Abstract
Upon remodeling of the ventricle after a provoking stimulus, such as hypertension, connections between adjacent myocytes may need to be “reformatted” to preserve a synchronization of excitation of the remodeling heart. In the mammalian heart, the protein connexin forms the gap junctions that allow electrical and chemical signaling communication between neighboring cells. We aim to elucidate whether mechanical load, in isolation, potentially changes the expression of connexin 43 (Cx43), the major isoform of the connexin family in the ventricle, and its phosphorylation. Cx43 expression levels and contractile function of multicellular rabbit cardiac preparations were assessed in a newly developed in vitro system that allows for the study of the transition of healthy multicellular rabbit myocardium to hypertrophied myocardium. We found that in mechanically loaded cardiac trabeculae, Cx43 levels remained stable for about 12 h and then rapidly declined. Phosphorylation at Ser368 declined much faster, being almost absent after 2 h of high-load conditions. No-load conditions did not affect Cx43 levels, nor did phosphorylation at Ser368. The downregulation of Cx43 under mechanical load did not correspond with the contractile changes that were observed. Furthermore, blocking paracrine activity of the muscle could only partially prevent the downregulation of Cx43. Additionally, no effect of mechanical loading on the expression of N-cadherin and zonula occludens-1 was observed, indicating a specificity of the connexin response. High mechanical load induced a rapid loss of Cx43 phosphorylation, followed by a decrease in Cx43 protein levels. Paracrine factors are partly responsible for the underlying mechanism of action, whereas no direct correlation to contractile ability was observed.
Keywords: contractility, gap junction, rabbit, trabeculae
ventricular remodeling after a provoking stimulus, such as hypertension or an infarct, may induce the reformation of the connections between adjacent myocytes to preserve the synchronization of excitation of the heart. When the myocyte grows in size, the adherence to the extracellular matrix needs to be maintained for the transduction of mechanical activity. In addition, cell-to-cell connections need to be maintained or established to preserve the electrical syncytium. In the mammalian heart, the protein connexin forms the gap junction channels that allow electrical and chemical signaling communication between neighboring cells. Three major isoforms of connexin have been detected in cardiomyocytes: connexin 40 (Cx40), Cx43, and Cx45 (48, 51). Among these, Cx43 predominantly expresses in ventricular myocytes (51), and the degree of its expression reflects the conduction velocity of the heart (15, 47). A significant downregulation of Cx43 protein levels has been observed in both ischemic and nonischemic forms of human heart failure (16, 34, 42). However, in a sizeable body of the literature, increases, no changes, and decreases in Cx43 expression have been reported in different hypertrophic models (26, 34, 45). Peters et al. (34) showed a decreased Cx43 localization on cell surface areas in hypertrophied hearts from patients who suffered from aortic valvular stenosis. In an animal model of hypertension-induced cardiac hypertrophy, a reduced gap junction density at intercalated disks was also detected; however, there was no change in the expression of total myocardial Cx43 protein (45). Kostin et al. (26) suggested a biphasic expression of Cx43 at various stages of patients with aortic stenosis, by which an upregulation of Cx43 was demonstrated in compensated [ejection fraction (EF) > 50%] cardiac hypertrophy, but a downregulation was detected in decompensated (EF < 30%) hypertrophy hearts. Although there is a clear relationship between hypertrophied development and Cx43 expression and localization, their correlation remains incompletely understood.
An in vitro induced upregulation of Cx43 has been demonstrated in cultured neonatal cardiac myocytes by either chemical- or mechanical-induced hypertrophic signaling stimulations (11, 36, 38, 53, 54). α- and β-Adrenergic agonists as well as endothelin-1 (ET-1) and angiotensin II (ANG II) all have been shown to be able to induce an increase in Cx43 expression within 24 h in cultured neonatal myocytes (36, 38). The mechanical stretch also rapidly activated an increase in Cx43 expression as high as twofold within 1 h in neonatal cardiac myocytes (54). With the use of tissue engineering of a hybrid cardiac construction, an upregulation of Cx43 after 1 wk stretching was also found (11). Thus these in vitro studies also imply that the regulation of Cx43 levels is somehow cocoordinated by the development of hypertrophy.
The underlying mechanism(s) via which hypertrophy impacts on Cx43 levels is, however, unresolved. Since an excess of both mechanical and hormonal stimuli occurs in vivo during the development of pathological or pathophysiological hypertrophy, it is imperative to gain an insight into how these factors impact Cx43 levels. Understanding the impact of mechanical and hormonal stimuli on Cx43 levels is therefore paramount in unifying a common concept that could possibly explain seemingly controversial findings.
In the present study, we hypothesized that the response to a mechanical stress in adult cardiomyocytes in their in situ setting is a key factor in determining the diverse expression of Cx43. To further our understanding of the regulation of Cx43 protein expression, we followed the change in Cx43 expression during the early development of hypertrophy in a recently developed in vitro model of cultured adult myocardium. This model allows for the adult human multicellular myocardium to be stimulated to undergo hypertrophy, while contractile parameters are continuously assessed under controlled in vitro conditions (13, 21). In the present study, Cx43 protein expression was compared between cultured cardiac trabeculae subjected to various loading conditions. We demonstrate, in a temporal resolved manner, the changes in Cx43 protein expression induced by mechanical load.
MATERIALS AND METHODS
The present study conforms to the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, Revised 1996). All of the animals were handled and cared for according to protocols approved by the Institutional Animal Care and Use Committee of The Ohio State University.
Multicellular myocardial culture.
The cardiac trabeculae culture procedure has been detailed previously (24). New Zealand White rabbits (1.5–2.0 kg) were heparinized and anesthetized by intravenous infusion of pentobarbital sodium (50 mg/kg). The hearts were rapidly excised and retrogradely perfused in a modified Langendorff perfusion system with a 2,3-butanedione monoxime-containing, low-calcium, Krebs-Henseleit solution. Nonbranched trabeculae from the free wall of the right ventricle were dissected and then mounted between the force transducer and a micromanipulator screw in a semiclosed-circuit culture system (modified from Scientific Instruments). The solution was exchanged for modified cell culture medium, and the muscles were electrically stimulated to contract at 1 Hz at 37°C as previously described (24). The muscles were then subjected to different loading conditions by increasing the diastolic tension to predetermined values (13). For the no-load group (no-load muscles), the muscle was buckled and remained slack during contraction throughout the entire experiment. A second group mimicked the high preload by stretching to a passive tension of around 5–8 mN/mm2, representing a sarcomere length of about 2.2 μm, near end-diastolic values (30, 37). Because the muscles contract isometrically and are not allowed to shorten like in the ejecting heart, the mechanical load that they are exposed to exceeds the in vivo normal working load. Contractile activity was continuously monitored for the entire duration of the experiment, up to 48 h. We stimulated the muscles at 1 Hz, which is below the resting rate for the rabbit. This allowed for a full relaxation of the muscle in between beats (which due to the isometric nature is much longer than were the muscle allowed to unload during contraction), thus avoiding complications of the slowing of the twitch that can lead to a severe diastolic impairment of the muscle and a potential loss of quality during the latter phases of the culture period.
Protein electrophoresis.
With the use of only the middle part of the trabeculae (to avoid complications of damaged ends), immunoblot analysis was performed using standard protocols. Anti-total Cx43 (1:2,000) and anti-zonula occludens-1 (ZO-1, 1:250) antibodies were obtained from Zymed. Anti-phospho-Cx43 (Ser368, 1:1,000) and anti-N-cadherin (1:1,000) were from Cell Signaling. The protein expression density was normalized to the expression of cardiac troponin I (cTnI) of the same blot using anti-cTnI (1:10,000) from Imgenex. The amount of protein loading was set to 25 μg for the assessment of total Cx43, 50 μg for phospho-Cx43, and 75 μg for N-cadherin and ZO-1. Because of the very small amount of protein that can be isolated from these small muscles, it is in most cases not possible to analyze the protein content before gel loading, and the entire sample is loaded, requiring an internal standard to quantify protein level changes. We chose to quantify Cx43 expression versus TnI since this myofilament protein is expressed stoichiometrically with many other proteins in the cell and can run and be detected on the same gel. In previous studies, we showed that in these cultured trabeculae, TnI increased proportionally with myosin heavy chain and troponin-T and that this was within the same increase as observed in myocyte size (13, 21).
Pharmacological antagonists.
Several chemicals were used in this study. BQ-123, a selective ET type A (ETA) receptor antagonist, telmisartan, a non-peptide ANG II type 1 receptor antagonist, and SB-431542, a selective inhibitor of transforming growth factor-β (TGF-β) superfamily type I, were obtained from Sigma. PD-145065, a non-selective ET receptor antagonist, was from Calbiochem, and losartan, another ANG II type 1 receptor antagonist, was from Cayman Chemical. All inhibitor concentrations used in the study were 10 to 100 times their effective Kd value.
Data analysis and statistics.
Diastolic tension and developed force of contraction (both normalized to the initial cross-sectional area of the muscle), as well as contraction and relaxation kinetics, were recorded and calculated off-line using custom-designed software (written in LabView, National Instruments). Immunoblot densitometry was calculated by the ImageJ v. 1.37 program (NIH). Multiple group comparisons were performed using ANOVA, followed by Bonferroni post hoc analysis. Values are given as means ± SE. A two-tailed P value < 0.05 was taken to identify statistical significant differences.
RESULTS
Contractile function in response to mechanical overload.
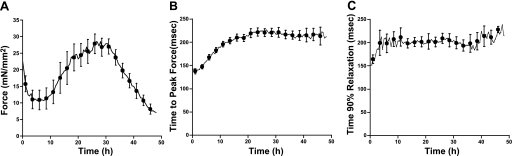
First, we subjected the muscles to a high preload condition (5–8 mN/mm2) with a continuous 1-Hz electrical stimulation at 37°C. Changes in force of contraction and twitch kinetics were recorded throughout 48 h of culture (Fig. 1). Developed force sharply decreased in the first couple of hours after stretching but then gradually increased after about 8 h. Developed force reached a maximum point at around 26 h, and the force increased 43 ± 11% of initial force (Fig. 1A). Time to peak force gradually increased and stabilized after 20 h (Fig. 1B). Time to 90% relaxation also increased in the first hours and then maintained constant over the remaining culture period (Fig. 1C). Both the quantity and timing of the changes in force are virtually identical to our previous study (13), indicating the reproducibility of the protocol.
Fig. 1.
A: active developed force of cultured rabbit cardiac trabeculae (n = 8), contracting at 1 Hz, continuously for 48 h at high load. B: time to peak force. C: 90% of relaxation time.
Expression of Cx43 in loaded muscles.
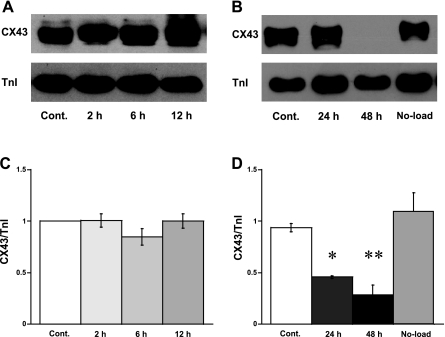
We next investigated the Cx43 levels and related proteins in these muscles subjected to high load. To do so, the middle part of the loaded and taut (no load) muscles was rapidly frozen in liquid nitrogen after various times in culture. The expression of Cx43 was quantified using immunoblot analysis. Because our previous results (13, 21) demonstrated that high loading or stretch induced the generation of new sarcomeres as well as an increase in myocyte size, the myofilament cTnI was chosen to normalize the amount of loading protein. Thus the relative arbitrary unit of Cx43 per cTnI also implied a ratio of Cx43 expression to myocyte size. High load did not affect the expression of Cx43 during the first 12 h of culture (Fig. 2, A and C). A significant downregulation of Cx43 (to 46 ± 13% of control, n = 5, P < 0.05) was observed after 24 h of culture, even though the developed force was still increasing as shown in Fig. 1. The expression of Cx43 was even lower at 48 h (29 ± 9% of control, n = 5, P < 0.01). Interestingly, the Cx43 expression in no-load muscles was not different from controls (Fig. 2, B and D). These latter control experiments confirmed that the downregulation of Cx43 was a result of the effect of high loading and not the culture technique itself.
Fig. 2.
A and B: immunoblot analysis demonstrates the expression of protein connexin 43 (Cx43) and relative amount of cardiac troponin-I (cTnI) under high-loading condition at 2, 6, 12, 24, and 48 h. Cont, control (a fresh muscle sample); No-load, a culture cardiac trabeculae contracting at 1 Hz for 48 h without pre-and afterload (i.e., slack). C and D: relative arbitrary unit of Cx43 vs. cTnI. C: each control and culture muscles were from the same heart (n = 10). D: control and culture samples were from the same and different hearts (n = 5). *P < 0.05 and **P < 0.01 vs. controls by Bonferroni's multiple comparisons after ANOVA.
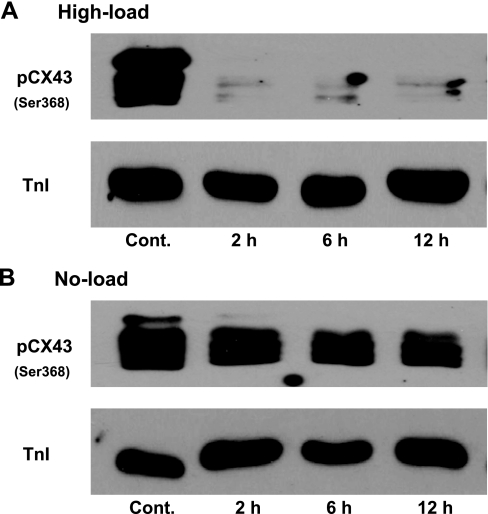
We next verified the effect of load on the Cx43 properties by measuring the level of Cx43 phosphorylation at Ser368. Immunoblot analysis demonstrated that the phosphorylation level of Cx43 (Ser368) slowly decreased over time in no-loaded muscles (Fig. 3B). On the other hand, under high loading, the phosphorylation level dropped much faster, to the extent that it was nearly undetectable after only 2 h (Fig. 3A). This sharp decrease in Cx43 phosphorylation at Ser368 in high-loaded muscles implies that the mechanical loading distresses the gap junction opening properties before a downregulation of the protein levels.
Fig. 3.
Comparison the phosphorylation level of Cx43 (pCx43) at Ser368 using immunoblot analysis of high-loading samples at 2, 6, and 12 h (A) vs. no-load samples at the same point (B). cTnI was used to indicate the amount of protein loading because no change in the ratio of Cx43 to cTnI was detected in 12 h of culture shown in Fig. 2. Each set of controls and cultured muscles were from the same hearts.
To test for a functional consequence of reduced Cx43 levels, we used an iontophoresis protocol (6, 50) on a subset of muscles that had undergone culture for 48 h under load. Iontophoresis is a technique used in our laboratory to load a single myocyte after impalement with a micropipette with the calcium indicator bis-fura-2. This indicator is loaded exclusively into the cytosol of a single cell and then diffuses through the gap junctions to neighboring cells, and thus via a single impalement, an entire trabeculae can be loaded. As this technique requires functional gap junctions to neighboring cells, we used it to determine whether fura-2 would diffuse normally. We observed that while we could successfully load a single cell of these trabeculae at the same rate that we have been able to do in control tissue, in loaded 48-h cultured tissue where Cx43 levels are severely reduced, the transport of fura-2 to neighboring cells was critically impaired. Only in one of nine tested muscles where we successfully loaded a central myocyte was a very small amount of dye detected in a few neighboring myocytes, whereas in nonloaded or control muscles, in nine out of nine muscles where we successfully loaded a central myocyte, we achieved a widespread loading of the indicator, in line with recent studies from our laboratory (31, 50). From these experiments we conclude that, at least at the level of a small molecule transport, a loss of Cx43 results in a loss of function.
Expression of other junction proteins.
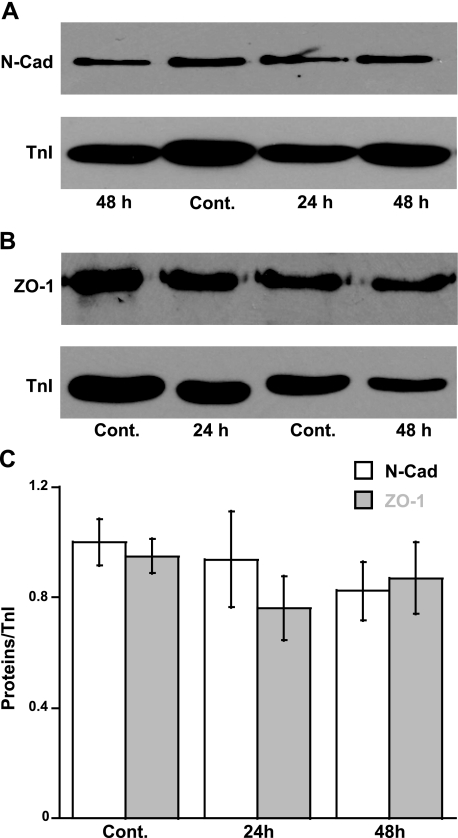
The expression of junction proteins and the organization of an intercalated disc are additional factors that affect the distribution and electromechanical function of gap junction (5, 8). We thus measured the expression of N-cadherin, a major protein forming an intercellular junction between cardiac myocytes. Results showed no significant difference in N-cadherin levels between controls and loaded muscles (Fig. 4, A and C). In addition, we also performed an immunoblotting of ZO-1 (a PDZ-MAGUK protein), a protein highly expressed at an intercalated disc that has binding interactions with Cx43 (20). Slight decreases in ZO-1 expression were detected after 24 h of high load (76 ± 11% of controls), but no significant difference was observed among groups by ANOVA (Fig. 4, B and C). These results implied that the required proteins associated with gap junction formation do not significantly alter their levels during the early response to mechanical overload.
Fig. 4.
Immunoblot analysis demonstrates the expression of N-cadherin (N-Cad; A) and zonula occludens-1 (ZO-1; B) vs. TnI. C: relative arbitrary unit of N-Cad vs. TnI (n = 8–10) and ZO-1 vs. TnI (n = 6 to 7). No significant difference among groups by ANOVA.
Pharmacological inhibition of load-induced paracrine and autocrine activities.
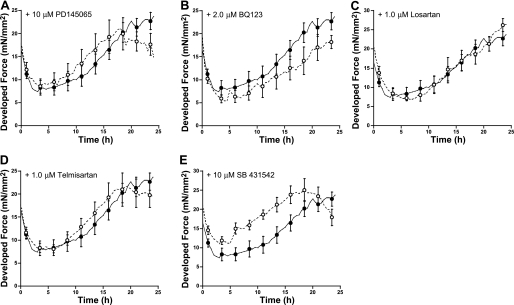
Previous studies indicated that mechanical loading activates paracrines released from non-myocyte cells in cardiac tissue, as well as releases autocrine compounds from the myocyte itself (4, 49). To investigate this mechanism, we proceeded to test whether the downregulation of Cx43 under high load was the effect of potential paracrine actions. We determined the involvement of potential candidates including ET-1, ANG II, and TGF-β using various specific inhibitors. Figure 5 represents changes in the developed force of contraction over 24 h of high-loaded muscles in the presence and in absence of inhibitors. Neither BQ-123, a specific ETA, nor PD-145065, a nonspecific ET-1 receptor blocker, significantly changed the pattern of force development in the high-load condition. High-load muscles treated with either losartan, telmisartan, or ANG II receptor blockers showed similar changes in force development as controls. In three control, unloaded experiments, the effects of losartan on Cx43 levels were assessed. We did not see any changes in the protein Cx43 levels, nor ZO-1 versus our previously collected controls. Loaded muscles incubated with SB-431542, a selective inhibitor of activin receptor-like kinase receptors, which inhibits TGF-β activity, showed a significantly higher developed force compared with that in controls. However, the increase in force of contraction over time was very similar to that in high-loaded controls measured in parallel.
Fig. 5.
Active developed force over 24 h of high-loaded culture cardiac trabeculae in the absence (•, n = 9) and presence (○, n = 6–8) of selective pharmacological antagonists. PD-145065 (A) is a nonselective endothelin receptor antagonist, BQ-123 (B) is a specific endothelin A receptor antagonist, losartan (C) and telmisartan (D) are angiotensin II receptor antagonists, and SB-431542 (E) is a selective inhibitor of activin receptor-like kinase receptors.
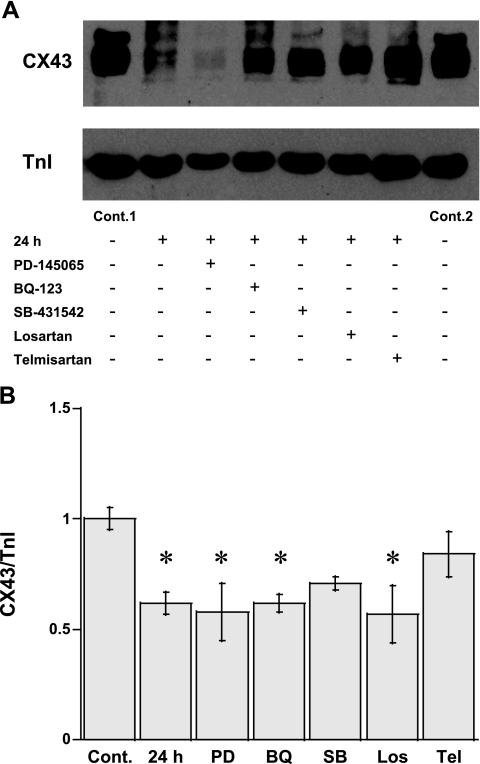
The muscles cultured in the presence of various inhibitors were also subjected to immunoblot analysis to determine the expression of Cx43 (Fig. 6). Densitometry measurements (Fig. 6B) also demonstrated a significant (P < 0.05) downregulation of Cx43 in high-loaded muscle in this experiment (62 ± 5% of controls, n = 6), as expected. Neither BQ-123 nor PD-145065 prevented the downregulation of Cx43 in high-loaded muscle (62 ± 4%, n = 5; and 58 ± 13%, n = 5, respectively). With the same target, telmisartan partially protected the suppression of Cx43 in this condition (84 ± 10% of controls, n = 7), whereas losartan did not (57 ± 13% of controls, n = 5). The expression of Cx43 in the presence of SB-431542 was not significantly different from controls (71 ± 3% of controls, n = 5). These results indicate the possibility that ANG II and TGF-β might play a part in the load-induced degradation of Cx43.
Fig. 6.
A: expression of Cx43 vs. cTnI of high-loaded muscle at 24 h in the absence and presence of selective pharmacological antagonists by immunoblot analysis. B: relative arbitrary unit of the ratio of Cx43 to TnI in each group was compared to Cont. PD, PD-145065; BQ, BQ-123; SB, SB-431542; Los, losartan; Tel, telmisartan. *P < 0.05 vs. controls by Bonferroni's multiple comparisons after ANOVA.
DISCUSSION
The results of the present study show for the first time that high mechanical loading of multicellular adult myocardium, in the absence of systemic neurohumoral stimulation, decreases the total myocardial Cx43 expression over time, and this results in a loss of function. In addition, we show that the phosphorylation of Cx43 is downregulated rapidly after an application of mechanical load and precedes the loss of protein. Conversely, without this high mechanical stress, but under otherwise identical conditions, the expression of Cx43 is maintained at a normal level for at least 48 h. Interestingly, the expression of Cx43 did not correspond with changes in developed force under a high mechanical load condition. Previous studies regarding the expression of Cx43 under the stress of mechanical tension have been performed in neonatal cardiac myocytes using bending silicone plates and engineered cardiac tissue as a model (19, 54). Although the results from these studies have advanced our knowledge of the biochemical properties and signaling of connexins, the effect of mechanical stress in the in situ situation (i.e., with connections to neighboring myocytes, endothelial cells, and fibroblasts) could not be determined in those experimental studies. First, there is a difference between developing (growing) and mature (adult) stages of cardiac myocytes. This factor might be the result of different protein phenotypes as well as sensitivities of cellular receptors between neonatal and adult tissues (43). A second factor is a difference between isolated (pure) myocytes versus multicellular preparations, the latter containing fibroblasts, endothelial cells, vascular smooth muscles, as well as myocytes, all in their in situ load-bearing composition. Because of this multitude of factors, neonatal myocytes are known to react differently to mechanical stress compared with adult myocytes. This led to the remaining questions, such as whether changes in Cx43 protein levels would occur in adult multicellular cardiac tissue upon mechanical load. The results from our study now indeed indicate that mechanical load induces a downregulation of Cx43 levels. In our study, we did not apply different loading conditions, as our principal goal was to determine whether load alone would be able to reduce Cx43 levels. The muscles that contracted at high preload contracted isometrically and thus also experienced a high afterload. In our study, we did not discriminate between these different load settings due to technical limitations of obtaining enough suitable trabeculae in parallel. Future studies, however, could be directed at a dissection between pre- and afterload, and the level of load, as has been technically established in rat myocardium (21).
The downregulation of Cx43 and changes in Cx43 phosphorylation suggest that this protein is influenced by mechanical overload. An increase in mechanical load is also a major cause of pathological hypertrophy and subsequent heart failure (18). From the temporal resolution of our experiments, we conclude that the expression of myocardial Cx43 is dependent on the stage of cardiac hypertrophy; we only found significant changes after 12 h, where functional changes had already occurred. Kostin and colleagues (26) showed that the upregulation of Cx43 was detected in compensated (EF > 50%) cardiac hypertrophy. An increase in Cx43 expression was also found in exercise-trained mouse hypertrophied hearts (9). On the other hand, a decrease in Cx43 was detected in decompensated (EF < 30%) hypertrophied hearts, a result similar to those detected in heart failure patients (16, 34, 42). When compared with the presently used model, we have previously demonstrated that high-load culture induces a rapid change in muscle mass with little or no change in the stoichiometric nature of the myofilament matrix (13). We also detected that a very high-loading condition activates cellular apoptotic signaling pathways (23). Combined, these studies indicate that the high-loading protocol can rapidly (within 24 h) induce cardiac hypertrophy which thereafter decompensates and is accompanied by apoptosis. Both decreases in the total Cx43 level and the level of Cx43 phosphorylation (Ser368) have also been confirmed in humans with end-stage dilated cardiomyopathy (1). In addition, the rapid decline in the phosphorylation level of Cx43 at Ser368 in high-loaded muscles may imply that a mechanical overload can directly, and within hours, alter the permeability of the gap junctions (7, 17). The phosphorylation of Cx43 is a key mechanism for regulating the gating of gap junction channels. Cx43 is phosphorylated at multiple serine residues, and the phosphorylation regulates channel function and turnover (27). Phosphorylation can lead to a reduction in intercellular communication and an alteration of single-channel behavior (17). Mutations at the phosphorylation site lead to the inability of the gap junction to respond to signaling cascades that would normally alter cell-to-cell communication (27). Phosphorylation of Cx43 at Ser368 in normal physiological conditions functions to decrease the channel conductance of signals during cell-to-cell communication. In our findings, this may imply that very early in the response, cell-to-cell communication is enhanced but subsequently lost when the levels of Cx43 are reduced. The loss of cell-to-cell communication can possibly cause cardiac arrhythmia during an early phase of cardiac hypertrophy. Unfortunately, our in vitro multicellular culture system does not, at present, allow for the study of arrhythmic properties, and thus the above possibility remains untested. Since our model is one of direct electrical stimulation (as opposed to field stimulation), we could speculate that the increased time to peak force after stretch might partly be a result of a cell-to-cell communication defect. However, many other possible mechanistic changes in excitation-contraction coupling must not be discarded. Noticeably, stretch has been known to activate PKC translocation from the cytosolic fraction to the membrane fraction and consequently activates many target cascades in cardiac myocytes (33, 52). This stretch-induced PKC activation may involve Ser368 on Cx43 as a target for this PKC (7, 27). However, a localization and isoform determination of PKC at the gap junction was deemed beyond the scope of this present study.
For adult cardiac muscle, mechanical loading affects gap junction protein expression. N-cadherin is a major protein that mediates adhesion in the intercalated disc at the termini of cardiac myocytes, thereby representing the construction of cell-to-cell connection. The absence of changes in the ratio of N-cadherin to myofilament proteins in the present study implies that no mismatch between myocyte size and the cell-to-cell junction was detected during early cardiac remodeling. In contrast, reports in neonatal cardiac myocytes demonstrated a rapid increase in N-cadherin expression by cyclic stretch. These controversial results between the present study and previous ones may imply that mechanical tension is a crucial factor in the formation of cell-to-cell junctions during cardiac development, but an excess tension in adult myocardium may be perceived as an adverse stimulus. In the present study, we also determined the expression of ZO-1, a PDZ domain-containing protein localized at the intercalated disc. Based on the binding interaction between the NH2-terminal domain of ZO-1 and the COOH-terminal domain of Cx43 and their colocalization, ZO-1 has been hypothesized to be involved in the regulation of Cx43 accretion and assembly (22, 44). The absence of change in ZO-1 expression in high-load muscles in the present study indicates, however, that ZO-1 expression was not a major cause of Cx43 erosion under these conditions. Recently, the ZO-1 required for PKC-driven disassembly of Cx43 has been reported in lens epithelial cells (3). However, little information on the ZO-1 and Cx43 interaction in cardiac muscles is currently available. Although we did not investigate whether the loss of Cx43 resulted in an altered expression of Cx40 or Cx45, from the loss of function we deduce that if compensatory upregulation of these alternate isoforms occurred, it was not sufficient to rescue the phenotypical loss of function.
To further investigate the mechanistic sensor and underlying signaling of mechanical loading-induced Cx43 degradation, we hypothesized that hormones released by stretch might activate a Cx43 downregulation. Many paracrine compounds released by mechanical stretch have been reported in cell culture experiments (4, 49). In the present study, we have investigated selected hormones that can have a potential hypertrophy-inducing effect, ET-1, ANG II, or TGF-β. We show that an increase in force of contraction in high-loaded muscles was not significantly affected by ET-1, ANG II, and TGF-β inhibitors. Only telmisartan and SB-431542 could partly prevent the reduction of Cx43 in high-loaded muscle after 24 h culture, but no investigated compound completely blocked the drop of Cx43 protein levels. The results reveal the lack of a direct relationship between an increase in the force of contraction and Cx43 expression (changes occur in a different temporal process), suggesting at least two separate mechanistic activations under high mechanical loading. In addition, a possibility of ANG II suppressing Cx43 expression through the activation TGF-β (40) might be one of the activated mechanism under high mechanical loading. Notably, losartan and telmisartan both are ANG II antagonists, but losartan did not show any effect on preventing Cx43 degradation. Although telmisartan is superior to losartan in reducing high blood pressure (32), a correlation with our data cannot unambiguously be determined at this point.
The lack of a preventive effect of ANG II antagonists and TGF-β inhibition on downregulation of Cx43 by mechanical loading in the present study is potentially in contrast to those hormonal effects that have been previously reported; the upregulation of Cx43 expression by ET-1, ANG II, and TGF-β has been demonstrated in cultured neonatal cardiac myocytes in a dose-dependent manner (35, 36). In the present study, we could expect a high concentration of those local hormones in high-loaded muscles. Instead of an increased Cx43 expression, as may have been expected based on neonatal data, the high load in adult myocardium as studied here decreased the expression of Cx43. Differences in responsiveness of endocrine receptors between neonatal and adult cardiac myocytes might very well be the explanation, and this has indeed been suggested previously (14). Based on the preventive action of ANG II antagonists in neonatal myocytes, it is therefore interesting to consider whether ANG II effects the expression and/or degradation of Cx43 in adult cardiac myocytes. Although ANG II is accepted as a hypertrophy activator, many previous studies revealed that ANG II also induces cellular apoptosis in both neonatal and adult cardiac myocytes (25, 29, 40). Leri et al. (29) demonstrated that stretch mediated a release of ANG II, which in turn increased the susceptibility of cardiac myocytes to undergo apoptosis. Sanders et al. (39) also demonstrated that ANG II mediated an upregulation of the ubiquitin-proteasome proteolytic pathway in cultured myoblasts. Since ubiquitination and proteasomal pathways are the main characteristics of gap junction degradation (10, 28), this evidence suggests that stretch-mediated ANG II might induce ubiquitination and downregulate Cx43. Nevertheless, we only observed a partial protection of telmisartan, indicating that other mechanisms, including other paracrines as well as hormonal-independent pathways, are also engaged in the stretch-induced downregulation of Cx43. A potential mechanism that may underlie the Cx43 regulation can only be speculated at this point. Mechanical overload may induce a stress on the extracellular matrix that then activates the integrin signaling transduction and subsequently induces cardiac Cx43 degradation. It is known that extracellular matrix-activated transmembrane protein is a major mechanical sensing pathway in cardiac function (2, 41, 46). Extracellular matrix proteins such as fibronectin and laminin are recognized by transmembrane protein integrins, and its intracellular domain associates with many signaling transductions (12). Therefore, it is a possibility that the inhibition of extracellular matrix transactivation might prevent the downregulation of Cx43 expression under a high-loading condition, although current data to prove this potential pathway is not available at present.
In conclusion, we established that in mechanically loaded adult multicellular myocardium from the rabbit under near-physiological conditions in vitro, Cx43 phosphorylation rapidly declines and the Cx43 protein level gradually declines, where both effects were not directly related to contractile performance of the myocardium. Blocking paracrine effects only partially prevented the drop in Cx43 protein levels, indicating that a complex regulation involving more than one mechanism is involved in the load-induced Cx43 regulation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-73816 and KO2-HL-083957 (to P. M. L. Janssen); American Heart Association Scientist Development Grant 0235045N (to P. M. L. Janssen); and American Heart Association Established Investigator Award 0740040N (to P. M. L. Janssen).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 96: 54–63, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa R, Nagai T, Kudoh S, Zou Y, Tanaka M, Tamura M, Akazawa H, Takano H, Nagai R, Komuro I. Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension 39: 233–238, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Akoyev V, Takemoto DJ. ZO-1 is required for protein kinase C gamma-driven disassembly of connexin 43. Cell Signal 19: 958–967, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez BV, Perez NG, Ennis IL, Camilion de Hurtado MC, Cingolani HE. Mechanisms underlying the increase in force and Ca2+ transient that follow stretch of cardiac muscle: a possible explanation of the Anrep effect. Circ Res 85: 716–722, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res 80: 88–94, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Backx PH, Ter Keurs HE. Fluorescent properties of rat cardiac trabeculae microinjected with fura-2 salt. Am J Physiol Heart Circ Physiol 264: H1098–H1110, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Bao X, Reuss L, Altenberg GA. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J Biol Chem 279: 20058–20066, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Barker RJ, Price RL, Gourdie RG. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res 90: 317–324, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Bellafiore M, Sivverini G, Palumbo D, Macaluso F, Bianco A, Palma A, Farina F. Increased cx43 and angiogenesis in exercised mouse hearts. Int J Sports Med 28: 749–755, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Berthoud VM, Minogue PJ, Laing JG, Beyer EC. Pathways for degradation of connexins and gap junctions. Cardiovasc Res 62: 256–267, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Boublik J, Park H, Radisic M, Tognana E, Chen F, Pei M, Vunjak-Novakovic G, Freed LE. Mechanical properties and remodeling of hybrid cardiac constructs made from heart cells, fibrin, and biodegradable, elastomeric knitted fabric. Tissue Eng 11: 1122–1132, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc Res 70: 422–433, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Bupha-Intr T, Holmes JW, Janssen PM. Induction of hypertrophy in vitro by mechanical loading in adult rabbit myocardium. Am J Physiol Heart Circ Physiol 293: H3759–H3767, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Burrell JH, Hegarty BD, McMullen JR, Lumbers ER. Effects of gestation on ovine fetal and maternal angiotensin receptor subtypes in the heart and major blood vessels. Exp Physiol 86: 71–82, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res 95: 1035–1041, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont E, Ko Y, Rothery S, Coppen SR, Baghai M, Haw M, Severs NJ. The gap-junctional protein connexin40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation 103: 842–849, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res 98: 1498–1505, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan D, Wannenburg T, de Tombe PP. Decreased myocyte tension development and calcium responsiveness in rat right ventricular pressure overload. Circulation 95: 2312–2317, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J 14: 669–679, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Giepmans BN Gap junctions and connexin-interacting proteins. Cardiovasc Res 62: 233–245, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Guterl KA, Haggart CR, Janssen PM, Holmes JW. Isometric contraction induces rapid myocyte remodeling in cultured rat right ventricular papillary muscles. Am J Physiol Heart Circ Physiol 293: H3707–H3712, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell 16: 5686–5698, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen PML, Hasenfuss G, Zeitz O, Lehnart SE, Prestle J, Darmer D, Holtz J, Schumann H. Load-dependent induction of apoptosis in multicellular myocardial preparations. Am J Physiol Heart Circ Physiol 282: H349–H356, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Janssen PML, Lehnart SE, Prestle J, Lynker JC, Salfeld P, Just H, Hasenfuss G. The trabecula culture system: a novel technique to study contractile parameters over a multiday time period. Am J Physiol Heart Circ Physiol 274: H1481–H1488, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol 29: 859–870, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Kostin S, Dammer S, Hein S, Klovekorn WP, Bauer EP, Schaper J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc Res 62: 426–436, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 149: 1503–1512, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leithe E, Rivedal E. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem 279: 50089–50096, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Leri A, Claudio PP, Li Q, Wang X, Reiss K, Wang S, Malhotra A, Kajstura J, Anversa P. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest 101: 1326–1342, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linke WA, Popov VI, Pollack GH. Passive and active tension in single cardiac myofibrils. Biophys J 67: 782–792, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monasky MM, Varian KD, Davis JP, Janssen PM. Dissociation of force decline from calcium decline by preload in isolated rabbit myocardium. Pflügers Arch 456: 267–276, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Neutel JM, Littlejohn TW, Chrysant SG, Singh A. Telmisartan/hydrochlorothiazide in comparison with losartan/hydrochlorothiazide in managing patients with mild-to-moderate hypertension. Hypertens Res 28: 555–563, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Paul K, Ball NA, Dorn GW, 2nd, Walsh RA. Left ventricular stretch stimulates angiotensin II-mediated phosphatidylinositol hydrolysis and protein kinase C epsilon isoform translocation in adult guinea pig hearts. Circ Res 81: 643–650, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation 88: 864–875, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Pimentel RC, Yamada KA, Kleber AG, Saffitz JE. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res 90: 671–677, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Polontchouk LO, Valiunas V, Haefliger JA, Eppenberger HM, Weingart R. Expression and regulation of connexins in cultured ventricular myocytes isolated from adult rat hearts. Pflügers Arch 443: 676–689, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez EK, Hunter WC, Royce MJ, Leppo MK, Douglas AS, Weisman HF. A method to reconstruct myocardial sarcomere lengths and orientations at transmural sites in beating canine hearts. Am J Physiol Heart Circ Physiol 263: H293–H306, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Salameh A Life cycle of connexins: regulation of connexin synthesis and degradation. Adv Cardiol 42: 57–70, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer 93: 425–434, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroder D, Heger J, Piper HM, Euler G. Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat. J Mol Med 84: 975–983, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Shanker AJ, Yamada K, Green KG, Yamada KA, Saffitz JE. Matrix-protein-specific regulation of Cx43 expression in cardiac myocytes subjected to mechanical load. Circ Res 96: 558–566, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol 139: 801–821, 1991. [PMC free article] [PubMed] [Google Scholar]
- 43.Syed F, Odley A, Hahn HS, Brunskill EW, Lynch RA, Marreez Y, Sanbe A, Robbins J, Dorn GW 2nd. Physiological growth synergizes with pathological genes in experimental cardiomyopathy. Circ Res 95: 1200–1206, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273: 12725–12731, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Uzzaman M, Honjo H, Takagishi Y, Emdad L, Magee AI, Severs NJ, Kodama I. Remodeling of gap junctional coupling in hypertrophied right ventricles of rats with monocrotaline-induced pulmonary hypertension. Circ Res 86: 871–878, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Valencik ML, Zhang D, Punske B, Hu P, McDonald JA, Litwin SE. Integrin activation in the heart: a link between electrical and contractile dysfunction? Circ Res 99: 1403–1410, 2006. [DOI] [PubMed] [Google Scholar]
- 47.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 109: 1048–1055, 2004. [DOI] [PubMed] [Google Scholar]
- 48.van Veen TA, van Rijen HV, Wiegerinck RF, Opthof T, Colbert MC, Clement S, de Bakker JM, Jongsma HJ. Remodeling of gap junctions in mouse hearts hypertrophied by forced retinoic acid signaling. J Mol Cell Cardiol 34: 1411–1423, 2002. [DOI] [PubMed] [Google Scholar]
- 49.van Wamel AJ, Ruwhof C, van der Valk-Kokshoom LE, Schrier PI, van der Laarse A. The role of angiotensin II, endothelin-1 and transforming growth factor-beta as autocrine/paracrine mediators of stretch-induced cardiomyocyte hypertrophy. Mol Cell Biochem 218: 113–124, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Varian KD, Janssen PM. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol 292: H2212–H2219, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Verheule S, van Kempen MJ, te Welscher PH, Kwak BR, Jongsma HJ. Characterization of gap junction channels in adult rabbit atrial and ventricular myocardium. Circ Res 80: 673–681, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Vincent F, Duquesnes N, Christov C, Damy T, Samuel JL, Crozatier B. Dual level of interactions between calcineurin and PKC-epsilon in cardiomyocyte stretch. Cardiovasc Res 71: 97–107, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Wang TL, Tseng YZ, Chang H. Regulation of connexin 43 gene expression by cyclical mechanical stretch in neonatal rat cardiomyocytes. Biochem Biophys Res Commun 267: 551–557, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Zhuang J, Yamada KA, Saffitz JE, Kleber AG. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res 87: 316–322, 2000. [DOI] [PubMed] [Google Scholar]