elevated in the kidney during late gestation in rats (3). Furthermore, the plasma level, metabolic production, and urinary excretion of cGMP, a second messenger of NO and a mediator of vascular smooth muscle (VSM) relaxation (33, 37), are increased during pregnancy (12). However, whether the AT2R is involved in the enhanced endothelium-dependent pathways of vascular relaxation and the reduction in BP during pregnancy is unclear.
The purpose of this study was to test the hypothesis that the decreased BP and vasoconstriction during pregnancy reflects changes in the amount/distribution of vascular AT2Rs and/or the downstream NO-dependent mechanisms of vascular relaxation. Aortic rings were isolated from virgin and late-pregnant rats and treated with AT2R agonists and antagonists to determine the following: 1) whether the decreased BP during pregnancy is associated with enhanced AT2R-mediated vascular relaxation and blunting of vascular contraction, 2) whether the enhanced vascular relaxation during pregnancy reflect changes in AT2R-mediated endothelium-dependent NO production, and 3) whether the enhanced vascular relaxation during pregnancy reflects changes in the amount/distribution of vascular AT2Rs.
MATERIAL AND METHODS
Animals.
Female timed-pregnant (day 19 of gestation, ∼350 g) and age-matched virgin (12 wk, ∼250 g) Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were housed individually and maintained on standard rat chow and tap water ad libitum on a 12:12-h light-dark cycle. Differences in weight between the pregnant and virgin rats were partly due to the gravid uterine weight but may also be related to the pregnancy-associated increase in plasma volume and extracellular fluid volume (40, 43). The specific stage of the estrous cycle in virgin rats was not determined in the present study. Synchronization of adult virgin rats at a specific stage of the estrous cycle would require the administration of exogenous estrogen and progesterone and abortifacient drugs such as PGF2α, which could change the vascular reactivity and thus would affect the measurements of the contractile response in the vascular rings. Therefore, adult virgin rats were studied using random selection regardless of the stage of the ovarian cycle. Since the ovarian cycle in rats is frequent (every 4–5 days) and the estrous stage is short (∼12 h), the average data from all adult rats should cancel out the effects of possible fluctuations in sex hormone levels at specific stages of the ovarian cycle and should, roughly, represent the average changes in vascular function during all stages of the ovarian cycle. All experimental procedures were approved by and performed in accordance with the guidelines of the Animal Care and Use Committee of Harvard Medical School and the American Physiological Society.
BP.
Systolic BP was measured using the tail-cuff electrosphygmomanometer system (ITTC, Woodland Hills, CA). After a 2-day training period, BP was measured on days 14–19 of gestation or the equivalent in age-matched virgin rats. All measurements were made between 8:00 and 11:00 AM. Rats were allowed to rest quietly in a Plexiglas restrainer placed in a warming chamber preset to 30°C. A 17-mm tail cuff with a pneumatic pulse transducer was applied to the base of the tail and programmed to inflate to a maximum pressure of 250 mmHg. The pressure cuff was gradually deflated, and the reappearance of pulsations was detected by a photoelectric sensor, amplified, and recorded on a two-channel polygraph. A rest period of 1 min was allowed between inflations. Results from the first three inflation cycles were discarded, and the average from the next three cycles was taken as the systolic BP of each individual rat on any given day.
Tissue preparation.
After BP was measured on day 19 of pregnancy in pregnant rats or the equivalent in age-matched virgin rats, rats were euthanized with CO2 inhalation, the thoracic and abdominal cavity was opened, and the thoracic aorta was rapidly excised. The aorta was placed in oxygenated Krebs solution, carefully dissected and cleaned of connective tissue under microscopic visualization, and cut into 3-mm-wide rings. Extreme care was taken throughout the tissue isolation and dissection procedure to minimize injury to the endothelium.
Isometric contraction.
Aortic rings were suspended between two tungsten wire hooks; one hook was fixed to the bottom of a tissue bath, and the other hook was connected to a Grass force transducer (FT03, Astro-Med, West Warwick, RI). Aortic rings were stretched under 2 g of resting tension and allowed to equilibrate for 45 min in a temperature-controlled, water-jacketed tissue bath filled with 50 ml Krebs solution continuously bubbled with 95% O2-5% CO2 at 37°C. The preliminary resting tension-KCl contraction relationship indicated that 2 g of resting tension produced maximal 96 mM KCl contraction. Further increases in resting tension did not produce further increases in KCl contraction. Changes in contraction/relaxation were recorded on a Grass 7D polygraph (Astro-Med).
After tissue equilibration, a control contraction to 96 mM KCl was elicited. Once maximum KCl contraction was reached, the tissue was rinsed with Krebs solution three times for 10 min each. The control KCl-induced contraction followed by a rinse in Krebs solution was repeated twice. Aortic rings were stimulated with increasing concentrations of phenylephrine (Phe; 10−9–10−5 M), concentration-response curves were constructed, and the maximal contraction was measured. Individual Phe concentration-response curves were further analyzed using a nonlinear regression curve (best-fit sigmoidal dose-response curve, SigmaPlot), and the effective concentration that produced half the maximal contraction (EC50) was measured. In other experiments, the Phe concentration-contraction relationship was constructed in vascular rings pretreated with the AT2R receptor antagonist PD-123319. The contractile response to ANG II (10−6 M) was also measured in untreated aortic rings or aortic rings treated with PD-123319. In another set of experiments, aortic rings were precontracted with Phe (10−6 M), increasing concentrations of the AT2R agonist CGP-42112A (10−10–10−5 M) were added, and the magnitude of vascular relaxation was then measured. Higher concentrations of CGP-42112A (>10−5 M) were not used to avoid potential stimulation of the AT1R, which could promote vascular contraction and thereby confound the vascular relaxation response. Control tissues were treated with vehicle (distilled water). The presence of functional endothelium was tested by eliciting a concentration-relaxation curve in response to acetylcholine (ACh). Although extreme care was taken throughout the procedure to minimize injury to the endothelium, the magnitude of the ACh relaxation varied, suggesting variable degrees of endothelium integrity among the vessels tested. However, none of the vessels tested showed complete relaxation to ACh (completely intact endothelium) or lack of relaxation to ACh (total damage of the endothelium). No exclusion criteria were implemented, and all ACh concentration-relaxation data were included in the analysis.
NO production.
NO production was measured using the NO-sensitive fluorescent dye 4-amino-5-methylamino-2′,7′-difluorescein (DAF-FM) (59). Aortic rings were placed in test tubes containing 2 ml Krebs solution and 7 μM DAF-FM and aerated with 95% O2-5% CO2 at 37°C for 45 min. Samples for the basal accumulation of NO were taken. Aortic rings were treated with ACh (10−5 M), CGP-4112A (10−6 M), or ANG II (10−6 M) + losartan (10−6 M) for 30 min, removed, dabbed dry with filter paper, and then weighed. The fluorescence of the incubation solutions was measured (50 μl, in triplicate) in a 96-well microplate using a SpectraMAX microplate reader (Molecular Devices, Sunnyvale, CA) at an excitation wavelength of 495 nm and an emission wavelength of 520 nm. DAF-FM fluorescence is linearly related to the NO concentration. NO production was expressed as steady-state DAF-FM fluorescence intensity minus its intensity before exposure to the released NO, and values were normalized to tissue weight.
Real-time RT-PCR analysis.
RNA was isolated from tissue samples using a RNeasy Fibrous Tissue Mini Kit (QIAGEN, Valencia, CA). Total RNA (1 μg) was used for reverse transcription to synthesize single-strand cDNA in a 20-μl reaction mixture according to the protocol of the First-Strand cDNA Synthesis Kit (Amersham Biosciences, Pittsburgh, PA). Two microliters of the cDNA dilution (1:5 for eNOS, AT1R, and AT2R and 1:25 for α-actin) of the reverse transcription product was applied to the 20-μl RT-PCR mixture. Quantification of gene expression was performed using a real-time quantitative RT-PCR machine (Mx4000, Multiplex Quantitative PCR System, Stratagene, La Jolla, CA) and using previously published oligonucleotide primers specific for eNOS, AT1R, and AT2R (Integrated DNA Technologies, Coralville, IA) and the Bio-Rad iQ SYBR Green Supermix, which employs the fluorescein compound SYBR Green for amplicon detection (Bio-Rad, Hercules, CA). The α-actin primer with an expected product length of 285 bp was included in the RT-PCR as an internal standard to normalize the results. The following primers and sequences were used: eNOS, forward 5′-TTCTGGCAAGACCGATTACACGACAT-3′ and reverse 5′-AAAGGCGGAGAGGACTTGTCCAAA-3′; AT1R, forward 5′-ACCTGCATCATCATCTGGCTAAT-3′ and reverse 5′-TGTGATATTGGTGTTCTCGATGAA-3′; and AT2R, forward 5′-TCTGGCTGTGGCTGACTTACTC-3′ and reverse 5′-CTTTGCACATCACAGGTCCAA-3′.
PCR was carried out with 1 cycle for 10 min at 95°C and then 40–45 cycles of 30 s of denaturation at 95°C, 45 s of annealing at 56°C, and 30 s of extension at 72°C followed by 1 min of final extension at 95°C. The number of PCR cycles varied according to the expression level of the target gene. The appropriate primer concentration and number of cycles was determined to ensure that the PCR took place in the linear range and thereby guaranteed a proportional relationship between input RNA and the cycle readout. The relative amount of gene expression was calculated by a comparison of cycle thresholds with the housekeeping gene α-actin.
Tissue homogenate.
Aortic rings were homogenized in a buffer containing 20 mM MOPS, 4% SDS, 10% glycerol, 2.3 mg DTT, 1.2 mM EDTA, 0.02% BSA, 5.5 μM leupeptin, 5.5 μM pepstatin, 2.15 μM aprotinin, and 20 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride using a 2-ml tight-fitting homogenizer (Kontes Glass). The homogenate was centrifuged at 10,000 g for 2 min, and the supernatant was used as the whole tissue fraction. The protein concentration was determined using a protein assay kit (Bio-Rad).
Western blot analysis.
The aortic tissue homogenate was subjected to electrophoresis on a 8% SDS-polyacrylamide gel and then transferred electrophoretically to a nitrocellulose membrane. The membrane was incubated in 5% dried nonfat milk for 1 h and then treated with monoclonal antibody to eNOS (1:500, BD Transduction Laboratories, San Diego, CA), phospho-eNOS (1:500, Cell Signaling Technology, Danvers, MA), or AT1R or AT2R (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) for 24 h. α-Actin was used as an internal control and was detected by anti-actin antibody (1:500,000, Sigma). Nitrocellulose membranes were washed five time for 15 min in Tris-buffered saline-Tween and then incubated in horseradish peroxidase-conjugated secondary antibody (1:1,000) for 1.5 h. Blots were visualized with ECL Western Blotting Detection Reagent (GE Healthcare Bio-Sciences, Piscataway, NJ), and the reactive bands were analyzed quantitatively by optical densitometry. Densitometry values represent the pixel intensity and were normalized to α-actin to correct for loading.
Histology and quantitative morphometry.
Cryosections (6 μm) of aortic rings from virgin and pregnant rats were placed on glass slides and prepared for staining with hematoxylin and eosin to assess the thickness of the intima, VSM layer, and adventitia. Stained sections were coded and labeled in a blinded fashion. Images were acquired on a light microscope (Olympus BX-2) with a digital camera mount and analyzed using MetaMorph Imaging System software (Universal Imaging, West Chester, PA). Outlines of the vessel lumen, internal elastic lamina, and external vascular wall were defined, and the intimal, medial, and total wall thickness were calculated.
Immunohistochemistry, immunofluorescence, and colocalization experiments.
The expression of angiotensin receptor subtypes in the aorta of virgin and pregnant rats was analyzed by immunohistochemistry. Cryosections of the aorta (6 μm thick) were thawed and fixed in ice-cold acetone for 10 min. Endogenous peroxidase was quenched in 1.5% H2O2 solution for 10 min, and nonspecific binding was blocked in 10% horse serum. Tissue sections were incubated with AT1R and AT2R antibodies (1:500, Santa Cruz Biotechnology). After being rinsed with PBS, tissue sections were incubated with biotinylated secondary antibody, rinsed with PBS, and then incubated with avidin-labeled peroxidase (VectaStain Elite ABC Kit, Vector Laboratories, Burlingame, CA). Positive labeling was visualized using diaminobenzadine and appeared as brown spots. Negative control slides were run simultaneously with no primary antibody. For colocalization experiments, a double-immunofluorescence strategy was used using a fluorescein readout for AT1R and AT2R labeling and Texas red readout for VSM α-actin. Fluorescent images were acquired using a Nikon Eclipse 300 microscope with an attached Quantix cooled charge-coupled device (Photometrics, Tucson, AZ) and analyzed using MetaMorph image-analysis software.
Solutions and drugs.
Krebs solution contained (in mM) 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose, 2.5 CaCl2, and 1.2 MgCl2 at pH 7.4 and was bubbled with 95% O2-5% CO2; 96 mM KCl was prepared as Krebs solution with equimolar substitution of NaCl with KCl. Stock solutions of Phe, ACh, ANG II, losartan, PD-123319, and CGP-42112A (10−1 M) were prepared in distilled water. All other chemicals were of reagent grade or better.
Statistical analysis.
Data were analyzed and presented as means ± SE. Student's t-test for unpaired and paired data was used for comparison of two means. Differences were considered statistically significant if P < 0.05.
RESULTS
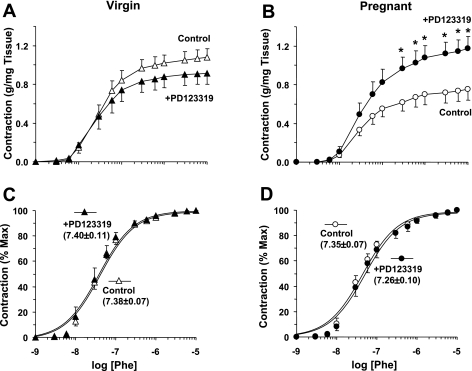
On day 19 of pregnancy or the equivalent in age-matched virgin rats, the average systolic BP was significantly lower in pregnant (103 ± 2 mmHg, n = 8) than virgin rats (113 ± 4 mmHg, n = 8, P = 0.042). In isolated aortic rings of virgin rats, Phe caused concentration-dependent contraction that reached a maximum at 10−5 M (Fig. 1). The Phe-induced contraction was significantly reduced in pregnant rats (maximum: 0.75 ± 0.11 g/mg tissue) compared with virgin rats (maximum: 1.08 ± 0.09 g/mg tissue, P = 0.033). Pretreatment of vascular rings of virgin rats with the AT2R antagonist PD-123319 (10−6 M) caused no significant change in Phe contraction (maximum: 0.91 ± 0.11 g/mg tissue; Fig. 1A). In contrast, in vascular rings of pregnant rats, pretreatment with PD-123319 caused significant enhancement of Phe contraction (maximum: 1.18 ± 0.2 g/mg tissue, P = 0.019; Fig. 1B). Presentation of the Phe contraction as a percentage of the maximum and calculation of EC50 indicated that Phe was equally potent in inducing contraction in PD-123319-treated compared with nontreated vascular rings of virgin and pregnant rats (Fig. 1, C and D).
Fig. 1.
Effect of angiotensin type 2 receptor (AT2R) blockade on phenylephrine (Phe)-induced contraction in aortic rings of virgin and pregnant (Preg) rats. Aortic rings were not treated (control) or pretreated with PD-123319 (10−6 M) for 15 min and then stimulated with increasing concentrations of Phe. The contractile response was measured and is presented as grams per milligram tissue weight (A and B) or as a percentage of the maximum Phe contraction (C and D). Numbers in parentheses indicate pEC50 (−log M). Data represent means ± SE; n = 8. *P < 0.05.
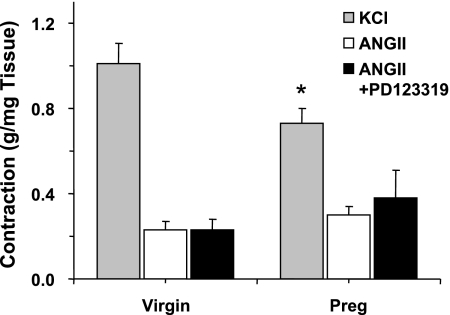
The control contractile response to membrane depolarization by KCl (96 mM) was significant and maintained and was significantly reduced in pregnant compared with virgin rats (P = 0.028; Fig. 2). In contrast, ANG II (10−6 M) caused a very small and transient contraction that was not significantly different between virgin and pregnant rats. Pretreatment of aortic rings with PD-123319 did not affect ANG II-induced contraction in virgin rats. Also, in aortic rings of pregnant rats, pretreatment with PD-123319 did not significantly enhance ANG II-induced contraction (Fig. 2).
Fig. 2.
KCl- and ANG II-induced contraction in aortic rings of virgin and pregnant rats. Aortic rings from virgin and pregnant rats were stimulated with KCl (96 mM) to elicit a control contraction. Tissues were either not treated or pretreated with PD-123319 (10−6 M) for 15 min and then stimulated with ANG II (10−6M). Data represent means ± SE; n = 8. *P < 0.05.
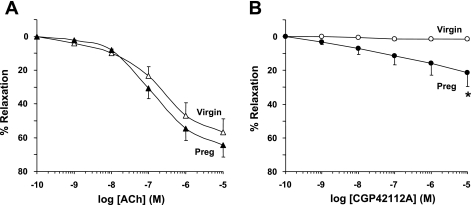
In aortic rings of virgin rats precontracted with Phe (10−6 M), ACh caused concentration-dependent relaxation that reached a maximum at 10−5 M (Fig. 3A). ACh also caused relaxation of aortic rings of pregnant rats that was not significantly greater than that in virgin rats. The AT2R agonist CGP-42112A caused no detectable relaxation in aortic rings of virgin rats. In contrast, CGP-42112A caused significantly greater relaxation of aortic rings of pregnant rats, with the maximal value recorded at 10−5 M (21.32 ± 8.36%, P = 0.040; Fig. 3B).
Fig. 3.
Acetylcholine (ACh)- and CGP-42112A-induced relaxation of Phe contraction in aortic rings of pregnant and virgin rats. Aortic rings were stimulated with Phe (10−6 M) to elicit a submaximal contraction. Increasing concentrations of ACh (A) or the AT2R agonist CGP-42112A (B) were added, and the percent relaxation of Phe contraction was measured. Data represent means ± SE; n = 6–12. *P < 0.05.
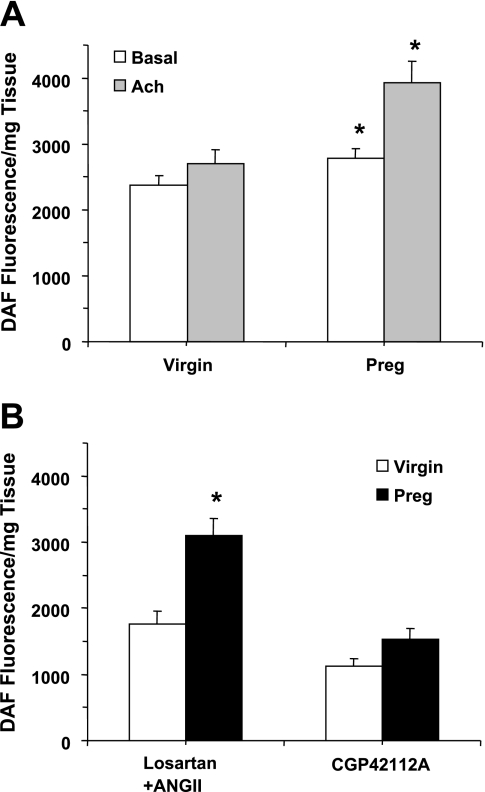
Measurements of NO production indicated that basal NO was significantly greater in aortic rings of pregnant compared with virgin rats (P = 0.029; Fig. 4A). Also, ACh (10−5) caused greater increases in NO production in vascular rings of pregnant compared with virgin rats (P = 0.003). The AT2R agonist CGP-42112A (10−6M) caused increases in NO production in aortic rings of pregnant rats that were not significantly greater than those in aortic rings of virgin rats (P = 0.052; Fig. 4B). Also, treatment of vascular rings with ANG II (10−6 M) plus the AT1R antagonist losartan (10−6 M) induced greater NO production in vascular rings of pregnant compared with virgin rats (P < 0.001). Since losartan is known to mainly block the AT1R, the observed effects of ANG II during AT1R blockade are likely mediated via other ANG II receptor subtypes, such as the AT2R.
Fig. 4.
Nitric oxide (NO) production in aortic rings of virgin and pregnant rats. Aortic rings were incubated in normal Krebs solution for 30 min, and basal NO production was measured using 4-amino-5-methylamino-2′,7′-difluorescein (DAF-FM) fluorescence (A). Tissues were then stimulated with ACh (10−5 M; A), the AT2R agonist CGP-42112A, or ANG II plus the angiotensin type 1 receptor (AT1R) antagonist losartan (B) for 30 min, and released NO was measured. Data represent means ± SE; n = 7–34. *Measurements in pregnant rats were significantly different (P < 0.05) from virgin rats.
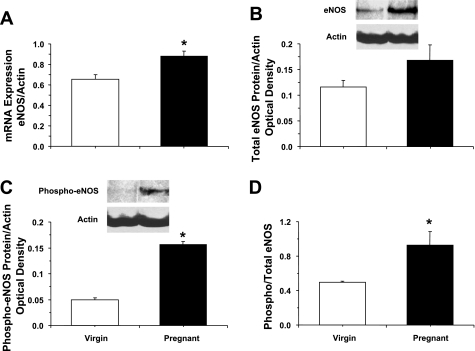
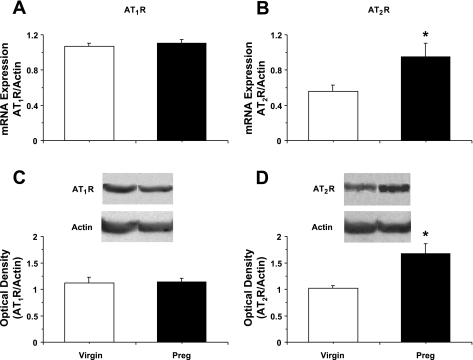
RT-PCR analysis indicated increased expression of eNOS mRNA in the aorta of pregnant compared with virgin rats (P = 0.007; Fig. 5A). Western blot analysis revealed no significant differences in the amount of total eNOS protein (Fig. 5B) but robust and significant increases in activated phospho-eNOS (P < 0.05) in pregnant compared with virgin rats (Fig. 5, C and D). RT-PCR analysis revealed no significant differences in AT1R mRNA expression (Fig. 6A) but significant increases in AT2R mRNA in the aorta of pregnant compared with virgin rats (P = 0.045; Fig. 6B). Western blot analysis revealed no differences in the amount of AT1R protein but significantly greater amounts of AT2R protein in the aorta of pregnant compared with virgin rats (P = 0.029).
Fig. 5.
Expression of endothelial NO synthase (eNOS) in the aorta of virgin and pregnant rats. RT-PCR and Western blot analysis were used to measure eNOS mRNA expression (A) and the amount of total eNOS protein (B), activated phospho-eNOS (C), and phospho-eNOS/total eNOS (D) in aortic tissue homogenates of virgin and pregnant rats. Data represent means ± SE; n = 3–6. *P < 0.05.
Fig. 6.
Expression of AT1Rs and AT2Rs in the aorta of virgin and pregnant rats. RT-PCR and Western blot analysis were used to measure AT1R and AT2R mRNA expression (A and B) and protein amount (C and D) in aortic tissue homogenates of virgin and pregnant rats. Data represent means ± SE; n = 3–5. *P < 0.05.
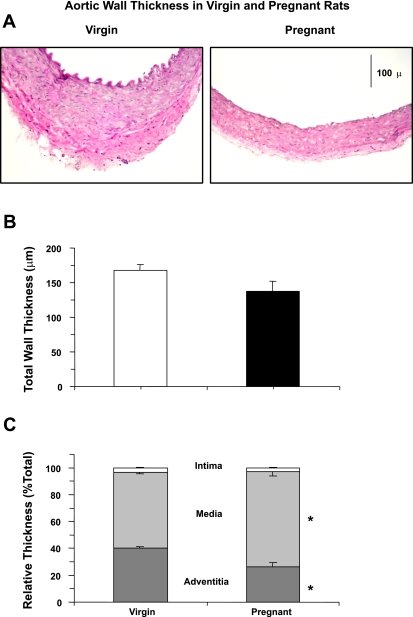
Histological analysis of the tissue sections indicated that the aortic wall thickness was similar in pregnant compared with virgin rats (Fig. 7, A and B). Additional morphometric analysis indicated that the tunica intima thickness as a percentage of the total thickness was similar in pregnant and virgin rats. In contrast, the percent tunica media thickness was significantly greater (P = 0.006) and the percent tunica adventitia thickness was significantly reduced (P = 0.006) in aortic tissue sections of pregnant compared with virgin rats (Fig. 7C).
Fig. 7.
Histology of the aorta of virgin and pregnant rats. Cryosections (6 μm) of the aorta were stained with hematoxylin and eosin (A). The total aortic wall thickness was measured (B). Aortic wall layers of the intima, media, and adventitia were defined, and their thickness is presented as a percentage of the total wall thickness (C). Total magnification: ×100. Data represent means ± SE; n = 4. *P < 0.05.
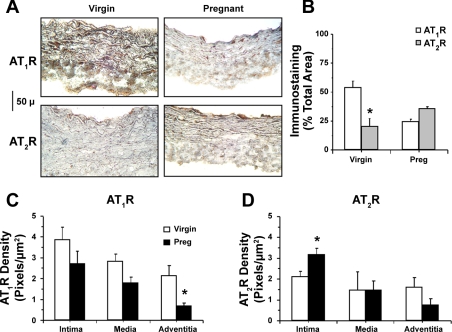
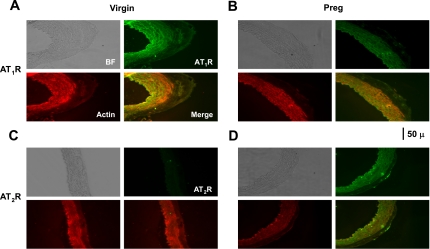
Immunohistochemical analysis revealed significant AT1R and AT2R staining in tissue sections of virgin and pregnant rats (Fig. 8, A and B). In virgin rats, AT1R staining was abundant and evenly distributed in the tunica media, VSM layer, and adventitia (Fig. 8C) and less AT2R staining could be detected (Fig. 8D). In pregnant rats, AT1R staining was not significantly reduced in the intima or media but was significantly reduced in the adventitia (P = 0.027; Fig. 8C), whereas AT2R staining in the tunica intima and endothelium was significantly enhanced (P = 0.039; Fig. 8D). Immunofluorescence analysis indicated that the total AT1R immunofluorescence intensity was 11.72 ± 2.97 units in pregnant rats compared with 26.29 ± 5.55 units in virgin rats, whereas the AT2R immunofluorescence intensity was 21.19 ± 7.1 units in tissue sections of pregnant rats compared with 16.25 ± 3.04 units in tissue sections of virgin rats. Double labeling with actin and colocalization experiments in tissue sections of virgin rats revealed that AT1Rs largely colocalized with actin in the VSM layer (Fig. 9A), whereas AT2Rs were mainly in the intima (Fig. 9C). In tissue sections of pregnant rats, the AT1R signal in the tunica media was reduced (Fig. 9B), whereas a prominent AT2R signal could be detected in the intima (Fig. 9D).
Fig. 8.
Distribution of AT1Rs and AT2Rs in the aorta of virgin and pregnant rats. Cryosections of the aorta were labeled with AT1R and AT2R antibodies, immunostained with the ABC Elite Kit, and counterstained with hematoxylin and eosin (A). Images of tissue sections were acquired and analyzed using the MetaMorph Imaging System. The total number of pixels in the tissue section image was first defined, and the number of brown spots (pixels) was then counted and presented as a percentage of the total pixels (B). The number of pixels in the specific vascular layer (intima, media, and adventitia) was also defined and transformed into the area (in μm2) using a calibration bar. The number of brown spots (pixels) representing the AT1R (C) and AT2R (D) in each vascular layer was then counted and presented as the number of pixels per micrometer squared. Total magnification: ×200. Data represent means ± SE; n = 4. *P < 0.05.
Fig. 9.
Immunofluorescence localization of AT1Rs (A and B) and AT2Rs (C and D) in the aorta of virgin and pregnant rats. Cryosections were labeled with primary AT1R and AT2R antibodies and a fluorsecein-tagged secondary antibody (green). Sections were also labeled with α-actin and Texas red-tagged secondary antibody (red). Bright-field (BF) images were acquired, and AT1R and AT2R fluorescent images were merged with those of actin to test for colocalization (yellow). Total magnification: ×200. Photomicrographs are representative of n = 4.
DISCUSSION
The main findings of the present study are as follows: 1) AT2R-mediated vascular relaxation is enhanced in vascular rings of pregnant rats, 2) the enhanced AT2R-mediated vascular relaxation during pregnancy is associated with increased eNOS mRNA expression and the amount of activated phospho-eNOS, and 3) the enhanced vascular relaxation during pregnancy is associated with increased amounts of vascular AT2Rs and dense distribution in the tunica intima.
Consistent with previous reports (16, 42), BP was found to be reduced in pregnant compared with virgin rats. Additionally, the α-adrenergic agonist Phe caused significant contraction that was reduced in aortic rings from pregnant compared with virgin rats. To test whether the decreased vascular contraction was specific to α-adrenergic receptors, vascular responses to other vasoconstrictor stimuli were examined. The contractile response to ANG II, which is largely mediated via the AT1R, was very small and somewhat difficult to analyze, likely due to the notoriously tachyphyalctic effects of ANG II. On the other hand, the contractile response to membrane depolarization by KCl, a receptor-independent response, was significantly reduced in pregnant compared with virgin rats. These findings are in agreement with previous studies (17, 43, 47, 53) that have shown that the myogenic tone and vascular responsiveness to vasoconstrictors are reduced during pregnancy. The decreased vascular contraction in pregnant rats could be explained by an enhanced NO-mediated vascular relaxation pathway because 1) RT-PCR revealed that eNOS mRNA expression was greater in vascular rings of pregnant compared with virgin rats; 2) Western blot analysis revealed that the protein amount of activated phospho-eNOS was greater in vascular rings of pregnant compared with virgin rats; 3) measurement of NO using DAF-FM fluorescence indicated that basal and ACh-induced NO production are greater in vascular rings of pregnant compared with virgin rats, suggesting increased NOS activity during pregnancy; and 4) prominent ACh-induced relaxation of Phe contraction was observed in aortic rings of pregnant rats. These findings are consistent with reports of increased expression of inducible NOS in the kidney and constitutive eNOS in the aorta of late pregnant rats (3, 27) and increased plasma levels, metabolic production, and urinary excretion of cGMP during pregnancy (12).
The potential upstream mechanisms that could increase the expression/activity of the NO vascular relaxation pathway during pregnancy were investigated. Studies (15, 48) have shown that Phe- and ANG II-induced vascular contraction are reduced in female rats compared with male or ovariectomized (OVX) female rats and not different between estrogen-replaced OVX females and intact female rats. Also, the amount of vascular AT2Rs has been reported to be greater in female compared with male spontaneously hypertensive rats, possibly due to an effect of estrogen on AT2R expression (60). Additionally, the AT2R is expressed heavily in fetal and placental tissues compared with adult tissues (9, 44, 68). Because AT2R-mediated responses are upregulated in the presence of estrogen, and because estrogen increases substantially during pregnancy, we reasoned that AT2R-mediated vascular responses are possibly upregulated during pregnancy due to increased expression/activity of vascular AT2Rs.
The present results suggest an increase in AT2R-mediated vascular relaxation during pregnancy because 1) the AT2R antagonist PD-123319 caused greater enhancement of Phe-induced contraction and enhanced ANG II contraction in vascular rings of pregnant but not virgin rats; 2) CGP-42112A, a highly selective AT2R agonist (36, 70), caused measurable and significant relaxation in the aorta of pregnant but not virgin rats; and 3) NO production in response to ANG II plus the AT1R antagonist losartan was greater in vascular rings of pregnant compared with virgin rats.
The increased AT2R-mediated vascular relaxation could reflect changes in the expression and/or tissue distribution of ANG II receptor subtypes. ANG II stimulates two receptor subtypes: AT1Rs and AT2Rs (24, 32, 39, 52, 62). Studies (30, 54, 65) have shown that the AT1R is widely expressed in blood vessels and the kidney and mediates most of the effects of ANG II. On the other hand, the AT2R is expressed in the kidney and heart as well as the aorta, mesenteric, coronary, and renal vessels of adult rats (9, 10, 60, 61, 68). Also, the AT2R is localized in the endothelium but not in VSM cells in coronary vessels of young rats. Furthermore, AT2R protein (∼44 kDa) is detectable in AT2R-transfected COS-7 cells and neonatal rat cardiac myocytes but not in fibroblasts or young rat aortic VSM cells (68). The present RT-PCR and Western blot experiments are consistent with previous reports and suggest that both AT1Rs and AT2Rs are expressed in the aorta of virgin rats. More importantly, the present study suggests little change in the mRNA expression and protein amount of vascular AT1Rs but substantial increase in AT2Rs during pregnancy.
The changes in the expression of ANG II receptor subtypes during pregnancy could affect both vascular and renal function and thereby affect BP. The AT2R mediates responses that counteract the effects of the AT1R (30, 49). The AT1R stimulates cell proliferation, whereas the AT2R inhibits proliferation and promotes cell differentiation (8, 49, 65). The AT1R mediates antinatriuretic and renal vasoconstrictor effects of ANG II (28, 34, 35, 50), whereas the AT2R opposes these effects and promotes natriuresis and renal vasodilation (8). The AT1R is a G protein-coupled receptor that causes the activation of various protein kinases and stimulation of cellular protein phosphorylation (18, 65). The AT2R is a G protein-coupled receptor with minimal sequence homology with the AT1R (7, 9) and causes the activation of various phosphatases and cellular protein dephosphorylation (4, 19).
Immunohistochemistry revealed a decrease in total AT1Rs and an increase in AT2Rs in the aortic wall of pregnant compared with virgin rats. The increase in AT2Rs in pregnant rats was more prominent in the intima. Immunofluorescence experiments confirmed the immunohistochemistry results and also revealed that AT1Rs colocalized mainly with α-actin in the aortic media, whereas AT2Rs localized mainly in the tunica intima. The present observation that the AT1R was mainly localized in the tunica media is consistent with its role in vascular contraction. On the other hand, the localization of the AT2R in the tunica intima is consistent with its potential effect on the endothelium. The observed dense distribution of the AT2R in the intima of aortic rings of pregnant rats is consistent with enhanced AT2R-mediated vascular relaxation during pregnancy.
The increased AT2R-mediated vascular relaxation may explain the decreased BP observed during pregnancy. It should be noted that the present experiments were conducted on the aorta, and whether similar pregnancy-associated increases in the AT2R-mediated vascular relaxation pathway also occur in small resistance vessels remains to be examined. Also, it is possible that other vascular relaxation pathways, such as AT2R-mediated bradykinin release and activation of PGI2-cAMP and/or EDHF pathways, may contribute to vascular relaxation during pregnancy. It has been shown that the activation of endothelial AT2Rs stimulates the release of bradykinin and thereby stimulates bradykinin B2 receptor-mediated mechanisms of vascular relaxation through increased NO, PGI2, and EDHF production (1, 8, 9, 41, 71). The AT2R is also coupled to eNOS activation and the NO-cGMP vascular relaxation pathway (1, 7, 8, 51, 57) as well as increased PGI2 synthesis and the release of EDHF (20, 38). A study (72) has also shown an increase in PGI2 production during late pregnancy. Additionally, EDHF may play a role in the enhanced ACh-induced relaxation of mesenteric and uterine arteries of pregnant rats (23, 26).
An important observation is that while the total aortic wall thickness may not be significantly different, the media/total thickness was greater in pregnant rats compared with virgin rats. These structural changes may be related to the increased cardiac output and hemodynamic forces during pregnancy. Whether these structural changes are also related to the changes in the expression of vascular AT1Rs and AT2Rs and their differential effects on vascular cell growth and proliferation during pregnancy should be examined in future studies.
In 5–10% of pregnancies, women develop preeclampsia, a condition characterized by proteinuria and hypertension, which represents a major cause of maternal and fetal morbidity and mortality (22, 29, 45, 55, 56). Hypertension in pregnancy is associated with increased vascular resistance, enhanced vascular contraction to ANG II, and reduced renal plasma flow (2, 29, 43, 63). Understanding the vascular mechanisms involved in the regulation of BP during normal pregnancy would help to delineate the pathological changes that occur during hypertension in pregnancy. The present experiments demonstrated that acute treatment of vascular strips with AT2R antagonist is associated with enhanced vascular contraction in pregnant rats. To further test the hypothesis that endothelial AT2R-mediated vascular relaxation mechanisms are enhanced during pregnancy, future studies should examine whether chronic infusion of pregnant rats with AT2R antagonist is associated with decreased vascular relaxation and increased contraction and BP, thus mimicking the conditions observed in hypertension in pregnancy and preeclampsia. Also, because of the difficulty in performing mechanistic studies in pregnant women, animal models of hypertension in pregnancy have been developed (5, 13, 14, 21, 42, 46). The present study highlights the exciting possibility that vascular AT2Rs could be downregulated in animal models of hypertension in pregnancy and in women with preeclampsia and should represent important areas for future investigations and translational research.
Thus, AT2R-mediated vascular relaxation is enhanced during normal pregnancy. The enhanced vascular relaxation could be related to increased AT2R-mediated signaling and increased eNOS activity. An increase in AT2R-mediated vascular relaxation may result in the decreased BP observed during normal pregnancy. Also, during normal pregnancy, the total amount and tissue distribution of ANG II receptor subtypes show a decrease in AT1Rs and an increase in AT2Rs, particularly in the tunica intima. The differential expression of vasodilator AT2Rs and vasoconstrictive AT1Rs between the vascular endothelium and smooth muscle during pregnancy may function as a compensatory mechanism for the increased hemodynamic forces and may in part explain the reduction of BP despite the upregulation of the renin-angiotensin system during pregnancy.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-65998 and HL-70659.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension 42: 600–604, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension 38: 742–745, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT, Miller MT, Kassab S, Novak J, Reckelhoff JF, Kruckeberg WC, Granger JP. Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats. Hypertension 33: 435–439, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Andresen BT, Romero GG, Jackson EK. AT2 receptors attenuate AT1 receptor-induced phospholipase D activation in vascular smooth muscle cells. J Pharmacol Exp Ther 309: 425–431, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Baylis C, Engels K. Adverse interactions between pregnancy and a new model of systemic hypertension produced by chronic blockade of endothelial derived relaxing factor (EDRF) in the rat. Clin Exp Hypertens B11: 117–129, 1992. [Google Scholar]
- 6.Cain AE, Khalil RA. Pathophysiology of essential hypertension: role of the pump, the vessel, and the kidney. Semin Nephrol 22: 3–16, 2002. [PubMed] [Google Scholar]
- 7.Carey RM, Howell NL, Jin XH, Siragy HM. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension 38: 1272–1277, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Carey RM, Jin X, Wang Z, Siragy HM. Nitric oxide: a physiological mediator of the type 2 (AT2) angiotensin receptor. Acta Physiol Scand 168: 65–71, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Carey RM, Jin XH, Siragy HM. Role of the angiotensin AT2 receptor in blood pressure regulation and therapeutic implications. Am J Hypertens 14: 98S–102S, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension 35: 155–163, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Chen K, Merrill DC, Rose JC. The importance of angiotensin II subtype receptors for blood pressure control during mouse pregnancy. Reprod Sci 14: 694–704, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Conrad KP, Vernier KA. Plasma level, urinary excretion, and metabolic production of cGMP during gestation in rats. Am J Physiol Regul Integr Comp Physiol 257: R847–R853, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Conrad KP Animal models of pre-eclampsia: do they exist? Fetal Med Rev 2: 67–88, 1990. [Google Scholar]
- 14.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion in pregnant rat. Hypertension 35: 367–372, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Crews JK, Murphy JG, Khalil RA. Gender differences in Ca2+ entry mechanisms of vasoconstriction in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 34: 931–936, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Crews JK, Novak J, Granger JP, Khalil RA. Stimulated mechanisms of Ca2+ entry into vascular smooth muscle during NO synthesis inhibition in pregnant rats. Am J Physiol Regul Integr Comp Physiol 276: R530–R538, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Davidge ST, McLaughlin MK. Endogenous modulation of the blunted adrenergic response in resistance-sized mesenteric arteries from the pregnant rat. Am J Obstet Gynecol 167: 1691–1698, 1992. [DOI] [PubMed] [Google Scholar]
- 18.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000. [PubMed] [Google Scholar]
- 19.de Gasparo M, Siragy HM. The AT2 receptor: fact, fancy and fantasy. Regul Pept 81: 11–24, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Dimitropoulou C, White RE, Fuchs L, Zhang H, Catravas JD, Carrier GO. Angiotensin II relaxes microvessels via the AT2 receptor and Ca2+-activated K+ (BKCa) channels. Hypertension 37: 301–307, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Eder DJ, McDonald MT. A role for brain angiotensin II in experimental pregnancy-induced hypertension in laboratory rats. Clin Exp Hyper Hyper Preg B6: 431–451, 1987. [Google Scholar]
- 22.Friedman SA, Lubarsky SL, Ahokas RA, Nova A, Sibai BM. Preeclampsia and related disorders. Clinical aspects and relevance of endothelin and nitric oxide. Clin Perinatol 22: 343–355, 1995. [PubMed] [Google Scholar]
- 23.Fulep EE, Vedernikov YP, Saade GR, Garfield RE. The role of endothelium-derived hyperpolarizing factor in the regulation of the uterine circulation in pregnant rats. Am J Obstet Gynecol 185: 638–642, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Gallinat S, Busche S, Raizada MK, Sumners C. The angiotensin II type 2 receptor: an enigma with multiple variations. Am J Physiol Endocrinol Metab 278: E357–E374, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 52: 2682–2689, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerber RT, Anwar MA, Poston L. Enhanced acetylcholine induced relaxation in small mesenteric arteries from pregnant rats: an important role for endothelium-derived hyperpolarizing factor (EDHF). Br J Pharmacol 125: 455–460, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz RM, Morano I, Calovini T, Studer R, Holtz J. Increased expression of endothelial constitutive nitric oxide synthase in rat aorta during pregnancy. Biochem Biophys Res Commun 205: 905–910, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Granger JP, Alexander BT, Llinas M. Mechanisms of pressure natriuresis. Curr Hypertens Rep 4: 152–159, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 9: 147–160, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Griendling KK, Tsuda T, Berk BC, Alexander RW. Angiotensin II stimulation of vascular smooth muscle cells. Secondary signalling mechanisms. Am J Hypertens 2: 659–665, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Griendling KK, Ushio-Fukai M, Lassègue B, Alexander RW. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension 29: 366–373, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Gross V, Obst M, Luft FC. Insights into angiotensin II receptor function through AT2 receptor knockout mice. Acta Physiol Scand 181: 487–494, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Gruetter CA, Gruetter DY, Lyon JE, Kadowitz PJ, Ignarro LJ. Relationship between cyclic guanosine 3′:5′-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther 219: 181–186, 1981. [PubMed] [Google Scholar]
- 34.Hall JE, Brands MW, Henegar JR. Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J Am Soc Nephrol 10, Suppl 12: S258–S265, 1999. [PubMed] [Google Scholar]
- 35.Hall JE Control of sodium excretion by angiotensin II: intrarenal mechanisms and blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 250: R960–R972, 1986. [DOI] [PubMed] [Google Scholar]
- 36.Hines J, Heerding JN, Fluharty SJ, Yee DK. Identification of angiotensin II type 2 (AT2) receptor domains mediating high-affinity CGP 42112A binding and receptor activation. J Pharmacol Exp Ther 298: 665–673, 2001. [PubMed] [Google Scholar]
- 37.Ignarro LJ, Kadowitz PJ. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol 25: 171–191, 1985. [DOI] [PubMed] [Google Scholar]
- 38.Jaiswal N, Tallant EA, Jaiswal RK, Diz DI, Ferrario CM. Differential regulation of prostaglandin synthesis by angiotensin peptides in porcine aortic smooth muscle cells: subtypes of angiotensin receptors involved. J Pharmacol Exp Ther 265: 664–673, 1993. [PubMed] [Google Scholar]
- 39.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signaling and function. Blood Press 12: 70–88, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Katada J, Majima M. AT2 receptor-dependent vasodilation is mediated by activation of vascular kinin generation under flow conditions. Br J Pharmacol 136: 484–491, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension 31: 1065–1069, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension 31: 1065–1069, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol 283: R29–R45, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Koukoulas I, Mustafa T, Douglas-Denton R, Wintour EM. Angiotensin II receptor (type 1 and 2) expression peaks when placental growth is maximal in sheep. Am J Physiol Regul Integr Comp Physiol 283: R972–R982, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Lindheimer MD HTN in pregnancy. Hypertension 22: 127–137, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Losonczy G, Brown G, Venuto RC. Increased peripheral resistance during reduced uterine perfusion pressure hypertension in pregnant rabbits. Am J Med Sci 303: 233–240, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Molnar M, Hertelendy F. Nω-nitro-l-arginine, an inhibitor of nitric oxide synthesis, increases blood pressure in rats and reverses the pregnancy-induced refractoriness to vasopressor agents. Am J Obstet Gynecol 166: 1560–1567, 1992. [DOI] [PubMed] [Google Scholar]
- 48.Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca2+]i in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol 278: C834–C844, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, Horiuchi M, Pratt RE, Dzau VJ. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci USA 92: 10663–10667, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navar LG, Harrison-Bernard LM, Imig JD, Cervenka L, Mitchell KD. Renal responses to AT1 receptor blockade. Am J Hypertens 13: 45S–54S, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Olson S, Oeckler R, Li X, Du L, Traganos F, Zhao X, Burke-Wolin T. Angiotensin II stimulates nitric oxide production in pulmonary artery endothelium via the type 2 receptor. Am J Physiol Lung Cell Mol Physiol 287: L559–L568, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Raizada MK, Lu D, Sumners C. AT1 receptors and angiotensin actions in the brain and neuronal cultures of normotensive and hypertensive rats. Adv Exp Med Biol 377: 331–348, 1995. [DOI] [PubMed] [Google Scholar]
- 53.Ramirez RJ, Novak J, Johnston TP, Gandley RE, McLaughlin MK, Hubel CA. Endothelial function and myogenic reactivity in small mesenteric arteries of hyperlipidemic pregnant rats. Am J Physiol Regul Integr Comp Physiol 281: R1330–R1337, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Reaves PY, Beck CR, Wang HW, Raizada MK, Katovich MJ. Endothelial-independent prevention of high blood pressure in l-NAME-treated rats by angiotensin II type I receptor antisense gene therapy. Exp Physiol 88: 467–473, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Roberts JM, Pearson G, Cutler J, Lindheimer M; NHLBI. Working Group on Research on Hypertension During Pregnancy. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 41: 437–445, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol 163: 460–465, 1990. [DOI] [PubMed] [Google Scholar]
- 57.Savoia C, Ebrahimian T, He Y, Gratton JP, Schiffrin EL, Touyz RM. Angiotensin II/AT2 receptor-induced vasodilation in stroke-prone spontaneously hypertensive rats involves nitric oxide and cGMP-dependent protein kinase. J Hypertens 24: 2417–2422, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Shah DM The role of RAS in the pathogenesis of preeclampsia. Curr Hypertens Rep 8: 144–152, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Sheng JZ, Wang D, Braun AP. DAF-FM (4-amino-5-methylamino-2′,7′-difluorofluorescein) diacetate detects impairment of agonist-stimulated nitric oxide synthesis by elevated glucose in human vascular endothelial cells: reversal by vitamin C and l-sepiapterin. J Pharmacol Exp Ther 315: 931–940, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Siragy HM, Carey RM. Angiotensin type 2 receptors: potential importance in the regulation of blood pressure. Curr Opin Nephrol Hypertens 10: 99–103, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Siragy HM AT1 and AT2 receptor in the kidney: role in health and disease. Semin Nephrol 24: 93–100, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Stennett AK, Khalil RA. Neurovascular mechanisms of hypertension in pregnancy. Curr Neurovasc Res 3: 131–148, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Takeda-Matsubara Y, Iwai M, Cui TX, Shiuchi T, Liu HW, Okumura M, Ito M, Horiuchi M. Roles of angiotensin type 1 and 2 receptors in pregnancy-associated blood pressure change. Am J Hypertens 17: 684–689, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Touyz RM, Berry C. Recent advances in angiotensin II signaling. Braz J Med Biol Res 35: 1001–1015, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Touyz RM, He G, Deng LY, Schiffrin EL. Role of extracellular signal-regulated kinases in angiotensin II-stimulated contraction of smooth muscle cells from human resistance arteries. Circulation 99: 392–399, 1999. [DOI] [PubMed] [Google Scholar]
- 67.Touyz RM The role of angiotensin II in regulating vascular structural and functional changes in hypertension. Curr Hypertens Rep 5: 155–164, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Wang ZQ, Moore AF, Ozono R, Siragy HM, Carey RM. Immunolocalization of subtype 2 angiotensin II (AT2) receptor protein in rat heart. Hypertension 32: 78–83, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens 12: 205S–213S, 1999. [DOI] [PubMed] [Google Scholar]
- 70.Whitebread SE, Taylor V, Bottari SP, Kamber B, de Gasparo M. Radioiodinated CGP 42112A: a novel high affinity and highly selective ligand for the characterization of angiotensin AT2 receptors. Biochem Biophys Res Commun 181: 1365–1371, 1991. [DOI] [PubMed] [Google Scholar]
- 71.Yayama K, Okamoto H. Angiotensin II-induced vasodilation via type 2 receptor: role of bradykinin and nitric oxide. Int Immunopharmacol 8: 312–318, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Ylikorkala O, Pekonen F, Viinikka L. Renal prostacyclin and thromboxane in normotensive and preeclamptic pregnant women and their infants. J Clin Endocrinol Metab 63: 1307–1312, 1986. [DOI] [PubMed] [Google Scholar]