Abstract
The objective of this experiment was to determine the effects on mammary carcinogenesis of similar limitations in energy availability either by energy expenditure due to moderate-intensity running (physical activity, PA) or by regulating dietary energy (RE) intake relative to a sedentary control (SC) group that ate ad libitum. A total of 90 female Sprague-Dawley rats were injected with 1-methyl-1-nitrosourea (50 mg/kg) and 7 days thereafter were randomized to either SC, a PA group given free access to a motorized running wheel, or a RE group whose food intake limited growth to the rate observed in PA. Compared with SC, mammary carcinogenesis was inhibited by RE or PA. Cancer incidence, 92.6%, 77.8%, and 66.7% (P = 0.06), and cancer multiplicity, 3.44, 2.11, and 1.62 cancers/rat (P = 0.006), in SC, RE, and PA, respectively, were reduced to a similar extent by RE and PA. Histological and Western blot analyses of mammary carcinomas provided evidence that RE and PA induced apoptosis via the mitochondrial pathway, that cell cycle progression was suppressed at the G1/S transition, and that intratumoral blood vessel density was reduced, although it remains to be determined whether PA and RE exert these effects via the same mechanisms.
Keywords: energy restriction, physical activity
when energy intake remains higher than energy expenditure over a sufficient period of time, body weight relative to stature will increase and can result in the occurrence of overweight or obesity (35). The number of individuals who are overweight or obese is increasing at an unprecedented rate, and the failure to prevent excessive weight gain that results in these conditions is associated with an increased risk for several types of cancer, including breast cancer (9). The guidance that is generally given to individuals attempting to prevent weight gain and obesity is to limit dietary energy intake and increase physical activity (22).
Because of the limitations inherent in the measurement of both energy intake and physical activity behaviors in human populations over the time course required for cancer to develop (15- to 20-yr latency), the investigation of their individual contributions to the prevalence of cancer and to cancer mortality in epidemiological studies remains problematic. The use of animal models for carcinogenesis and physical activity offers an alternative approach by which to systematically study the effects on cancer of alterations in energy balance by manipulation of either energy intake or energy expenditure (28). For this study, a well-characterized model for breast cancer, induced in the rat by 1-methyl-1-nitrosourea (MNU) (32), and a rodent wheel-running device newly developed by our laboratory were used. PA was accomplished by giving rats access to a motorized activity wheel that was linked, under computer control, to a food pellet dispenser so that wheel-running behavior was maintained by food reward. This permitted the comparative investigation of the effects of wheel running and limited energy intake on experimentally induced mammary carcinogenesis compared with a sedentary control group in which animals were allowed to eat ad libitum. As previously reported, sedentary rats allowed to eat ad libitum model overeating behavior associated with overweight and obesity, whereas animals whose energy availability was limited by physical activity or a regulated dietary energy model the targeted health state of normal weight for height (1, 16–20, 30, 31, 35).
MATERIALS AND METHODS
Chemicals
Primary antibodies used in this study were anti-cyclin D1 and anti-E2F-1 from Thermo Fisher Scientific (Fremont, CA), anti-retinoblastoma (Rb), anti-Bcl-2, anti-hILP/X-linked inhibitor of apoptosis protein (XIAP), and anti-Bax from BD Biosciences (San Diego, CA), anti-apoptosis protease-activating factor 1 (Apaf-1) from Millipore (Billerica, MA), and anti-rabbit immunoglobulin-horseradish peroxidase (HRP)-conjugated secondary antibody, as well as LumiGLO reagent with peroxide, all from Cell Signaling Technology (Beverly, MA). Anti-VEGF, anti-p21Cip1, and anti-mouse immunoglobulin-HRP -conjugated secondary antibody were from Santa Cruz (Santa Cruz, CA). Mouse anti-β-actin primary antibody was obtained from Sigma Chemical (St. Louis, MO). Rabbit anti-Ki-67, clone SP6 was from Labvision (Fremont, CA); goat anti-CD31 was from Santa Cruz Biotechnology. Biotinylated donkey anti-rabbit, donkey anti-goat secondary antibodies, and normal donkey serum were from Jackson Immuno Research (West Grove, PA); HRP-conjugated streptavidin was from Dako (Carpinteria, CA) and stable 3,3′-diaminobenzidine (DAB) from Invitrogen (Carlsbad, CA).
Animals and Physical Activity
Ninety female Sprague Dawley rats were obtained from Taconic Farms (Germantown, NY) at 20 days of age. At 21 days of age, rats were injected with 50 mg MNU/kg body wt ip as previously described (32). Rats were housed individually in solid-bottomed polycarbonate cages. Seven days following carcinogen injection, all rats were randomized into one of three groups, a sedentary control (SC) group, a physically active (PA) group, or a group given a regulated amount of food (RE). Rats were fed AIN-93G pellet diet (Research Diet, Brunswick, NJ).
The animals in the PA group were housed in cages equipped with a running wheel for PA and earned food according to the distance they ran. Each PA rat was paired to a RE rat that was also individually housed in a cage adjacent to the PA rat; pellet dispensers on each pair of cages were linked by cables so that each animal in a pair ate the designated amount of food that maintained comparable growth rates between the paired rats. Rats were weighed daily, and the ratio of food delivered to each RE and PA rat was adjusted using a programmable electronic transducer that linked the pellet dispensers. This instrument overcomes a number of problems that have limited the study of PA and cancer using preclinical models (reviewed in Refs. 7, 27). They include 1) inherent differences among running wheels in the resistance of the wheel to rotate; 2) the variable speed at which animals run in the wheels, thus affecting the intensity of PA; and 3) the well-documented decline in running activity over the time frame of most carcinogenesis experiments. Animal rooms were maintained at 22 ± 1°C with 50% relative humidity and a 12:12-h light-dark cycle. Rats were palpated for detection of mammary tumors twice per week starting from 29 days postcarcinogen. The work reported was reviewed and approved by the Colorado State University Institutional Animal Care and Use Committee and conducted according to the committee guidelines.
Necropsy
Rats were killed over a 3-h time interval via inhalation of gaseous carbon dioxide followed by cervical dislocation. Rats were then skinned, and the skin to which the mammary gland chains were attached was examined under translucent light for detectable mammary pathologies. All grossly detectable mammary gland lesions were excised for histological classification. When size was sufficient, a piece of each tumor was also immediately frozen in liquid nitrogen for the future molecular biological analysis.
Immunohistochemistry
Formalin-fixed paraffin sections were cut at 4 μm, floated onto charged slides, and heat-immobilized at 70°C for 30 min. Slides were deparaffinized in xylene, 3 × 10 min, and hydrated to water using a series of graded ethanols. Slides were immersed in 10 mM citrate buffer, pH 6.0 (Biogenex, San Ramon, CA) and heat-induced epitope retrieval (HIER) performed using a pressure cooker (Biocare Medical, Concord, CA). Following HIER, slides were allowed to cool in citrate buffer for 30 min, and all remaining incubations were carried out at room temperature. Endogenous peroxidase activity was quenched by immersing slides in 3% H2O2 for 5 min. Slides were rinsed in PBS, pH 7.4, and loaded onto an Autostainer (Dako). Normal donkey serum (10%) was applied to all sections for 20 min. Excess serum was drained and primary antibody applied, for 60 min. Negative controls were treated with PBS in lieu of primary antibody. Slides were rinsed in PBS 3× between each of the following reagent applications: target biotinylated secondary antibody, 30 min; HRP-conjugated streptavidin, 30 min; and stable DAB, 10 min. Slides were removed from the Autostainer, rinsed in water, counterstained in dilute hematoxylin for 2 min, dehydrated using a series of graded ethanols, cleared in xylene, and mounted using a synthetic resin.
Image Analysis
Ki-67 stained sections were analyzed using a CAS-200 image analysis system (Bacus Labs, Lombard, IL) as described previously (12). All images were captured at a magnification of 400× using a fixed field of 120 × 80 μm or 9,600 μm2. Ten dots were placed randomly on each slide analyzed using a green marker before review under the microscope to avoid selection bias. Images were captured as close to each dot as possible.
Apoptosis index of images of corresponding hematoxylin- and eosin-stained serial sections were acquired using a Zeiss Axioskop II at a magnification of 400× and evaluated for evidence of apoptosis. Ten random dots were marked on each slide before review under the microscope to avoid selection bias. Images were captured as close to each dot as possible and cropped to a fixed field area encompassing 9,600 μm2, identical to that used for measurement on the CAS system. Apoptotic and normal cells were marked and counted using the manual tag tools in ImagePro Plus 4.5 (Media Cybernetics, Bethesda, MD).
CD31-stained sections were acquired using a Zeiss Axioskop II at a magnification of 100×. Three contiguous microscopic fields were captured and assembled into a single composite TIF image using Adobe Photoshop CS3, version 10.0.1 (Adobe Systems, San Jose, CA). CD31 stained blood vessels were circumscribed and analyzed in ImagePro Plus 4.5 (Media Cybernetics).
Western Blotting
Mammary carcinomas were homogenized in lysis buffer [40 mM Tris·HCl (pH 7.5), 1% Triton X-100, 0.25 M sucrose, 3 mM EGTA, 3 mM EDTA, 50 μM β-mercaptoethanol, 1 mM phenylmethylsulfony fluoride, and complete protease inhibitor cocktail (Calbiochem, San Diego, CA)]. The lysates were centrifuged at 7,500 g for 10 min in a tabletop centrifuge at 4°C, and clear supernatant fractions were collected and stored at −80°C. The protein concentration in the supernatants was determined by the Bio-Rad protein assay (Bio-Rad, Hercules, CA).
Western blotting was performed as described previously (10). Briefly, 40 μg of protein lysate per sample was subjected to 8–16% SDS-PAGE after being denatured by boiling with SDS sample buffer [63 mM Tris·HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mM DTT, and 0.01% bromophenol blue] for 5 min, and the proteins were transferred to a nitrocellulose membrane. The levels of cyclin D1, E2F-1, Rb, p21Cip1, Bcl-2, hILP/XIAP, Bax Apaf-1, VEGF, and β-actin were determined using specific primary antibodies, followed by treatment with the appropriate peroxidase-conjugated secondary antibodies and visualized by LumiGLO reagent Western blotting detection system. The chemiluminescence signal was captured using a ChemiDoc densitometer (Bio-Rad) run under the control of Quantity One software (Bio-Rad) and analyzed by the software. The actin-normalized scanning density data were reported.
Caspase 3 Activity Assay
Caspase 3 activity was determined using EnChek Caspase Assay Kit no. 2 (Invitrogen) following the instruction provided by the company. Briefly, samples were prepared in 1× lysis buffer (pH 7.5, Invitrogen), and 50 μl of the sample was incubated with caspase-3 substrate Z-DEVD-R110 (Invitrogen) at room temperature for 30 min. The caspase 3 activity was quantified by measuring the fluorescence with excitation at 496 nm and emission at 520 nm using 96-well plate reader SpectraMax M5 (Molecular Devices, Sunnyvale, CA). The protein concentration in the sample was determined by the Bio-Rad protein assay (Bio-Rad). The caspase 3 activity was expressed as nanomolar per minute per milligram protein of rhodamine 110, which is a caspase 3-catalyzed product of Z-DEVD-R110 substrate, by measuring the rhodamine 110 as reference standard at the same time.
Statistical Analyses
Differences among groups in the incidence and multiplicity of mammary carcinomas were evaluated, respectively, by chi-square analysis (25) or ANOVA after square root transformation of tumor count as recommended in Ref. 26. Effects on cancer latency were evaluated by survival analysis (2). Differences among groups in the body weight and caspase-3 activity were analyzed by ANOVA (5). For Western blots, representative Western bands were shown in the figures. The data shown in the tables were either the actin-normalized scanning data for proteins involved in cell cycle, apoptosis, or the ratio of the actual scanning units derived from the densitometric analysis of each Western blot for the phosphoproteins involved in cell proliferation. For statistical analyses, the actin-normalized scanning density data obtained from the ChemiDoc scanner using Quantity One (Bio-Rad) or the ratio data from the immunohistochemical and histological analyses for rates of cell proliferation, apoptosis, and angiogenesis were first rank transformed. The ranked data were then subjected to either ANOVA or multivariate ANOVA (14). All analyses were performed using Systat statistical analysis software version 12 (Systat Software, San Jose, CA).
RESULTS
Physical Activity and Growth
A total of 30 sets of rats (one set included one PA, one RE, and one SC rat) were assigned to the study. Of the 30 rats that were randomized to wheel running, 27 sets (90%) were judged to be compliant to running in response to food reward and completed the experiment. The three rats eliminated either refused to run or ran to a limited extent and failed to gain weight during the first week of the study. The rats paired (set) to those three PA rats were also removed from the experiment. The average distance run per day by the 27 PA rats that completed the study was 5,048 ± 430 m. SC rats ate 19% more than RE rats and 12% more than PA; SC rats had an average final body weight 8% higher than either PA or RE (P < 0.001, Table 1).
Table 1.
Effect of physical activity and regulated energy on final body weight and the carcinogenic response in the mammary gland
| Treatment | Carcinogenic Response |
Final Body Weight, g | Total Food Intake, g | ||
|---|---|---|---|---|---|
| Incidence, % | Multiplicity, number per rat | Latency, days | |||
| Sedentary control | 92.6 | 3.44±0.47 | 41 (38, 44) | 215±3.6 | 635±11 |
| Regulated energy | 77.8* | 2.11±0.38* | 46 (43, 49)* | 197±3.5* | 532±8* |
| Physically active | 66.7* | 1.62±0.31* | 45 (42, 47)* | 199±3.5* | 569±10*† |
| Overall P value | 0.06 | 0.006 | 0.022 | <0.001 | <0.001 |
Final body weight values are expressed as means ± SE. Carcinogenic response values are expressed as percentages for cancer incidence, as means ± SE (n =27/group) for cancer multiplicity, and as mean time to first palpable mammary tumor histologically confirmed to be an adenocarcinoma (with 95% confidence intervals) for cancer latency.
P < 0.05 compared with sedentary control.
P < 0.05 compared with regulated energy.
Carcinogenic Response
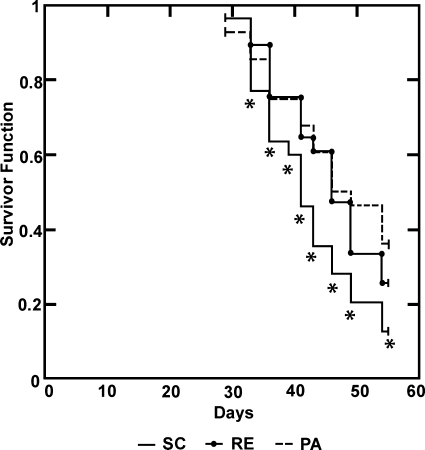
Mammary cancer incidence and multiplicity were reduced to a similar extent by RE and PA. Cancer incidence was 92.6%, 77.8%, and 66.7%, respectively, in SC, RE, and PA (Table 1, P = 0.06). Cancer multiplicity was 3.44, 2.11, and 1.62 cancers/rat, P = 0.006, in SC, RE, and PA, respectively. Cancer latency was prolonged by either RE or PA relative to SC (Table 1 and Fig. 1, P = 0.022).
Fig. 1.
Effect of regulated energy (RE) and physical activity (PA) on the latency to detection of palpable mammary tumors that were subsequently confirmed histologically to be adenocarcinomas relative to sedentary control rats that ate ad libitum (SC). PA and RE prolonged tumor latency relative to SC (trend, P = 0.022). *P < 0.05 at different days postcarcinogen.
Analyses for Cell Proliferation, Apoptosis, and Angiogenesis
Cell proliferation.
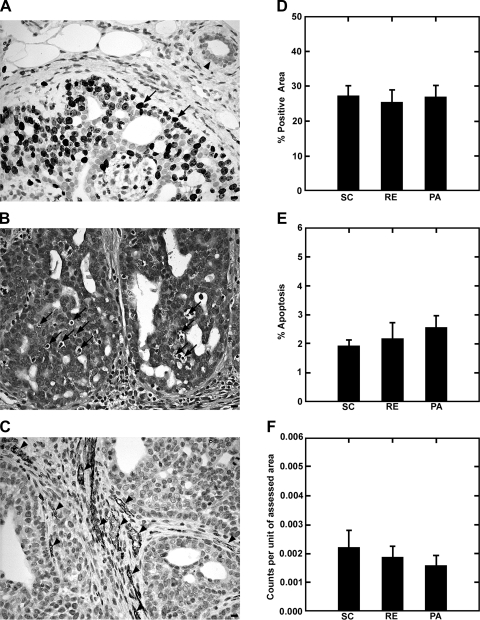
Cell growth fraction was assessed by Ki-67 staining using a random counting method. As shown in Fig. 2, A and D, a small numerical decrease was observed in the growth fraction in RE and PA relative to SC, but the intergroup differences were not statistically significant (P > 0.10). The table included in Fig. 3B provides the average relative absorbance values, normalized to β-actin, as a measurement of the amount of each protein assessed for cell proliferation. Levels of cyclin D1, hypo- and hyperphosphorylated Rb, E2F-1, and p21Cip1 were evaluated. E2F-1 was significantly reduced by PA and RE (P = 0.02). The relative abundance of hypo- to hyperphosphorylated Rb was increased by PA (P = 0.02).
Fig. 2.
Effect of RE and PA on growth fraction, apoptosis, and blood vessel density in mammary carcinomas relative to SC rats. A: growth fraction was determined by Ki-67 staining showing low to no activity in normal mammary duct (arrowhead) and high activity in mammary adenocarcinoma (arrows). B: apoptosis was evaluated by counting apoptotic cells detected in hematoxylin- and eosin-stained sections (arrows). C: angiogenesis was measured by CD31 staining (arrowheads). All digital images were acquired at high magnification (×400); bars, 10 μm. Bar graphs in D (growth fraction), E (apoptosis), and F (angiogenesis) show the patterns observed across treatment groups; n = 9 carcinomas per treatment (each bar in a graph is mean ± SE).
Fig. 3.
Levels of cell cycle regulatory proteins in mammary carcinomas from SC, RE, and PA rats: cyclin D1, E2F-1, retinoblastoma (Rb), and p21Cip1. Representative Western blot images (A) and table (B) for the levels of proteins (relative absorbance unit × 104) or the ratio of hyperphosphorylated Rb (ppRb) to Rb (ppRb/Rb) are shown as means ± SE; n = 7 per treatment group. Compared with SC: *P < 0.05. Compared with RE: †P < 0.05. pRb, hypophosphorylated Rb. [Note added in proof: Figure 3A is a digital composite of representative bands from a series of gels. In each case, the analytes represented by each lane were assessed in a single lysate, and equal loading was confirmed by assessment of actin (same is true for Fig. 4A).]
Apoptosis.
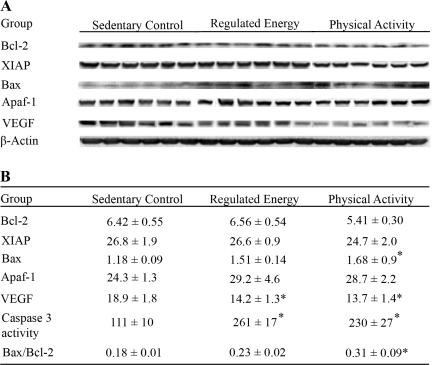
Figure 2, B and E, shows the data obtained for apoptosis index using the original method and criteria developed by Kerr et al. (11) for its detection. A pattern of increased apoptosis in either PA or RE relative to SC was observed, although the magnitude of the difference was not statistically significant. Figure 4B shows that the activity of caspase 3, which was determined by enzymatic reaction, was increased approximately twofold by PA and RE (P < 0.001). Western blots for proteins involved in apoptosis are also shown in Fig. 4. Mammary carcinomas from PA or RE rats had lower levels of Bcl-2 and XIAP and higher levels of Bax and Apaf than found in carcinomas from SC rats, and the ratio of Bax to Bcl-2 was increased (P < 0.01). This pattern of protein expression is consistent with PA and RE inducing a proapoptotic environment (Hotelling-Lawley multivariate statistic, P = 0.01). Univariate statistics from the multivariate analysis indicated that the effects of PA and RE on Bax (P < 0.001) were significant.
Fig. 4.
Levels of proteins involved in the regulation of apoptosis in mammary carcinomas from SC, RE, and PA rats: Bcl-2, human X-linked inhibitor of apoptosis protein (XIAP), Bax and apoptosis protease-activating factor 1 (Apaf-1). Representative Western blot images (A) and table (B) for the levels of proteins (relative absorbance unit × 104) are shown as means ± SE; n = 7 per treatment group. Data also shown for caspase 3 activity as an alternative indicator of apoptosis and VEGF as a marker for angiogenesis. The caspase 3 activity was expressed as nM·min−1·mg protein−1 of rhodamine 110, which is a caspase 3-catalyzed product of Z-DEVD-R110 substrate, by measuring the rhodamine 110 as reference standard at the same time. The ratio of Bax to Bcl-2 was calculated. Compared with SC: *P < 0.05. [Note added in proof: Figure 4A is a digital composite of representative bands from a series of gels. In each case, the analytes represented by each lane were assessed in a single lysate, and equal loading was confirmed by assessment of actin (same is true for Fig. 3A).]
Angiogenesis.
Figure 2, C and F, shows the CD31 staining used to detect the presence of blood vessels within the mammary carcinomas using the approach previously published by our laboratory (13). As shown in Fig. 2F and detailed in Table 2, blood vessel density was reduced in PA or RE vs. SC for all vessels greater than category 1. While the multivariate statistic for the overall effect of PA and RE on tumor vascularization was not significant, the univariate statistics for vessel size categories above 25 μm2 were P < 0.05. Western blots for VEGF are also shown in Fig. 4. VEGF was reduced in PA and RE relative to SC (P = 0.04).
Table 2.
Effect of physical activity and regulated energy on intratumoral vascular density
| Vessel Size Category, μm2 | Unit of Measure* | Treatment Group |
||
|---|---|---|---|---|
| SC | RE | PA | ||
| Cat. 1: ≤10 | Count | 14.4±25.3 (4.0, 7.0, 10.0) | 15.4±18.5 (3.0, 5.0, 24.0) | 10.8±12.1 (2.0, 5.0, 15.0) |
| Cat. 2: >10 and ≤25 | Count | 33.6±20.1 (22.0, 35.0, 42.0) | 36.8±26.8 (16.0, 33.0, 39.0) | 31.0±22.2 (7.0, 30.0, 51.0) |
| Cat. 3: >25 and ≤50 | Count | 33.1±13.73 (31.0, 38.0, 42.0) | 24.0±13.4 (11.0, 26.0, 29.0) | 18.7±8.2 (9.0, 19.0, 25.0) |
| Cat. 4: >50 and ≤75 | Count | 12.3±5.1 (11.0, 14.0, 17.0) | 7.8±6.6 (3.0, 7.0, 10.0) | 6.6±5.9 (4.0, 5.0, 8.0) |
| Cat. 5: >75 | Count | 20.8±14.2 (12.0, 17.0, 28.0) | 13.1±8.8 (5.0, 15.0, 17.0) | 12.4±8.8 (3.0, 14.0, 21.0) |
| Total | Count | 114.2±59.8 (87.0, 118.0, 125.0) | 97.1±52.7 (63.0, 88.0, 125.0) | 79.4±39.5 (69.0, 75.0, 89.0) |
| Cat. 1: ≤10 | Area | 99.3±167.0 (30.6, 51.9, 73.9) | 107.8±121.6 (28.3, 34.0, 176.1) | 80.4±83.1 (14.3, 44.3, 119.3) |
| Cat. 2: >10 and ≤25 | Area | 591.4±331.3 (413.0, 667.2, 737.1) | 610.8±418.8 (318.6, 538.2, 667.2) | 502.1±351.1 (129.4, 443.9, 845.5) |
| Cat. 3: >25 and ≤50 | Area | 1,164.0±491.9 (1,021.3, 1,442.1, 1672.6) | 846.3±475.7 (391.2, 866.3, 1,129.6) | 659.6±293.1 (325.6, 683.4, 894.3) |
| Cat. 4: >50 and ≤75 | Area | 743.7±313.6 (641.5, 858.1, 1,017.8) | 466.5±402.5 (179.8, 410.9, 585.6) | 398.8±355.4 (241.5, 311.6, 485.1) |
| Cat. 5: >75 | Area | 4,623.2±3,650.4 (1,332.8, 3,741.3, 10,345.3) | 3,763.0±3,606.3 (955.1, 2,687.5, 6,705.3) | 3,164.7±3,011.9 (403.4, 2,817.8, 4,588.3) |
| Total | Area | 7,221.6±4,184.9 (4,700.3, 7,030.0, 10,589.8) | 5,794.3±4,369.4 (1,725.0, 4,076.0, 9,872.5) | 4,805.6±3,307.4 (2,030.9, 4,172.2, 6,848.3) |
Values are means ± SD.
Count data are average number of blood vessels/mm2; area data are average area in μm2/mm2. Nos. in parentheses are (25th percentile, median, 75th percentile); n = 9 carcinomas in each treatment group. SC, sedentary control group; RE, regulated energy group; PA, physically active group; Cat. 1–Cat. 5, categories 1–5. Total density of intratumoral blood vessels was reduced by PA (P = 0.03) or RE (P = 0.09). The reduction was significant for vessel size categories above 25 μm2, P < 0.05.
DISCUSSION
While the most common guidance given for maintenance of an appropriate body weight for height is to be physically active and to limit food intake (22), little is known about the effects on the carcinogenic process of limiting weight gain by physical activity-associated energy expenditure vs. reduced caloric intake. For the study reported herein, a newly developed motor-driven rodent activity wheel designed and built in our laboratory was used. The motorized wheel permitted PA intensity to be held constant while PA duration was self-determined by each rat. This PA model reinforced running behavior by delivering food via a pellet dispenser and also limited the food intake of another animal paired to each PA rat. As shown in Table 1 and Fig. 1, limitation in energy availability by RE or PA reduced mammary cancer incidence and multiplicity and prolonged cancer latency relative to SC rats that ate ad libitum. At the gross level, all of the animals in this experiment were in positive energy balance, meaning that they all grew without weight loss. The SC rats ate 12 and 19% more than PA or RE rats, respectively, and SC had body weights that were 8% higher than either PA or RA. Given that our previous work indicates that SC rats are likely to have higher body fat content than RE or PA (3), it will be important to determine if body composition plays a role in explaining the observed effects on the carcinogenic response. However, emerging evidence from mice that lack white adipose tissue indicates that body fat per se is unlikely to be an obligatory factor in accounting for differences in the carcinogenic response associated with body composition (8, 15). Relative to whether PA and RE exerted quantitatively similar effects on mammary carcinogenesis [cancer incidence differed by 11.1% (66.7% vs. 77.8%) and cancer multiplicity by 0.49 cancers/rat (1.62 vs. 2.11 cancers/rat), respectively, in PA vs. RE], the data presented herein can only be interpreted as indicating that PA and RE had comparable effects. However, additional work is needed to investigate these approaches to weight control more fully. While the differences observed may in fact be due to expected random variation in the carcinogenic response among treatment groups, PA and RE resulting in the same growth rate are likely to exert dissimilar effects on energy metabolism, inflammation, and muscle metabolism that have the potential to exert differential effects on the carcinogenic response.
There are a number of reasons that a difference in the carcinogenic response could be exerted by PA vs. RE. Other than effects on body composition, PA induces a greater flux of energy through the system than RE, which may induce differences in redox-sensitive cell signaling via reactive oxygen species generated during oxidative phosphorylation. On the other hand, RE reduces circulating glucose and mitochondrial oxidative phosphorylation, both of which could influence the metabolic reprogramming associated with carcinogenesis. PA, depending on its type, intensity, duration, and frequency, induces either pro- or anti-inflammatory responses, both of which have the potential to affect the carcinogenic process in a number of organ sites, whereas reduced energy intake is associated with suppression of inflammation via increasing corticosterone and other hormones. Relative to whether PA could exert effects on the carcinogenic process that are related to bodily movement per se, i.e., to muscle contraction, is consistent with an experiment reported over three decades ago indicating that the metabolism of contracting skeletal muscle produces a factor(s) that inhibits tumor cell growth throughout the initiated and progressed carcinogenesis (6). Moreover, emerging evidence indicates that contracting muscle releases cytokines, referred to as myokines, that have endocrine activity (21); this finding has important implications in that it suggests that skeletal muscle represents the largest endocrine organ in the body.
Tissue Size Homeostasis
Given that tumors arise due to the misregulation of cell proliferation, apoptosis, and angiogenesis (4), and that our studies of dietary energy restriction on carcinogenesis imply that these processes are likely to be affected (29, 33, 34), mammary carcinomas from each experimental group were analyzed.
Cell Proliferation
The expression of the human Ki-67 protein is strictly associated with cell proliferation. The fact that the Ki-67 protein is present during all active phases of the cell cycle (G1, S, G2, and mitosis) but is absent from resting cells (G0) makes it an excellent marker for determining the so-called growth fraction of a given cell population(23). As shown in Fig. 2, there was no evidence to indicate that PA or RE affected growth fraction in the mammary carcinomas assessed, i.e., the proportion of cells in G0, a finding that is consistent with an earlier report on the effects of dietary energy restriction in mammary carcinomas (10). However, when the data in Fig. 3 were used to determine the ratio of hypophosphorylated to hyperphosphorylated Rb, the ratio was increased by PA (P = 0.02). This implies that PA suppressed the rate of passage of cells from G1 to S even though the growth fraction was not affected. The fact that levels of E2F-1 were reduced by PA and RE also is consistent with the Rb data in that E2F-1 is normally bound to hypophosphorylated Rb and is released when Rb is progressively phosphorylated by cyclin-dependent kinases 2 and 4. Free E2F-1 is a critical transcriptional factor that promotes the expression of genes involved in the G1/S transition and the synthesis of DNA (24).
Apoptosis
The rate of apoptosis was measured histologically using the criteria initially used by Kerr et al. (11). Recognizing that apoptosis is a very rapid process and that small differences in apoptotic rate can have a major impact on tumor growth, the effect reflected in gross morphological evidence of apoptosis, while not statistically significant (Fig. 2), was judged to merit further analysis using more sensitive molecular techniques. As shown in Fig. 4, the activity of the caspase 3, which is an executioner caspase, was about twofold higher in PA or RE relative to SC. Further investigation of Bcl-2 family members indicated that at least a component of the proapoptotic effect was being mediated by the intrinsic or mitochondrial pathway involved in cell death induction. Additional work is necessary to determine if the receptor-mediated pathway might also be involved. Moreover, given the increasing evidence that changes in intracellular energy availability may induce autophagy and that IL-6, a myokine that is released by contracting muscle, can play a role in activating autophagy, and that autophagy can prompt apoptosis, our observations provide evidence that additional mechanistic studies of cell death induction by PA and RE are warranted.
Angiogenesis
As shown in Fig. 2 and Table 2, vascular density was lower in mammary carcinomas from either PA or RE rats relative to SC. While the effects on small blood vessels (categories 1 and 2) did not reach the level of statistical significance, density of large vessels was reduced, and overall both the number of blood vessels and blood vessel area were lower in PA or RE. These findings are consistent with the reduced level of VEGF, determined by Western blotting, in either PA or RE. These data provide a rationale for additional studies that elucidate the mechanism(s) through which PA and RE downregulate blood vessel growth since it is well recognized that at least in muscle, PA is expected to exert proangiogenic effects.
Summary
A reduction in energy availability relative to sedentary control animals that ate ad libitum, whether due to PA-associated energy expenditure or reduced food intake, significantly inhibited the postinitiation phase of mammary carcinogenesis. In the tumors that did occur, evidence was obtained indicating that PA and RE suppressed passage of transformed cells through the G1/S stage of the cell cycle, induced apoptosis resulting in activation of caspase 3 via the mitochondrial pathway of cell death induction, and decreased tissue levels of VEGF and blood vessel density. For each of these cellular processes, additional studies are needed to define specific signaling events responsible for these effects. Those studies should be directed, at least initially, to signal transduction pathways that been shown to have the capacity to exert coordinated effects on cell cycle transit, cell death induction, and blood vessel formation and maintenance.
NOTE ADDED IN PROOF
Figures 3A and 4A are digital composites of representative bands from a series of gels. In each case, the analytes represented by each lane were assessed in a single lysate, and equal loading was confirmed by assessment of actin.
GRANTS
This work was supported by U.S. Public Health Services Grants CA-100693 and U54-CA-116847 from the National Cancer Institute.
Supplementary Material
Acknowledgments
We thank Nicholas Fernandez, Vanessa Fitzgerald, Elizabeth Neil, Andre Powell, Jennifer Price, Denise Rush, Jennifer Sells, and Jay Waterman for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Boissonneault GA, Elson CE, Pariza MW. Dietary fat and neoplasia—the role of net energy in enhancement of carcinogenesis: effects of fat and calories on the immune system. Adv Exp Med Biol 206: 85–98, 1986. [DOI] [PubMed] [Google Scholar]
- 2.Collett D Modelling Survival Data in Medical Research. London: Chapman & Hall, 1994.
- 3.Gillette CA, Zhu Z, Westerlind KC, Melby CL, Wolfe P, Thompson HJ. Energy availability and mammary carcinogenesis: effects of calorie restriction and exercise. Carcinogenesis 18: 1183–1188, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg Y, Tamhane AC. Multiple Comparison Procedures. New York: Wiley, 1987.
- 6.Hoffman SA, Paschkis KE, Debias DA, Cantarow A, Williams TL. The influence of exercise on the growth of transplanted rat tumors. Cancer Res 22: 597–599, 1962. [PubMed] [Google Scholar]
- 7.Hoffman-Goetz L Physical activity and cancer prevention: animal-tumor models. Med Sci Sports Exerc 35: 1828–1833, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Hursting SD, Nunez NP, Varticovski L, Vinson C. The obesity-cancer link: lessons learned from a fatless mouse. Cancer Res 67: 2391–2393, 2007. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer/World Health Organization. Weight Control and Physical Activity. IARC Handbooks of Cancer Prevention, vol. 6. Lyon: IARC, 2002.
- 10.Jiang W, Zhu Z, Thompson HJ. Effect of energy restriction on cell cycle machinery in 1-methyl-1-nitrosourea-induced mammary carcinomas in rats. Cancer Res 63: 1228–1234, 2003. [PubMed] [Google Scholar]
- 11.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26: 239–257, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGinley JN, Knott KK, Thompson HJ. Effect of fixation and epitope retrieval on BrdU indices in mammary carcinomas. J Histochem Cytochem 48: 355–362, 2000. [DOI] [PubMed] [Google Scholar]
- 13.McGinley JN, Knott KK, Thompson HJ. Semi-automated method of quantifying vasculature of 1-methyl-1-nitrosourea-induced rat mammary carcinomas using immunohistochemical detection. J Histochem Cytochem 50: 213–222, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Morrison DF Multivariate Statistical Methods. New York: McGraw-Hill, 1990.
- 15.Nunez NP, Oh WJ, Rozenberg J, Perella C, Anver M, Barrett JC, Perkins SN, Berrigan D, Moitra J, Varticovski L, Hursting SD, Vinson C. Accelerated tumor formation in a fatless mouse with type 2 diabetes and inflammation. Cancer Res 66: 5469–5476, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Pariza MW Calorie restriction, ad libitum feeding, and cancer. Proc Soc Exp Biol Med 183: 293–298, 1986. [PubMed] [Google Scholar]
- 17.Pariza MW Calories and energy expenditure in carcinogenesis. Bol Asoc Med PR 78: 456–458, 1986. [PubMed] [Google Scholar]
- 18.Pariza MW Dietary fat, calorie restriction, ad libitum feeding, and cancer risk. Nutr Rev 45: 1–7, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Pariza MW Fat, calories, and mammary carcinogenesis: net energy effects. Am J Clin Nutr 45: 261–263, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Pariza MW, Boutwell RK. Historical perspective: calories and energy expenditure in carcinogenesis. Am J Clin Nutr 45: 151–156, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen BK, Akerstrom TCA, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol 103: 1093–1098, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Dept. of Health and Human Services, 2008. [DOI] [PubMed]
- 23.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311–322, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev 18: 2699–2711, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Snedecor GW, Cochran WG. Statistical Methods. Ames, IA: Iowa State Univ. Press, 1989.
- 26.Sokal RR, Rohlf FJ. Biometry. The Principles and Practice of Statistics in Biological Research. New York: Freeman, 1995.
- 27.Thompson HJ Effects of physical activity and exercise on experimentally-induced mammary carcinogenesis. Breast Cancer Res Treat 46: 135–141, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Thompson HJ Pre-clinical investigations of physical activity and cancer: a brief review and analysis. Carcinogenesis 27: 1946–1949, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Thompson HJ, Jiang W, Zhu Z. Mechanisms by which energy restriction inhibits carcinogenesis. Adv Exp Med Biol 470: 77–84, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Thompson HJ, Jiang W, Zhu Z. Mechanisms associating obesity with cancer incidence: animal models. In: Cancer Prevention and Management through Exercise and Weight Control, edited by McTiernan A. Boca Raton, FL: CRC Taylor and Francis, 2006, p. 329–340.
- 31.Thompson HJ, Jiang W, Zhu Z. Obesity as a cancer risk factor: potential mechanisms of action. In: Nutrition and Cancer Prevention, edited by Awad AB and Bradford PG. Boca Raton, FL: CRC Taylor and Francis, 2006, p. 565–578.
- 32.Thompson HJ, McGinley JN, Rothhammer K, Singh M. Rapid induction of mammary intraductal proliferations, ductal carcinoma in situ and carcinomas by the injection of sexually immature female rats with 1-methyl-1-nitrosourea. Carcinogenesis 16: 2407–2411, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Thompson HJ, McGinley JN, Wolfe P, Spoelstra NS, Knott KK. Targeting angiogenesis for mammary cancer prevention: factors to consider in experimental design and analysis. Cancer Epidemiol Biomarkers Prev 13: 1173–1184, 2004. [PubMed] [Google Scholar]
- 34.Thompson HJ, Strange R, Schedin PJ. Apoptosis in the genesis and prevention of cancer. Cancer Epidemiol Biomarkers Prev 1: 597–602, 1992. [PubMed] [Google Scholar]
- 35.Thompson HJ, Zhu Z, Jiang W. Weight control and breast cancer prevention: are the effects of reduced energy intake equivalent to those of increased energy expenditure? J Nutr 134: 3407S–3411S, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.