Abstract
Hydrogen sulfide (H2S) is one of three endogenous gases, along with carbon monoxide (CO) and nitric oxide (NO), that exert a variety of important vascular actions in vivo. Although it has been demonstrated that CO or NO can trigger the development of a preconditioned phenotype in postischemic tissues, it is unclear whether H2S may also induce protection in organs subsequently exposed to ischemia-reperfusion (I/R). In light of these observations, we postulated that preconditioning with the exogenous H2S donor sodium hydrosulfide (NaHS-PC) would inhibit leukocyte rolling (LR) and adhesion (LA) induced by I/R. We used intravital microscopic techniques to demonstrate that NaHS-PC 24 h, but not 1 h, before I/R causes postcapillary venules to shift to an anti-inflammatory phenotype in wild-type (WT) mice such that these vessels fail to support LR and LA during reperfusion. The protective effect of NaHS-PC on LR was largely abolished by coincident pharmacological inhibition of NO synthase (NOS) in WT animals and was absent in endothelial NOS-deficient (eNOS−/−) mice. A similar pattern of response was noted in WT mice treated concomitantly with NaHS plus p38 mitogen-activated protein kinase (MAPK) inhibitors (SB 203580 or SK-86002). Whereas the reduction in LA induced by antecedent NaHS was attenuated by pharmacological inhibition of NOS or p38 MAPK in WT mice, the antiadhesive effect of NaHS was still evident in eNOS−/− mice. Thus NaHS-PC prevents LR and LA by triggering the activation of an eNOS- and p38 MAPK-dependent mechanism. However, the role of eNOS in the antiadhesive effect of NaHS-PC was less prominent than its effect to reduce LR.
Keywords: ischemia, reperfusion, sodium hydrosulfide, endothelial nitric oxide synthase-deficient mice, leukocyte rolling, leukocyte adhesion
preconditioning refers to a phenomenon whereby antecedent exposure to a particular stimulus confers protection against the deleterious effects induced by subsequent exposure to prolonged ischemia-reperfusion (I/R). Preconditioning stimuli induce two phases of protection in I/R. Acute- or early-phase preconditioning develops rapidly (within minutes), involves activation of preexisting effector molecules, and is short-lived, conferring protection for 2–6 h before disappearing. Delayed acquisition of tolerance to ischemia (late-phase preconditioning) arises 12–24 h after the initial preconditioning stimulus is applied, is longer lived (24 h or longer), and requires the expression of new gene products to mediate cardioprotection. Many of these preconditioning stimuli appear to promote the production of the gaseous monoxide nitric oxide (NO), phosphorylation and activation of p38 MAPK, and activation of plasmalemmal ATP-sensitive potassium (KATP) channels as initial triggering events in the acquisition of tolerance to I/R (10, 46), whereas formation of the diatomic gas carbon monoxide (CO) and activation of mitochondrial KATP channels appear to serve as important effectors of protection during I/R (5, 34, 40, 51). In addition, preconditioning with NO donors, p38 MAPK activators, and KATP channel agonists prevents postischemic leukocyte infiltration and exerts infarct-sparing effects in postischemic tissues (53).
Several effects of hydrogen sulfide (H2S) suggest a potential role for this gaseous signaling molecule as a preconditioning stimulus. H2S produces vasorelaxation by activation of KATP channels and can enhance the vasorelaxant effects of NO (21). Conversely, the production of H2S can be upregulated by NO (57). Both NO and KATP channel activation produce preconditioned states. H2S also exhibits antiadhesive effects, preventing leukocyte rolling and adhesion to mesenteric venules induced by coincident exposure to inflammatory mediators (13, 56). On the basis of these observations, we hypothesized that H2S exposure, using the H2S donor sodium hydrosulfide (NaHS), either during reperfusion or as a preconditioning stimulus 1 (acute phase) or 24 h (late phase) before I/R, would prevent postischemic leukocyte rolling and adhesion. Since the results of these initial experiments indicated that H2S failed to prevent postischemic leukocyte-endothelial adhesive interactions when administered 1 h before I/R (acute-phase preconditioning) or during reperfusion but was remarkably effective at preventing these responses when administered as a preconditioning stimulus 24 h before I/R, we focused the remainder of our work on the mechanisms involved in triggering entrance into this anti-inflammatory state. We hypothesized that late-phase NaHS preconditioning (NaHS-PC) 24 h before I/R would induce the development of an anti-inflammatory phenotype by an endothelial NO synthase (eNOS)- and p38 mitogen-activated protein kinase (MAPK)-dependent mechanism.
MATERIALS AND METHODS
Animals
Wild-type (WT) male C57BL/6J mice or eNOS−/− mice (6–7 wk of age) were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were maintained on standard mouse chow and used at 8–12 wk of age. The experimental procedures described were performed according to the criteria outlined in the National Institutes of Health guidelines and were approved by the University of Missouri Institutional Animal Care and Use Committee.
Surgical Procedures and Induction of I/R
The mice were anesthetized initially with a mixture of ketamine (150 mg/kg body wt ip) and xylazine (7.5 mg/kg body wt ip). The right carotid artery was cannulated, and systemic arterial pressure was measured with a Statham P23A pressure transducer (Gould) connected to the carotid artery catheter. Systemic blood pressure was recorded continuously with a personal computer (Power Macintosh 8600; Apple) equipped with an analog-to-digital converter (MP 100; Biopac Systems). Carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Molecular Probes, Eugene, OR) was dissolved in DMSO at a stock concentration of 5 mg/ml, divided into 25-μl aliquots, and stored in light-tight containers at −20°C until further dilution immediately before intravenous injection. The left jugular vein was cannulated for administration of CFDA-SE. A midline abdominal incision was then performed, and the superior mesenteric artery (SMA) was occluded with a microvascular clip for 0 (sham) or 45 min. After the ischemic period, the clip was gently removed and leukocytes were labeled with CFDA-SE by intravenous administration of the fluorochrome solution (250 μg/ml saline) at 20 μl/min for 5 min. During preparation, storage, and administration of CFDA-SE, care was taken to minimize light exposure. Leukocyte-endothelial cell adhesive interactions were quantified over 30–40 and 60–70 min of reperfusion, as described below.
Intravital Fluorescence Microscopy
The mice were positioned on a 20 × 30-cm Plexiglas board in a manner that allowed a selected section of small intestine to be exteriorized and placed carefully and gently over a glass slide covering a 4 × 3-cm hole centered in the Plexiglas. The exposed small intestine was superfused with warmed (37°C) bicarbonate-buffered saline (BBS; pH 7.4) at 1.5 ml/min using a peristaltic pump (model M312; Gilson). The exteriorized region of the small bowel was covered with BBS-soaked gauze to minimize tissue dehydration, temperature changes, and the influence of respiratory movements. The superfusate was maintained at 37 ± 0.5°C by pumping the solution through a heat exchanger warmed by a constant-temperature circulator (model 1130; VWR). Body temperature of the mice was maintained between 36.5 and 37.5°C by using a thermostatically controlled heat lamp. The board was mounted on the stage of an inverted microscope (Diaphot TMD-EF; Nikon), and the intestinal microcirculation was observed through a ×20 objective lens. Intravital fluorescence images of the microcirculation (excitation wavelength, 420–490 nm; emission wavelength, 520 nm) were detected with a charge-coupled device (CCD) camera (XC-77; Hamamatsu Photonics), a CCD camera control unit (C2400; Hamamatsu Photonics), and an intensifier head (M4314; Hamamatsu Photonics) attached to the camera. Microfluorographs were projected on a television monitor (PVM-1953MD; SONY) and recorded on digital video using a digital video recorder (DMR-E50; Panasonic) for off-line quantification of measured variables during playback of the recorded image. A video time-date generator (WJ810; Panasonic) displayed the stopwatch function on the monitor.
The intravital microscopic measurements described below were obtained during minutes 30–40 and 60–70 of reperfusion or at equivalent time points in the sham control groups. The intestinal segment was scanned from the oral to aboral section, and 10 single, unbranched venules (20–50 μm in diameter, 100 μm in length) were observed, each for 30 s. Leukocyte-endothelial cell interactions (the numbers of rolling and firmly adherent leukocytes) were quantified in each of the 10 venules, followed by calculation of the mean value, which was used in the statistical analysis of the data. Circulating leukocytes were considered to be firmly adherent if they did not move or detach from the venular wall for a period ≥30 s. Rolling cells are defined as cells crossing an imaginary line in the microvessel at a velocity that is significantly lower than centerline velocity; their numbers are expressed as rolling cells per minute. The numbers of rolling or adherent leukocytes were normalized by expressing each as the number of cells per square millimeter of vessel area.
Effect of NaHS on Phosphorylation of eNOS and p38 MAPK in Endothelial Cells
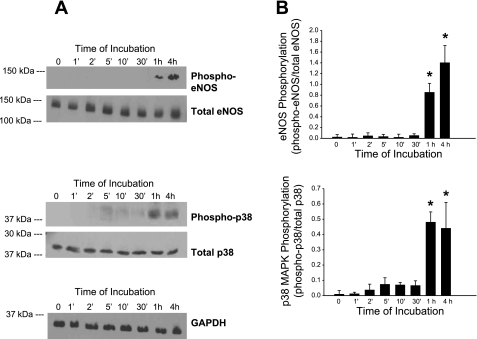
Human dermal microvascular endothelial cells were obtained from the Centers for Disease Control (2). They were cultured in MCDB 131 medium (Sigma Chemical, St. Louis, MO) supplemented with glutamine (10 mM; Sigma), EGF (10 ng/ml; BD Biosciences, San Jose, CA), hydrocortisone (1 μg/ml; Sigma), and 10% heat-inactivated fetal calf serum (FCS; Atlanta Biological, Atlanta, GA) in an atmosphere of 5% CO2 at 37°C. Medium was changed every 3–4 days, and cells were passaged once per week. For experiments, cells were grown to confluence in 100-mm cell culture dishes and used for experiments 3–4 days postconfluence. Before experiments, cells were serum starved (medium with 0.1% FCS) overnight (16–18 h). They were then gently washed with Hanks' buffered saline solution (HBSS) and preincubated for 15 min in HBSS + 0.1% BSA + 10 mM HEPES + 10 nM okadaic acid (Sigma). NaHS from a freshly-prepared stock dissolved in HBSS was then added to the final concentration of 100 μM for various periods from 1 min to 4 h (see Fig. 3). At the end of the experimental period, cells were placed on ice, gently washed with ice-cold PBS, and then harvested by scraping in SDS-PAGE reducing buffer, supplemented with phosphatase inhibitors (cocktails I and II; Sigma), protease inhibitor cocktail (Sigma), and PMSF (1 μM). Unboiled lysates were immediately subjected to SDS-PAGE and Western blotting.
Fig. 3.
Effect of NaHS on phosphorylation of endothelial NO synthase (eNOS) and p38 MAPK. Human microvascular endothelial cells (HMEC-1) were incubated with NaHS (100 μM) for the indicated times, lysed, and subjected to SDS-PAGE and Western blotting for both total and phospho-eNOS (Ser1177), and total and phospho-p38 MAPK (Thr180/Tyr182), and GAPDH. A: representative blot showing results from a single experiment from among the full dataset. B: densitometric quantitation of phosphorylation of eNOS and p38 normalized to total expression of eNOS and p38, respectively. Values are means ± SE of ratios of arbitrary densitometric units of phosphorylated vs. total immunoreactivity for each enzyme, obtained by scanning bands from separate repeated experiments (eNOS, n = 6; p38, n = 5). *P < 0.001, values significantly different from time 0.
Immunoblots were probed using primary antibodies specific for total eNOS (BD Biosciences), total p38 MAPK and phospho-eNOS and -p38 (Cell Signaling Technology, Danvers, MA), and GAPDH (Chemicon, Temecula, CA), with the appropriate horseradish peroxidase-coupled secondary antibodies. Blotted signals were obtained by developing the blots in a chemiluminescent detection system (Supersignal West Pico kit; Pierce, Rockford, IL). Films were scanned optically; semiquantitative analysis of relative expression of total and phospho-specific forms of eNOS and p38 MAPK was performed using Quantity One software (Bio-Rad, Hercules, CA). Results presented are for 5–6 separate experiments for p38 MAPK and eNOS, respectively.
Experimental Protocols
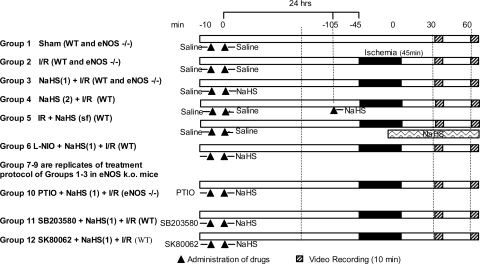
The general design of the experimental protocols for each group in the study is described below and in Fig. 1.
Fig. 1.
Diagram of the experimental protocols assigned to each group. The numbers at top in minutes refer to the timeline of the protocol on day 1, 24 h before ischemia-reperfusion (I/R), and day 2, the day of I/R. Hatched bars indicate digital video recording (10 min). Solid black bars indicate the 45-min period of ischemia during which the superior mesenteric artery had no blood flow. Triangles indicate administration of drug. See text for further details and definition of groups.
Group 1: sham.
As a time control for the effects of experimental duration, the mesentery and intestinal wall of each mouse in this group (n = 6) were superfused with BBS. The SMA was exposed but not subjected to occlusion, with leukocyte-endothelial cell adhesive interactions quantified at time points comparable to those described for mice subjected to 45 min of intestinal ischemia followed by 70 min of reperfusion (see group 2 below).
Group 2: I/R alone.
Mice in this group (n = 6) were treated as described for group 1 above except that I/R was induced by occlusion of the SMA for 45 min followed by reperfusion for 70 min. Leukocyte rolling and adhesion were quantified during minutes 30–40 and 60–70 of reperfusion.
Groups 3, 4, and 5: NaHS + I/R.
To determine whether H2S can act as a preconditioning stimulus and prevent I/R-induced leukocyte rolling and adhesion, a solution of NaHS was used as a H2S donor and given at 24 h before I/R [NaHS(1) + I/R, group 3] (late-phase preconditioning), 1 h before I/R [NaHS(2) + I/R, group 4] (early-phase preconditioning), or during reperfusion [I/R + NaHS(sf), group 5]. NaHS (Sigma Chemical, St. Louis, MO) was diluted in saline to produce a concentration of 1.4 mM and administered as a bolus at a dose reported to maximally produce H2S in intestinal tissues (n = 6; 14 μmol/kg ip; volume of solution injected was 0.3 ml/30 g mouse).
Group 6: l-NIO + NaHS + I/R.
To determine whether the effects of late-phase H2S preconditioning (H2S-PC) to prevent postischemic leukocyte rolling and adhesion were initiated by an NO-dependent signaling mechanism, we treated mice with the specific but non-isoform-selective NOS inhibitor l-N5-(1-iminoethyl)ornithine (l-NIO). Mice in this group (n = 3) were treated as described for group 3 except that l-NIO (100 mg/kg ip) was administered 10 min before NaHS on day 1 and then subsequently exposed to I/R 24 h later (day 2). We have previously used this l-NIO dosing protocol to demonstrate a role for NO as a trigger for late-phase preconditioning induced by ethanol ingestion or adenosine (30, 51).
Groups 7–9: sham (eNOS−/−), I/R (eNOS−/−), and NaHS + I/R (eNOS−/−).
Because l-NIO is a specific but non-isoform-selective NOS inhibitor, we repeated the studies outlined for groups 1–3 in eNOS-deficient mice [sham (eNOS−/−), group 7; I/R (eNOS−/−), group 8; and NaHS + I/R (eNOS−/−), group 9; respectively] to determine whether eNOS was the isoform responsible for triggering H2S-PC.
Group 10: PTIO + NaHS + I/R (eNOS−/−).
Mice in this group (n = 8) were treated in a fashion identical to those described for group 6 except that the NO scavenger 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide (PTIO; 100 μM in 0.5 ml) was administered instead of l-NIO 10 min before NaHS in eNOS−/− mice.
Groups 11 and 12: SB 203580 + NaHS + I/R and SK-86002 + NaHS + I/R.
To determine whether the effects of late phase H2S-PC were initiated by a p38 MAPK-dependent signaling mechanism, mice were treated with two different p38 MAPK inhibitors, SB 203580 or SK-86002. Mice in these groups (n = 6 per group) were treated as described for group 6 except that the p38 MAPK inhibitors (10 mg/kg ip), rather than l-NIO, were administered 10 min before NaHS.
Statistical Analysis
Data were analyzed using standard statistical analysis, i.e., ANOVA with Sheffé's (post hoc) test for multiple comparisons. All values are means ± SE. Statistical significance was defined at P < 0.05.
RESULTS
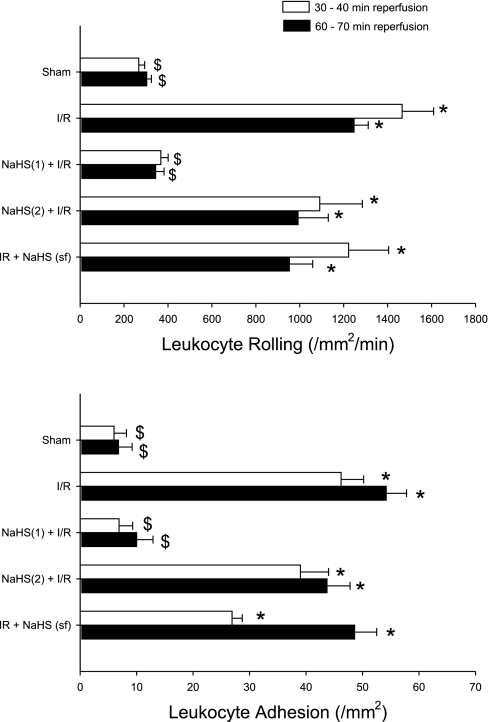
Figure 2 illustrates the average numbers of rolling (top) and adherent (bottom) leukocytes in postcapillary venules of the murine small intestine exposed to I/R alone (group 2), exposed to the H2S donor either 1 h [NaHS(2) + I/R, group 3] or 24 h [NaHS(1) + I/R, group 4] before I/R, or only during reperfusion (I/R + NaHS, group 5) relative to nonischemic controls (sham, group 1). I/R induced marked increases in the numbers of rolling and adherent leukocytes after 30 and 60 min of reperfusion, proadhesive effects that were largely abolished by preconditioning with the H2S donor NaHS when the donor was given 24 h before I/R [NaHS(1) + I/R]. Interestingly, the protective effects of H2S were not elicited when the donor was given 1 h before I/R [NaHS(2) + I/R] (early-phase preconditioning) or when the intestine was exposed to the H2S donor via the superfusate during reperfusion (I/R + NaHS). [NaHS was given without I/R, as a control, and had no effect on leukocyte rolling or adhesion at 24 h (data not shown)]. Together, these results indicate that NaHS treatment can induce late- but not early-phase preconditioning and is ineffective in reducing leukocyte rolling and adhesion when administered only during reperfusion.
Fig. 2.
Effects of preconditioning with the hydrogen sulfide (H2S) donor sodium hydrosulfide (NaHS) 1 h [NaHS-PC(2) + I/R] or 24 h before I/R [NaHS-PC(1) + I/R] on postischemic leukocyte rolling (top) and adhesion (bottom) compared with superfusion of the bowel during I/R with NaHS [I/R + NaHS (sf)] relative to sham (no NaHS, no I/R) controls or I/R alone. *P < 0.05, mean values statistically different from sham control. $P < 0.05, mean values statistically different from I/R alone.
Figure 3 illustrates the time course for the appearance of phospho-eNOS and phospho-p38 MAPK in representative Western blots (A) obtained in human microvascular endothelial cells (HMEC) exposed to NaHS, with densitometric quantitation of eNOS and p38 MAPK phosphorylation normalized to the total expression of eNOS and p38 MAPK, respectively (B). NaHS exposure was associated with the appearance of phospho-eNOS and phospho-p38 MAPK within 1 h and remained elevated 4 h after exposure. These observations led us to postulate that NaHS exposure may trigger the acquisition of delayed or late-phase tolerance to I/R by activating eNOS and p38 MAPK.
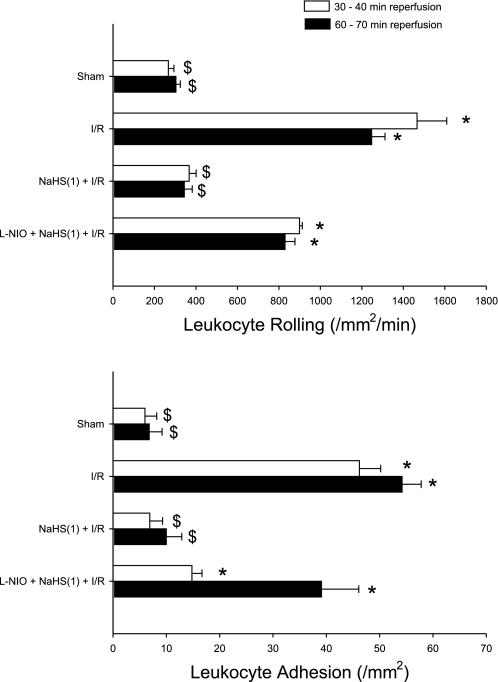
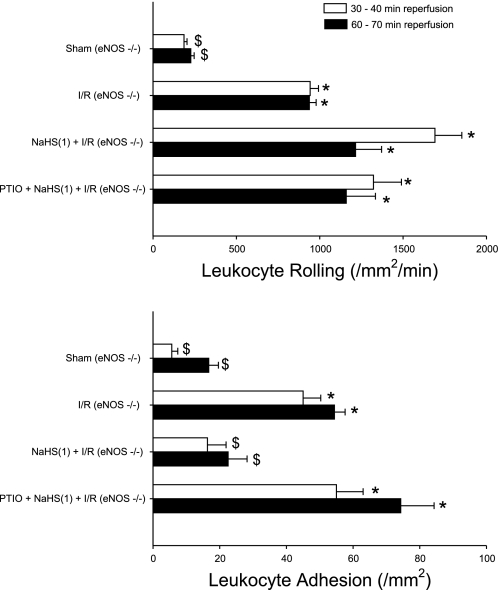
To address the role of eNOS as an initiator of NaHS-PC, we conducted two groups of studies. In the first, postischemic leukocyte rolling and adhesion were quantified in WT mice treated with the NOS inhibitor l-NIO coincident with NaHS administration on day 1. NOS inhibition attenuated the effect of antecedent H2S to prevent postischemic leukocyte rolling (Fig. 4, top). NOS inhibition only marginally influenced the effect of NaHS-PC to reduce leukocyte adhesion after 30 min of reperfusion but was associated with a marked increase in adhesion at 60 min (Fig. 4, bottom). [l-NIO was given alone, without NaHS or I/R, as a control group, but had no effect at 24 h (data not shown)]. To further explore this question, we examined the effectiveness of NaHS-PC in eNOS-deficient mice. I/R increased leukocyte rolling and adhesion in eNOS−/− mice (Fig. 5, top and bottom, respectively) to levels similar to those noted in WT animals (Fig. 4, top and bottom, respectively). Preconditioning with the H2S donor failed to limit postischemic leukocyte rolling in eNOS−/− mice (Fig. 5, top), a result that supports the concept that H2S-dependent eNOS activation is essential for the development of this anti-inflammatory effect in I/R. However, the ability of NaHS-PC to prevent postischemic leukocyte adhesion persisted in eNOS−/− mice (Fig. 5, bottom). Taken together with NOS inhibition data presented in Fig. 4, the results obtained in eNOS-deficient mice (Fig. 5) indicate that the effect of antecedent NaHS to limit postischemic leukocyte rolling occurs by an eNOS-dependent mechanism. However, the role of eNOS in the effect of NaHS to prevent stationary leukocyte adhesion is less prominent and suggests that other NOS isoforms (which are inhibited by l-NIO) may contribute to this latter effect or that compensatory alterations occur in eNOS-deficient mice. Indeed, when the NO scavenger PTIO was administered before NaHS-PC in eNOS-deficient mice subjected to IR, the protective effects of NaHS-PC were completely abolished (Fig. 5). This further supports our hypothesis that other NOS isoforms may also contribute to the antiadhesive effects elicited by NaHS.
Fig. 4.
Effects of I/R or I/R following pretreatment 24 h before I/R with a H2S donor [NaHS(1) + I/R] or NOS inhibitor l-N5-(1-iminoethyl)ornithine (l-NIO) just before treatment with H2S donor [l-NIO + NaHS(1) +I/R] on postischemic leukocyte rolling (top) or stationary leukocyte adhesion (bottom) determined after 30 and 60 min of reperfusion. *P < 0.05, mean values statistically different from sham control. $P < 0.05, mean values statistically different from I/R alone.
Fig. 5.
Effects of H2S preconditioning 24 h before I/R in eNOS−/− mice on postischemic leukocyte rolling (top) or stationary leukocyte adhesion (bottom) determined after 30 and 60 min of reperfusion. Groups shown include sham, I/R alone, I/R following pretreatment with a H2S donor [NaHS(1) + I/R], and NO scavenger 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide (PTIO) given just before H2S donor [PTIO + NaHS(1) + I/R] in eNOS−/− mice. *P < 0.05, mean values statistically different from sham control. $P < 0.05, mean values statistically different from I/R alone.
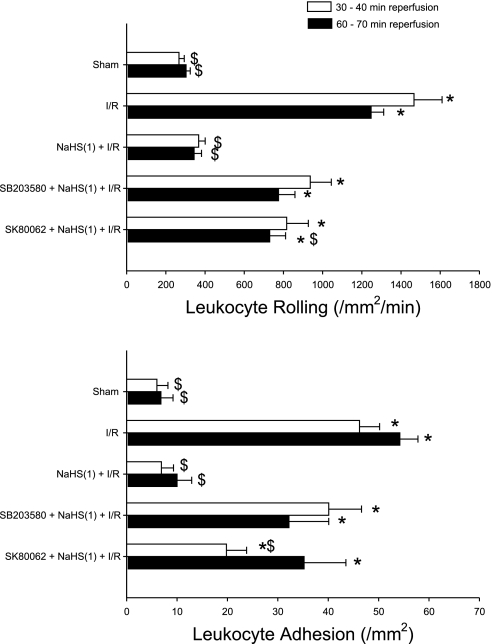
To determine whether p38 MAPK also participates as an initiator of the anti-inflammatory actions elicited by H2S donor treatment, we evaluated the effects of two different p38 MAPK inhibitors, SB 203580 and SK-86002, administered just before NaHS to prevent leukocyte rolling and adhesion induced by I/R 24 h later (Fig. 6). [As control groups, neither inhibitor alone, without NaHS or I/R, showed any effect on leukocyte rolling or adhesion at 24 h (data not shown)]. Both inhibitors attenuated the effectiveness of NaHS as a preconditioning stimulus to reduce postischemic leukocyte rolling and adhesion.
Fig. 6.
Effects of p38 MAPK activation as a trigger of NaHS preconditioning 24 h before I/R. Groups include I/R alone or I/R plus treatment with a p38 MAPK inhibitor 10 min before H2S donor preconditioning. MAPK inhibition was achieved using 2 different p38 MAPK inhibitors, SB 203580 [SB203580 + NaHS(1) + I/R] or SK-80062 [SK-80062 + NaHS(1) + I/R]. Data include postischemic leukocyte rolling (top) or stationary leukocyte adhesion (bottom) determined after 30 and 60 min of reperfusion. *P < 0.05, mean values statistically different from sham control. $P < 0.05, mean values statistically different from I/R alone.
DISCUSSION
Although the noxious effects of H2S have long been recognized, it is now clear that this gaseous environmental toxicant is produced endogenously by the vasculature and many tissues, is present in micromolar concentrations in blood and brain (50), and modulates tissue function. Endogenous H2S is formed by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE; also referred to as cystathionase). These enzymes exhibit a differential tissue distribution with CBS being highly expressed in the central nervous system (1, 27), whereas CSE (but not CBS) is expressed in the vascular wall of the aorta, tail artery, pulmonary artery, mesenteric artery, and portal vein (21, 59). Each of these enzymes utilizes l-cysteine as a substrate to produce H2S, which exists in physiological solutions as H2S and HS− in a 30%:70% ratio. Because the molecular targets for these sulfide species are not well-described, we use H2S to refer to H2S and HS−. A third H2S-producing enzyme, 3-mercaptosulfurtransferase, has been demonstrated in the heart (25).
As an environmental pollutant, high concentrations (>250 μM) of H2S are toxic by virtue of its ability to complex with Fe3+ of mitochondrial cytochrome oxidase (thereby impeding cellular oxidative metabolism) and to inhibit carbonic anhydrase and tyrosine aminotransferase (50). Endogenous production of H2S results in plasma levels of 10–100 μM, much lower concentrations that allow this sulfide to subserve signaling functions that regulate vascular tone, lower baseline leukocyte adhesion, and modulate neuronal activity (13, 50, 55, 56). In addition, exogenous administration of NaHS, a H2S donor, has been shown to relax vascular smooth muscle, attenuate the increase in leukocyte-endothelial cell adhesive interactions when administered just before or during exposure to proinflammatory mediators, and exert infarct-sparing effects in models of myocardial I/R (48, 50, 60). In this regard, H2S modulates cardiovascular function in a manner similar to the other two endogenously produced gaseous signaling molecules, NO and CO.
The results of this study provide the first evidence that preconditioning by exposing the small bowel to the H2S donor NaHS induces the development of an anti-inflammatory phenotype in murine small intestine such that postcapillary venules fail to support leukocyte rolling and adhesion when subjected to I/R 24 h later. Interestingly, treatment with NaHS 1 h before I/R or continuous superfusion with this donor during reperfusion failed to elicit these antiadhesive responses. These observations suggest that H2S is effective at inducing delayed acquisition of tolerance to I/R (late-phase preconditioning) but does not instigate early-phase preconditioning and does not exert a direct effect to limit postischemic leukocyte rolling and adhesion when the bowel is exposed to this donor during reperfusion. Similarly, Sivarajah et al. (44) have shown that preconditioning elicited by endotoxin is regulated via endogenous H2S in the rat. Our data add the exciting possibility that manipulation of endogenous mechanisms with exogenously administered H2S donors could also be effective as a preconditioning stimulus and yield powerful pharmaceutical options in the field of ischemia and reperfusion injury.
These observations are important because I/R is now well-recognized as one form of acute inflammation in which leukocytes play a key role (35). Recognition of the importance of the inflammatory process to the pathogenesis of I/R injury has led to an intensive research effort directed at identifying strategies to prevent leukocyte infiltration into postischemic tissues. Indeed, work conducted over the past 20 years has led to the development of the concept that oxidant-induced leukocyte-endothelial cell interactions are largely responsible for the microvascular dysfunction induced by reperfusion (18–20, 38, 39). Preconditioning with H2S donors may represent a promising new avenue for prevention of the microvascular complications of I/R.
In view of the powerful antiadhesive effects of NaHS treatment 24 h before I/R, but not when administered 1 h before induction of ischemia or during reperfusion, we focused our attention on the mechanisms that may be involved in eliciting late-phase preconditioning by this H2S donor. Because it has been demonstrated that exposing the mesentery to exogenous H2S attenuates leukocyte rolling and adhesion induced by proinflammatory stimuli such as TNF-α (15), it is possible that the protective effects noted in the present study were due to the continued presence of sulfides 24 h after administration. However, this explanation is highly unlikely because of the inherent instability of H2S in our experimental conditions due to oxidation in mitochondria, methylation in the cytosol, and scavenging by metalloproteins, disulfide-containing proteins, and heme compounds (46). In addition, H2S is subject to rapid catabolism via thiol S-methyltransferase and rhodenese, both of which are expressed in the small intestine (50). Indeed, Doeller et al. (11) designed a polaragraphic H2S probe that is highly sensitive and specific for H2S and were then able to eloquently show that H2S exists in a dynamic steady state in mammalian cells. Finally, our data indicating that NaHS treatment 1 h before I/R or ordinary reperfusion was not effective in preventing postischemic leukocyte rolling or adhesion indicate that late-phase H2S preconditioning was not due to a direct effect of this gas on these adhesive events.
The aforementioned observations in our studies suggest that the protective actions of H2S preconditioning are initiated by activation of downstream signaling mechanisms in a manner analogous to other short-lived preconditioning stimuli, such as adenosine and NO. Two distinct mechanisms for triggering the development of this protective effect were implicated by our studies. First, the effect of late-phase NaHS-PC to prevent the postischemic increases in leukocyte rolling appears to be triggered by an eNOS-dependent mechanism. Second, inhibition of p38 MAPK coincident with NaHS administration attenuated the effectiveness of this H2S donor in preventing leukocyte rolling and adhesion noted on exposure to I/R 24 h later.
A growing body of evidence indicates that H2S exerts a variety of effects that may limit I/R-induced injury and inflammation. For example, H2S produces vasorelaxation by activating KATP channels (8, 14, 21, 26, 47, 58, 59) and can enhance the vasodilatory effects of NO (21, 57), actions that may improve tissue perfusion in I/R. In addition, this gaseous signaling molecule reduces mitochondrial respiration, which may conserve ATP levels in ischemic tissues (12). H2S also exerts antioxidant effects and limits oxidative stress by virtue of its actions to raise intracellular glutathione levels by enhancing the activity of γ-glutamylcysteine synthetase and upregulating cysteine transport (28). In addition, H2S enhances the ability of superoxide dismutase to scavenge superoxide (42). Furthermore, H2S reduces apoptosis induced by inhibition of caspase-3 cleavage and p38 MAPK phosphorylation (41), which may contribute to potential infarct-sparing effects in I/R. The redox potential of H2S in the vascular tissue may also be related to the recent finding that H2S mediates vasoactivity in an oxygen-dependent manner (29). Moreover, inhibition of endogenous H2S synthesis reduces leukocyte rolling velocity and increases leukocyte adherence to mesenteric postcapillary venules under baseline conditions, whereas NaHS treatment prevents leukocyte-endothelial cell adhesive interactions following exposure to TNF-α (13, 15). The former results indicate that basal H2S production serves as an endogenous modulator of leukocyte-endothelial cell adhesive interactions, whereas the latter observation indicates that H2S donors may be effective in preventing adhesive responses induced by proinflammatory mediators.
The first in vivo study that suggested a possible protective role of H2S in I/R was conducted by Jeon and Lee (23) in the liver, where they found that S-adenosylmethionine, an H2S precursor, protected against mitochondrial injury, prevented mitochondrial oxidant stress, and improved ischemia-induced hepatic energy metabolism. Subsequent work provided more direct support for the concept that H2S itself can protect against I/R injury and inflammation in cardiac myocytes and gastric mucosa, respectively (6, 13, 17a, 37). Pan et al. (37) used an isolated rat ventricular myocyte preparation to show that NaHS-induced cardioprotection occurs in two time windows (∼1 h and 16–28 h).
In light of the aforementioned observations, we hypothesized that exposing the small bowel to the H2S donor NaHS during reperfusion would limit the increases in leukocyte rolling and adhesion. However, this treatment protocol failed to prevent the proinflammatory effects of I/R (Fig. 2). We then turned our attention to the postulate that antecedent NaHS treatment might induce the development of an anti-inflammatory phenotype on subsequent exposure to I/R 1 or 24 h later, similar to the biphasic protective actions (early- and late-phase preconditioning) elicited by ischemic preconditioning, ingestion of ethanol, or exposure to a variety of pharmacological agents such as NO donors, bradykinin, p38 MAPK activators, opioids, and KATP channel agonists. Our results provide the first evidence that exogenous H2S may also induce protection in I/R by instigating the development of a preconditioned, anti-inflammatory phenotype such that postcapillary venules fail to support leukocyte rolling and adhesion in tissues exposed to I/R 24 h after treatment with this gaseous signaling molecule (Fig. 2). However, in contrast to the preconditioning stimuli listed above, antecedent NaHS induced the development of an anti-inflammatory phenotype only when administered 24 h, but not 1 h, before I/R. These results indicate that NaHS induces late- but not early-phase acquisition of tolerance to ischemia in the small intestine, a property unique to this form of preconditioning.
In previous studies (16, 22, 43, 52, 53), we have demonstrated that a variety of late-phase preconditioning stimuli, including antecedent ethanol ingestion, short bouts of ischemia, adenosine A2 receptor agonists, exogenous calcitonin gene-related peptide (CGRP) or bradykinin, and AMP-activated protein kinase (AMPK) activators, induce the development of an anti-inflammatory phenotype that is triggered by eNOS-dependent NO release. Although we found no data to support NOS-dependent H2S vasodilation, the inverse relationship has been shown: H2S can enhance the vasorelaxant effects of NO (21). In addition, the production of H2S can be upregulated by NO (49, 57). Most recently, Geng et al. (17) have shown that eNOS (vs. inducible NOS and neuronal NOS) may be the specific target of H2S regulation in rat aortas (50). Thus we evaluated the role of eNOS in the vasculoprotection afforded by late-phase H2S-PC by employing a pharmacological inhibitor approach in WT animals and using an eNOS knockout model. The ability of H2S-PC to prevent postischemic leukocyte rolling was completely absent in eNOS-deficient mice and was markedly attenuated by NOS inhibition with l-NIO in WT animals (Figs. 4 and 5).
These results were surprising in view of our earlier work demonstrating that eNOS plays an essential role in preventing both leukocyte rolling and adhesion when preconditioning is induced by antecedent ethanol ingestion, short bouts of ischemia, exogenous CGRP or bradykinin, and adenosine A2 receptor agonists 24 h before I/R (43, 52, 53). In addition, preconditioning with NO donors also prevented both leukocyte rolling and adhesion during I/R in both WT and eNOS-deficient mice. The latter result is important because it indicates that the signaling mechanisms that are induced by NO downstream from eNOS activation remain intact in eNOS−/− mice. Interestingly, we (16) recently demonstrated that preconditioning with the AMPK agonist 5-aminoimidazole-4-carboxamide 1-β-d-furanoside (AICAR) also prevents postischemic leukocyte rolling and adhesion but that eNOS appeared to be involved only in the effect of this agent on leukocyte rolling, not leukocyte adhesion. The similarity in responses suggests the possibility that H2S-PC may signal through AMPK to produce its salutary effect on leukocyte rolling. Clearly, much additional work is required to evaluate this intriguing hypothesis. Nevertheless, our PTIO data in eNOS−/− mice (Fig. 5) clearly supports a role for NO, derived from eNOS as well as other NOS isoforms, in triggering the antiadhesive effects of NaHS-PC.
A number of recent studies implicate the activation of a number of kinases, such as p38 MAPK, in the development of preconditioned states (3, 4, 24). At least six different isoforms of p38 MAPK have been described. This MAPK is activated by dual phosphorylation on a Thr-Gly-Tyr motif in response to a number of stimuli implicated as triggers for preconditioning, including antecedent exposure to short bouts of ischemia or NO donors (9, 30, 32, 33, 36). Since H2S has been shown to increase p38 MAPK phosphorylation (54), we postulated that the anti-inflammatory effects induced by exogenous H2S might be elicited through activation of this kinase. Our results show that pharmacological inhibition of p38 MAPK by either SB 203580 or SK-80062 abolished the protective effects of late-phase NaHS-PC to limit leukocyte rolling and leukocyte adhesion induced by I/R 24 h after preconditioning (Fig. 6).
It is unclear whether H2S directly activates eNOS and/or p38 MAPK or targets other molecular elements upstream from these signaling enzymes. H2S can induce dithiol reduction and ligand displacement from heme iron, posttranslational protein modifications that may subserve this function. In addition, H2S can interact with the oxygen binding site of cytochrome c oxidase in the respiratory chain, leading to enhanced mitochondrial production of reactive oxygen species (ROS). The latter possibility is particularly appealing, since ROS have been implicated in the triggering mechanism for other preconditioning stimuli, such as ethanol and short bouts of ischemia.
In summary, our results provide the first evidence that treatment with NaHS, an exogenous source of H2S, induces the development of an anti-inflammatory phenotype in postcapillary venules, preventing both postischemic leukocyte rolling and adhesion when given 24 h before I/R. Since the protection afforded by NaHS-PC on postischemic leukocyte rolling was prevented by NOS inhibition and was absent in eNOS-deficient mice, it appears that eNOS activation triggers the appearance of this protective effect. However, the role of eNOS activation in the effect of NaHS-PC to limit postischemic leukocyte adhesion appears to be less prominent. On the other hand, NaHS-dependent p38 MAPK activation may play an essential role in preventing the increases in both leukocyte rolling and adhesion induced by subsequent exposure to I/R 24 h later.
Our results contribute to a growing body of evidence indicating that H2S, the third and most recently discovered endogenous gaseous signaling molecules, can be protective in the vascular system. Specifically, our data are novel to the field of preconditioning in that we have demonstrated that antecedent H2S induces powerful anti-inflammatory effects that become apparent during I/R 24 h later. In addition, our work provides new insights into pharmacological therapy, through exogenous H2S donors, that may be beneficial to I/R injury syndromes. Furthermore, our data highlight a new signaling mechanism to the field of H2S-induced signaling, the downstream activation of p38 MAPK.
GRANTS
This work was supported by National Institutes of Health Grants AA-14945 and HL-82816.
Acknowledgments
Meifang Wang, Alaina Boyett, and Christine Korthuis contributed to the completion of this work, and their help is greatly appreciated.
Present address of K. Kamada: Department of Inflammation and Immunology, Kyoto Prefectural University of Medicine, Graduate School of Medical Science, Kyoto 602-8566, Japan.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 99: 683–690, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong SC Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res 61: 427–436, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Ballard-Croft C, Kristo G, Yoshimura Y, Reid E, Keith BJ, Mentzer RM, Lasley RD. Acute adenosine preconditioning is mediated by p38 MAPK activation in discrete subcellular compartments. Am J Physiol Heart Circ Physiol 288: H1359–H1366, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Beresewicz A, Maczewski M, Duda M. Effect of classic preconditioning and diazoxide on endothelial function and O2- and NO generation in the post-ischemic guinea-pig heart. Cardiovasc Res 63: 118–129, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316: 670–678, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Dana A, Skarli M, Papakrivopoulou J, Yellon DM. Adenosine A1 receptor induced delayed preconditioning in rabbits: induction of p38 mitogen-activated protein kinase activation and Hsp27 phosphorylation via a tyrosine kinase- and protein kinase C-dependent mechanism. Circ Res 86: 989–997, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Das S, Otani H, Maulik N, Das DK. Redox regulation of angiotensin II preconditioning of the myocardium requires MAP kinase signaling. J Mol Cell Cardiol 41: 248–255, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR Jr, Darley-Usmar VM, Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem 341: 40–51, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 129: 1210–1224, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 42: 539–548, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, Santucci L, Cirino G, Wallace JL. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br J Pharmacol 150: 996–1002, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaskin FS, Kamada K, Yusof M, Korthuis RJ. 5′-AMP-activated protein kinase activation prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol 292: H326–H332, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Geng B, Cui Y, Zhao J, Yu F, Zhu Y, Xu G, Zhang Z, Tang C, Du J. Hydrogen sulfide downregulates the aortic l-arginine/nitric oxide pathway in rats. Am J Physiol Regul Integr Comp Physiol 293: R1608–R1618, 2007. [DOI] [PubMed] [Google Scholar]
- 17a.Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effect on cardiac function. Biochem Biophys Res Commun 313: 362–368, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Granger DN, Korthuis RJ. Physiologic mechanisms of postischemic tissue injury. Annu Rev Physiol 57: 311–332, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol Cell Biochem 179: 169–187, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Gute DC, Korthuis RJ. Role of leukocyte adherence in reperfusion-induced microvascular dysfunction and tissue injury. In: Physiology and Pathophysiology of Leukocyte Adhesion, edited by Granger DN and Schmid-Schönbein GW. Oxford, UK: Oxford University Press, 1995, p. 359–380.
- 21.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T, Yarimizu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock 8: 86–94, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Jeon BR, Lee SM. S-adenosylmethionine protects post-ischemic mitochondrial injury in rat liver. J Hepatol 34: 395–401, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, Molkentin JD. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J Biol Chem 279: 15524–15530, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Kamoun P Endogenous production of hydrogen sulfide in mammals. Amino Acids 26: 243–254, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kimura H Hydrogen sulfide as a neuromodulator. Mol Neurobiol 26: 13–19, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Kimura H Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun 267: 129–133, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18: 1165–1167, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR Jr, Doeller JE, Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol 292: H1953–H1960, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Korthuis RJ, Dayton C, Yamaguchi T. Early and late preconditioning prevent ischemia/reperfusion injury: signaling pathways mediating the adaptive metamorphosis to a protective phenotype in preconditioned tissues. In: Molecular Mechanisms of Microvascular Disorders, edited by Schmid-Schönbein GW and Granger DN. New York: Springer, 2001.
- 31.Korthuis RJ, Granger DN, Townsley MI, Taylor AE. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ Res 57: 599–609, 1985. [DOI] [PubMed] [Google Scholar]
- 32.Lochner A, Marais E, Genade S, Huisamen B, Moolman JA. Events downstream of p38MAPK (p38) in ischaemic preconditioning (PC): HSP27 and CREB. Cardiovasc J S Afr 15: S4–S5, 2004. [Google Scholar]
- 33.Marais E, Genade S, Salie R, Huisamen B, Maritz S, Moolman JA, Lochner A. The temporal relationship between p38 MAPK and HSP27 activation in ischaemic and pharmacological preconditioning. Basic Res Cardiol 100: 35–47, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Moses MA, Addison PD, Neligan PC, Ashrafpour H, Huang N, Zair M, Rassouli A, Forrest CR, Grover GJ, Pang CY. Mitochondrial KATP channels in hindlimb remote ischemic preconditioning of skeletal muscle against infarction. Am J Physiol Heart Circ Physiol 288: H559–H567, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Ogita H, Liao J. Endothelial function and oxidative stress. Endothelium 11: 123–132, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Okubo S, Xi L, Bernardo NL, Yoshida K, Kukreja RC. Myocardial preconditioning: basic concepts and potential mechanisms. Mol Cell Biochem 196: 3–12, 1999. [PubMed] [Google Scholar]
- 37.Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol 40: 119–130, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Panes J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology 114: 1066–1090, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Peart JN, Gross GJ. Sarcolemmal and mitochondrial KATP channels and myocardial ischemic preconditioning. J Cell Mol Med 6: 453–464, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajesh KG, Sasaguri S, Suzuki R, Xing Y, Maeda H. Ischemic preconditioning prevents reperfusion heart injury in cardiac hypertrophy by activation of mitochondrial KATP channels. Int J Cardiol 96: 41–49, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, Vitale M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest 86: 391–397, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Searcy DG, Whitehead JP, Maroney MJ. Interaction of Cu,Zn superoxide dismutase with hydrogen sulfide. Arch Biochem Biophys 318: 251–263, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Postischemic anti-inflammatory effects of bradykinin preconditioning. Am J Physiol Heart Circ Physiol 280: H441–H454, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock 26: 154–161, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Sohn H, Kuriyama H. The role of amino acids in the regulation of hydrogen sulfide production during ultradian respiratory oscillation of Saccharomyces cerevisiae. Arch Microbiol 176: 69–78, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Stein AB, Guo Y, Tan W, Wu WJ, Zhu X, Li Q, Luo C, Dawn B, Johnson TR, Motterlini R, Bolli R. Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J Mol Cell Cardiol 38: 127–134, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol 137: 139–145, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology 132: 261–271, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Wang R The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal 5: 493–501, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Wang R Two's company, three's a crowd—can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Wei K, Min S, Long C. Cardioprotective effects of mitochondrial KATP channels activated at different time. Chin Med J (Engl) 117: 647–651, 2004. [PubMed] [Google Scholar]
- 52.Yamaguchi T, Dayton C, Shigematsu T, Carter P, Yoshikawa T, Gute DC, Korthuis RJ. Preconditioning with ethanol prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol 283: H1019–H1030, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi T, Kamada K, Dayton C, Gaskin FS, Yusof M, Yoshikawa T, Carter P, Korthuis RJ. Role of eNOS-derived NO in the postischemic anti-inflammatory effects of antecedent ethanol ingestion in murine small intestine. Am J Physiol Heart Circ Physiol 292: H1435–H1442, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J 18: 1782–1784, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20: 2118–2120, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Zhi L, Moochhala SM, Moore PK, Bhatia M. Endogenous hydrogen sulfide regulates leukocyte trafficking in cecal ligation and puncture-induced sepsis. J Leukoc Biol 82: 892–905, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 81: 848–853, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 283: H474–H480, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, Moore PK. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol 102: 261–268, 2007. [DOI] [PubMed] [Google Scholar]