Abstract
This article summarizes perspectives on how reactive oxygen species (ROS) and redox signaling mechanisms participate in regulating vascular smooth muscle function that have resulted from our studies over the past 25 years in areas including oxygen sensing and the regulation of cGMP production by soluble guanylate cyclase (sGC) that were presented in the Robert M. Berne Distinguished Lectureship at the 2008 Experimental Biology Meeting. It considers mechanisms controlling the activity of sources of ROS including Nox oxidases and mitochondria by physiological stimuli, vascular diseases processes, and metabolic mechanisms linked to NAD(P)H redox and hypoxia. Metabolic interactions of individual ROS such as hydrogen peroxide with cellular peroxide metabolizing enzymes are viewed as some of the most sensitive ways of influencing cellular signaling systems. The control of cytosolic NADPH redox also seems to be a major contributor to bovine coronary arterial relaxation to hypoxia, where its oxidation functions to coordinate the lowering of intracellular calcium, whereas increased cytosolic NADPH generation in pulmonary arteries appears to maintain elevated Nox oxidase activity, and relaxation to hydrogen peroxide, which is attenuated by hypoxia. The sensitivity of sGC to nitric oxide seems to be regulated by thiol and heme redox systems controlled by cytosolic NADPH. Heme biosynthesis and metabolism are also important factors regulating the sGC system. The signaling pathways controlling oxidases and their colocalization with redox-regulated systems enables selective activation of numerous regulatory mechanisms influencing vascular function in physiological processes and the progression of aging-associated vascular diseases.
Keywords: guanylate cyclase, Nox oxidases, oxygen sensing, redox signaling
reactive oxygen species (ROS) are now known to have many important roles in the control of vascular function. If we look back to the field that existed before realizing contributions of ROS to vascular regulation, the work of two individuals stands out as having a tremendous impact on our own work and the field as we know it today. Early studies by Fridovich (24, 25) on agents that altered an oxygen-dependent reduction of cytochrome c by xanthine oxidase evolved into the discovery of the enzyme superoxide dismutase (SOD) that metabolizes superoxide generated by this enzyme (43) and a modeling of the biological properties of superoxide biosynthesis and metabolism. The initial interests of Chance in detecting how enzymes such as catalase metabolize peroxide (14) evolved into developing an integrated understanding of many key aspects of cellular peroxide metabolism that included areas such as how the redox status of components of the mitochondrial electron transport chain influence ROS generation (15). While the existence and importance of NAD(P)H oxidases or Nox oxidases in vascular regulation is a relatively new area of investigation, early studies recognized the major importance of microsomal NAD(P)H oxidase activities as cellular sources of peroxide in tissues such as the liver (9, 27). Currently, there is substantial evidence that Nox oxidases and mitochondria are significant sources of ROS generation in vascular smooth muscle (3, 35, 41, 70, 74). In addition, the endothelium can be a source of these and additional enzymes such as nitric oxide synthase, xanthine oxidase, cyclooxygenase, and cytochrome P-450, which can potentially generate and/or release ROS in amounts that have a major influence on the control of vascular function (39, 55). Early studies by groups lead by Kontos (38) and others showing how endogenously produced ROS can regulate microvascular contractile function contributed to developing the evolving field of how these systems regulate blood flow in a diversity of physiological and pathophysiological processes (40, 55). A major source of the variability in vascular regulation seen in arteries and veins, and the microcirculation of different organ systems during the progression of aging and disease processes, originates from changes in ROS and their interactions with systems controlling endothelial mediator release and vascular function. An early hypothesis for the mechanism of pulmonary hypoxic vasoconstriction by Archer and Weir (6) highlights initial thoughts on ways the modulation of ROS by changes in oxygen tension could influence cytosolic NAD(P)H redox in a manner that regulates thiol redox-controlled systems such as potassium channels. Our current understanding of how redox-regulated systems are organized to control signaling remains rather incomplete, and it is an evolving field of science. This article provides a concise overview of some of the known ways the metabolism of ROS and function of key cellular redox control mechanisms potentially result in interactions with signaling systems that control vascular function. Perspectives on ROS-redox signaling gained from our work in areas including oxygen sensing and the regulation of cGMP production by soluble guanylate cyclase (sGC) in vascular smooth muscle are emphasized in the context of where they are potentially contributing factors to physiological regulation and the progression of aging-associated vascular disease.
Cellular Generation and Metabolism of ROS
There appears to be a basal rate of production of ROS in essentially all cells from oxidases such as Nox oxidases and components of the mitochondrial electron transport chain, and many types of physiological and pathophysiological stimuli can cause acute increases in the rates of ROS production by influencing the activities of the oxidases that are expressed. Most oxidases begin the production of ROS by donating an electron to oxygen to generate superoxide anion (O2•−), and this electron often originates from cellular systems utilizing reduced pyridine nucleotides such as NADPH and/or NADH as the electron donors. In the absence of superoxide metabolizing systems, the rates of superoxide production by oxidases in cells will probably generate concentrations of superoxide in the nanomolar range based on the known levels of oxidase activities that are present in biological systems and the spontaneous rate of reaction of superoxide with itself, which generates hydrogen peroxide (H2O2) and oxygen. The amounts of different forms of SOD thought to be present in intracellular regions should lower the levels of superoxide into the picomolar concentration range (25). Based on the apparent rates of generation of peroxide and its metabolism by key scavenging systems, including glutathione peroxidase and catalase, peroxide levels in the cytosolic region of cells are thought to be in the low nanomolar concentration range (15). While superoxide reacts with nitric oxide (NO) three to four times faster than it reacts with SOD, this reaction only becomes a significant biological regulatory factor when superoxide levels rise or when NO levels are very high and approach the concentrations of SOD that are present. This reaction of NO with superoxide generates peroxynitrite (ONOO−), a reactive molecule that causes the modification of multiple other biological molecules and key sites on proteins potentially involved in their regulation. Initially, hydroxyl radical was thought to be a key regulatory species generated from reactions of peroxide with ferrous iron (Fe2+) and from the decomposition of peroxynitrite (61). However, because of the extreme chemical reactivity of hydroxyl radical, its importance in biological regulation seems to have been questioned, and evidence for alternative reactions of peroxide and peroxynitrite has evolved. For example, the reaction of peroxide with metal sites on proteins such as heme or bound iron is more likely to be involved in biological regulation. In addition, reactions associated with nitric oxide oxidation generating other reactive radicals such as nitrogen dioxide (·NO2) and carbonate radical are potential contributing factors in responses initially thought to result from peroxynitrite formation.
Mechanisms for Interactions of ROS with Cellular Signaling Systems
The relatively low intracellular concentrations of superoxide because of the presence of SOD may limit its direct interactions with signaling systems. However, regulation resulting from superoxide generation may originate from either the efficient formation of peroxide by SOD or as a result of its interaction with NO (see Fig. 1), and perhaps other targets that superoxide readily reacts with. Some of the most efficient ways peroxide can interact with signaling systems appear to result from the consequences of its enzymatic metabolism by proteins including catalase, glutathione peroxidase, and peroxiredoxins. Early observations documented that exposure of microvascular preparations to peroxide elicited responses that were often dominated by prostaglandin (PG)-mediated mechanisms (64, 75). These observations and studies on cyclooxygenase evolved into recognizing the important role of peroxide metabolism by the heme peroxidase reaction of this enzyme in the physiological stimulation of prostaglandin generation (42). Variation in activities of PGH2-metabolizing enzymes that are present in different segments of the circulation in normal and pathophysiological states seems to be a major contributor to the expression of both prostaglandin-mediated contractile and relaxing responses to conditions that expose vascular tissue to peroxide. We observed that, although catalase was metabolizing peroxide, it was able to stimulate sGC either with purified enzymes or under conditions present in pulmonary arteries that were associated with relaxation to peroxide (11, 12, 18). It is well established that the glutathione peroxidase reaction generates oxidized glutathione (GSSG) while it metabolizes peroxide and that GSSG is a substrate for enzymes that appear to regulate the redox status of thiols on proteins in ways that have potential regulatory roles (23, 72). The metabolism of peroxide by peroxiredoxins is usually linked to the generation of oxidized thioredoxin thiols, and thioredoxin reductase/thioredoxin systems are well established as regulators of protein thiol redox sites that often have important regulatory roles. For example, the status of these cellular thiol redox systems in subcellular regions potentially influences signaling systems through enzymatic reactions such as glutaredoxin and protein disulfide isomerases, which reversibly modify the redox of protein thiol groups in a manner dependent on the localized ratio of cofactors such as reduced glutathione (GSH) to GSSG. Although there are many hypotheses assuming peroxides interact with signaling systems by directly reacting with thiol groups presumably in their more reactive anionic form, the slow rates that have been reported for these reactions suggest alternative processes may be involved. One possibility is that protein-binding interactions might put the catalytic site of oxidases together with thiols in a manner that exposes them to the high concentrations of peroxide needed to drive direct reactions of peroxide with thiols. Alternatively, it could be hypothesized that catalysts such as transition metals might promote peroxide reactions with reactive protein thiol groups. Oxidized forms of NO, including peroxynitrite and nitrogen dioxide, have chemical properties that are potentially involved in regulation through their reactions with protein sites such as thiols and tyrosine, unsaturated fatty acids, and other molecules (GSH, tetrahydrobiopterin, etc.) that these species readily react with (60). Most of the other known direct chemical reactions of superoxide, peroxide, and other ROS with targets linked to signaling systems have rates suggesting they would not be very significant or selective at the concentrations of these species potentially present in cells under biological conditions. However, there is evidence for distinct subcellular localizations for oxidases that may enable signaling systems colocalized with the oxidases to be exposed to very high concentrations of ROS (16, 35).
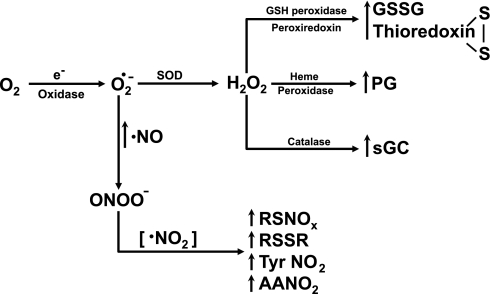
Fig. 1.
Interactions of the metabolism of reactive O2 species with vascular signaling systems. Redox changes in thiols and heme resulting from the fast rates of metabolism of peroxide by peroxidases and catalase are hypothesized to be some of the most sensitive ways reactive oxygen species (ROS) generation by oxidases interacts with signaling systems that control vascular contractile function such as the generation of prostaglandins (PGs) by cyclooxygenase and the production of cGMP by soluble guanylate cyclase (sGC). As nitric oxide (NO) levels increase, it competes with superoxide dismutase (SOD) for the metabolism of superoxide, forming reactive species, including peroxynitrite (ONOO−) and nitrogen dioxide (·NO2), which directly modify tissue thiols (RSH), unsaturated fatty acids [e.g., arachidonic acid (AA)] and reactive protein tyrosine groups (Tyr) through oxidation, nitrosation, or nitration reactions linked to signaling mechanisms that influence vascular function.
An extremely important, but rather poorly understood, aspect of signaling by ROS is that there are specific signaling mechanisms regulating the activities of the different oxidases in cells, and there is likely to be selective activation of redox-regulated signaling systems that are localized to the subcellular regions of the oxidases being regulated. For example, Griendling and colleagues (35) initially documented that Nox1 and Nox4 had specific subcellular localizations in vascular smooth muscle cells and suggested this was an important factor in determining the signaling processes that these ROS-generating systems were involved in. An endoplasmic reticulum localization of Nox4 in endothelium has recently been shown by the Keaney group (16) to be essential for observing its ability to inhibit protein tyrosine phosphatase 1B in epidermal growth factor receptor (EGFR) signaling. Thus, while a molecule such as hydrogen peroxide could influence the function of many different signaling systems, there will be selective regulation of specific cellular functions as a result of the unique ways each oxidase-generating peroxide is controlled by biological processes and colocalized with redox-regulated signaling systems.
Cytosolic NAD(P)H Redox Metabolic Control Mechanisms
Pathways of glucose metabolism and aspects of mitochondrial function are potentially major regulators of cellular redox control through their influence on the redox status of NADPH and NADH [or ratios of reduced NAD(P)H to oxidized NAD(P)+] in subcellular regions (see Fig. 2). The major source of NADH generation in the cytosolic region appears to be glyceraldehyde 3-phosphate dehydrogenase in glycolysis. However, the efficiency of mitochondrial shuttles and the equilibrium-like behavior of the lactate dehydrogenase reaction generally function in the direction of removing cytosolic NADH and keeping the cytosolic NAD(H) system primarily in its oxidized NAD+ form. In contrast, most studies suggest cells maintain greater levels of NADPH than NADP+ in subcellular regions such as cytosol and mitochondria. It is generally thought that glucose-6-phosphate dehydrogenase (G6PD) and phosphogluconate dehydrogenase, the subsequent enzyme in the pentose phosphate pathway (PPP) of glucose metabolism, are the primary sites generating NADPH in the cytosolic region of vascular tissue. An observation consistent with this interpretation is evidence that inhibitors of G6PD promote the oxidation of NADPH in vascular smooth muscle (28). Whereas the control of cytosolic NADPH by a variety of biosynthetic enzymes, oxidases, and enzymes maintaining thiol redox which consume NADPH is not well understood, it is likely that the activities of these enzymes determine their influence on NADPH redox in subcellular regions.
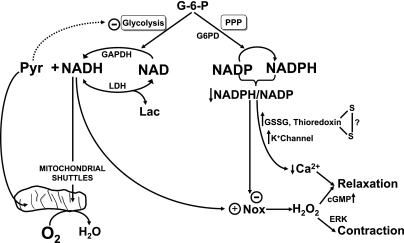
Fig. 2.
Metabolic control of vascular redox signaling. The redox status of cytosolic NADH generated primarily by the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in glycolysis and cytosolic NADPH derived from glucose-6-phosphate (G-6-P) metabolism by glucose-6-phosphate dehydrogenase (G6PD) and the pentose phosphate pathway (PPP) are hypothesized to control signaling mechanisms through their influence in providing NAD(P)H to support ROS generation by Nox oxidases and processes directly regulated by NADP(H) redox. Note that the lactate dehydrogenase (LDH) reaction and mitochondrial shuttles normally function to maintain very low levels of cytosolic NADH, allowing high levels of lactate (Lac) to regulate signaling through increasing NADH and ROS generation by Nox oxidases. Some of the signaling mechanisms we have observed to be regulating contractile function in bovine arteries by these metabolic redox control systems, including cGMP signaling, extracellular signal-regulated kinase (ERK), potassium (K+) channels, and processes controlled by NADP(H) redox (NADP/NADPH) that coordinate the lowering of cytosolic calcium (Ca2+), are included in the model shown.
Interactions of Cytosolic NAD(P)H Redox with Vascular Signaling Systems
A variety of regulatory systems are influenced by NAD(P)H redox in a manner that could potentially be important in the control of vascular function. The key roles of NADPH as both a source of vascular ROS generation and as a controller of the redox status of thiol redox systems involved in peroxide metabolism and oxidant-associated signaling suggest that the control of NADPH redox in cytosolic-linked cellular regions will be a major factor in the expression of signaling and responses that are observed. Because both NADH and NADPH appear to be substrates for Nox oxidases, the availability of these electron donors for superoxide generation can influence ROS-regulated signaling. We first recognized the potential importance of cytosolic NADH as a substrate for vascular Nox oxidases as a result of discovering that lactate increased superoxide and caused a peroxide-mediated relaxation in endothelium-removed bovine arteries (53, 59). Our emphasis in studying the role of substrates for Nox oxidases may have initially been biased toward focusing on NADH because measurements of superoxide generation using high concentrations of lucigenin could have contained a redox cycling artifact enhancing NADH-derived superoxide production compared with NADPH. However, our subsequent studies in coronary arteries developed by Gupte et al. (28) with inhibition of NADPH generation by G6PD detected a decrease in superoxide and peroxide release from bovine coronary arteries, suggesting that NADPH was normally the primary substrate supporting Nox oxidase activity.
Studies on the mechanism of a ROS-independent relaxation that resulted from inhibition of G6PD evolved into the realization that cytosolic NADPH oxidation appears to function as a coordinator of multiple mechanisms that lower intracellular calcium (28, 32). Some of the systems that seem to have roles in the relaxation of bovine coronary arteries when cytosolic NADPH oxidizes include stimulation of calcium uptake by the sarco(endo)plasmic reticulum calcium ATPase pump and the opening of voltage- and calcium-regulated potassium channels. Inhibition of G6PD also promotes oxidation of glutathione and has actions similar to the thiol oxidant diamide in promoting relaxation through what appears to be a thiol redox-regulated mechanism of controlling calcium influx through both voltage-regulated calcium channels and other receptor-regulated calcium channels (28, 36). Figure 3 shows some of the ways cytosolic NADPH oxidation potentially coordinates the lowering of intracellular calcium. However, the poorly understood interactive nature of systems regulating calcium around the sarcoplasmic reticulum and evidence for potential redox regulation of most calcium and potassium channels makes it difficult to sort out which redox-regulated sites are most important. Oxidation of cytosolic NADPH also appears to inhibit nitric oxide-elicited stimulation of sGC and vascular relaxation through thiol and heme redox-associated mechanisms that are discussed below. Thus, as nitric oxide-elicited relaxation becomes impaired, cytosolic NADPH oxidation appears have an important role in additional processes promoting vasodilation through coordinating multiple mechanisms of lowering intracellular calcium.
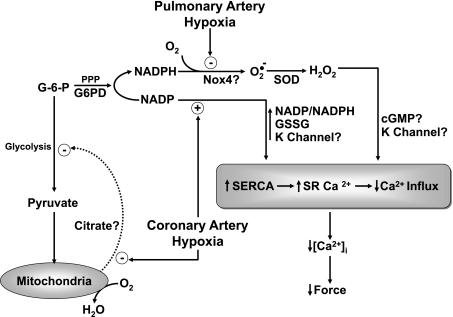
Fig. 3.
Model showing processes we hypothesize to participate in mediating relaxation of bovine coronary and contraction of bovine pulmonary arterial smooth muscle to hypoxia. In coronary arteries, a metabolic stress caused by hypoxia is hypothesized to impair a mitochondrial pyruvate metabolism-controlled feedback inhibition of glycolysis (26, 32). This feedback mechanism functions to sustain G-6-P availability for metabolism by G6PD and the PPP for the generation and maintenance of cytosolic NADPH. The observed hypoxia-elicited increase in the ratio of cytosolic NADP/NADPH appears to elicit relaxation through multiple redox-regulated processes that seem to coordinate the lowering of intracellular calcium ([Ca2+]i). Studies probing the mechanism of the relaxation of bovine coronary arteries to hypoxia suggest that systems, including the opening of potassium (K+) channels, increased sarcoplasmic reticulum (SR) uptake of calcium by the sarco(endo)plasmic reticulum calcium ATPase (SERCA) pump, decreased SR calcium release, and decreased calcium influx across the plasma membrane, all appear to participate in the response that is observed. However, the presence of multiple thiol redox sites potentially controlling key processes regulating interactive systems influencing SR calcium and calcium influx makes it difficult to establish the actual sequence of processes mediating the lowering [Ca2+]i and relaxation elicited by increased NADP/NADPH redox. The presence of increased levels of G6PD expression and activity in bovine pulmonary arteries (30) appears to maintain elevated levels of cytosolic NADPH available for the generation of superoxide by endogenous Nox oxidase activity, and to prevent the relaxation to hypoxia that is observed in coronary arteries. Hypoxia appears to promote contraction in bovine pulmonary arteries through removing a relaxation originating from peroxide potentially derived from the metabolism of superoxide by Cu,Zn-SOD (2, 53). Although there is evidence justifying hypothesizing removal of relaxing mechanisms potentially coordinated by processes regulated by cGMP protein kinase (12, 13) and decreased NADP-to-NADPH ratios (31), the actual mechanism mediating the increase in force elicited by hypoxia remains to be established. Although we find evidence for Nox2 and mitochondria as potential sources of ROS in bovine pulmonary arteries, our most recent studies suggest a role for Nox4 as the Po2-sensitive source of peroxide that is regulating the pulmonary vascular response to hypoxia (1, 2).
The control of thiol redox on proteins is thought to be an important component of the redox regulation of multiple signaling systems. Thiol redox changes involve processes such as the formation of sulfenic acids, S-nitrosated thiols, S-nitrated thiols, disulfide formation between protein cysteine thiols or with glutathione to form S-thiolated or S-gluthionated adducts with protein cysteines, and the disruption of iron-sulfur or zinc sulfur centers (23, 60, 61, 72). NAD(P)H and thiol redox potentially influence key sites regulating calcium reuptake and release from the sarcoplasmic reticulum, channels involved in capacitive- and voltage-regulated calcium influx across the plasma membrane, ion channels controlling membrane potential, and contractile-enhancing systems that participate in controlling vascular smooth muscle force generation. The role of NADPH in influencing redox effects of peroxide metabolism by glutathione peroxidase and peroxiredoxins is potentially a major route for ROS to interact with thiol redox-associated signaling. NADPH is used by glutathione reductase and thioredoxin reductase reactions to metabolize peroxide through its role in maintaining glutathione and thioredoxin in their reduced forms. Oxidation of the glutathione and thioredoxin systems is thought to be a key cofactors in controlling the status of thiol sites on proteins that are involved in redox regulation of their function through their actions in controlling sites undergoing thiol oxidation or S-thiolation. The reversible or chemical equilibrium-like behavior of NADPH-dependent enzymes, including glutathione and thioredoxin reductases controlling the thiol redox status of the glutathione and thioredoxin systems, is likely to be an important contributing factor in the localized redox control of proteins involved in cellular signaling. Although the balance in subcellular regions between metabolic production of NADPH and its consumption by oxidase, peroxidase, thiol-reducing, and other metabolic systems could be hypothesized to be a major factor in determining which signaling processes are regulated, minimal consideration has been given to the interactive properties of these redox systems. Thus the influence of vasoactive stimuli on the interactive balance between peroxide metabolism and thiol redox control systems linked to NADPH redox in subcellular regions should be considered for its potential role as a key factor in determining how specific signaling systems are regulating vascular function.
Influence of Hypoxia on Cytosolic NADPH Redox and Basal Levels of ROS are Potentially Major Factors in Vascular Oxygen-Sensing Mechanisms
Mechanisms involving changes in ROS and redox have been key components of most current hypotheses for linking the sensing of changes in oxygen tension to the regulation of vascular tissue (54, 69, 74). However, substantial controversy currently exists regarding the role of ROS and redox changes in modulating contractile function elicited by exposing the vascular smooth muscle of pulmonary and systemic arteries to hypoxia. For example, in pulmonary arteries, there is evidence for hypoxia promoting vasoconstriction through either decreasing or increasing vasoactive ROS derived from mitochondrial and/or Nox oxidase sources. In systemic arteries, hypoxia has been reported to increase mitochondrial ROS, and to elicit relaxation through oxidative redox changes in systems such as cytosolic NADPH and/or thiol redox regulation of potassium channels that are either ROS-dependent (44) or ROS-independent (32, 49, 52).
Our recent studies in bovine calf coronary and pulmonary arteries have provided evidence supporting potential explanations for the origins of some key controversies in this field. Initially, the studies of Mohazzab-H et al. (52) found that hypoxia seemed to lower superoxide detected by lucigenin chemiluminescence and peroxide based on its ability to convert methanol to formaldehyde by endogenous catalase in precontracted endothelium-denuded coronary arteries, and Nox-type oxidases dependent on NAD(P)H appeared to be the major source of basal superoxide in this vascular segment. However, the relaxation of these arteries to hypoxia did not appear to involve superoxide-derived ROS such as hydrogen peroxide (49, 52). Recent work by Gao and Wolin (26) has provided evidence that we can detect hypoxia-eliciting increases in mitochondrial superoxide detected by mitosox in coronary arteries. Surprisingly, when the arteries are contracted with 30 mM KCl, they show decreases in mitochondrial superoxide. In addition, inhibition of mitochondrial electron transport in bovine coronary arteries under normoxic conditions with probes such as rotenone and antimycin increases mitochondrial superoxide, but these treatments do not decrease force generation or mimic the effects of hypoxia (26, 28). Superoxide and/or hydrogen peroxide from extramitochondrial sources that appear to be Nox oxidases also seem to be decreased by hypoxia in the absence or presence of contraction (26, 52). Our studies suggest that increases in superoxide detected by mitosox are observed when hypoxia or mitochondrial electron transport inhibitors raise mitochondrial NAD(P)H redox above the basal level, and this was observed to be associated with mitochondrial membrane hyperpolarization (26). However, when the arteries are exposed to 30 mM KCl, the increased intracellular calcium release and/or metabolic stress associated with contraction alters the balance of processes controlling mitochondrial NAD(P)H redox in a manner that prevents the increase caused by hypoxia, and these conditions are associated with a decrease in superoxide (26). Thus differences in experimental conditions such as the absence or presence of force generation could be factors in influencing if mitochondria in vascular smooth muscle show increases or decreases in superoxide or ROS generation under hypoxic conditions.
Studies by Gupte and Wolin (32) have provided evidence for a key role of hypoxia in promoting relaxation of bovine coronary arteries through a metabolic stress (see Fig. 3) that decreases the activity of the G6PD reaction of the PPP, and this seems to promote the oxidation of cytosolic NADPH and relaxation through the coordination of calcium-lowering processes regulated by this system. Because ATP levels are maintained under hypoxia, the metabolic stress does not seem to be related to a deficiency in energy metabolism. Pulmonary arteries maintain higher levels of NADPH and G6PD [but not glucose-6-phosphate (G-6-P)], and this seems to prevent hypoxia from causing cytosolic NADPH oxidation and relaxation (30, 31). Interestingly, pyruvate inhibits relaxation and changes such a lowering of G-6-P and NADPH elicited by hypoxia, suggesting that a metabolic stress on glycolysis or process promoting NADPH oxidation could be a key factor (32). Because hypoxia lowers endogenous pyruvate, a mitochondrial-dependent metabolic pathway of pyruvate linked to maintaining cytosolic NADPH redox could be a fundamental process in the mechanism of sensing hypoxia by coronary arteries (26). Thus a metabolic impairment of the ability of mitochondrial-associated pathways of pyruvate metabolism to maintain cytosolic NADPH by the G6PD reaction of the PPP appears to be an oxygen-sensing mechanism that regulates the relaxation of coronary arteries by hypoxia.
Early studies by Burke and Wolin (11–13) developed evidence for a hypothesis that hypoxia promoted vasoconstriction of bovine calf pulmonary arteries through removing a peroxide-dependent relaxing mechanism, where a suppression of cGMP signaling seemed to be a contributing factor to the response that was observed. Although this seemed to be an alternative to the initial redox hypothesis for hypoxic pulmonary vasoconstriction of Archer et al. (4, 6) linking hypoxia to removing a ROS-dependent hyperpolarization depressing force through a loss of ROS-mediated oxidation of cytosolic NAD(P)H and thiol redox systems maintaining the opening of potassium channels, subsequent work is providing evidence that components of both hypotheses may be important contributing factors. The detection of NAD(P)H oxidases as major sources of superoxide-derived peroxide in bovine pulmonary arteries led us to consider the oxygen dependence of Nox oxidases as the oxygen sensor for hypoxia (50, 53). In contrast, the influence of mitochondrial inhibitors on responses of rat pulmonary arterial smooth muscle to hypoxia promoted consideration of mitochondrial sources of ROS as key contributors to the redox hypothesis (4, 44). Although it is possible that either source of ROS generation could be important, our studies in bovine pulmonary arteries have led us in the direction of considering Nox4 as the dominant oxygen-regulated source of ROS linked to force regulation by hypoxia. Studies of Gupte et al. (30) have provided evidence that Nox2 and Nox4 are the major forms of this oxidase present in the smooth muscle of bovine pulmonary arteries. Our group and Archer and Weir have evidence from studies using pharmacological probes (1) and knockout mice (5) suggesting that, while Nox2 may be an important source of ROS in the pulmonary vasculature, the oxidants generated by this oxidase system do not seem to be a significant contributing factor to vasoconstriction elicited by hypoxia. We hypothesize that Nox4 is the important source of peroxide influencing hypoxic vasoconstriction in bovine pulmonary arteries based on recent work developed by Ahmad et al. (1, 2) suggesting that pharmacological or small-interfering RNA inhibition of Nox4 activity and increasing Nox4 expression by organ culture with transforming growth factor-β influences the magnitude of contractions to hypoxia in a manner consistent with a role for changes in ROS generation by this oxidase (Ahmad M and Wolin MS, unpublished observations). In contrast, while mitochondrial inhibitors including rotenone and antimycin decrease superoxide in bovine arteries (1) in a manner similar to changes seen in the rat pulmonary vasculature (4, 44), we find that these inhibitors do not alter the magnitude of contraction to hypoxia in the bovine pulmonary arteries. Gupte and colleagues (31) have observed that hypoxia increases NADPH in rat pulmonary arteries under conditions where contraction is observed, suggesting that a contributing factor could also be removal of relaxing mechanisms controlled by cytosolic NADP/NADPH redox. For example, increasing cytosolic NADPH redox could result in the closing of potassium channels and/or lowering intracellular calcium. Because decreased NADPH consumption by Nox oxidases and the metabolism of peroxide are components of the hypotheses for hypoxic pulmonary vasoconstriction being discussed, much of the evidence on the response of pulmonary arterial smooth muscle to hypoxia being considered here seems to be converging on what appears to be a signaling mechanism involving hypoxia decreasing a peroxide-controlled NADPH redox-regulated relaxing mechanism.
sGC and cGMP Signaling are Major Targets of Regulation by ROS and Cytosolic NADPH Redox
Many redox systems seem to regulate sGC- and/or cGMP-associated signaling systems designed to coordinate processes involved in vascular smooth muscle relaxation. As discussed previously, it is well established that superoxide anion attenuates sGC stimulation by NO as a result the efficiency of the diffusion-controlled reaction between these two free radical species. Our studies by Cherry et al. (17) and Omar et al. (58) developed evidence for the important role of Cu,Zn-SOD activity in protecting the stimulation of sGC by NO from being impaired by the basal levels of superoxide generated in pulmonary and coronary arteries, respectively. Peroxide generated from the scavenging of superoxide by Cu,Zn-SOD derived from sources including Nox oxidases (11–13, 49, 52, 53) seems to be able to stimulate sGC activity when it is metabolized by catalase. However, several factors can make it difficult to observe relaxation responses through this mechanism. Relaxation to peroxide is readily inhibited by both superoxide and NO through their ability to inhibit catalase (11, 18, 51). It addition, peroxide can also simultaneously stimulate contractile-enhancing mechanisms such as those activated by stimulation of extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase, which seems to promote a functional antagonism of relaxation (56, 57). Peroxynitrite and nitrogen dioxide can interact with thiols in a manner that generates donors of NO, and the release of NO over time can promote a prolongation of relaxation (22). In addition, peroxynitrite appears to be able to oxidize the heme of sGC from its ferrous (Fe2+) NO-binding form to its ferric (Fe3+) form, which seems to be resistant to participating in stimulating sGC activity, and potentially associated with a loss of heme and the degradation of sGC (63). Thus the levels of NO and individual ROS and NO-derived species in the proximity of sGC are likely to determine how the production of cGMP by this enzyme is regulated.
Our more recent studies by Gupte and Mingone have provided evidence that cytosolic NADPH redox also has an important role in controlled thiol and heme redox systems, which have a major influence on the ability of NO to stimulate sGC through mechanisms that are shown in Fig. 4. It is well established that NO stimulates cGMP production by binding the ferrous (Fe2+) heme of sGC (19, 71, 76). Recent studies suggest that the heme of sGC is readily oxidized to its ferric (Fe3+) form under vascular pathophysiological conditions associated with impaired NO stimulation of sGC (63). Our studies have detected a cytosolic NADPH redox-controlled methemoprotein reductase activity that appears to have an important role in maintaining the heme of sGC in its ferrous oxidation state (33). When inhibitors of G6PD cause oxidation of cytosolic NADPH associated with increased GSSG, it appears that an enzymatic reaction utilizes GSSG to promote what appears to be a thiol redox-mediated inhibition of sGC activation by NO in a manner that does not appear to inhibit the basal sGC (46). Interestingly, peroxide has been shown to cause a thiol oxidation-mediated dimerization of cGMP-dependent protein kinase (PKG) associated with its activation and relaxation of coronary arteries (10), and recently reproduced these observations in the bovine pulmonary arteries studied in our laboratory (Neo BH and Wolin MS, unpublished observations). Thus cytosolic NADPH redox also appears to have important roles in regulating the vascular function through altering the sensitivity of sGC to NO through its influence on thiol and heme groups of sGC. In addition, redox regulation of PKG could be a contributing factor or alternative mechanism for oxidant-elicited vascular relaxation.
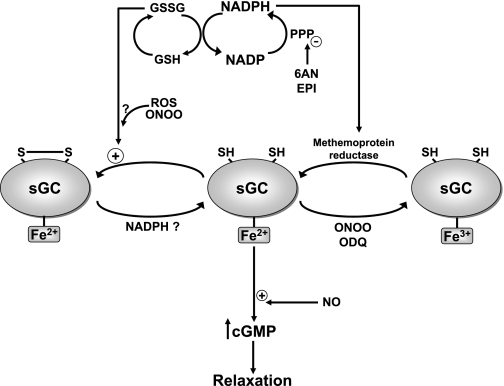
Fig. 4.
Relationships between cytosolic NADPH redox and the control of sGC by thiol and heme redox mechanisms. Our studies with inhibitors of G6PD [6-aminonicotinamide (6AN) and epiandrosterone (Epi)] that decrease glucose metabolism through the PPP and the generation of cytosolic NADPH detected evidence that NADPH functions to prevent inhibition of NO stimulation of sGC by thiol and heme oxidant mechanisms (33, 46). NADPH oxidation increases the ratio of reduced glutathione (GSH) to oxidized GSH (GSSG), and an enzymatic process enables GSSG to inhibit NO stimulation of sGC. In addition, both peroxynitrite and the sGC inhibitor ODQ are thought to oxidize the heme of sGC to its ferric (Fe3+) form. An NADPH-dependent methemoprotein reductase activity appears to restore the Fe3+-heme of sGC to the ferrous (Fe2+) form that binds NO. These systems potentially function to prevent the loss of NO stimulation of sGC under conditions of oxidative stress associated with increased ROS and ONOO.
Pathways controlling the biosynthesis of heme and its metabolism by heme oxygenase (HO) also have a major influence on the generation of cGMP by sGC. Heme biosynthesis is obviously needed to maintain the availability of heme for its role in enabling NO to bind and stimulate sGC. The biosynthetic precursor to heme, protoporphyrin IX, is an activator of sGC that is thought to function through replacing the heme on sGC in a manner that reproduces interactions elicited by NO (76), such as the loss of a heme iron-histidine coordination associated with stimulation of cGMP production (71). Recently developed drugs designed to stimulate sGC through the protoporphyrin IX binding appear to be resistant to some of the oxidant processes that impair sGC regulation in disease models that impair NO-mediated vascular relaxation through this system (63). Although exposure of vascular tissue to δ-aminolevulinic acid can cause the accumulation of protoporphyrin IX in amounts that stimulate sGC and promote relaxation (47), there is an absence of evidence that this activator of sGC accumulates under physiological conditions in amounts that regulate vascular function. The HO reaction also seems to regulate sGC through several potential mechanisms. There is evidence that its product carbon monoxide is a stimulator of sGC at micromolar concentrations and an inhibitor of NO stimulation of sGC; however, we have not been successful in finding evidence for these effects in our studies on bovine arteries (45). Generation of biliverdin and bilirubin by the HO reaction seems to have antioxidant-like actions that could have effects on systems regulating sGC (73). In addition, our recent studies suggest that the induction of heme oxygenase-1 (HO-1) can impair sGC regulation by NO under conditions where it depletes heme in vascular smooth muscle. The induction of HO-1 also seems to lower superoxide through increasing the expression of the extracellular form of SOD, and this may have an important beneficial role in restoring NO-mediated responses in diseases such as diabetes (65). Thus heme metabolism has multiple interactions with redox processes that regulate the activity of sGC.
Stimuli of Vasoconstriction Can Function Through Promoting Oxidant Signaling
There is some evidence that many agents or stimuli associated with promoting vasoconstriction also cause the generation of ROS. The generation of ROS as a result of ANG II (AT) stimulating the AT1 receptor on vascular smooth muscle is one of the most-studied systems. Griendling and colleagues (62) have documented that stimulation of the AT1 receptor initially promotes a p47phox stimulation of Nox oxidase activity in rat aortic smooth muscle cells. The peroxide generated by this pathway appears to further stimulate Nox oxidase activity by activating Src kinase, which promotes a EGFR transactivation of phosphatidylinositol-3-kinase, which is linked to rac binding to Nox oxidase. Our studies by Richard Oeckler on a stretch-induced enhancement of force generation in bovine coronary arteries associated with a p47phox-mediated activation of superoxide generation by Nox oxidase resulted in evidence for a peroxide-mediated Src kinase-EGFR transactivation of ERK MAP kinase in the response observed (57). This mechanism was also observed in bovine pulmonary arteries, and it could be activated by increased lactate, which was presumably functioning through increasing the availability of cytosolic NADH for utilization as a substrate for Nox oxidase (56). We have observed in collaborative work by Ungvari et al. (66) that acute increases in luminal pressure are also a direct stimulus for increasing superoxide in both endothelium and vascular smooth muscle of rat femoral arteries. Studies by Gupte et al. (29) on a contraction of bovine coronary arteries elicited by the phorbol 12,13-dibutyrate activator of protein kinase C has resulted in the detection of a p47phox kinase activation of Nox2, suggesting that the activation of this system could be an important factor in the action of contractile agents that function through stimulating protein kinase C. Webb and colleagues (37) have provided evidence that Rho kinase may also be an important factor in regulating the mechanisms of contraction elicited by ROS. We suspect that the subcellular colocalization of signaling and spilling over of oxidant stress into other subcellular regions will be major factors in determining the regulation of signaling systems in responses that are observed. It is likely that differences in expression of components regulating oxidases and the actions of ROS on signaling systems influencing endothelial mediator release and vascular force are major contributors to regional differences in vascular regulation, and changes associated with vascular disease processes.
ROS and Redox Controlled Signaling Mechanisms are Potentially Major Factors in Aging-Associated Vascular Diseases
Studies published in the literature provide an extensive documentation of roles for changes in ROS and the function of redox-regulated systems in cardiovascular diseases associated with aging. Our laboratory has gained much insight into how ROS and redox controlled signaling mechanisms are potentially involved in aging and aging-associated cardiovascular diseases (e.g., hypertension, heart failure, diabetes) as a result of collaborations with colleagues at our institution, including Gabor Kaley, Thomas Hintze, Akos Koller, Zoltan Ungvari, Alberto Nasjletti, and others we have published with. Although the scientific literature has provided extensive documentation for increased superoxide generation functioning to attenuate endothelial regulation of vascular smooth muscle relaxation by nitric oxide in aging-associated vascular diseases and the ability of the endothelium to generate vasoactive levels of hydrogen peroxide, there appear to be many additional aspects of ROS and redox regulation in these diseases that remain to be defined. Models for each disease have provided evidence for different aspects of processes increasing oxidant production in the endothelium and vascular smooth muscle. In addition, the Harrison group (55) has highlighted the potential importance of a feedforward mechanism where the initial activation of Nox oxidases by pathophysiological mediators such as ANG II promotes the activation of other oxidases. Each major ROS-generating system shown in the endothelium of the model in Fig. 5 has ways of being activated by oxidants derived from superoxide and NO to produce further increases in ROS. For example, peroxynitrite oxidizes tetrahydrobiopterin and uncouples NO synthase (55); xanthine oxidase activity is increased by multiple oxidant-linked processes, including p38 MAP kinase activation, thiol oxidation, and oxidant-stimulated proteolysis (8); oxidants activate ROS production by cyclooxygenase (COX) by a combination of promoting release of its substrate arachidonic acid and by oxidizing the heme of COX to a form that promotes prostaglandin biosynthesis and oxidant generation by its peroxidase reaction (38, 42), and oxidant conditions are also known to promote mitochondrial superoxide generation (34). In addition, Nox oxidases have previously discussed roles for oxidants in their activation of superoxide production. Because PGI2 synthase is readily inactivated by peroxynitrite through a tyrosine nitration reaction, oxidant stress of vascular disease processes is often associated with a shift from PG-mediated relaxation responses by PGI2 to constrictor responses elicited by the accumulation of PGH2 (77). Increases in hormones such as ANG II, cytokines, pressure in the vasculature, turbulence of blood flow (shear), and metabolic stresses such as elevated glucose and low-density lipoprotein all have the ability to increase ROS in the vessel wall in a manner that is generally associated with alterations in endothelial regulation of vascular function.
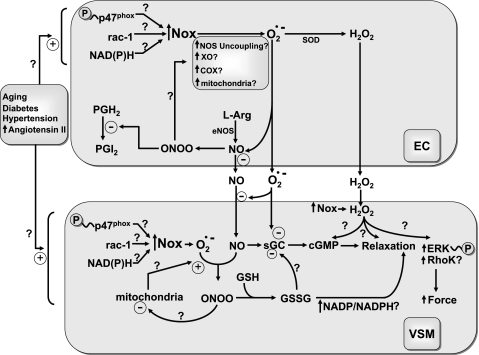
Fig. 5.
Model showing potential consequences of Nox oxidase activation in endothelium (EC) and vascular smooth muscle (VSM) during aging and aging-associated vascular diseases. Aging-associated vascular diseases appear to have a common property of increasing the expression and/or activation of Nox oxidases in EC and VSM through processes including protein kinase C-mediated phosphorylation of its p47phox subunit, a ROS-associated src kinase activation of rac-1 binding, and by increasing the availability of NAD(P)H. This results in increased peroxynitrite (ONOO) formation as superoxide reacts with NO and oxidant signaling in the endothelium begins to cause additional ROS to be produced through processes that may include tetrahydrobiopterin oxidation-mediated uncoupling of nitric oxide synthase (NOS), peroxidase-associated stimulation of PGG2/PGH2 biosynthesis by cyclooxygenase (COX), increased conversion of xanthine dehydrogenase its oxidase (XO) form, and alterations in the function of the mitochondrial electron transport chain. These changes contribute to decreasing the release of endothelium-derived relaxing mediators through processes that often include attenuating the release of NO, increasing the generation of H2O2 and ONOO, and inactivating PGI2 synthase through tyrosine nitration. Aging-associated vascular diseases also expose VSM to increased ROS in a manner that often attenuates relaxation by NO stimulation of sGC and promotes increased reactivity to vasoconstrictors through enhancing activation of systems, including protein kinase C, extracellular regulated kinase (ERK), and Rho kinase (RhoK). However, it is important to note that little is known about how the progression of aging-associated vascular diseases alters sGC regulation, cGMP-protein kinase activity, cytosolic NADP/NADPH, and many other aspects of redox-controlled signaling systems influencing VSM function that are considered in this review.
Although some aspects of the effects of increased oxidant production in vascular smooth muscle have been studied, we believe that many aspects of the regulation that exists remains to be defined. Aging itself and models of hypertension, diabetes, and elevated angiotensin appear to increase Nox oxidase activity in vascular smooth muscle (7, 20, 21, 62, 66–68). In addition, some of these disease models also seem to be associated with increased mitochondrial oxidant production. While some disease models show a superoxide-mediated impairment of NO-mediated vascular smooth muscle relaxation, there are additional systems regulating vascular function that are likely to be influenced by the observed increases in oxidants. Systems such as NAD(P)H redox and ROS-linked signaling mechanisms associated with oxygen sensing, regulating sGC/PKG, and other relaxant and contractile mechanisms, are also likely to be contributing factors to changes in vascular regulation related to aging. For example, Gutterman and colleagues (48) have shown that diabetes impairs the relaxation of human coronary arterioles to hypoxia. However, the processes responsible for this change remain to be defined. Because evidence is emerging for subcellular colocalization of specific pathways of oxidase activation and redox-controlled signaling mechanisms regulated by oxidase activation, it is likely that each metabolic stress or pathophysiological condition has unique ways of influencing adaptive regulation through the diversity of oxidant-signaling mechanisms discussed in this article. While we have emphasized acute responses influencing the control of vascular force, it is important to emphasize that many processes, including inflammatory responses and remodeling of the vessel wall, are also regulated by the time-dependent function of many of the ROS redox-signaling mechanisms we have considered. Thus factors regulating redox and oxidant signaling are likely to be major determining aspects in the progression of vascular adaptation and pathophysiology seen in aging and vascular disease processes.
GRANTS
Recent studies from the author's laboratory have been funded by National Institutes of Health Grants HL-31069, HL-43023, and HL-66331.
REFERENCES
- 1.Ahmad M, Kaminski PM, Wolin MS. Modulation of the contractile response of bovine pulmonary arteries to hypoxia by plumbagin, an inhibitor of Nox oxidase-4 (Abstract). FASEB J 21: A922, 2007. [Google Scholar]
- 2.Ahmad M, Zhao X, Kelly MR, Kaminski PM, Wolin MS. TGF β-1 mediated increase in Nox-4 expression enhances hypoxic pulmonary vasoconstriction in bovine pulmonary arteries (Abstract). FASEB J 22: 1152.22, 2008. [Google Scholar]
- 3.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JGN, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 294: H570–H578, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res 73: 1100–1112, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weir EK. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci USA 96: 7944–7949, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer SL, Will JA, Weir EK. Redox status in the control of pulmonary vascular tone. Herz 11: 127–141, 1986. [PubMed] [Google Scholar]
- 7.Bagi Z, Koller A, Kaley G. Superoxide-NO interaction decreases flow- and agonist-induced dilations of coronary arterioles in Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol 285: H1404–H1410, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Boueiz A, Damarla M, Hassoun PM. Xanthine oxidoreductase in respiratory and cardiovascular disorders. Am J Physiol Lung Cell Mol Physiol 294: L830–L840, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J 128: 617–630, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317: 1393–1397, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol Heart Circ Physiol 252: H721–H732, 1987. [DOI] [PubMed] [Google Scholar]
- 12.Burke-Wolin TM, Wolin MS. H2O2 and cGMP may function as an O2 sensor in the pulmonary artery. J Appl Physiol 66: 167–170, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Burke-Wolin TM, Wolin MS. Inhibition of cGMP-associated pulmonary arterial relaxation to H2O2 and O2 by ethanol. Am J Physiol Heart Circ Physiol 258: H1267–H1273, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Chance B On the reaction of catalase peroxides with acceptors. J Biol Chem 182: 649–658, 1950. [Google Scholar]
- 15.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605, 1979. [DOI] [PubMed] [Google Scholar]
- 16.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherry PD, Omar HA, Farrell KA, Stuart JS, Wolin MS. Superoxide anion inhibits cGMP-associated bovine pulmonary arterial relaxation. Am J Physiol Heart Circ Physiol 259: H1056–H1062, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Cherry PD, Wolin MS. Ascorbate activates soluble guanylate cyclase via H2O2-metabolism by catalase. Free Radic Biol Med 7: 485–490, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Craven PA, DeRubertis FR. Restoration of the responsiveness of purified guanylate cyclase to nitrosoguanidine, nitric oxide, and related activators by heme and hemeproteins. Evidence for involvement of the paramagnetic nitrosyl-heme complex in enzyme activation. J Biol Chem 253: 8433–8443, 1978. [PubMed] [Google Scholar]
- 20.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783–797, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary artery function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Davidson CA, Kaminski PM, Wolin MS. Nitric oxide elicits prolonged relaxation of bovine pulmonary arteries via endogenous peroxynitrite generation. Am J Physiol Lung Cell Mol Physiol 273: L437–L444, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287: C246–C256, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Fridovich I Reflections of a fortunate biochemist. J Biol Chem 276: 28629–28636, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Fridovich I Superoxide dismutases. Annu Rev Biochem 44: 147–159, 1975. [DOI] [PubMed] [Google Scholar]
- 26.Gao Q, Wolin MS. Effects of hypoxia on relationships between cytosolic and mitochondrial NAD(P)H redox and superoxide generation in coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol 295: H978–H989, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillette JH, Brodie BB, La Du BN. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther 119: 532–540, 1957. [PubMed] [Google Scholar]
- 28.Gupte SA, Arshad M, Viola S, Kaminski PM, Ungvari Z, Rabbani G, Koller A, Wolin MS. Pentose phosphate pathway coordinates multiple redox-controlled relaxing mechanisms in bovine coronary arteries. Am J Physiol Heart Circ Physiol 285: H2316–H2326, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Gupte SA, Hintze TH, Wolin MS. Hydrogen peroxide derived from Nox-2 mediates protein kinase C-induced contraction of bovine coronary artery and mouse aorta (Abstract). FASEB J 20: A724, 2006. [Google Scholar]
- 30.Gupte SA, Kaminski PM, Floyd B, Agarwal R, Ali N, Ahmad M, Edwards J, Wolin MS. Cytosolic NADPH may regulate differences in basal Nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am J Physiol Heart Circ Physiol 288: H13–H21, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Gupte SA, Okada T, McMurtry IF, Oka M. Role of pentose phosphate pathway-derived NADPH in hypoxic pulmonary vasoconstriction. Pulm Pharmacol Ther 19: 303–309, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Gupte SA, Wolin MS. Hypoxia promotes relaxation of bovine coronary arteries through lowering cytosolic NADPH. Am J Physiol Heart Circ Physiol 290: H2228–H2238, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Gupte SA, Rupawalla T, Phillibert D Jr, Wolin MS. NADPH and heme redox modulate pulmonary artery relaxation and guanylate cyclase activation by NO. Am J Physiol Lung Cell Mol Physiol 277: L1124–L1132, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: The emerging role in signal transduction in vascular cells. Circ Res 99: 924–932, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Iesaki T, Wolin MS. Thiol oxidation activates a novel redox-regulated coronary vasodilator mechanism involving inhibition of Ca2+ influx. Arterioscler Thromb Vasc Biol 20: 2359–2365, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 287: H1495–H1500, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Kontos HA, George E. Brown memorial lecture. Oxygen radicals in cerebral vascular injury. Circ Res 57: 508–516, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res 59: 612–619, 1986. [DOI] [PubMed] [Google Scholar]
- 40.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol 287: R1014–R1030, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology 21: 269–280, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Marshall PJ, Kulmacz RJ, Lands WE. Constraints on prostaglandin biosynthesis in tissues. J Biol Chem 262: 3510–3517, 1987. [PubMed] [Google Scholar]
- 43.McCord JM, Fridovich I. Superoxide dismutase. An enzymatic function for erythrocupfein (hemocuprein). J Biol Chem 244: 6049–6055, 1969. [PubMed] [Google Scholar]
- 44.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res 90: 1307–1315, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Mingone CJ, Ahmad M, Gupte SA, Chow JL, Wolin MS. Heme oxygenase-1 induction depletes heme and attenuates pulmonary artery relaxation and guanylate cyclase activation by nitric oxide. Am J Physiol Heart Circ Physiol 294: H1244–H1250, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mingone CJ, Gupte SA, Ali N, Wolin MS. Thiol oxidation inhibits nitric oxide-mediated pulmonary artery relaxation and guanylate cyclase stimulation. Am J Physiol Lung Cell Mol Physiol 290: L549–L557, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Mingone CJ, Gupte SA, Chow JL, Abraham NG, Wolin MS. Protoporphyrin IX generation from δ-aminolevulinic acid elicits pulmonary artery relaxation and soluble guanylate cyclase activation. Am J Physiol Lung Cell Mol Physiol 291: L337–L344, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Miura H, Wachtel RE, Loberiza FR, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res 92: 151–158, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Mohazzab-H KM, Agarwal R, Wolin MS. Influence of glutathione peroxidase on coronary artery responses to alterations in Po2 and H2O2. Am J Physiol Heart Circ Physiol 276: H235–H241, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Mohazzab-H KM, Fayngersh RP, Kaminski PM, Wolin MS. Potential role of NADH oxidoreductase-derived reactive O2 species in calf pulmonary arterial Po2-elicited responses. Am J Physiol Lung Cell Mol Physiol 269: L637–L644, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Mohazzab-H KM, Fayngersh RP, Wolin MS. Nitric oxide inhibits pulmonary artery catalase and H2O2-associated relaxation. Am J Physiol Heart Circ Physiol 271: H1900–H1906, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Mohazzab-H KM, Kaminski PM, Fayngersh RP, Wolin MS. Oxygen-elicited responses in calf coronary arteries: role of H2O2 production via NADH-derived superoxide. Am J Physiol Heart Circ Physiol 270: H1044–H1053, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Mohazzab-H KM, Wolin MS. Properties of a superoxide anion-generating microsomal NADH oxidoreductase, a potential pulmonary artery Po2 sensor. Am J Physiol Lung Cell Mol Physiol 267: L823–L831, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol 98: 390–403, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Mueller CFH, Laude K, McNally JS, Harrison DG. Redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol 25: 274–278, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Oeckler RA, Acruino E, Wolin MS. Cytosolic NADH redox and thiol oxidation regulate pulmonary arterial force through ERK MAP Kinase. Am J Physiol Lung Cell Mol Physiol 288: L1017–L1025, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res 92: 23–31, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Omar HA, Cherry PD, Mortelliti MP, Burke-Wolin T, Wolin MS. Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and independent nitrovasodilator relaxation. Circ Res 69: 601–608, 1991. [DOI] [PubMed] [Google Scholar]
- 59.Omar HA, Mohazzab-H KM, Mortelliti MP, Wolin MS. O2-dependent modulation of calf pulmonary artery tone by lactate: potential role of H2O2 and cGMP. Am J Physiol Lung Cell Mol Physiol 264: L141–L145, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJA. Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol 291: R491–R511, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, Arun Kumar HSA, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Müller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Müller-Esterl W, Schmidt HHHW. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tate RM, Morris HG, Schroeder WR, Repine JE. Oxygen metabolites stimulate thromboxane production and vasoconstriction in isolated saline-perfused rabbit lungs. J Clin Invest 74: 608–613, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turkseven S, Kruger A, Mingone C, Kaminski P, Inaba M, Rodella IF, Ikehara S, Wolin MS, Abraham NG. The antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol 289: H701–H707, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via a protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253–1258, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic High pressure-induced oxidative stress. Involvement of protein kinase C-dependent NAD(P)H oxidase and local rennin-angiotensin system. Am J Pathol 165: 219–226, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ungvari Z, Wolin MS, Csiszar A. Mechanosensitive production of reactive oxygen species in endothelial and smooth muscle cells: role in microvascular remodeling? Antiox Redox Signal 8: 1121–1129, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Waypa GB, Schumacker PT. Hypoxic pulmonary vasoconstriction: redox events in oxygen sensing. J Appl Physiol 98: 404–414, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Waypa GB, Schumacker PT. Oxygen sensing in hypoxic pulmonary vasoconstriction: using new tools to answer an age-old question. Exp Physiol 93: 133–138, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Winger JA, Derbyshire ER, Marletta MA. Dissociation of nitric oxide from soluble guanylate cyclase and heme-nitric oxide/oxygen binding domain constructs. J Biol Chem 282: 897–907, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Wolin MS Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 20: 1430–1442, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Wolin MS, Abraham NA. Heme oxygenase-1 inhibition of Nox oxidase activation is a microvascular endothelial antioxidant effect of NO. Hypertension 48: 826–827, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Wolin MS, Ahmad M, Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: Basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol 289: L159–L173, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Wolin MS, Rodenburg JM, Messina EJ, Kaley G. Oxygen metabolites and vasodilator mechanisms in rat cremasteric arterioles. Am J Physiol Heart Circ Physiol 252: H1159–H1163, 1987. [DOI] [PubMed] [Google Scholar]
- 76.Wolin MS, Wood KS, Ignarro LJ. Guanylate cyclase from bovine lung. A kinetic analysis of the regulation of the purified soluble enzyme by protoporphyrin IX, heme, and nitrosyl-heme. J Biol Chem 257: 13312–13320, 1982. [PubMed] [Google Scholar]
- 77.Zou MH, Bachschmid M. Hypoxia-reoxygenation triggers coronary vasospasm in isolated bovine coronary arteries via tyrosine nitration of prostacyclin synthase. J Exp Med 190: 135–140, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]